Abstract
Human glioblastoma multiforme (GBM) accounts for the majority of human brain gliomas. Several TMEM proteins, such as TMEM 45A, TMEM 97, and TMEM 140, are implicated in human brain gliomas. However, the roles of TMEM168 in human GBM remain poorly understood. Herein we found that mRNA levels of TMEM168 were overexpressed in GBM patients (n = 85) when compared with healthy people (n = 10), which was also supported by data from The Cancer Genome Atlas (TCGA). Kaplan–Meier analysis of Gene Expression Omnibus dataset GSE16011 suggested that enhanced TMEM168 expression was associated with shorter survival time. To investigate whether and how TMEM168 functioned in the tumorigenesis of human GBM cells, two human GBM cell lines (U87 and U373) were used for study. Lithium chloride (LiCl), an activator for Wnt/β-catenin pathway, was used for the treatment. Our data suggested that siRNA-TMEM168 (siTMEM168) prevented viability of U87 and U373 cells, induced cell cycle arrest (G0/G1 phase) and promoted apoptosis, and the mechanisms involved in blocking Wnt/β-catenin pathway, as evidenced by reducing expression of β-catenin, C-myc, cyclin D1, and survivin. Furthermore, the inhibited effect of siTMEM168 on human GBM cell growth was significantly alleviated with additional LiCl treatment, substantiating the involvement of the Wnt/β-catenin pathway in this process. In summary, our data demonstrated that TMEM168 may represent a therapeutic target for the treatment of human GBM.
Key words: Human glioblastoma multiforme (GBM), Transmembrane 168 (TMEM168), U87 cells, U373 cells, Wnt/β-catenin pathway
INTRODUCTION
To date, glioblastoma multiforme (GBM) is identified as the most aggressive, deadly, and common type (15%) of human malignant brain tumor1 with an average survival of only 14 months after diagnosis2. The term “multiforme” reflects the histological features of this disease, that is to say, cellular and morphological heterogeneity3. In 2013, Smoll et al. reported that only a few (2.2%) patients with GBM can survive 3 years or more2. Over the past decade, more efforts have been taken to block GBM. Unfortunately, GBM treatment is still limited and insufficiently studied4; moreover, difficult overall prognosis, undesirable surgical resection, and radio- or chemoresistance make this disease more troublesome5. Thus, a better understanding of GBM and its underlying pathogenic mechanisms will provide us new possibilities for more specific and satisfactory therapies.
Transmembrane (TMEM) protein family is involved in some basic physiological processes, especially in plasma membrane ion channels, signal transduction pathways, as well as cell chemotaxis, adhesion, apoptosis, and autophagy. Among the TMEM protein family members, TMEM45A6, TMEM977, and TMEM1408 have been indicated in human GBM. TMEM168, known as a TMEM protein, is localized on the cell membrane and showed clinical significance in sensorimotor gating, anxiety schizophrenia, or other addiction disorder-like symptoms9. However, currently, the roles of TMEM168 in human GBM remain undefined.
The Wnt/β-catenin signaling pathway plays important roles in neurogenesis10, neuronal differentiation and migration11, as well as synapse and axonal growth12. The Wnt/β-catenin pathway prevents glioma angiogenesis in mouse glioma models13, influences the growth of malignant gliomas, and exerts oncogenic activities on human malignant glioma U87 glioma cells11. TMEM 64, TMEM 88, and TMEM 170B blocked the Wnt/β-catenin pathway in osteoblast, adipocyte, human hepatic stellate cells, or breast cancer cells14–16. However, the roles of TMEM168 and its relationship with Wnt/β-catenin pathway in GBM remain poorly understood.
In this article, we found that mRNA levels of TMEM168 were upregulated in GBM and predicated a poor survival. We established small interfering RNA (siRNA)-indicated TMEM168 silencing within GBM U87 and U373 cells. Techniques included cell proliferation assay, cell apoptosis assay, and cell cycle assay. Expression of β-catenin (the core component of the Wnt/β-catenin signaling pathway) and its down targets (C-myc, cyclin D1, and survivin) were measured. In addition, lithium chloride (LiCl) was used to active the Wnt/β-catenin signaling pathway. Our data suggested that knockdown of TMEM168 prevented the tumorigenesis of human GBM cells and the mechanisms involved in blocking the Wnt/β-catenin pathway.
MATERIAL AND METHODS
Bioinformatics Analysis
In 2017, 10 healthy people and a total of 85 patients who were confirmed for GBM (WHO grade IV) and aged between 14 and 79 were recruited by Huzhou Central Hospital. To study whether TMEM168 was involved in human GBM, mRNA levels of TMEM168 were detected by real-time (RT)-PCR in the brain tissue of GBM patients and healthy people. Our investigation strictly obeyed the Helsinki Declaration and was permitted by the Human Ethics Committee of Huzhou Central Hospital. Written informed agreement was obtained from each person involved in this study. In parallel, a total of 529 expression data of TMEM168 in GBM patients and 10 expression data of TMEM168 in healthy people were also downloaded in The Cancer Genome Atlas (TCGA; https://cancergenome.nih.gov/cancersselected/glioblastomamultiforme) to make further confirmation of the results.
In addition, 116 GBM cases including 80 males and 36 females with a mean age of 51.6 ± 13.5 were downloaded from NCBIs Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) with accession No. GSE1601117. Then overall survival analysis was evaluated for mRNA expression of TMEM168 using the Kaplan–Meier method.
Cell Culture, Treatment, and siRNA-TMEM168 (siTMEM168) Transfection
Human GBM cell lines (U87 and U373; ATCC, Manassas, VA, USA) were placed in DMEM/4.0 mM l-glutamine medium (SH30243.01; HyClone, Logan, UT, USA) with fetal bovine serum [10% (v/v); 16000-044; Gibco, Carlsbad, CA, USA], as well as 100 U/ml penicillin and 0.1 mg/ml streptomycin (P1400-100; Solarbio, Beijing, P.R. China) at 37°C under 5% CO2 atmosphere. Cells in logarithmic growth were digested using trypsin (T1300-100; Solarbio) and suspended as 3 × 104 cells/ml in culture medium.
Three siRNA sequences targeting TMEM168 (GenBank No. NM_001287497.1) were designed as shown in Table 1. Cells were transfected with siRNAs (20 μmol/L) via Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to a reported study18. After 48 h, silencing efficacy of TMEM168 gene was detected by RT-PCR and Western blot, with the best knockdown effect being seen in Table 1 (Label 1), which was chosen in our following study. A scramble siRNA was identified as a negative control (siRNA-NC).
Table 1.
siRNA-TMEM168 Sequences
| Label 1 | |
| TMEM168 | 5′-GGATGTGACTATTCCACAA-3′, start point 1,873 |
| siRNA forward | 5′-CCGGGGATGTGACTATTCCACAACTCGAGTTGTGGAATAGTCACATCCTTTTT-3′ |
| siRNA reverse | 5′-AATTAAAAAGGATGTGACTATTCCACAACTCGAGTTGTGGAATAGTCACATCC-3′ |
| Label 2 | |
| TMEM168 | GGTCCTGATTAGAAAGAAA, start point 2,846 |
| siRNA forward | 5′-CCGGGGTCCTGATTAGAAAGAAACTCGAGTTTCTTTCTAATCAGGACCTTTTT-3′ |
| siRNA reverse | 5′-AATTAAAAAGGTCCTGATTAGAAAGAAACTCGAGTTTCTTTCTAATCAGGACC-3′ |
| Label 3 | |
| TMEM168 | 5′-CACAAGTGATGATAGTTAA-3′, start point 6,121 |
| siRNA forward | 5′-CCGGCACAAGTGATGATAGTTAACTCGAGTTAACTATCATCACTTGTGTTTTT-3′ |
| siRNA reverse | 5′-AATTAAAAACACAAGTGATGATAGTTAACTCGAGTTAACTATCATCACTTGTG-3′ |
To study whether the Wnt/β-catenin pathway was involved, lithium chloride (LiCl), a Wnt activator inhibitor, was used for treatment. In our study, U87 cells with siRNA-TMEM168 (siTMEM168) or siRNA-NC (siNC) transfection were treated with LiCl [10 mmol/L; L130113; Aladdin (http://www.aladdin-e.com)] or vehicle [phosphate-buffered saline (PBS)], and then cells were cultured normally as mentioned above.
Cell Proliferation
In 96-well plates, 3.0 × 103 cells/well was cultured overnight before treatment. After transfection, cell proliferation at 0, 24, 48, and 72 h was measured, respectively, using the Cell Counting Kit-8 (CCK-8; CP002; SAB, Shanghai, P.R. China). Briefly, a mixture of 10% cell suspension/90% CCK-8 solution (v/v; total 100 μl) was induced for an additional 1 h, and then optical density (OD, 450 nm) was measured. The larger the OD value, the higher the cell viability.
Cell Cycle Analysis
In 96-well dishes, 3.0 × 103 cells/well was cultured overnight before treatment. After transfection, cells were conventionally trypsinized, washed with PBS, and then maintained in 70% ethanol at 4°C for 24 h. The samples were resuspended with RNAase (AR8020-25; Solarbio) and exposed to 50 μg/ml of propidium iodide (PI; 400 μl; C001-200; 7Sea Biotech, Shanghai, P.R. China). DNA content and cell cycle were analyzed using flow cytometry (BD Biosciences, San Jose, USA) and FlowJo software (Tree Star, Ashland, OR, USA).
Cell Apoptosis Assessment
Fluorescein isothiocyanate-labeled recombinant (Annexin-V–FITC) Apoptosis Detection Kit (C1062; Beyotime, Shanghai, P.R. China) was performed to detect cell apoptosis following the provided protocol. Cells were incubated with 5 μl of annexin V–FITC followed by 5 μl of PI for 15 min in darkness, respectively, and apoptotic cells were measured by a flow cytometer (Accuri C6; Becton Dickinson, San Jose, CA, USA). Dead cells were PI+ and presented in the upper left quadrant; healthy cells were annexin−/PI− and presented in the lower left quadrant; late apoptotic cells were annexin V++/PI++ and presented in the upper right quadrant; early apoptotic cells were annexin+ and presented in the lower right quadrant.
Quantitative Real-Time (RT)-PCR and Western Blotting
Routinely extracted total RNA, by TRIzol regent (1596-026; Invitrogen), underwent quantitative RT-PCR analysis using SYBR Green PCR Kit (No. K0223, Thermo Fisher Scientific, Waltham, MA, USA) and ABI Prism 7300 SDS Software (Applied Biosystem, Foster City, CA, USA) with programmed reaction conditions as follows: 95°C, 10 min for (95°C, 15 s; 60°C, 45 s) ×40 cycles, 95°C for 15 s, and then 60°C for 1 min. The primer pairs targeting TMEM168 (GenBank NM_001287497.1) were: 5′-GCCCACTTTACTAACCAC-3′ (forward); 5′-CATAGCCAGAGCTACAAC-3′ (reverse); position: 933–1,050; product size: 118 bp, GC 44%. The primer pairs targeting glyceraldehyde-3-phosphate dehydrogenase (GADPH; GenBank NM_001256799.2), an internal standard, were: 5′-AATCCCATCACCATCTTC-3′ (forward); 5′-AGGCTGTTGTCATACTTC-3′ (reverse); position: 436–653; product size: 218 bp, GC 56%.
Total protein (30 μg) in the supernatant of cell lysis underwent Western blotting according to a reported study18. SDS-PAGE gel (10%–15%) combined with a nitrocellulose membrane (NC; Millipore, Bedford, MA, USA) was used for separation. Antibodies used in our study were antibody against TMEM168 (22527-1-AP; Proteintech, Wuhan, P.R. China), antibody against β-catenin [ab32572; Abcam (https://www.abcam.cn)], antibody against proliferation-associated cell nuclear antigen (PCNA; ab92552; Abcam), antibody against C-myc (ab32072; Abcam), antibody against cyclin D1 (ab134175; Abcam), antibody against survivin (ab76424; Abcam), antibody against Bax (ab32503; Abcam), antibody against GAPDH (No. 5174; CST, Beverly, MA, USA), and horseradish peroxidase (HRP)-conjugated secondary antibody (Beyotime).
Statistical Analysis
GraphPad Prism 7.0 and MedCalc software were adopted for graphing and statistical analysis. Data mentioned in our study were expressed as mean ± standard error of the mean (SEM; n = 3). Statistical methods used for between groups include the Kaplan–Meier method, t-test, and one-way analysis of variance (ANOVA). A value of p < 0.05 was considered statistically significant.
RESULTS
Elevated TMEM168 Was Associated With Poor Prognosis in Human GBM
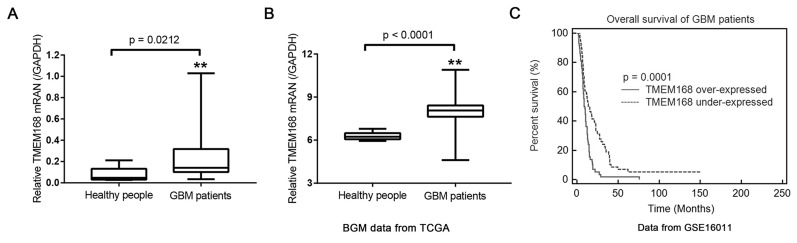
To study whether TMEM168 was involved in human GBM, mRNA levels of TMEM168 were determined using RT-PCR in brain tissues of GBM patients (n = 85) and corresponding healthy people (n = 10) from Huzhou Central Hospital. Our data demonstrated that TMEM168 was significantly enhanced in GBM patients when compared with that in healthy people (p < 0.05) (Fig. 1A). Similar result was also obtained based on data from TCGA dataset (p < 0.01) (Fig. 1B).
Figure 1.
Correlation between mRNA level of transmembrane 168 (TMEM168) and survival time of glioblastoma multiforme (GBM) patients. (A) mRNA level of TMEM168 was significantly increased in the brain tissue of GBM patients (n = 85) treated at Huzhou Central Hospital when compared with healthy people (n = 10). (B) mRNA level of TMEM168 was much higher in the brain tissue of GBM patients from The Cancer Genome Atlas (TCGA) dataset (n = 529, p < 0.05) when compared with the corresponding healthy people (n = 10). (C) The overall survival time of 116 patients with GBM from GEO dataset GSE16011 (n = 116). **p < 0.01 versus healthy people.
We then studied whether overexpression of TMEM168 was involved in GBM prognosis. Among the 116 GBM patients from GEO dataset (Access ID: GSE16011), the median mRNA expression of TMEM168 was 9.08, thus those patients were divided into two groups: TMEM168 overexpression group (n = 58, relative mRNA levels of TMEM168 > 9.08) and TMEM168 low expression group (n = 58, relative mRNA levels of TMEM168 < 9.08). Kaplan–Meier analysis (Fig. 1C) indicated that the patients with low expression of TMEM168 had significantly longer survival time (median survival 13.92 months) than that with high expression of TMEM168 (median survival 8.76 months). Above all, our data elucidated that increased TMEM168 expression in GBM patients was correlated with poor survival.
Knockdown of TMEM168 Within Human GBM Cells Inhibited Cell Proliferation, Promoted Apoptosis, and Induced Cell Cycle Arrest
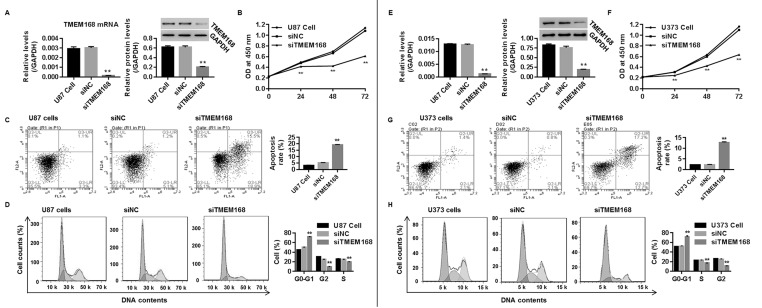
To study whether TMEM168 deficiency represented a therapy in human GBM in vitro, we injected siTMEM168 into two human GBM cell lines (U87 and U373). As shown in Figure 2A and E, significant decrease in mRNA and protein levels of TMEM168 were found in the siTMEM168 group when compared with siNC, demonstrating a successful establishment of TMEM168 silencing within those two cells.
Figure 2.
TMEM168 silencing suppressed tumorigenesis of human GBM cells. After transfection for 48 h, protein and mRNA levels of TMEM168 in human (A) U87 cells and (E) U373 cells were measured by Western blotting and real-time (RT)-PCR, respectively. Knockdown of TMEM168 within U87 and U373 cells time dependently inhibited cell proliferation, assessed by the Cell Counting Kit-8 (CCK8) method (B and F); promoted cell apoptosis, analyzed by annexin V/PI staining (C and G); and increased the proportion of cells in G0/G1 phase, while reducing the proportion of cells in G2 and S phases, assessed by flow cytometry (D and H). **p < 0.01 versus siNC group.
The effect of siTMEM168 on U87 and U373 cell proliferation, cell apoptosis, and cell cycle were assessed by CCK-8, annexin V/PI staining, and flow cytometry, respectively. As shown in Figure 2B and F, siTMEM168 prevented the viability of U87 and U373 cells in a time-dependent manner with inhibited proliferation being 15%, 39%, and 46% at 24, 48, and 72 h in U87 cells, and 20%, 32%, and 45% at 24, 48, and 72 h in U373 cells, respectively, when compared with siNC. Figure 2C and G suggested that siTMEM168 promoted cell apoptosis by approximately threefold in U87 cells and fivefold in U373 cells when compared with siNC. Besides, Figure 2D and H shows that siTMEM168 obviously induced a cell cycle arrest at G0/G1 phase within those two cells when compared with the corresponding control (all p < 0.05).
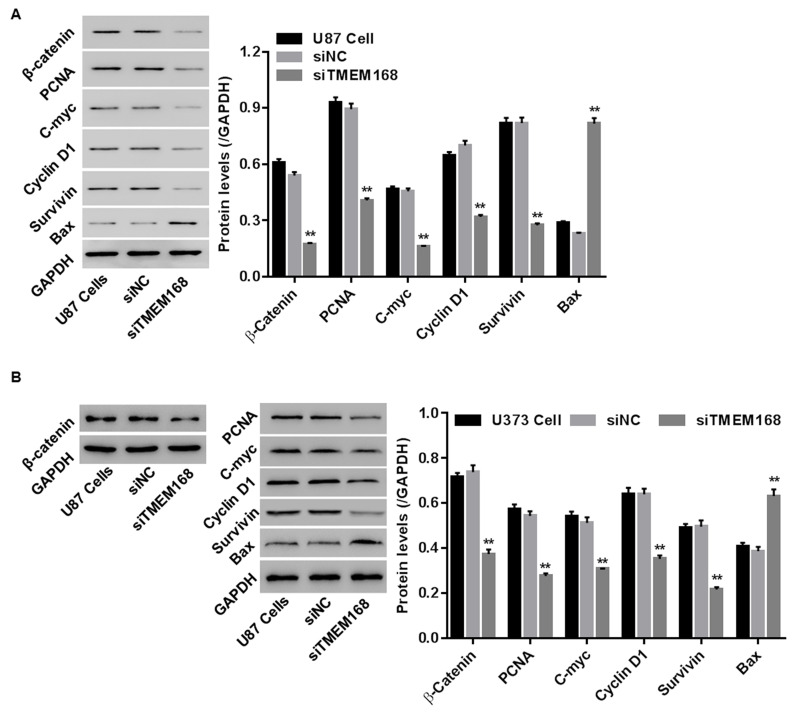
To substantiate the underlying molecular mechanism, protein levels of PCNA (a cell proliferative maker), Bax (a pro-apoptotic factor), survivin (an antiapoptotic factor), as well as C-myc and cyclin D1 (a positive regulatory factor in G1/GS progression) were detected by Western blotting in U87 and U373 cells. Our data showed that siTMEM168 reduced PCNA, decreased survivin, and increased Bax, as well as inhibited cyclin D1 (Fig. 3) within those two cells, confirming the anticancer effect of siTMEM168 in U87 cells at a molecular level.
Figure 3.
siTMEM168 regulated expressions of cell proliferation-related [proliferation-associated cell nuclear antigen (PCNA)], apoptosis-related (survivin and Bax), cell cycle-related (cyclin D1), and Wnt/β-catenin pathway-related (β-catenin, C-myc, cyclin D1, and survivin) proteins in (A) U87 cells and (B) U373 cells. **p < 0.01 versus siNC group.
Knockdown of TMEM16 Inhibited the Wnt/β-Catenin Pathway in Human GBM Cells
To study whether the Wnt/β-catenin pathway was engaged in anticancer effect of siTMEM168 in human GBM cells, after transfection for 48 h, protein levels of β-catenin and its down targets (C-myc, cyclin D1, and survivin) were assessed in U87 and U373 cells. We found that siTMEM168 significantly reduced the expressions of β-catenin, C-myc, cyclin D1, and survivin when compared with that in the siNC group within those two cells (Fig. 3), suggesting that siTMEM168 blocked the activation of the Wnt/β-catenin pathway in human GBM cells.
siTMEM168 Blocked the Wnt/β-Catenin Pathway to Regulate U87 Cell Proliferation
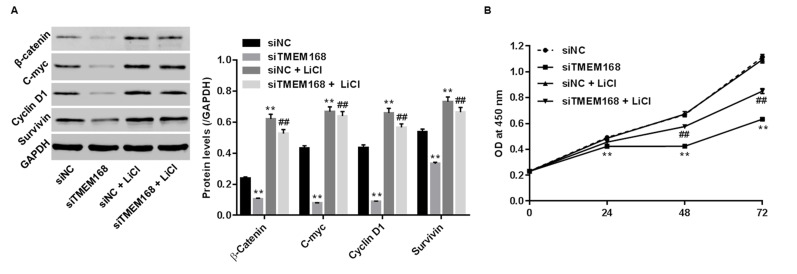
To substantiate the involvement of the Wnt/β-catenin pathway in the anticancer effect of siTMEM168 on U87 cells, LiCl was used to active the Wnt/β-catenin pathway. As shown in Figure 4A, LiCl significantly elevated the expressions of β-catenin (the core component of the Wnt/β-catenin signaling pathway) and its down targets (C-myc, cyclin D1, and survivin) in siNC/siTMEM168-transfected cells, confirming the activation of the Wnt/β-catenin pathway. Treatment with LiCl alone had no effect on proliferation of siRNA-injected cells (Fig. 4B). On the contrary, siTMEM168 significantly suppressed Wnt/β-catenin activation and inhibited proliferation of U87 cells, the changes of which were obviously reversed with additional LiCl treatment (Fig. 4), demonstrating the reverse effect of LiCl in siTMEM168-transfected U87 cells.
Figure 4.
Wnt/β-catenin pathway was the mechanism by which TMEM168 exerted its oncogenic activities in human GBM cells. siTMEM168 induced changes in (A) protein levels of β-catenin, C-myc, cyclin D1, and survivin, and (B) proliferative changes were significantly reversed by a Wnt/β-catenin activator LiCl in U87 cells. **p < 0.01 versus siNC; ##p < 0.01 versus siTMEM168.
DISCUSSION
TMEM168 protein, consisting of 697 amino acid residues, is expressed in both humans and mice19. Upregulated TMEM168 expression promotes sensorimotor gating deficit, anxiety schizophrenia, or other addiction disorder-like symptoms9; however, whether and how TMEM168 functions in human GBM have been scarcely reported. In this study, we identified that TMEM168 was upregulated in the brain tissue of patients with GBM (Fig. 1A), the results of which were also confirmed by data from TCGA dataset (Fig. 1B), demonstrating the involvement of TMEM168 in human GBM.
GBM represents about 80% of all glioblastomas20 and commonly occurs in adults aged 45–7021 with poor prognostic analysis. Currently, many TMEM proteins, such as TMEM4, TMEM48, TMEM173, can serve as prognostic makers in pancreatic cancer22, lung carcinoma23, and hepatocellular carcinoma24. In this study, Kaplan–Meier analysis was performed to study the relationship between expression level of TMEM168 and survival time of GBM patients. Our data first elucidated that the survival time of GBM patients with TMEM168 low expression was significantly prolonged compared with TMEM168 high expression (Fig. 1C), demonstrating that TMEM168 may serve as a prognostic factor of survival time for human GBM.
To study whether knockdown of TMEM168 would prevent the tumorigenesis of human GBM in vitro, siTMEM168 was injected into two human GBM cell lines (U87 and U373 cells) to reduce mRNA and protein expressions of endogenous TMEM168 (Fig. 2A), and then cell proliferation, cell cycle, and apoptosis were assessed. We found that TMEM168 depletion significantly inhibited proliferation of U87 and U373 cells (Fig. 2B), which was accompanied with reduced protein levels of PCNA. The growth inhibitory effect was mediated by cell cycle arrest (G0/G1 phase) (Figs. 2D and H and 3). Furthermore, siTMEM168 remarkably promoted apoptosis of glioma U87 cells, which could be attributed to increased expression of Bax and decreased expression of survivin (Figs. 2C and G and 3). Taken together, these results suggest that downregulation of TMEM168 expression suppressed the tumorigenesis of human GBM cells. TMEM168 may be a potential therapeutic target for human GBM.
The Wnt/β-catenin signaling pathway exerts oncogenic activities on U87 glioma cells by modulating cell proliferation, cell cycle, and apoptosis11. In this study, we investigated whether the Wnt/β-catenin pathway participated in the anticancer effect of siTMEM16 in human GBM cells. We found that TMEM168 depletion blocked the Wnt/β-catenin pathway in U87 and U373 cells, as proven by reduced protein expressions of β-catenin, C-myc, cyclin D1, and survivin (Fig. 3). However, the effect of siTMEM168 on the tumorigenesis of U87 cells (only proliferation was assessed in our intervention studies) was remarkably attenuated with additional LiCl, a specific Wnt/β-catenin activator (Fig. 4), verifying the involvement of the Wnt/β-catenin signaling pathway in the anticancer effect of siTMEM168.
In conclusion, our data suggested that TMEM168 was overexpressed in brain tissues of GBM patients and also could be used as a prognostic marker for survival in GBM patients. The tumorigenesis of glioma U87 and U373 cells was increased following TMEM168 silencing, and TMEM168 silencing was associated with Wnt/β-catenin pathway inhibition. We propose that TMEM168 may be a potential therapeutic target for GBM. Moreover, further studies on other GBM cells, such as BB184, DA66, JM94, T98G, and U118, should be conducted.
ACKNOWLEDGMENTS
This work was supported by grant 2016GYB38 from Huzhou General Science and Social Development Project.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Pooladi M, Rezaeitavirani M, Hashemi M, Hesamitackallou S, Khaghaniraziabad S, Moradi A, Zali AR, Mousavi M, Firozidalvand L, Rakhshan A. Cluster and principal component analysis of human glioblastoma multiforme (GBM) tumor proteome. Iran J Cancer Prev. 2014;7(2):87–95. [PMC free article] [PubMed] [Google Scholar]
- 2. Smoll NR, Schaller K, Gautschi OP. Long-term survival of patients with glioblastoma multiforme (GBM). J Clin Neurosci. 2013;20(5):670–5. [DOI] [PubMed] [Google Scholar]
- 3. Baronchelli S, Bentivegna A, Redaelli S, Riva G, Butta V, Paoletta L, Isimbaldi G, Miozzo M, Tabano S, Daga A. Delineating the cytogenomic and epigenomic landscapes of glioma stem. PLoS One 2016;8(2):e57462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Urbańska K, Sokołowska J, Szmidt M, Sysa P. Glioblastoma multiforme—An overview. Contemp Oncol. (Pozn) 2014;18(5):307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nikiforova ZN, Kudryavtsev IA, Arnotskaya NE, Bryukhovetskiy IS, Shevchenko VE. Tumor stem cells from glioblastoma multiforme. Uspehi Molekulârnoj Onkologii 2016;3(2):26–33. [Google Scholar]
- 6. Sun W, Qiu G, Zou Y, Cai Z, Wang P, Lin X, Huang J, Jiang L, Ding X, Hu G. Knockdown of TMEM45A inhibits the proliferation, migration and invasion of glioma cells. Int J Clin Exp Pathol. 2015;8(10):12657–67. [PMC free article] [PubMed] [Google Scholar]
- 7. Qiu G, Sun W, Zou Y, Cai Z, Wang P, Lin X, Huang J, Jiang L, Ding X, Hu G. RNA interference against TMEM97 inhibits cell proliferation, migration, and invasion in glioma cells. Tumour Biol. 2015;36(10):8231–8. [DOI] [PubMed] [Google Scholar]
- 8. Li B, Huang MZ, Wang XQ, Tao BB, Zhong J, Wang XH, Zhang WC, Li ST. TMEM140 is associated with the prognosis of glioma by promoting cell viability and invasion. J Hematol Oncol. 2015;8(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fu K, Miyamoto Y, Sumi K, Saika E, Muramatsu SI, Uno K, Nitta A. Overexpression of transmembrane protein 168 in the mouse nucleus accumbens induces anxiety and sensorimotor gating deficit. PLoS One 2017;12(12):e0189006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang L, Yang X, Yang S, Zhang J. The Wnt/β-catenin signaling pathway in the adult neurogenesis. Eur J Neurosci. 2011;33(1):1–8. [DOI] [PubMed] [Google Scholar]
- 11. Gao L, Chen B, Li J, Yang F, Cen X, Liao Z, Long X. Wnt/β-catenin signaling pathway inhibits the proliferation and apoptosis of U87 glioma cells via different mechanisms. PLoS One 2017;12(8):e0181346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inestrosa NC, Varela-Nallar L. Wnt signalling in neuronal differentiation and development. Cell Tissue Res. 2015;359(1):215–23. [DOI] [PubMed] [Google Scholar]
- 13. Reis M, Czupalla CJ, Ziegler N, Devraj K, Zinke J, Seidel S, Heck R, Thom S, Macas J, Bockamp E. Endothelial Wnt/β-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J Exp Med. 2012;209(9):1611–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeong BC, Kim TS, Kim HS, Lee SH, Choi Y. Transmembrane protein 64 reciprocally regulates osteoblast and adipocyte differentiation by modulating Wnt/β-catenin signaling. Bone 2015;78(9):165–73. [DOI] [PubMed] [Google Scholar]
- 15. Xu T, Pan LX, Ge YX, Li P, Meng XM, Huang C, Li J. TMEM88 mediates inflammatory cytokines secretion by regulating JNK/P38 and canonical Wnt/β-catenin signaling pathway in LX-2 cells. Inflammopharmacology 2018;26(5):1339–48. [DOI] [PubMed] [Google Scholar]
- 16. Li M, Han Y, Zhou H, Li X, Lin C, Zhang E, Chi X, Hu J, Xu H. Transmembrane protein 170B is a novel breast tumorigenesis suppressor gene that inhibits the Wnt/β-catenin pathway. Cell Death Dis. 2018;9(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gravendeel LA, Kouwenhoven MC, Gevaert O, de Rooi JJ, Stubbs AP, Duijm JE, Daemen A, Bleeker FE, Bralten LB, Kloosterhof NK. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009;69(23):9065–72. [DOI] [PubMed] [Google Scholar]
- 18. Yi Y, Ma J, Jianrao L, Wang H, Zhao Y. WISP3 prevents fibroblast-myofibroblast transdifferentiation in NRK-49F cells. Biomed Pharmacother. 2018;99:306–12. [DOI] [PubMed] [Google Scholar]
- 19. Fu K, Miyamoto Y, Otake K, Sumi K, Saika E, Matsumura S, Sato N, Ueno Y, Seo S, Uno K. Involvement of the accumbal osteopontin-interacting transmembrane protein 168 in methamphetamine-induced place preference and hyperlocomotion in mice. Sci Rep. 2017;7(1):13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ulutin C, Fayda M, Aksu G, Cetinayak O, Kuzhan O, Ors F, Beyzadeoglu M. Primary glioblastoma multiforme in younger patients: A single-institution experience. Tumori 2006;92(5):407–11. [DOI] [PubMed] [Google Scholar]
- 21. Kleinschmidtdemasters BK, Meltesen L, Mcgavran L, Lillehei KO. Characterization of glioblastomas in young adults. Brain Pathol. 2010;16(4):273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sho M, Adachi M, Taki T, Hashida H, Konishi T, Huang CL, Ikeda N, Nakajima Y, Kanehiro H, Hisanaga M. Transmembrane 4 superfamily as a prognostic factor in pancreatic cancer. Int J Cancer 1998;79(5):509–16. [DOI] [PubMed] [Google Scholar]
- 23. Qiao W, Han Y, Wei J, Mi T, Pei C, Jie M, Hu H, Xu B, Zhu W, Xiong L. Overexpression and biological function of TMEM48 in non-small cell lung carcinoma. Tumour Biol. 2016;37(2):2575–86. [DOI] [PubMed] [Google Scholar]
- 24. Bu Y, Liu F, Jia QA, Yu SN. Decreased expression of TMEM173 predicts poor prognosis in patients with hepatocellular carcinoma. PLoS One 2016;11(11):e0165681. [DOI] [PMC free article] [PubMed] [Google Scholar]