Abstract
Triptolide, an extract of Tripterygium wilfordii, has been shown to have a potent anticancer activity. In the present study, it was found that triptolide could effectively induce apoptosis and inhibit proliferation and invasion in malignant MDA-MB-231 breast cancer cells. The study focused on its effect on inhibiting invasion, which has not been extensively reported to date. We predicted that triptolide may change invasion activity via microRNAs (miRNAs), which have been recognized as important regulators of gene expression. miRNAome variation in MDA-MB-231 cells with or without triptolide treatment demonstrated that miR-146a was upregulated following treatment with triptolide. Our previous studies have shown that miR-146a can inhibit migration and invasion by targeting RhoA in breast cancer. This time, we found that miR-146a can target Rac1, another key member of the Rho GTPase family. Luciferase reporter containing Rac1 3′-UTR was constructed to prove this hypothesis. In addition, following treatment with triptolide, the expression of RhoA and Rac1 was found to be decreased. These results indicated that triptolide exerts its anti-invasion activity through a miRNA-mediated mechanism, which indirectly regulates the expression of Rho GTPase. Triptolide combined with miR-146a could improve the effect of triptolide treatment on breast cancer.
Key words: Breast cancer, Triptolide, miR-146a, Rho A, Rac1
INTRODUCTION
Breast cancer is one of the most common types of cancer among women. Tumor recurrence and metastasis are the main causes of mortality in patients with breast cancer. Recent statistics show that breast cancer has become the first cause of cancer-related mortality among female cancer patients in China1. Therefore, preventing and treatment of breast cancer metastasis and the clarification of its metastasis mechanisms are of high clinical significance. Research on antitumor therapy and the contribution of traditional Chinese medicine to it have received extensive attention recently. Several reports have found that certain components of Chinese herbal medicine can effectively battle cancer. Triptolide is the main active ingredient of the compound extracted from the root of traditional Chinese herb Tripterygium wilfordii 2. Triptolide has received attention for its antitumor activity since the 1970s3,4.
In vivo and in vitro experiments have shown that triptolide has an antitumor effect in several types of cancer, including pancreatic cancer, lung cancer, breast cancer, and neuroblastoma5–8. Triptolide achieves this antitumor effect mainly by inhibiting tumor cell proliferation and inducing tumor cell apoptosis9–11. However, recent studies have also identified an antimetastatic activity of tripolide6,12. To date, in vivo studies have indicated that triptolide protects against breast cancer metastasis, but its specific mechanism of action requires further study8.
The mechanism through which microRNAs regulate tumor cell invasion and metastasis has become central in the field of microRNA (miRNA) research13,14. miRNAs bind to target mRNA sequences through canonical base pairing between nucleotides 2 to 8 in the 3′-UTR of target mRNA15,16. Previous reports have confirmed that miR-146a has an inhibitory effect on tumor occurrence, development, and metastasis, but its specific mechanism of action remains to be elucidated17–19. Recent studies have shown that miR-146a is closely associated with the prognosis of breast cancer and plays an important role in the proliferation, invasion, and metastasis of breast cancer cells19–21.
Rho GTPases are members of the small G protein superfamily, which has GTP enzyme activity22,23. RhoA and Rac1 are important members of the GTPase Rho family. RhoA and Rac1 are overexpressed in lung cancer, colon cancer, breast cancer, and other types of cancer cells. They have also been demonstrated to play an important role in tumor invasion and metastasis through their mediation of the cytoskeleton reorganization of key regulatory proteins24–26.
In the present study, it was found that triptolide exerts an antitumor activity through inducing breast cancer cell apoptosis and inhibiting their proliferation, migration, and invasion. In this study, miR-146a was found to be upregulated in triptolide-treated breast cancer cells. Previous data from our laboratory showed that miR-146a could inhibit migration and invasion by targeting RhoA in breast cancer27. In addition, miR-146a was found to target Rac1, another key member of the Rho GTPase family. A reverse correlation was observed between Rac1 and miR-146a. miR-146a was also found to target Rac1 3′-UTR directly, thereby downregulating breast cancer cell migration and invasion. Triptolide could specifically induce RhoA and Rac1 downregulation. A comprehensive analysis of these results led us to the conclusion that triptolide may inhibit breast cancer cell metastasis through inducing the expression of miR-146a, a negative regulator of Rho GTPase. In the present study, we want to preliminarily elucidate the association between miR-146a and Rho GTPase, and search for potential antimetastatic agents and promising molecular targets that could be used in breast cancer treatment for the inhibition of metastasis.
MATERIALS AND METHODS
Cell Culture and Drug Treatment
Human breast cancer cell line MDA-MB-231 was maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Life Technologies) and 1% antibiotics (Gibco, Life Technologies). The cells were incubated at 37°C in a 5% CO2 humidified atmosphere until 75% confluent. Triptolide (≥98%; Yuanye Bio-Technology, Shanghai, P.R. China) was stored in a stock solution in dimethyl sulfoxide (DMSO; Sangon, Shanghai, P.R. China) at −20°C and diluted to various concentrations with serum-free culture medium when used.
Cell Counting Kit-8 (CCK-8) Assay
Cell growth and viability were measured using Cell Counting Kit-8 (CCK-8; KeyGEN, Nanjing, Jiangsu, P.R. China). Cells at a density of 1 × 105 cells/well in medium were placed into 96-well plates and were continuously cultured for 24 h. Then the cells were treated with triptolide (0, 5, 10, 15, 20, and 25 ng/ml). At the harvest time, 10 μl of CCK-8 was added into each well, and after 1-h incubation cell viability was determined by measuring the absorbance of the converted dye at 450 nm. The experiments were performed in triplicate.
Flow Cytometry Analysis of Cell Apoptosis
Apoptotic cells were identified by FITC-conjugated Annexin-V/propidium iodide (PI) staining (KeyGEN) according to the manufacturer’s instructions and analyzed with Cytomics TM FC 500 (Beckman, Brea, CA, USA). Both early apoptosis (annexin V+, PI−) and late apoptosis (annexin V+ and PI+) cells were included in cell death measures.
Cell Migration Assay
Transwell invasion assays were performed by Transwell chamber (Corning, New York, NY, USA) on MDA-MB-231 cells, which were treated with triptolide and control for 24 h. Then the cells were resuspended in serum-free DMEM and placed in the top chamber with Matrigel-coated membrane (BD, San Jose, CA, USA). The lower portion of the chamber contained 20% FBS as a chemoattractant. After the cells were cultured for 24 h, we washed the chambers twice by phosphate-buffered saline (PBS) and stained the chambers by crystal violet. Five fields were selected randomly and photographed from each chamber. Then chambers were decolonized by glacial acetic acid, adding 1 ml 33% glacial acetic acid into each chamber for 15 s and then removing the liquid. Quantitative analysis was carried out using the enzyme marker at 570 nm wavelength. All independent experiments were carried out in duplicate.
Wound Healing Assay
When cell confluence was less than 90% at 24 h after transfection, wounds were created in confluent cells using a 200-μl pipette tip, removing any free-floating cells and debris by PBS. Medium was then added, and culture plates were incubated at 37°C. Wound healing within the scrape line was observed at 24-h time points, and representative scrape lines for each cell line were photographed. The optical distance of sound was measured using ImageJ software. All independent experiments were carried out in duplicate.
miRNA Microarray and miR-146a Quantitative Assay
The miRNA microarray was analyzed by Ribobio (Guangzhou, Guangdong, P.R. China). For analysis of miR-146a expression, the TaqMan One-step RT-PCR Master Mix Reagents kit and TaqMan MicroRNA Assays kit were purchased from Applied Biosystems (ABI, Foster City, CA, USA) to detect miR-146a and the control miRNA (U6 snRNA). The protocol is two steps, requiring reverse transcription with miRNA-specific primers, followed by reverse transcription quantitative polymerase chain reaction (qRT-PCR) with TaqMan probes (ABI). Amplification and detection were performed using StepOne Plus QRT-PCR system (ABI). Quantitative assay was calculated based on the following equation: RQ = 2 − ΔΔCt.
Plasmid Constructs and miR-146a Mimic Synthesize
The 3′-UTR of the human Rac1 gene (NM_006908) was PCR amplified from human genomic DNA. The primers were digested and cloned into the pmiR-RB-REPORT™ Vector (RiboBio) at XhoI and NotI sites, which was named pmiR-Rac1-wt. Site-directed mutagenesis with miR-146a target sites in the Rac1 was carried out using site-directed mutagenesis kit (TaKaRa, Dalian, Liaoning, P.R. China), with pmiR-Rac1-wt as a template, which was named pmiR-Rac1-mut. Mimic miR-146a, control oligo was chemically synthesized by RiboBio. All transfections were carried out using Lipofectamine 2000 reagents (Invitrogen, Carlsbad, CA, USA), according to the reagent manufacturer’s instructions.
Dual-Luciferase Reporter Assay and 3′-UTR-Binding Site Mutagenesis
MDA-MB-231 cells were seeded in a 24-well plate 24 h before transfection. They were transfected with 0.4 μg various pmiR-REPORT plasmids, 20 μM mimic miR-146a, or control oligo using Lipofectamine 2000. Firefly and Renilla luciferase activities were measured consecutively by using Dual Luciferase Assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions; 0.02 μg of the Renilla luciferase vector pRL-TK (Promega) was used for normalization. All independent experiments were performed in triplicate.
Quantitative Analysis of RhoA and Rac1
Total RNA was prepared using TRIzol (TaKaRa), and cDNA was generated by PrimerScript RT reagent kit (TaKaRa) and amplified using RhoA or Rac1 primers with SYBR Premix Ex-Taq™ kit (TaKaRa). The primer sequences for RhoA were 5′-TTTGGAGGTGGCATAGCCTT-3′ (forward) and 5′-ATGTTTAGTCAGCTGGAGAGAAGAG-3′ (reverse); the primer sequences for Rac1 were 5′-TGCATTGTTGTGCCGAGAAC-3′ (forward) and 5′-GAGGCATGGCAGGTGTAAGA-3′ (reverse); and the primer sequences for β-actin were 5′-CCCTGGACTTCGAGCAAGAG-3′ (forward) and 5′-AATGCCAGGGTACATGGTGG-3′ (reverse). Amplification and detection were performed using StepOne Plus QRT-PCR system (ABI). β-Actin was used to normalize the expression of RhoA and Rac1. Quantitative assays were calculated based on the following equation: RQ = 2−ΔΔCt.
Western Blot Analysis
Protein was extracted from breast cancer cell lines using RIPA buffer [150 mM NaCl, 1% NP-40, 50 mM Tris-HCl (pH 7.4), 1 μg/ml leupeptin, 1 mM deoxycholic acid, 1 mM phenylmethysulfonyl fluoride, and 1 mM EDTA] including a protease inhibitor cocktail and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO, USA), following the manufacturers’ instructions. Equal amounts of protein sample (50 μg) were separated by 12% SDS-PAGE and transferred to a PVDF membrane using the Bio-Rad semidry transfer system (Bio-Rad Laboratories, Hercules, CA, USA). Western blotting was performed using anti-β-actin, anti-RhoA, and anti-Rac1 (CST, Denver, CO, USA). The signals were detected with an ECL kit (Beyotime, Nantong, Jiangsu, P.R. China), according to the manufacturer’s instructions.
Statistical Analysis
All data are presented as mean ± SD of three independent experiments. Assays for characterizing phenotypes of cells were analyzed by Students t-test or one-way ANOVA. Values of p < 0.05 were considered as statistically significant. All statistical analyses were performed using the SPSS 22.0 software package (SPSS Inc., Chicago, IL, USA).
RESULTS
Triptolide Inhibited Cell Proliferation and Induced Cell Apoptosis in MDA-MB-231 Cells
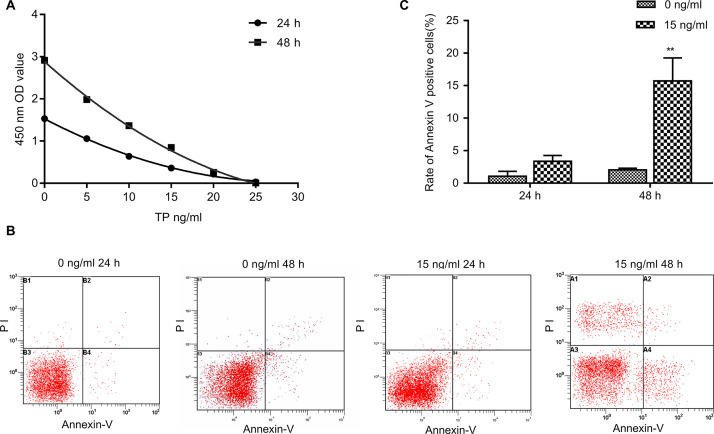
CCK-8 was used to detect breast cancer cell proliferation and access the effects of different triptolide concentrations and treatment times (Fig. 1A). Flow cytometry was used to detect the changes in the apoptotic activity of breast cancer cells following treatment with 15 ng/ml triptolide for 48 and 24 h. It was found that the apoptotic activity of cells at 48 h was significantly increased (p < 0.01) (Fig. 1B and C). These results indicated that triptolide can inhibit cell proliferation and induce apoptosis in breast cancer cells. To study the anti-invasion effect of triptolide in MDA-MB-231 cells and avoid the interference of cell apoptosis and necrosis, the cell death rate of <50% dose (<15 ng/ml) was selected as the study dose, and 24 h was selected as the observation time point in the following experiments.
Figure 1.
Effect of triptolide treatment on the cell proliferation and apoptosis of breast cancer cells. (A) MDA-MB-231 cells treated with triptolide (5, 10, 15, 20, and 25 ng/ml) for 24 and 48 h. The level of cell proliferation was detected using the Cell Counting Kit (CCK-8). (B, C) The percentage of apoptotic cells was determined by FACS analysis using annexin V/propidium iodide (PI) staining. Data are presented as the mean ± SD. n = 3. **p < 0.01 versus 0 ng/ml group.
Triptolide Inhibits Invasion in MDA-MB-231
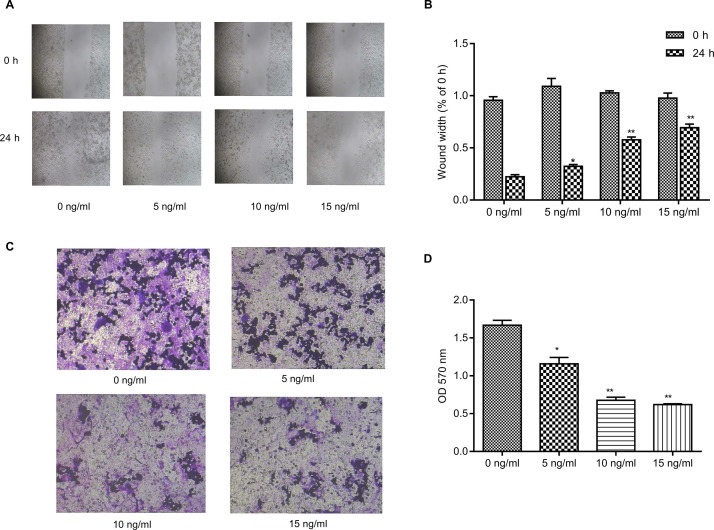
Wound healing and Transwell cell invasion assays were used to detect the metastasis of cells treated with different drug concentrations for 24 h. The results showed that the metastatic activity of breast cancer cells was inhibited following treatment with 5 ng/ml triptolide and significantly inhibited following treatment with 10 and 15 ng/ml triptolide for 24 h (Fig. 2).
Figure 2.
Triptolide inhibits invasion in MDA-MB-231 cells. (A) Effect of triptolide on MDA-MB-231 cell migration was determined by a wound healing assay. MDA-MB-231 cells were plated in six-well plates and allowed to grow to 90% confluence in complete medium. Cell monolayers were wounded using a sterile micropipette tip (200 μl) and were further treated with triptolide (0, 5, 10, and 15 ng/ml) for 24 h and migrated into the wound surface following transfection. Images were acquired at 0 and 24 h after wounding. (B) The wound width was quantified. (C) Effect of triptolide on cell invasion was determined by the Transwell assay. MDA-MB-231 cells pretreated with triptolide (0, 5, 10, and 15 ng/ml) for 24 h were plated in Transwell chambers coated with Matrigel at the same density and then incubated for another 24 h. Photographs of the cell invasion were captured through the polycarbonate membrane stained with crystal violet dye (original magnification, ×200). (D) The OD570 of the crystal violet stain is shown. Data are presented as the mean ± SD. n = 3. OD, optical density. *p < 0.05 and **p < 0.01 versus 0 ng/ml group.
Triptolide Alters the MicroRNAome of MDA-MB-231
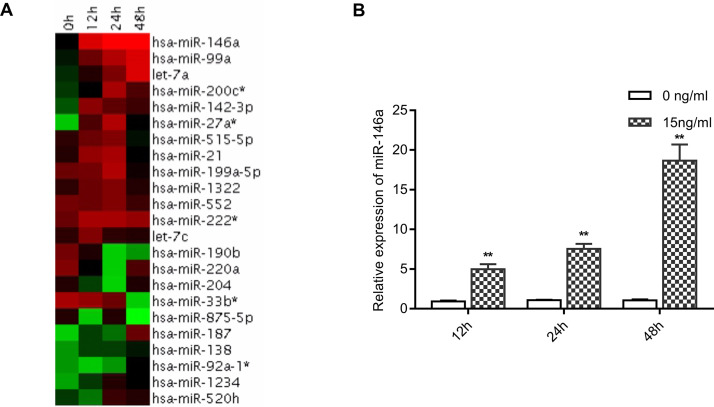
miRNA gene chip was used to detect the variation of miRNAome in breast cancer cells treated with 15 ng/ml triptolide for 12, 24, and 48 h. Various expressions of miRNAs were observed following triptolide treatment, and this variety was verified by RT-qPCR. In particular, miR-146a was significantly upregulated in triptolide-treated breast cancer cells. These results indicated that triptolide can induce miR-146a expression in breast cancer cells (Fig. 3).
Figure 3.
Triptolide-regulated microRNAs in MDA-MB-231 cells. (A) Heatmap representation of differentially expressed human microRNAs (miRNAs) following triptolide treatment (15 ng/ml for 0, 12, 24, or 48 h) in MDA-MB-231 cells was measured using microarray analysis. Only miRNAs with a strong differential expression (t-test between 0 and 24 h replicate time points; p < 0.05) are shown. (B) miR-146a expression [as assessed using reverse transcription quantitative polymerase chain reaction (RT-qPCR)] in MDA-MB-231 cells following 12, 24, and 48 h of 15 ng/ml triptolide treatment. The expression of miR-146a was normalized against U6. Bars represent the mean ± SD. n = 3. **p < 0.01 versus 0 ng/ml group.
Rac1 3′-UTRs Were Potential miR-146a Target Genes
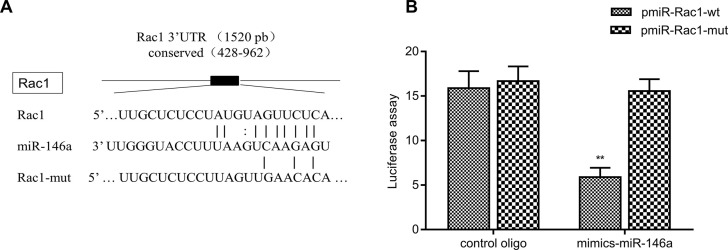
The target genes affecting tumor cell migration and invasion were predicted using the microRNA.org database. In a candidate gene study, it was found that the 3′-UTRs of the Rac1 gene have a complementary region with miR-146a (Fig. 4A). The Rac1 3′-UTRs may be miR-146a binding sites of target genes. The identified miR-146a expression suggests that miR-146a may directly or indirectly regulate the Rac1 genes. Furthermore, a luciferase reporter plasmid and mutant plasmid containing Rac1 3′-UTR was constructed. The luciferase activity of each group of cells was detected following transfection with Promega dual reporter assay kit. The reporter activity was found to be significantly reduced by the ectopic expression of miR-146a (p < 0.001), while no significant change was observed in the mutant plasmid (Fig. 4B). These results indicated that the 3′-UTRs of Rac1 were functional targets of miR-146a in MDA-MB-231 cells.
Figure 4.
Rac1 3′-UTRs are potential targets of miR-146a. (A) A luciferase reporter of Rac1 3′-UTR was constructed, which contained the binding sites of miR-146a, as predicted by bioinformatics analysis. A luciferase reporter of Rac1 3′-UTR containing mutated binding sites of miR-146a was also constructed. (B) miR-146a inhibited the luciferase activity of the reporter containing the Rac1 3′-UTR in MDA-MB-231 cells. However, miR-146a could not inhibit the luciferase activity of the reporter containing a mutated sequence of the Rac1 3′-UTR. **p < 0.01 versus control oligo group.
The Expression of Rho GTPase Was Changed in Triptolide-Treated MDA-MB-231 Cells
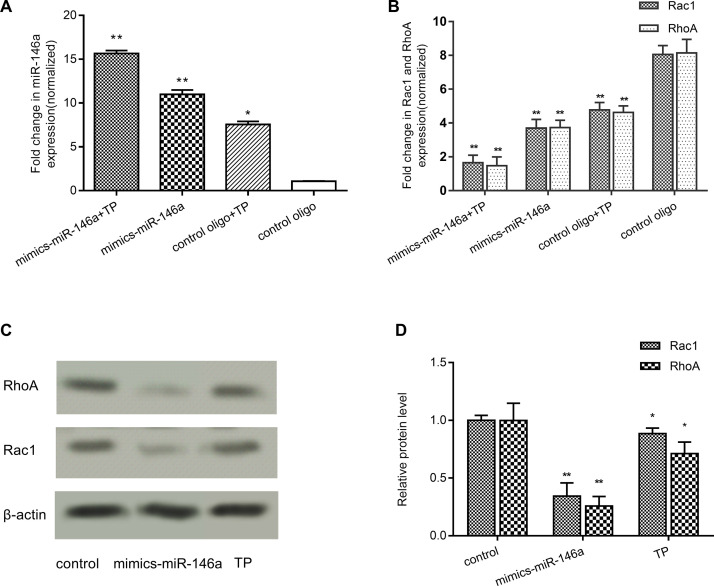
The cells were transfected with miR-146a mimics, which led to the upregulation of the miR-146a expression, after treatment with or without triptolide (Fig. 5A). The gene expression of RhoA and Rac1 in triptolide-treated MDA-MB-231 cells was assessed using RT-qPCR (Fig. 5B). Cells in the miR-146a mimic and triptolide-treated groups exhibited a lower RhoA and Rac1 gene expression than those in the blank control group (p < 0.001). According to the Western blot analysis results (Fig. 5C and D), the RhoA and Rac1 protein concentration decreased (p < 0.01) upregulating miR-146a and treating with triptolide. These results indicated that both triptolide and miR-146a could regulate RhoA and Rac1 expression. These results also confirmed that miR-146a can target RhoA and Rac1 genes, thus affecting breast cancer cell invasion and metastasis.
Figure 5.
Both miR-146a and triptolide can affect the Rac1 and RhoA protein expression in MDA-MB-231 cells. (A) MDA-MB-231 cells were pretreated with miR-146a-mimics, followed by further treatment with or without triptolide. The expression of miR-146a in MDA-MB-231 cells was measured by RT-qPCR. The gene expression level was normalized to U6. (B) MDA-MB-231 cells were pretreated with miR-146a-mimics, followed by further treatment with or without triptolide. The expression of RhoA and Rac1 mRNA in MDA-MB-231 cells was measured using RT-qPCR. The gene expression level was normalized to β-actin. (C, D) MDA-MB-231 cells were treated with miR-146a-mimics or triptolide. The relative protein expression levels of RhoA and Rac1 were measured by Western blot analysis and normalized to β-actin. Data are presented as the mean ± SD. n = 3. *p < 0.05 and **p < 0.01 versus control group.
DISCUSSION
Triptolide is the main active ingredient of the traditional Chinese herb T. wilfordii. In recent years, triptolide has been found to exert a potent anticancer activity in several types of cancer5–8. However, the underlying mechanism remains unclear. Existing research has mainly focused on triptolide-induced apoptosis, and certain studies have identified triptolide-induced inhibition of metastasis in vivo, but its specific mechanism of action requires further study8. The experimental results indicated that triptolide can inhibit proliferation and apoptosis in breast cancer cells, which is consistent with previous studies11,28–30. The present results also identified a triptolide-induced antimetastatic activity and early apoptosis in breast cancer cells, when triptolide was used in low concentrations. This suggests that triptolide can inhibit the invasion and metastasis of breast cancer cells in vitro. However, the antimetastasis mechanism of triptolide also requires further elucidation.
In recent years, miRNAs have been in the center of biological research, since they are involved in the occurrence, development, metastasis, and elimination of tumors by regulating, among others, gene and protein synthesis, transcription, and posttranscriptional modification15,16. Our current study found that the expressions of miRNAs were varied, after treatment with triptolide in MDA-MB-231 cells. miR-146a was found significantly upregulated following treatment. In our previous study, we found that a change in the expression of miR-146a can influence the migration and invasion abilities of breast cancer cells27, which suggested that triptolide may inhibit the metastasis of breast cancer cells by inducing the expression of miR-146a.
miRNAs targeting the regulation of gene 3′-UTRs is one of the important means in which miRNAs participate in the regulation of cell biology15,16. The target genes affecting tumor cell migration and invasion were predicted using bioinformatics analysis. In a candidate gene study, it was found that the 3′-UTRs of both RhoA and Rac1 genes have complementary regions (Fig. 4A and C), which may be the binding sites of miR-146a and target genes. RhoA and Rac1 are important members of the Rho GTPase family associated with tumor progression and metastasis24–26. Therefore, it can be speculated that miR-146a and RhoA and Rac1 genes may have a fine regulatory relationship, which impacts the migration and invasion of tumor cells. Previous data from our laboratory showed that miR-146a could inhibit migration and invasion by targeting RhoA in breast cancer27. To verify the regulatory relationship between miR-146a and Rac1, we constructed Rac1 luciferase reporters and validated the miR-146a-targeting regulation using the dual-luciferase reporter system with Rac1. In addition, miR-146a overexpression was induced through the transfection of an miR-146a mimic. Next, the expression of Rac1 genes was detected. The results indicated that miR-146a plays an important role in regulating Rac1 gene, which is similar to its effect on RhoA.
In conclusion, triptolide can induce the expression of miR-146a in breast cancer cells. The secreted miR-146a reduced the expression of RhoA and Rac1 mRNA, effectively reducing the metastatic activity of breast cancer cells. The aim of the present study was to elucidate the mechanism of the antitumor activity of triptolide and to find the connection between the triptolide-induced inhibition of breast cancer cell metastasis and the mechanism of miR-146a target regulating Rho GTPase expression. The protective effect of triptolide against invasion and metastasis in breast cancer cells was verified. Triptolide influenced breast cancer cell migration and invasion by inducing the expression of miR-146a, which regulated two important Rho GTPases: RhoA and Rac1. The results of the present study could serve as a reference for the association between miR-146a and Rho GTPase and assist in the search for promising molecular targets that could inhibit breast cancer metastasis.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (No. 81602822, No. 81774026), Science and Technology Project of Zhejiang Province Grant (No. 2018C37008), and Wuhan Scientific Research Projects Grant (No. WX15C22). The authors would like to thank Dr. Zhijian Cai for providing the MDA-MB-231 cell line, as well as the members of our lab for their technical assistance and useful suggestions. The authors declared no conflicts of interest.
REFERENCES
- 1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. [DOI] [PubMed] [Google Scholar]
- 2. Yu DQ, Zhang DM, Wang HB, Liang XT. Structure modification of triptolide, a diterpenoid from Tripterygium wilfordii. Yao Xue Xue Bao 1992;27(11):830–6. [PubMed] [Google Scholar]
- 3. Kupchan SM, Schubert RM. Selective alkylation: A biomimetic reaction of the antileukemic triptolides? Science 1974;185(4153):791–3. [DOI] [PubMed] [Google Scholar]
- 4. Kupchan SM, Court WA, Dailey RG, Jr, Gilmore CJ, Bryan RF. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc. 1972;94(20):7194–5. [DOI] [PubMed] [Google Scholar]
- 5. Ma JX, Sun YL, Wang YQ, Wu HY, Jin J, Yu XF. Triptolide induces apoptosis and inhibits the growth and angiogenesis of human pancreatic cancer cells by downregulating COX-2 and VEGF. Oncol Res. 2013;20(8):359–68. [DOI] [PubMed] [Google Scholar]
- 6. Reno TA, Kim JY, Raz DJ. Triptolide inhibits lung cancer cell migration, invasion, and metastasis. Ann Thorac Surg. 2015;100(5):1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang J, Song X, Yang J, Lei K, Ni Y, Zhou F, Sun L. Triptolide inhibits proliferation and migration of human neuroblastoma SH-SY5Y cells by upregulating microRNA-181a. Oncol Res. 2018;26(8):1235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Liu R, Yang Y, Huang Y, Li X, Liu R, Shen X. Triptolide-induced in vitro and in vivo cytotoxicity in human breast cancer stem cells and primary breast cancer cells. Oncol Rep. 2014;31(5):2181–6. [DOI] [PubMed] [Google Scholar]
- 9. Meng C, Zhu H, Song H, Wang Z, Huang G, Li D, Ma Z, Ma J, Qin Q, Sun X, Ma J. Targets and molecular mechanisms of triptolide in cancer therapy. Chin J Cancer Res. 2014;26(5):622–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu H, Huang G, Wang H, Li X, Wang X, Feng Y, Tan B, Chen T. Inhibition effect of triptolide on human epithelial ovarian cancer via adjusting cellular immunity and angiogenesis. Oncol Rep. 2018;39(3):1191–6. [DOI] [PubMed] [Google Scholar]
- 11. Messina ME Jr, Halaby R. Does triptolide induce lysosomal-mediated apoptosis in human breast cancer cells? Med Hypotheses 2011;77(1):91–3. [DOI] [PubMed] [Google Scholar]
- 12. Sangwan V, Banerjee S, Jensen KM, Chen Z, Chugh R, Dudeja V, Vickers SM, Saluja AK. Primary and liver metastasis-derived cell lines from KrasG12D; Trp53R172H; Pdx-1 Cre animals undergo apoptosis in response to triptolide. Pancreas 2015;44(4):583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abrantes JL, Tornatore TF, Pelizzaro-Rocha KJ, de Jesus MB, Cartaxo RT, Milani R, Ferreira-Halder CV. Crosstalk between kinases, phosphatases and miRNAs in cancer. Biochimie 2014;107(Pt B):167–87. [DOI] [PubMed] [Google Scholar]
- 14. Luo D, Wilson JM, Harvel N, Liu J, Pei L, Huang S, Hawthorn L, Shi H. A systematic evaluation of miRNA:mRNA interactions involved in the migration and invasion of breast cancer cells. J Transl Med. 2013;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136(2):215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shukla GC, Singh J, Barik S. MicroRNAs: Processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3(3):83–92. [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Z, Zhang Y, Sun XX, Ma X, Chen ZN. MicroRNA-146a inhibits cancer metastasis by downregulating VEGF through dual pathways in hepatocellular carcinoma. Mol Cancer 2015;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao Q, Cao Z, Tu C, Zhao Y, Liu H, Zhang S. MicroRNA-146a acts as a metastasis suppressor in gastric cancer by targeting WASF2. Cancer Lett. 2013;335(1):219–24. [DOI] [PubMed] [Google Scholar]
- 19. Wang D, Liu D, Gao J, Liu M, Liu S, Jiang M, Liu Y, Zheng D. TRAIL-induced miR-146a expression suppresses CXCR4-mediated human breast cancer migration. FEBS J. 2013;280(14):3340–53. [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Zhao L, Shi B, Ma S, Xu Z, Ge Y, Liu Y, Zheng D, Shi J. Functions of miR-146a and miR-222 in tumor-associated macrophages in breast cancer. Sci Rep. 2015;5:18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stuckrath I, Rack B, Janni W, Jager B, Pantel K, Schwarzenbach H. Aberrant plasma levels of circulating miR-16, miR-107, miR-130a and miR-146a are associated with lymph node metastasis and receptor status of breast cancer patients. Oncotarget 2015;6(15):13387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sadok A, Marshall CJ. Rho GTPases: Masters of cell migration. Small GTPases 2014;5:e29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reymond N, Riou P, Ridley AJ. Rho GTPases and cancer cell transendothelial migration. Methods Mol Biol. 2012;827:123–42. [DOI] [PubMed] [Google Scholar]
- 24. Oleinik NV, Helke KL, Kistner-Griffin E, Krupenko NI, Krupenko SA. Rho GTPases RhoA and Rac1 mediate effects of dietary folate on metastatic potential of A549 cancer cells through the control of cofilin phosphorylation. J Biol Chem. 2014;289(38):26383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li C, Gao S, Li X, Li C, Ma L. Procaine inhibits the proliferation and migration of colon cancer cells through inactivation of the ERK/MAPK/FAK pathways by regulation of RhoA. Oncol Res. 2018;26(2):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenblatt AE, Garcia MI, Lyons L, Xie Y, Maiorino C, Desire L, Slingerland J, Burnstein KL. Inhibition of the Rho GTPase, Rac1, decreases estrogen receptor levels and is a novel therapeutic strategy in breast cancer. Endocr Relat Cancer 2011;18(2):207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Q, Wang W, Yang X, Zhao D, Li F, Wang H. MicroRNA-146a inhibits cell migration and invasion by targeting RhoA in breast cancer. Oncol Rep. 2016;36(1):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao H, Zhang Y, Dong L, Qu XY, Tao LN, Zhang YM, Zhai JH, Song YQ. Triptolide induces autophagy and apoptosis through ERK activation in human breast cancer MCF-7 cells. Exp Ther Med. 2018;15(4):3413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng X, Shi W, Zhao C, Zhang D, Liang P, Wang G, Lu L. Triptolide sensitizes human breast cancer cells to tumor necrosis factor alpha induced apoptosis by inhibiting activation of the nuclear factor kappaB pathway. Mol Med Rep. 2016;13(4):3257–64. [DOI] [PubMed] [Google Scholar]
- 30. Shao H, Ma J, Guo T, Hu R. Triptolide induces apoptosis of breast cancer cells via a mechanism associated with the Wnt/beta-catenin signaling pathway. Exp Ther Med. 2014;8(2):505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]