Abstract
The lncRNA AFAP1-AS1, oriented from an antisense direction to the protein-coding gene AFAP1 in the opposite strand, was upregulated in a variety of tumors and associated with poor prognosis, including lung cancer, breast cancer, ovarian cancer, and so on. However, the biological role of AFAP1-AS1 in clear cell renal cell carcinoma (ccRCC) is still unknown. We observed that AFAP1-AS1 expression was significantly upregulated in ccRCC tissues and that patients with high-level expression of AFAP1-AS1 had a shorter overall survival. Knockdown of AFAP1-AS1 markedly suppressed the progression of proliferation, invasion, migration, and EMT in ccRCC cells. Downregulation of AFAP1-AS1 resulted in an increase in E-cadherin and a decrease in vimentin. Noticeably, we found that PTEN has a negative correlation with the lncRNA AFAP1-AS1 expression. Further studies verified that PTEN deficiency effectively attenuated the ability of AFAP1-AS1 in promoting ccRCC cell proliferation, invasion, migration, and EMT. Moreover, the similar biological response of silencing AFAP1-AS1 was observed in our ccRCC mice model. Knockdown of AFAP1-AS1 evidently suppressed tumor growth. Taken together, our results provide the evidences that silencing of AFAP1-AS1 inhibits cell proliferation, EMT, and metastasis through PTEN-dependent signaling, and our findings elucidate a novel potential therapeutic target or biomarker for the treatment of ccRCC.
Key words: Long noncoding RNAs (lncRNAs), AFAP1-AS1, Clear cell renal cell carcinoma (ccRCC), Metastasis, Phosphatase and tensin homolog (PTEN)
INTRODUCTION
Renal cell carcinoma (RCC) ranks among the top 10 lists of the most frequent neoplasms in Western countries, with over 63,000 estimated new cases in the US in 20171. Clear cell renal cell carcinoma (ccRCC) is the most common histological subtype, accounting for more than 70% of RCC2. Due to resistance to traditional chemo- and radiotherapy, after the initial radical surgery, advanced ccRCC has a poor survival rate and may result in metastasis or recurrence3–5. Moreover, the lack of specific prognostic biomarker prevents the development of specific therapy6. Thus, it is urgent to discover a novel biomarker/therapeutic target for the prediction and treatment of ccRCC.
The long noncoding RNA (lncRNA) AFAP1-AS1 (actin filament-associated protein 1 antisense RNA) is oriented from an antisense direction to the protein-coding gene AFAP1 in the opposite strand. AFAP1-AS1, as an oncogene, was upregulated in a variety of tumors, including hepatocellular carcinoma, esophageal carcinoma, pancreatic ductal adenocarcinoma, colorectal cancer, cholangiocarcinoma, gallbladder cancer, and so on7–12. AFAP1-AS1 is upregulated in hepatocellular carcinoma, and its higher expression is associated with tumor size, TNM stage, vascular invasion, and poor prognosis. Silencing AFAP1-AS1 significantly reduces cell proliferation, clonal growth, cell migration, and invasion in hepatocellular carcinoma and gastric cancer8,10. However, the expression and precise biological role of AFAP1-AS1 in ccRCC is still unknown. Thus, we evaluate the expression of AFAP1-AS1 in ccRCC tissues and investigate the potential biological function of AFAP1-AS1 on cell growth and metastasis in our in vitro and in vivo ccRCC model.
MATERIALS AND METHODS
Human Tissue Samples
Patients with ccRCC who were diagnosed, treated, and followed up at the Liaoning Cancer Hospital and Institute were included in the study. This study was approved by the Board of China Medical University, and written informed consent was obtained from all patients. All protocols were reviewed by the Joint Ethics Committee of China Medical University Health Authority and performed following national guidelines. Tissue samples were collected at the time of surgery, immediately frozen in liquid nitrogen, and stored until total RNA or proteins were extracted.
Cell Lines and Cell Culture
Human kidney epithelial cell HK2 and ccRCC cell lines, including 786-O, Caki-1, ACHN, and A498 cells, were maintained in RPMI-1640 medium (Gibco, Waltham, MA, USA), supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2.
Vector Construction and Cell Transfection
As previously described13, cell transfection was performed using Lipofectamine 2000 (Invitrogen-Life Technologies, Carlsbad, CA, USA). For screening, puromycin (10 μg/ml) was added to the medium 72 h after transfection. The medium was changed every 2 days for 2–3 weeks. Caki-1 and ACHN cells with high-level endogenous AFAP1-AS1 were selected for silencing. The expression of AFAP1-AS1 was measured by quantitative real-time (qRT)-PCR. The sequence of AFAP1-AS1 shRNA and scrambled control shRNA were as follows: shAF, 5′-CCG GAG CGG T CT CAG CCG AAT GAC TCT CGA GAG TCA TTC GGC TGA GAC CGC TTT TTTG-3′ (forward) and 5′-AAT TCA AAA AAG CGG TCT CAG CCG AAT GAC TCT CGA GAG TCA TTC GGC TGA GAC CGC T-3’ (reverse); control shRNA (shNC), 5′-CCG GTT TCT CCG AAC GTG TCA CGT CTC GAG ACG TGA CAC GTT CGG AGA ATT TTTG-3′ (forward) and 5′-AAT TCA AAG TTC TCG AAC GTG TCA CGT CTC GAG ACG TGA CAC GTT CGG AGAA-3′ (reverse).
Cell Proliferation Assay
Cell viability was determined using CCK-8 assay. Briefly, 1,000 cells/well were seeded into 96-well plates. According to the manufacturer’s instruction, the absorptions of the cells will be measured using a CCK-8 kit (Beyotime Institute of Biotechnology, Jiangsu, P.R. China) at 0, 24, 48, and 72 h. Data were from at least three separate experiments.
Cell Migration and Invasion Assay
Twenty-four-well insert Transwell chambers (8.0 μm; Corning, Corning, NY, USA) were used for cell migration and invasion assays. For migration assay, 2 × 104 cells were added on top of the Transwell membrane in the upper chamber. For the invasion assay, 5 × 104 cells were added on top of the Transwell membrane in the upper chamber coated with Matrigel (BD Biosciences, San Jose, CA, USA). Complete medium was added to the bottom wells as a stimulation of migration or invasion. The cells were then incubated for 48 h at 37°C, and the cells on the upper surface were washed away, while the cells on the bottom surface were fixed with 20% methanol and stained with 0.1% crystal violet. The number of invaded cells was counted in five randomly selected fields using a microscope.
Quantitative Real-Time-PCR Analysis
As previously described14, the sequences of primers were as follows: AFAP-AS1: 5′-TCG CTC AAT GGA GTG ACG GCA-3′, 5′-CGG CTG AGA CCG CTGA GAA CTT-3′; PTEN: 5′-TTT GAA GAC CAT AAC CCA CCAC-3′, 5′-ATT ACA CCA GTT CGT CCC TTTC-3′; and GAPDH: 5′-CAC CCA CTC CTC CAC CTT TG-3′, 5′-CCA CCA CCC TGT TGC TGT AG-3′ (normalized as control). Results were expressed as the threshold cycle (Ct). The 2−ΔΔCt method was used to analyze the relative changes in gene expression. Control experiments without reverse transcription were performed to confirm that the total RNA was not contaminated with genomic DNA.
Western Blot Analysis
Western blot analysis for specific protein expression was performed as previously described15. Antibodies against PTEN (#9188), AKT (#9272), p-AKT (#9271), proliferating cell nuclear antigen (PCNA) (#8580), E-cadherin (#3195), vimentin (#5741), and β-actin (#8457) were purchased from Cell Signaling Technology (Beverly, MA, USA). After incubation, the membranes were washed three times in TBST, followed by incubation with HRP-conjugated secondary antibodies for 1 h at room temperature, and washed extensively before detection. The membranes were subsequently developed using ECL (FujiFilm, Tokyo, Japan) reagent (Applygen Technologies Inc., Beijing, P.R. China) and exposed to film according to the manufacturer’s protocol. Quantitation of signal intensities was performed by densitometry on a scanner using ImageJ software.
Xenograft Mice Model
All the animal procedures were performed in accordance with local institutional ethical guidelines. Ethical approval was obtained from the Experimental Animal Ethics Committee of the China Medical University Health Authority. Caki-1 cells (1 × 107) with stably transfected shAF or shNC were suspended in 0.1 ml of PBS and injected into the left flank of 4-week-old female BALB/c athymic nude mice, six mice for each group. Tumor size (length and width in mm) was measured by calipers every week after the injection, and tumor volumes were calculated using the following formula: tumor volume (mm3) = 1/2(length × width2)16,17. At 21 days, mice were sacrificed, and tumor volume and weight were recorded.
Statistical Analysis
All experimental data are presented as mean ± SD. Survival rate was analyzed using the Kaplan–Meier method. Pearson test was used for correlation analysis. Statistical analysis of the data was performed using one-way ANOVA by SPSS 13.0 software. A value of p < 0.05 was considered statistically significant.
RESULTS
AFAP1-AS1 Is Upregulated in ccRCC
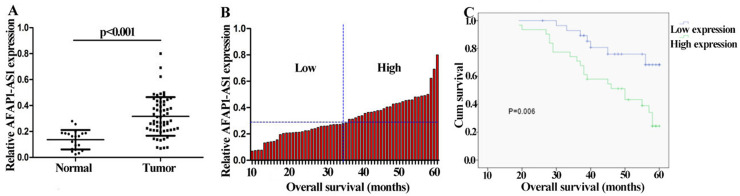
We first evaluated the relative expression of AFAP1-AS1 in ccRCC tissues (n = 60) compared with that of the corresponding adjacent normal tissues (n = 20) using qRT-PCR. As seen in Figure 1A, AFAP1-AS1 expression was significantly increased in ccRCC samples compared with that in the adjacent normal tissues (p < 0.001). According to AFAP1-AS1 expression (median value), ccRCC samples were classified into high and low level of AFAP1-AS1 (Fig. 1B and Table 1), and Kaplan–Meier plotter was performed. The Kaplan–Meier curve is shown in Figure 1C. The expression level of AFAP1-AS1 is positively correlated with ccRCC progression (p = 0.006); patients with a high-level expression of AFAP1-AS1 had a shorter overall survival. Noticeably, the expression level of AFAP1-AS1 is positively correlated with the TNM stage and lymph node metastasis (p < 0.05).
Figure 1.
Actin filament-associated protein 1 antisense RNA (AFAP1-AS1) is upregulated in patients with clear cell renal cell carcinoma (ccRCC). (A) Comparison of AFAP1-AS1 relative expression level in ccRCC tissues (n = 60) with that of adjacent normal tissues (n = 20). AFAP1-AS1 expression was determined using quantitative real-time (qRT)-PCR and normalized to GAPDH (p < 0.001). (B) According to median expression level AFAP1-AS1, high and low level of AFAP1-AS1 was analyzed by Pearson test. (C) Kaplan–Meier curve was performed using overall survival and AFAP1-AS1 expression in ccRCC patient. The survival time of patients with low AFAP1-AS1 expression was longer than that in patients with high AFAP1-AS1 expression.
Table 1.
Association of lncRNA AFAP1-AS1 Expression With Clinicopathologic Features in Clear Cell Renal Cell Carcinoma (ccRCC) Patients
| Parameter | Case | AFAP1-AS1 Expression | p Value | |
|---|---|---|---|---|
| Low | High | |||
| Gender | 0.599 | |||
| Female | 31 | 16 | 15 | |
| Male | 29 | 13 | 16 | |
| Age (years) | 0.951 | |||
| ≤55 | 37 | 18 | 19 | |
| >55 | 23 | 13 | 10 | |
| Tumor size (cm) | 0.297 | |||
| ≤7 | 31 | 17 | 14 | |
| >7 | 29 | 8 | 21 | |
| TNM stage | 0.008 | |||
| I–II | 35 | 22 | 13 | |
| III–IV | 25 | 7 | 18 | |
| Lymph node metastasis | 0.009 | |||
| No | 33 | 21 | 12 | |
| Yes | 27 | 8 | 19 | |
| Smoker | 0.586 | |||
| No | 33 | 17 | 16 | |
| Yes | 27 | 12 | 15 | |
Effect of AFAP1-AS1 on Growth of ccRCC Cells
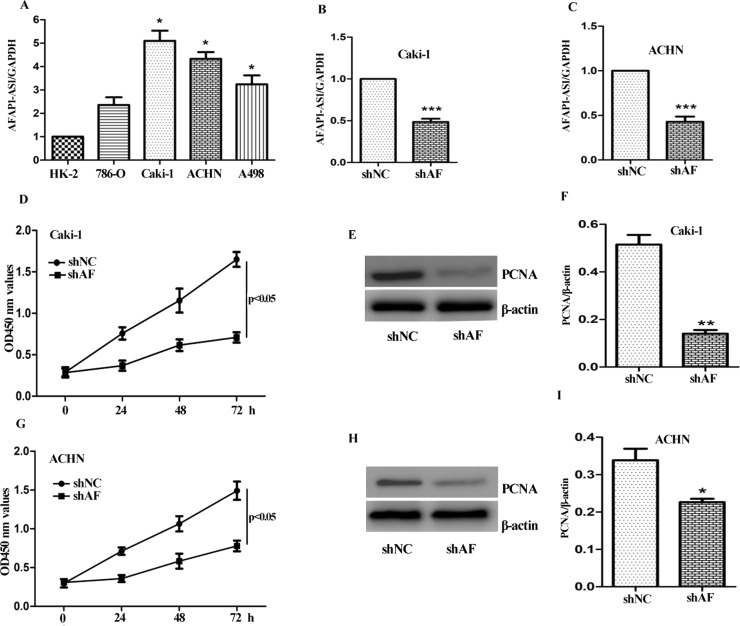
First, the expression level of AFAP1-AS1 in ccRCC cell panel, including 786-O, Caki-1, ACHN, and A498 cells was detected using qRT-PCR. Meanwhile, HK2 cells (normal human kidney epithelial cells) were used as control. As shown in Figure 2A, AFAP1-AS1 was detectable in all cell lines. Noticeably, the expression level of AFAP1-AS1 in ccRCC cells was significantly higher than that in HK2 cells. Caki-1 and ACHN exhibited high-level expression of AFAP1-AS1.
Figure 2.
Effect of AFAP1-AS1 on growth of ccRCC cells. (A) The expression level of AFAP1-AS1 in ccRCC cells was much higher than that in normal kidney epithelial HK-2 cells. (B, C) Knockdown of AFAP1-AS1 expression using specific shRNA. ShAF dramatically suppressed AFAP1-AS1 expression in Caki-1 and ACHN cells, which was determined by qRT-PCR compared with the control shRNA (shNC). (D, G) Caki-1 and ACHN cells transfected with shAF and shNC for 24, 48, and 72 h, cell growth was determined using CCK-8 assay. Effect of knockdown of AFAP1-AS1 on proliferating cell nuclear antigen (PCNA) expression was measured by Western blot analysis. Representative blots in Caki-1 (E, F) and ACHN (H, I) cells are shown. The data are shown as mean ± SD of three separate times. Significant difference between the shNC and shAF group was compared, *p < 0.05; **p < 0.01; ***p < 0.001.
Next, specific shRNA targeting AFAP1-AS1 was designed. As seen in Figure 2B and C, the expression level of AFAP1-AS1 in Caki-1 and ACHN cells was markedly decreased (∼50%) compared to the control group (shNC). Furthermore, the cell viability was measured by CCK-8 assay. After transfection with shAF for 24, 48, and 72 h, knockdown of AFAP1-AS1 significantly suppressed the growth of Caki-1 and ACHN cells (p < 0.05) (Fig. 2D and G). PCNA is a cell proliferation biomarker used in ccRCC. The effect of shAF on the expression of PCNA was also measured. As shown in Figure 2E and H, the expression level of PCNA was markedly decreased in the shAF group.
Effect of AFAP1-AS1 on Invasion, Migration, and EMT of ccRCC Cells
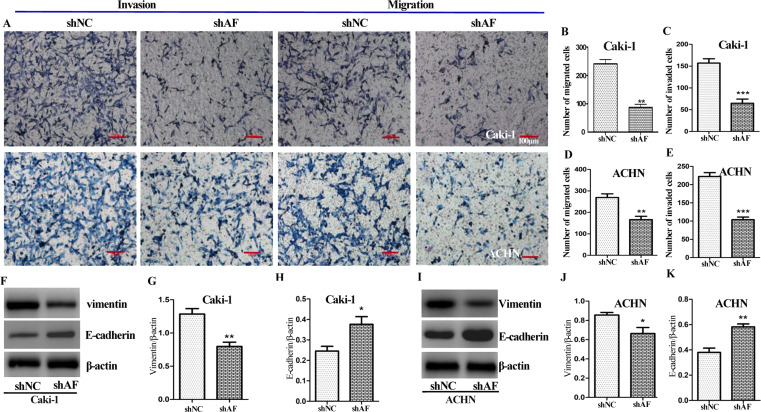
To evaluate the ability of AFAP1-AS1 on invasion and migration of ccRCC cells, Transwell assay was performed. The positive staining cell number in the shAF group was much less than that of the control group (Fig. 3A–C). Meanwhile, we investigated the effect of AFAP1-AS1 on cell migration by Transwell assay without Matrigel coating in the upper compartment. Compared with the cells transfected with shNC, we observed that knockdown of AFAP1-AS1 could attenuate the migratory ability of both Caki-1 and ACHN cells (Fig. 3A, D, and E). Thus, silencing of AFAP1-AS1 significantly suppressed the ability of invasion and migration of ccRCC cells.
Figure 3.
Effect of AFAP1-AS1 on cell migration, invasion, and epithelial–mesenchymal transition (EMT) in ccRCC cells. (A, B, C) Effect of shAF or shNC on cell invasion was determined using Transwell assays with Matrigel. (D, E) Effect of knockdown of AFAP1-AS1 on cell migration of Caki-1 and ACHN cells using Transwell assays without Matrigel. The effect of knockdown of AFAP1-AS1 on the protein expression of EMT-related genes (including vimentin and E-cadherin) was detected by immunoblot analysis. Representative blots in Caki-1 (F) and ACHN (I) cells. Values are presented as mean ± SD from at least three experiments (G, H, J, K). Scale bars: 100 μm. Significant difference between shNC group and shAF group was compared, * p < 0.05, ** p < 0.01, *** p < 0.001.
EMT (epithelial–mesenchymal transition) is essential for initiation of metastasis in tumor progression18. We observed that high level of AFAP1-AS1 promotes proliferation and invasion of Caki-1 and ACHN cells. Based on these evidences, AFAP1-AS1 may affect EMT-related genes, including E-cadherin and vimentin. As seen in Figure 3F and I, silencing of AFAP1-AS1 resulted in an increase in E-cadherin and decrease in vimentin in both Caki-1 and ACHN cells. This result is consistent with our previous observation that AFAP1-AS1 promoted cell invasion and migration.
AFAP1-AS1 Is Negatively Correlated With PTEN
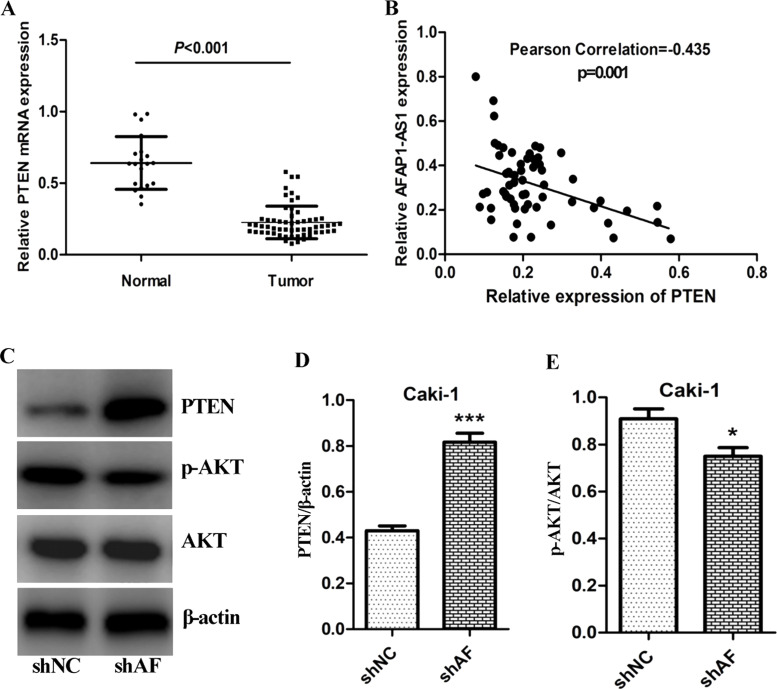
Previous studies showed that lncRNAs is associated with PTEN expression19. Thus, we next investigated the relationship of AFAP1-AS1 and PTEN expression in ccRCC. First, the mRNA level of PTEN in ccRCC (n = 60) and adjutant normal tissues (n = 20) was determined by RT-PCR. As seen in Figure 4A, the expression level of PTEN in ccRCC was significantly downregulated, compared with the adjutant normal tissues (p < 0.001). Furthermore, the correlation of PTEN and AFAP1-AS1 was analyzed by Pearson correlation test. The expression level of AFAP1-AS1 is negatively correlated with PTEN mRNA level (Pearson correlation r = −0.435, p = 0.001) (Fig. 4B). Given the negative correlation of AFAP1-AS1 and PTEN, we further determined whether knockdown of AFAP1-AS1 affects PTEN/AKT signaling. As seen in Figure 4C, silencing of AFAP1-AS1 evidently upregulated PTEN expression and inactivated AKT in Caki-1 cells. This result also verified that AFAP1-AS1 negatively regulated PTEN/AKT signaling.
Figure 4.
Negative correlation of AFAP1-AS1 and PTEN. (A) The comparison of PTEN expression in ccRCC tissues (n = 60) with that of adjacent normal tissues (n = 20), the mRNA expression level of PTEN determined by qRT-PCR analysis. (B) The correlation of PTEN and AFAP1-AS1 was analyzed by Pearson test, and Pearson correlation r = −0.435, p < 0.001. (C, D, E) Effect of knockdown of AFAP1-AS1 on expression of PTEN and pAKT, and AKT was detected by immunoblot analysis. β-Actin was used as loading control, *p < 0.05; ***p < 0.001.
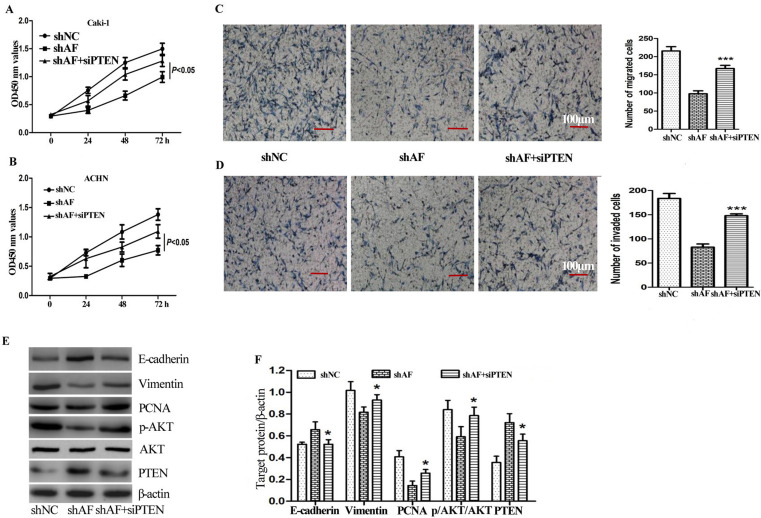
PTEN Deficiency Attenuates the Ability of AFAP1-AS1 to Promote Growth and Metastasis of ccRCC Cells
To further study the effect of PTEN on the negative regulating AFAP1-AS1-promoted cell growth, si-PTEN was transfected into Caik-1 and ACHN cells. Similar to our previous result, knockdown of AFAP1-AS1 alone significantly inhibited proliferation of Caik-1 and ACHN cells, while silencing PTEN expression together evidently reversed the biological function of shAF on cell growth (Fig. 5A and B).
Figure 5.
PTEN deficiency attenuates the ability of AFAP1-AS1 to promote growth and metastasis of ccRCC cells. (A, B) The effect of shNC, shAF with or without siPTEN on growth of Caki-1 (A) and ACHN (B) cells at 24, 48, and 72 h was determined using CCK-8 assay. (C) Cell migration was measured in Caki-1 cells transfected with shNC, shAF with or without siPTEN through Transwell assays without Matrigel. (D) Cell invasion was measured in Caki-1 cells transfected with shNC, shAF with or without siPTEN using Transwell assays with Matrigel. (E, F) The effect of shAF with or without siPTEN on expression of EMT-related targets (including E-cadherin and vimentin), and PTEN, p-AKT, AKT, and PCNA were determined by Western blotting analysis. A significant difference between the shAF group and shAF plus siPTEN group was compared, *p < 0.05; ***p < 0.001.
We also evaluated the effect of PTEN deficiency on AFAP1-AS1-mediated invasion and migration in ccRCC cells. Knockdown of AFAP1-AS1 significantly suppressed cell invasion and migration. Moreover, we observed that si-PTEN resulted in an increase in invasive cell number (Fig. 5C and D).
In addition, the effect of si-PTEN on AFAP1-AS1-mediated EMT was also investigated. shAF lead to increase in E-cadherin and decrease in vimentin in Caki-1 cells, but the opposite regulation of E-cadherin and vimentin expressions was observed in combination with si-PTEN (Fig. 5E and F).
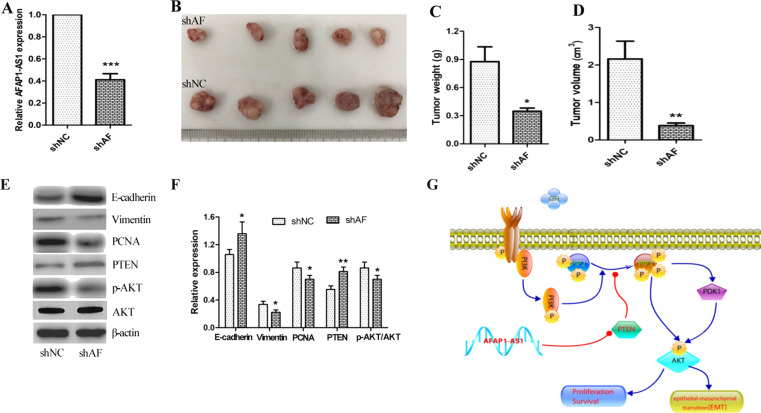
In Vivo Biological Role of AFAP1-AS1 on Tumor Growth of ccRCC
Given the ability of AFAP1-AS1 in promoting cell growth, invasion, migration, and EMT in ccRCC cells, we next evaluated the biological role of AFAP1-AS1 in vivo using xenograft mice (n = 6) bearing shNC and shAF-transfected Caki-1cells. At the end of the experimental period, tumor tissue was obtained on day 21, and expression level of AFAP1-AS1 was determined using qRT-PCR. As shown in Figure 6A, AFAP1-AS1 expression level was significantly decreased in the shAF group compared with shNC xenograft mice (p < 0.001). Tumor image is shown in Figure 6B. Tumor weight was also measured. The knockdown of AFAP1-AS1 leads to a decrease in tumor weight (Fig. 6C). No gross toxicities were evident as determined by body weight. Noticeably, tumor volume was decreased by 68%, compared with that of the shNC group (Fig. 6D). We then determined the levels of protein expression of PTEN, E-cadherin, vimentin, PCNA, pAKT, and total-AKT expression. PTEN and E-cadherin were significantly increased, and vimentin, PCNA, and p-AKT/AKT were markedly decreased (E-cadherin, p < 0.05; vimentin, p < 0.05; PCNA, p < 0.05; p-AKT, p < 0.05; PTEN, p < 0.01) (Fig. 6E and F). These in vivo findings correlated nicely with the in vitro results presented in Figures 2 and 3.
Figure 6.
In vivo biological role of AFAP1-AS1 on tumor growth of ccRCC. Nude mice were transplanted subcutaneously with Caki-1 cells transfected with shAF or the control shNC (n = 6). After 21 days, the mice were sacrificed. (A) Relative expression of AFAP1-AS1 in tumor tissue was determined by qRT-PCR. (B) A representative picture of the morphology of tumor xenografts after excision at 21 days of treatment. (C) Tumor weights were measured after the mice were sacrificed. (D) Tumor volume was also calculated by the formula in the Materials and Methods section. (E, F) The effect of knockdown of AFAP1-AS1 on the expression of EMT-related target proteins in xenograft tumors was analyzed by Western blot analysis. A representative immunoblot is shown. Values are presented as mean ± SD from three separate experiments. (G) Silencing of AFAP1-AS1 inhibits growth and metastasis of ccRCC via negative regulation of PTEN/AKT signaling. Significant difference between the shNC group and the shAF group was compared, *p < 0.05; **p < 0.01; *** p < 0.001.
DISCUSSION
Currently, aberrant expression or dysfunctional activity of lncRNAs occurs in many tumor types, and emerging evidence indicates that lncRNAs participate in tumor initiation, development, and metastasis20. lncRNAs can be used as a novel therapeutic target or prognostic tumor biomarkers21. lncRNA AFAP1-AS1 is oriented in an antisense direction to the protein-coding gene AFAP1 in the opposite strand. High-level expression of AFAP1-AS1 is associated with tumor initiation, metastasis, and poor prognosis. In gallbladder cancer, hepatocellular carcinoma, tongue squamous cell carcinoma, and gastric cancer, AFAP1-AS1 is upregulated, and its higher expression is associated with tumor progression and poor prognosis. Knockdown of AFAP1-AS1 significantly inhibited cell proliferation, migration, and invasion. AFAP1-AS1 also promoted tumor growth and invasion of cholangiocarcinoma8,10,22. In this study, we found that AFAP1-AS1 expression was significantly increased in ccRCC tissues and that patients with high-level expression of AFAP1-AS1 had a shorter overall survival. Silencing AFAP1-AS1 by shAF significantly suppressed proliferation, invasion, and migration of ccRCC cells. EMT plays a key role in regulation of cancer development and metastasis. Downregulation of the epithelial marker E-cadherin to disassemble cell junctions and upregulation of the mesenchymal markers N-cadherin and vimentin to increase cell protrusions and motility is known as the main step to acquire invasive properties23–25. In both Caki-1 and ACHN cells, silencing AFAP1-AS1 resulted in an increase in E-cadherin and decrease in vimentin. Moreover, the similar effect of the knockdown of AFAP1-AS1 on vimentin and E-cadherin expression in our ccRCC xenograft mice model was also observed. This result is consistent with our previous observation that AFAP1-AS1 promoted cell invasion and migration. In addition, it was reported that men have higher incidence of developing RCC than women, and men present with larger, higher-stage, higher-grade RCC than women26. Thus, the limitation of our study is only a female mice model was used for evaluation of AFAP1-AS1 on tumor growth and metastasis.
The PI3K/AKT pathway is commonly activated in ccRCC. PTEN, known as a tumor suppressor, could downregulate AKT signaling27–29. In addition, some studies reported that AFAP1-AS1 promotes cell proliferation, invasion, and metastasis through the PTEN/AKT signaling axis in non-small cell lung cancer30. In the present study, we confirmed that AFAP1-AS1 negatively regulated PTEN/AKT signaling. What is more, PTEN deficiency significantly attenuated the ability of AFAP1-AS1 in promoting ccRCC cell proliferation, invasion, migration, and EMT.
In conclusion, we first observed that AFAP1-AS1 expression was significantly upregulated in ccRCC tissues. High-level expression of AFAP1-AS1 is associated with malignant behavior and poor prognosis in ccRCC. Biological function experiments showed that knockdown of AFAP1-AS1 by shRNA significantly suppressed proliferation, invasion, migration, and EMT in ccRCC cells. Moreover, a similar biological response of silencing AFAP1-AS1 was observed in our ccRCC mice model. Knockdown of AFAP1-AS1 markedly suppressed tumor growth and metastasis. In addition, we found that PTEN is negatively correlated with the expression level of AFAP1-AS1. Further studies verified that PTEN deficiency effectively attenuated the ability of AFAP1-AS1 in promoting ccRCC cell proliferation, invasion, migration, and EMT (Fig. 6G). Based on our findings, AFAP1-AS1 plays a significant role in mediating growth and metastasis signaling in ccRCC and may represent a novel therapeutic target for the treatment of ccRCC.
ACKNOWLEDGMENTS
We thank all technicians in our department for their excellent technical assistance. This work was supported by grants (No. 81501436) from China National Natural Sciences Foundation and from Liaoning Provincial Natural Science Foundation of China (No. 20170540560). The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. Dr. Z. Y. Mu and Dr. D. Dong mainly performed the experiments and drafted the manuscript. W. Wang, M. L. Sun, and C. H. Zhao played an important role in coordinating the study and performing the analysis with constructive discussions. S. Yue, J. Gao, P. Yin, D. Dong, and Z. Y. Mu performed Western blot and prepared the samples. M. L. Sun, N. Wei, and C. H. Zhao conceived the study and revised the manuscript. All authors discussed the results and contributed to the writing and editing of the manuscript.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Lopez JI. Renal tumors with clear cells. A review. Pathol Res Pract. 2013;209(3):137–46. [DOI] [PubMed] [Google Scholar]
- 3. Palsdottir HB, Hardarson S, Petursdottir V, Jonsson A, Jonsson E, Sigurdsson MI, Einarsson GV, Gudbjartsson T. Incidental detection of renal cell carcinoma is an independent prognostic marker: Results of a long-term, whole population study. J Urol. 2012;187(1):48–53. [DOI] [PubMed] [Google Scholar]
- 4. Yu-Li Su H. Reintroducing pazopanib reverses the primary resistance of nivolumab in a patient with metastatic clear-cell renal cell carcinoma. Clin Genitourin Cancer 2018;16(2):114–6. [DOI] [PubMed] [Google Scholar]
- 5. Rojas JD, Lin F, Chiang YC, Chytil A, Chong DC, Bautch VL, Rathmell WK, Dayton PA. Ultrasound molecular imaging of VEGFR-2 in clear-cell renal cell carcinoma tracks disease response to antiangiogenic and notch-inhibition therapy. Theranostics 2018;8(1):141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verbiest A, Couchy G, Job S, Caruana L, Lerut E, Oyen R, de Reynies A, Tosco L, Joniau S, Van Poppel H, Van Raemdonck D, Van Den Eynde K, Wozniak A, Zucman-Rossi J, Beuselinck B. Molecular subtypes of clear-cell renal cell carcinoma are prognostic for outcome after complete metastasectomy. Eur Urol. 2018;74(4):474–80. [DOI] [PubMed] [Google Scholar]
- 7. Luo HL, Huang MD, Guo JN, Fan RH, Xia XT, He JD, Chen XF. AFAP1-AS1 is upregulated and promotes esophageal squamous cell carcinoma cell proliferation and inhibits cell apoptosis. Cancer Med. 2016;5(10):2879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo JQ, Li SJ, Guo GX. Long noncoding RNA AFAP1-AS1 promotes cell proliferation and apoptosis of gastric cancer cells via PTEN/p-AKT pathway. Dig Dis Sci. 2017;62(8):2004–10. [DOI] [PubMed] [Google Scholar]
- 9. Wang F, Ni H, Sun F, Li M, Chen L. Overexpression of lncRNA AFAP1-AS1 correlates with poor prognosis and promotes tumorigenesis in colorectal cancer. Biomed Pharmacother. 2016;81:152–9. [DOI] [PubMed] [Google Scholar]
- 10. Zhang JY, Weng MZ, Song FB, Xu YG, Liu Q, Wu JY, Qin J, Jin T, Xu JM. Long noncoding RNA AFAP1-AS1 indicates a poor prognosis of hepatocellular carcinoma and promotes cell proliferation and invasion via upregulation of the RhoA/Rac2 signaling. Int J Oncol. 2016;48(4):1590–8. [DOI] [PubMed] [Google Scholar]
- 11. Lu X, Zhou C, Li R, Deng Y, Zhao L, Zhai W. Long noncoding RNA AFAP1-AS1 promoted tumor growth and invasion in cholangiocarcinoma. Cell Physiol Biochem. 2017;42(1):222–30. [DOI] [PubMed] [Google Scholar]
- 12. Zhang F, Li J, Xiao H, Zou Y, Liu Y, Huang W. AFAP1-AS1: A novel oncogenic long non-coding RNA in human cancers. Cell Prolif. 2018;51(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei N, Chu E, Wipf P, Schmitz JC. Protein kinase d as a potential chemotherapeutic target for colorectal cancer. Mol Cancer Ther. 2014;13(5):1130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Sun H, Xiao Z, Zhang D, Bao X, Wei N. XWL-1-48 exerts antitumor activity via targeting topoisomerase II and enhancing degradation of Mdm2 in human hepatocellular carcinoma. Sci Rep. 2017;7(1):9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang YX, Liu XM, Wang J, Li J, Liu Y, Zhang H, Yu XW, Wei N. Inhibition of AKT/FoxO3a signaling induced PUMA expression in response to p53-independent cytotoxic effects of H1: A derivative of tetrandrine. Cancer Biol Ther. 2015;16(6):965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei N, Liu GT, Chen XG, Liu Q, Wang FP, Sun H. H1, a derivative of tetrandrine, exerts anti-MDR activity by initiating intrinsic apoptosis pathway and inhibiting the activation of Erk1/2 and Akt1/2. Biochem Pharmacol. 2011;82(11):1593–603. [DOI] [PubMed] [Google Scholar]
- 17. Wei N, Li J, Fang C, Chang J, Xirou V, Syrigos NK, Marks BJ, Chu E, Schmitz JC. Targeting colon cancer with the novel STAT3 inhibitor bruceantinol. Oncogene 2018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18. Papageorgis P. TGFbeta signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015:587193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng Q, Lin Z, Xu J, Lu Y, Meng Q, Wang C, Yang Y, Xin X, Li X, Pu H, Gui X, Li T, Xiong W, Lu D. Long noncoding RNA MEG3 suppresses liver cancer cells growth through inhibiting beta-catenin by activating PKM2 and inactivating PTEN. Cell Death Dis. 2018;9(3):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murugan AK, Munirajan AK, Alzahrani AS. Long noncoding RNAs: Emerging players in thyroid cancer pathogenesis. Endocr Relat Cancer 2018;25(2):R59–82. [DOI] [PubMed] [Google Scholar]
- 21. Kondo Y, Shinjo K, Katsushima K. Long non-coding RNAs as an epigenetic regulator in human cancers. Cancer Sci. 2017;108(10):1927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang ZY, Hu M, Dai MH, Xiong J, Zhang S, Wu HJ, Zhang SS, Gong ZJ. Upregulation of the long non-coding RNA AFAP1-AS1 affects the proliferation, invasion and survival of tongue squamous cell carcinoma via the Wnt/beta-catenin signaling pathway. Mol Cancer 2018;17(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Begemann D, Anastos H, Kyprianou N. Cell death under epithelial-mesenchymal transition control in prostate cancer therapeutic response. Int J Urol. 2018;25(4):318–26. [DOI] [PubMed] [Google Scholar]
- 24. Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer 2018;18(2):128–34. [DOI] [PubMed] [Google Scholar]
- 25. Smigiel JM, Parameswaran N, Jackson MW. Targeting pancreatic cancer cell plasticity: The latest in therapeutics. Cancers (Basel) 2018;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aron M, Nguyen MM, Stein RJ, Gill IS. Impact of gender in renal cell carcinoma: An analysis of the SEER database. Eur Urol. 2008;54(1):133–40. [DOI] [PubMed] [Google Scholar]
- 27. Moch H, Montironi R, Lopez-Beltran A, Cheng L, Mischo A. Oncotargets in different renal cancer subtypes. Curr Drug Targets 2015;16(2):125–35. [DOI] [PubMed] [Google Scholar]
- 28. Azim H, Azim HA Jr, Escudier B. Targeting mTOR in cancer: Renal cell is just a beginning. Target Oncol. 2010;5(4):269–80. [DOI] [PubMed] [Google Scholar]
- 29. Lee HJ, Lee HY, Lee JH, Lee H, Kang G, Song JS, Kang J, Kim JH. Prognostic significance of biallelic loss of PTEN in clear cell renal cell carcinoma. J Urol. 2014;192(3):940–6. [DOI] [PubMed] [Google Scholar]
- 30. Deng J, Liang Y, Liu C, He S, Wang S. The up-regulation of long non-coding RNA AFAP1-AS1 is associated with the poor prognosis of NSCLC patients. Biomed Pharmacother. 2015;75:8–11. [DOI] [PubMed] [Google Scholar]