Abstract
Background
Refractometry is used to assess transfer of passive immunity (TPI), but studies evaluating different refractometers and appropriate thresholds for recommended target immunoglobulin G (IgG) concentrations for beef calves are limited.
Objectives
To evaluate test performance of digital (DSTP) and optical (OSTP) serum total protein (STP) refractometers and a digital Brix (DBRIX) refractometer for assessment of passive immunity in beef calves.
Animals
A total of 398 beef calves from 6 herds, 1 to 7 days of age.
Methods
Serum IgG concentration was estimated by DSTP, OSTP, and DBRIX, and measured by radial immunodiffusion (RID). Correlation coefficients (r) among results were calculated. Optimal STP and Brix thresholds for identification of IgG <10, <16, and <24 g/L were determined using interval likelihood ratios. Refractometer performance and agreement were assessed using areas under the curve (AUC), diagnostic test characteristics, Cohen's kappa (κ), and Bland‐Altman analysis.
Results
Refractometer results were highly correlated with RID (r = 0.82‐0.91) and with each other (r = 0.91‐0.95), and overall test performance was excellent (AUC = 0.93‐0.99). The STP concentrations of ≤5.1, ≤5.1, and ≤5.7 g/dL and Brix percentages of ≤7.9%, ≤8.3%, and ≤8.7% indicated IgG concentrations <10, <16, and <24 g/L, respectively. Agreement of refractometers with RID was variable (κ = 0.46‐0.80) and among refractometers was substantial (κ = 0.62‐0.89).
Conclusions and Clinical Importance
All refractometers showed good utility as monitoring tools for assessment of TPI in beef calves.
Keywords: beef calves, Brix, immunoglobulin G, refractometer, serum total protein, transfer of passive immunity
Abbreviations
- AUC
area under the curve
- CI
confidence interval
- DBRIX
digital Brix refractometer
- DSTP
digital serum total protein refractometer
- IgG
immunoglobulin G
- LHR
likelihood ratio
- NPV
negative predictive value
- PPV
positive predictive value
- OSTP
optical serum total protein refractometer
- QAL
Quality Assurance Laboratory
- r
correlation coefficient
- RID
radial immunodiffusion
- ROC
receiver operating characteristic
- SD
standard deviation
- Se
sensitivity
- Sp
specificity
- STP
serum total protein
- TPI
transfer of passive immunity
- κ
Cohen's kappa
1. INTRODUCTION
Transfer of passive immunity (TPI) in calves occurs by ingestion of colostrum within the first 24 hours postpartum. 1 Inadequate transfer of immunoglobulins, mainly immunoglobulin G (IgG), leaves calves vulnerable to disease and increases their risk of mortality. 1 , 2 , 3 , 4 Therefore, determination of TPI status is essential for assessment of the effectiveness of on‐farm colostrum management practices and crucial to minimize negative health and performance outcomes. 1
Serum IgG concentrations can be assessed using direct and indirect testing methods. Radial immunodiffusion (RID), which is the current reference test for direct IgG determination, is impractical for clinical use because of its relatively high cost, need for skilled laboratory technicians, and a turn‐around time of 24 to 48 hours. 1 Other ways to directly quantify serum IgG concentrations in calves are ELISA 5 , transmission infrared spectroscopy 6 , and automated turbidimetric immunoassay, 7 all of which either have limited availability, are cost‐prohibitive, or are impractical for clinical application. Substantial research efforts have been made to develop and validate indirect tests that estimate IgG concentrations based on measurement of substrates that are highly correlated with serum IgG concentration. 8 Digital and optical serum total protein (DSTP and OSTP) or Brix refractometers have received much interest from researchers, veterinarians, and producers. 8 These refractometers measure STP (g/dL) or total solids (% Brix) in nonsucrose‐containing fluids such as colostrum or serum. A recent systematic review, however, identified an overall lack of reported studies evaluating the accuracy of optical and digital refractometers against the current reference test RID, or comparing different refractometers with each other. 8 Furthermore, appropriate STP and Brix thresholds to determine failed or inadequate TPI are limited for beef calves 9 , 10 , perhaps because consensus on the definition of adequate TPI in beef calves is lacking. It is generally accepted that IgG concentrations <10 g/L indicate failed TPI. 1 , 8 However, IgG concentrations ≥16 and ≥24 g/L are associated with decreased morbidity and mortality in beef calves 2 , 4 and therefore may be more appropriate for the definition of adequate TPI. Regardless, a single, generic IgG target recommendation may be somewhat misleading, because it likely would not be universally applicable given the many factors involved in passive immunity and the incidence of disease within a herd. It therefore may be useful to determine STP and Brix thresholds not only for confirmation of failed TPI (IgG <10 g/L), but also for detection of inadequate TPI using higher potential IgG target concentrations (eg, IgG <16 g/L 4 , 9 or < 24 g/L 2 , 4 ).
Our overall aim was to evaluate the performance of DSTP and OSTP refractometry and digital Brix refractometry (DBRIX) to assess TPI in neonatal beef calves. The specific objectives were to (a) assess the correlation among results obtained by DSTP, OSTP, DBRIX, and RID; (b) establish STP concentrations and Brix percentages that predict IgG <10, <16, and <24 g /L; and (c) assess agreement among results obtained by DSTP, OSTP, DBRIX, and RID for detection of IgG <10, <16, and <24 g /L.
2. MATERIALS AND METHODS
2.1. Serum samples
A convenience sample of 398 serum samples was available for this diagnostic test evaluation. Samples were collected between February and May 2018 for a study investigating specific antibody titers in neonatal beef calves (data not published). Sampling was conducted in accordance with guidelines established by the Canadian Council on Animal Care and the study was approved by the University of Calgary Veterinary Sciences Animal Care Committee (AC16‐0209). Samples originated from 6 cow‐calf operations located in Alberta, Canada. Herd characteristics (ie, herd size and predominant breed type) and peri‐parturient factors (ie, calving ease, colostrum source, route of colostrum delivery) were recorded. All blood samples were collected from apparently healthy (based on visual examination) beef calves at 1 to 7 days of age by research personnel or trained ranch staff. Calves that were overtly dehydrated or undergoing treatment for any morbidity on the day of blood collection were not enrolled. Whole blood was collected into sterile 10‐mL vacuum tubes without anticoagulant (BD Vacutainer tubes, Becton Dickinson, Franklin Lakes, New Jersey) by jugular venipuncture and, if necessary, samples were refrigerated on farm before being transported on ice to the laboratory at the University of Calgary. Serum was separated by centrifugation at 3000 × g for 15 minutes at room temperature and samples were frozen in triplicate at −80°C until analysis. Samples were transported on ice to the Saskatoon Colostrum Company Ltd. Quality Assurance Laboratory (QAL; Saskatoon, Saskatchewan, Canada) for analysis.
2.2. Laboratory analysis
The STP concentration was determined using both a digital handheld (DSTP; Misco Palm Abbe PA203, MISCO Refractometer, Solon, Ohio) and an optical handheld (OSTP; J‐0351, Jorvet temperature compensated refractometer, Jorgensens Labs, Loveland, Colorado) refractometer. The DSTP and OSTP precisions indicated by the manufacturers were 0.1 and 0.2 g/dL, respectively. A DBRIX (PAL‐1, Atago Co. Ltd, Bellevue, Washington) was used to determine Brix percentages as a measure of total solids. The DBRIX had a precision of 0.1% Brix. All refractometry tests were performed according to manufacturer's instructions at room temperature, zeroed with distilled water before each use, and the prism cleaned with distilled water before each sample reading. All samples were tested once only. An in‐house RID assay 11 was performed at the QAL as the reference test for this study with modifications as described previously. 12 The RID and DSTP assays were performed concurrently at the QAL, and the OSTP and DBRIX were performed concurrently at the University of Calgary. The personnel performing the analyses were blinded to results of other tests.
2.3. Statistical analysis
Statistical analysis was performed using STATA 16.1 (StataCorp, College Station, Texas) and R software (R Core Team, 2019; version 3.5.3), with results considered significant at P < .05. Normality of the data was assessed visually using histograms and normality probability plots. Descriptive statistics for STP concentrations determined by DSTP and OSTP, Brix percentages determined by DBRIX, and IgG concentrations determined by RID subsequently were calculated as means and standard deviations (SD).
2.3.1. Correlation coefficients
Results obtained by different refractometers (g/dL or % Brix) were plotted against the IgG concentration measured by RID (g/L) and against each other in scatter plots. Pearson correlation coefficients were calculated to assess the correlation of results obtained by each of the 3 refractometers and RID, as well as the correlation of results among the 3 refractometers. The statistical difference between any 2 correlation coefficients was investigated using the cocor package (R Core Team, 2019; version 3.5.3). 13
2.3.2. Overall test performance and threshold determination
Because of the low number of samples with IgG <10 g/L in the study population (16/398), a power analysis was performed using the pwr package (R Core Team, 2019; version 3.5.3) and determined that the number of serum samples with IgG <10 g/L was sufficient to give adequate power (>80%) for subsequent statistical analyses. 14
Receiver operating characteristic (ROC) curves were constructed for each of the refractometers and for 3 IgG conditions (<10, <16, and <24 g/L). Areas under the curve (AUC) were examined to assess the overall test accuracy of each refractometer to determine TPI at the evaluated IgG target conditions across the range of STP and Brix value thresholds. An AUC of 0.7 to 0.9 was considered moderately accurate, an AUC of >0.9 highly accurate, and an AUC of 1 perfect. 15 Differences in the AUC of DSTP, OSTP, and DBRIX were compared for the 3 applications using the roc.test function in pROC package (R Core Team, 2019; version 3.5.3). 16
Likelihood ratios (LHRs) were considered more clinically useful for this study compared with the traditional approach of selecting a single cut‐point based solely on the highest sum of sensitivity and specificity because LHR use more information in a given dataset and minimize the risk of distortion. 17 , 18 Calves were assigned to 1 of the following strata based on their STP concentration: ≤4.5, 4.6 to 5.1, 5.2 to 5.7, 5.8 to 6.3, 6.4 to 6.9, ≥7 g/dL. Likewise, calves were assigned to 1 of the following strata based on their serum Brix percentages: ≤7.9%, 8.0% to 8.3%, 8.4% to 8.7%, 8.8% to 9.1%, 9.2% to 9.5%, 9.6% to 9.9%, 10.0% to 10.3%, ≥10.4% Brix. These strata were chosen to examine biologically important differences for both indirect measures while avoiding the lack of precision that can occur if the chosen intervals and subsequent numbers of calves in each stratum are too small. 17 Serum IgG concentration was dichotomized as follows for the 3 evaluated IgG concentrations: IgG <10 or ≥10 g/L, <16 or ≥16 g/L, or <24 or ≥24 g/L.
Interval LHRs were calculated for each interval range using the following modified formula, as described previously 15 :
where P (test result | IgG condition present) is the probability of a refractometry result (g/dL or % Brix) in serum that truly contains IgG <10, <16, or <24 g/L, and P (test result | IgG condition absent) is the probability of a refractometry result in serum that does not contain IgG <10, <16, or <24 g/L. Thus, LHR was defined as the likelihood that a calf with a STP concentration or Brix % in a given stratum would contain IgG <10, <16, or <24 g/L, respectively. A LHR of >1 was interpreted as indicative for the target condition (ie, IgG <10, <16, or <24 g/L), whereas a LHR <1 was interpreted as protective for the target condition, and a LHR of 1 was considered to have no effect. The confidence intervals (CI) for LHR were calculated as described elsewhere 19 and a LHR with a CI including 1 was considered not statistically significant. Whenever a stratum contained 0 calves, 1 count was added to each stratum for that calculation. The upper limit of the last interval ratio with a LHR >1 and a CI not including 1 was chosen as a threshold for detection of IgG <10, <16, or <24 g/L. To investigate if being fed a colostrum product in addition to consuming maternal colostrum had undue influence on the study results 20 , 21 , data from these calves were removed from the dataset and statistical analysis repeated.
Epidemiologic test characteristics (sensitivity, specificity, Youden index, positive and negative predictive values, and accuracy) were calculated for the thresholds selected by LHR. Sensitivity (Se) was defined as the proportion of serum samples containing IgG <10, <16, or <24 g/L, respectively, that were correctly detected by the respective refractometer. Specificity (Sp) was defined as the proportion of serum samples with IgG ≥10, ≥16, or ≥24 g/L correctly detected by the respective refractometer. The Youden index (J) was calculated by using the following formula:
Positive predictive value (PPV) was defined as the proportion of calves classified by refractometry to contain IgG <10, <16, or <24 g/L that truly had IgG concentrations <10, <16, or <24 g/L based on RID. Negative predictive value (NPV) was defined as the proportion of calves classified by refractometry to contain IgG ≥10, ≥16, and ≥24 g/L that truly contained IgG ≥10, ≥16, and ≥24 g/L based on RID. The PPV and NPV were calculated for the prevalence of IgG <10, <16, and <24 g/L in this study population, as well as the prevalence of these IgG concentrations in beef calves reported in recent, comparable literature to increase external validity. 4 , 9 Previously, a prevalence of 16% was reported for calves with IgG <16 g/L 4 , 9 , and a prevalence of 33% was reported for calves with IgG <24 g/L. 4 Although prevalences of IgG <10 g/L in beef calves are lacking, prevalences of 6% to 14% were reported for IgG concentrations <8 g/L in other studies from North America. 2 , 4 Based on these considerations, we chose a 14% prevalence to imitate a herd scenario with a higher prevalence of IgG <10 g/L compared to our study population. Lastly, accuracy was defined as the percentage of serum samples that were correctly classified (true positive and true negative) by refractometry as compared to RID.
2.3.3. Agreement between tests using selected thresholds
To assess the level of agreement between refractometer results and results obtained by RID, as well as agreement among the 3 refractometers, Cohen's kappa (κ) was calculated for the selected STP and Brix thresholds indicative of IgG <10, <16, and <24 g/L. Bland‐Altman analysis was performed to further assess the agreement between DSTP and OSTP. 22
3. RESULTS
3.1. Sample population
Enrolled calves originated from 6 commercial herds consisting mostly of crossbred cattle and were largely Angus, Charolais, Hereford, and Simmental based. Herd sizes ranged from 466 to 1026 calving dams. Most calves were born by unassisted delivery (n = 319), whereas a small number of calves was born by assisted delivery (n = 33). Calving ease was not recorded for 37 calves. All calves were confirmed by trained farmed personnel to have nursed maternal colostrum by 24 hours, but shortly after birth 23 calves also received a commercially available colostrum‐derived colostrum product with total IgG masses ranging from 30 to 100 g. Whether or not a colostrum product was administered shortly after birth was not recorded for 13 calves.
3.1.1. Descriptive statistics
The frequency distributions of STP concentrations, Brix %, and serum IgG concentrations are shown in Figure 1 and all followed a normal distribution. Mean STP concentration measured by DSTP was 6.6 g/dL (SD, 1.1) with a range of 4.1 to 9.5 g/dL. Similarly, mean STP concentration measured by OSTP was 6.4 g/dL (SD, 1.0) with a range of 4 to 9.2 g/dL. Mean Brix % was 9.8% (SD, 1.3) with a range of 6.7% to 13.2% Brix. Mean serum IgG concentration was 40.1 g/L (SD, 16.8) with a range of 0.5 to 88.7 g/L. Based on RID analysis, 4% (16/398) of calves in the study population had serum IgG concentrations <10 g/L, 8.5% (34/398) had serum IgG concentrations <16 g/L, and 17.6% (70/398) had serum IgG concentrations <24 g/L.
FIGURE 1.
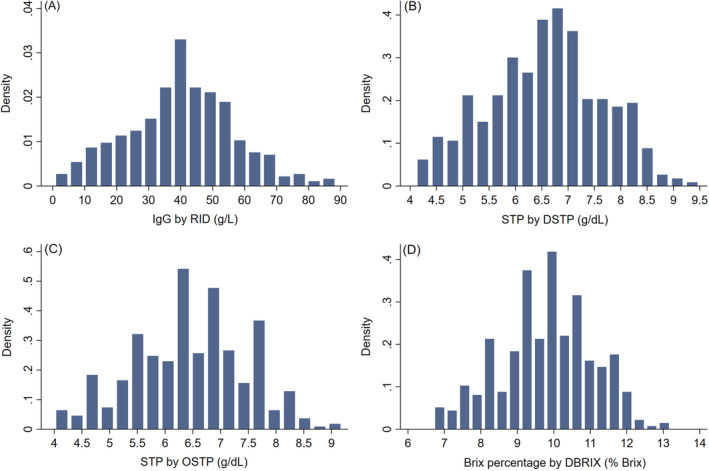
Frequency distributions of serum IgG obtained by (A) RID assay, STP obtained by (B) DSTP and (C) OSTP, and Brix percentages obtained by (D) DBRIX in 398 serum samples of neonatal beef calves age 1 to 7 days. DBRIX, digital Brix refractometer; DSTP, digital serum total protein refractometer; IgG, immunoglobulin G; OSTP, optical serum total protein refractometer; STP, serum total protein
When calves fed a colostrum product in addition to maternal colostrum (n = 23) were removed and the correlation, overall test performance, and LHR analyses repeated, results were not notably different (data not shown) and thus these calves were included in all analyses.
3.1.2. Correlation coefficients
The STP concentrations and Brix % determined by the different refractometers were highly correlated with IgG results obtained by RID, with correlation coefficients of 0.91, 0.85, and 0.82 for DSTP, OSTP, and DBRIX, respectively (Figure 2A‐C). Results obtained by the 3 different refractometers showed very high correlations with each other (r = 0.91‐0.95, Figure 2D‐F). The correlation between DSTP and RID was significantly (P < .0001) higher than the correlation between OSTP or DBRIX and RID, and the correlation between OSTP and RID was significantly (P < .0001) higher than that between DBRIX and RID.
FIGURE 2.

Scatter plots illustrating the relationships between serum IgG concentration measured by the reference test (RID) and estimated by refractometry (A‐C), and among the 3 different refractometers(D‐F), in serum samples obtained from 398 neonatal beef calves. Correlation between (A) DSTP and RID results, (B) OSTP and RID results, (C) DBRIX and RID results, (D) DSTP and DBRIX results, (E) OSTP and DBRIX results, and (F) DSTP and OSTP results. DBRIX, digital Brix refractometer; DSTP, digital serum total protein refractometer; IgG, immunoglobulin G; OSTP, optical serum total protein refractometer; r; Pearson correlation coefficient; RID, radial immunodiffusion
3.1.3. Overall test performance and threshold determination
The ROC curves and corresponding AUC for prediction of IgG concentrations <10, <16, and <24 g/L by the different refractometers are shown in Figure 3. The AUC, and thus overall test accuracy, for all 3 refractometers was >0.9 and therefore can be considered high. 23 For detection of IgG <10 g/L, no significant difference was found between the AUC of DSTP and OSTP (P = .27) or OSTP and DBRIX (P = .1). However, a significant difference was found between the AUC of DSTP and DBRIX (P = .001). For detection of IgG <16 g/L, the AUC among all 3 refractometers were not significantly different (DSTP and OSTP, P = .09; DSTP and DBRIX, P = .07; OSTP and DBRIX, P = .14). The AUC among all 3 refractometers were significantly different for detection of IgG <24 g/L (DSTP and OSTP, P = .02; DSTP and DBRIX, P < .001; OSTP and DBRIX, P = .01). Despite the statistical significance, the clinical importance of these finding likely is negligible, given that all AUC values were very high (≥0.93).
FIGURE 3.
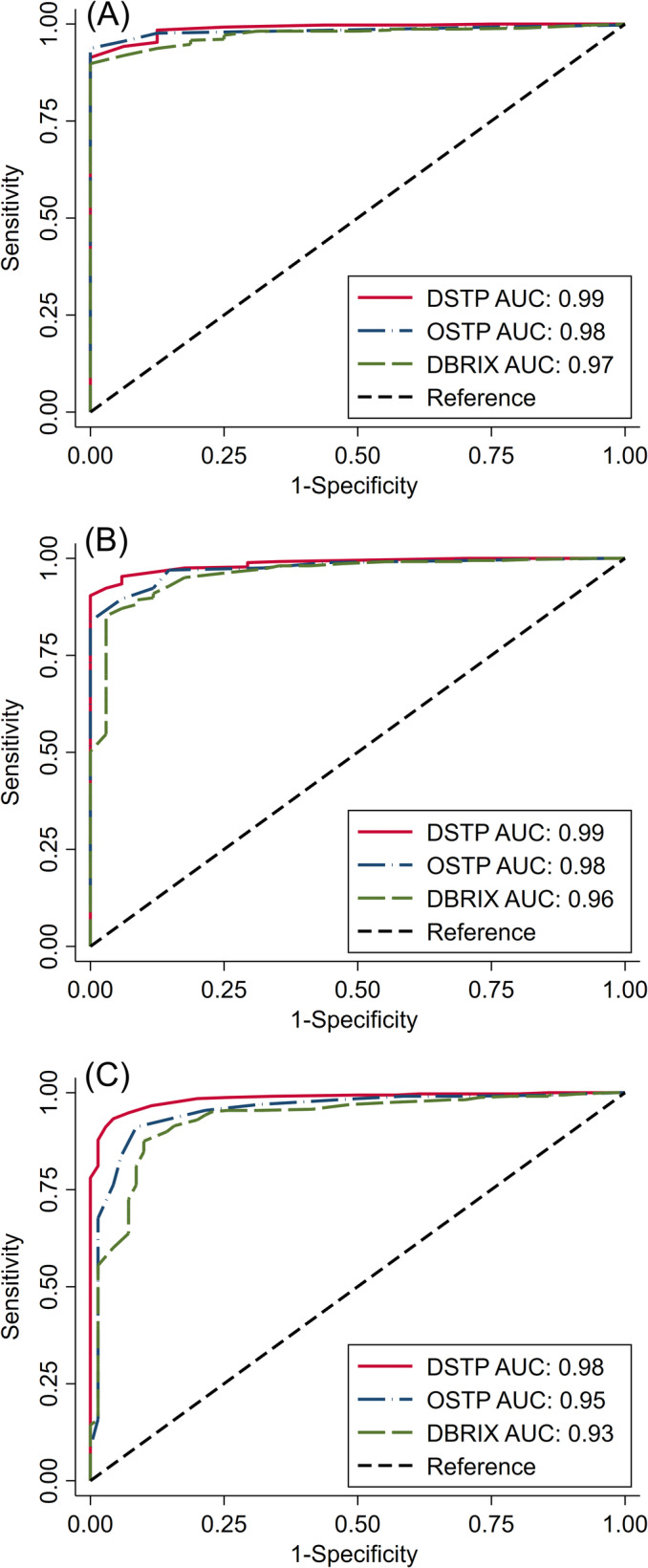
Receiver operating characteristic curves and associated AUC for prediction of serum IgG concentrations (A) <10 g/L, (B) <16 g/L, and (C) <24 g/L in 398 neonatal beef calves using three different refractometers. AUC, area under the curve; DBRIX; digital Brix refractometer; DSTP, digital serum total protein refractometer; IgG, immunoglobulin G; OSTP, optical serum total protein refractometer
The interval LHR for different STP concentrations and serum Brix % are presented in Table 1. Serum total protein concentrations ≤5.1, ≤5.1, and ≤5.7 g/dL were strongly indicative of serum IgG concentrations <10, <16, and <24 g/L, respectively, for both DSTP and OSTP. The STP thresholds indicative of IgG <10 and <16 g/L determined by LHR were the same. However, the likelihood of a sample with STP concentration between 4.6 and 5.1 g/dL being <16 g/L was much higher (LHR = 10.3 to 18.5) than the likelihood of such a sample being <10 g/L (LHR = 2.8 to 8.8), and therefore this threshold is more robust for IgG <16 g/L. Brix % of ≤7.9%, ≤8.3%, and ≤8.7% Brix were indicative of serum IgG concentrations <10, <16, and <24 g/L, respectively.
TABLE 1.
Number of serum samples in different strata of STP concentrations and Brix percentages obtained by DSTP, OSTP, and DBRIX, and corresponding interval LHR based on serum samples of 398 beef calves age 1 to 7 days
| Strata | Number of calves in stratum | IgG a <10 g/L | IgG a <16 g/L | IgG a <24 g/L | |||
|---|---|---|---|---|---|---|---|
| Number of calves with IgG <10 g/L | LHR (95% CI) | Number of calves with IgG <16 g/L | LHR (95% CI) | Number of calves with IgG <24 g/L | LHR (95% CI) | ||
| DSTP (g/dL) | |||||||
| ≤4.5 | 15 | 12 | 57.32 (20.36‐161.37) | 14 | 69.38 (16.46‐292.49) | 14 | 32.96 (7.70‐141.11) |
| 4.6‐5.1 | 34 | 4 | 2.84 (1.23‐6.60) | 18 | 10.34 (5.86‐18.23) | 32 | 48.34 (15.23‐153.49) |
| 5.2‐5.7 | 40 | 0 | 0.43 (0.06‐2.98) | 2 | 0.71 (0.23‐2.20) | 21 | 4.83 (2.78‐8.40) |
| 5.8‐6.3 | 72 | 0 | 0.24 (0.04‐1.66) | 0 | 0.13 (0.02‐0.89) | 3 | 0.25 (0.10‐0.67) |
| 6.4–6.9 | 91 | 0 | 0.19 (0.03‐1.31) | 0 | 0.10 (0.01‐0.70) | 0 | 0.05 (0.01‐0.34) |
| ≥7 | 146 | 0 | 0.12 (0.02‐0.82) | 0 | 0.06 (0.01‐0.44) | 0 | 0.03 (0.004‐0.21) |
| OSTP (g/dL) | |||||||
| ≤4.5 | 12 | 7 | 23.52 (8.94‐61.87) | 10 | 33.92 (9.87‐116.53) | 10 | 16.11 (4.61‐56.36) |
| 4.6–5.1 | 28 | 9 | 8.82 (4.72‐16.49) | 19 | 18.50 (9.32‐36.72) | 25 | 28.57 (10.27‐79.44) |
| 5.2–5.7 | 53 | 0 | 0.33 (0.05‐2.25) | 5 | 1.13 (0.52‐2.48) | 29 | 5.27 (3.30‐8.43) |
| 5.8–6.3 | 82 | 0 | 0.21 (0.03‐1.46) | 0 | 0.11 (0.02‐0.78) | 5 | 0.34 (0.15‐0.75) |
| 6.4–6.9 | 89 | 0 | 0.20 (0.03‐1.34) | 0 | 0.10 (0.02‐0.72) | 0 | 0.05 (0.01‐0.35) |
| ≥7 | 134 | 0 | 0.13 (0.02–0.89) | 0 | 0.07 (0.01‐0.48) | 1 | 0.07 (0.02‐0.26) |
| DBRIX (% Brix) | |||||||
| ≤7.9 | 33 | 13 | 10.83 (6.34‐18.52) | 23 | 19.32 (10.21‐36.59) | 26 | 14.54 (6.87‐30.76) |
| 8.0‐8.3 | 30 | 3 | 2.32 (0.89‐6.08) | 7 | 2.95 (1.42‐6.15) | 22 | 11.01 (5.30‐22.85) |
| 8.4–8.7 | 16 | 0 | 0.96 (0.13‐6.88) | 2 | 1.77 (0.54‐5.87) | 8 | 4.31 (1.77‐10.50) |
| 8.8–9.1 | 34 | 0 | 0.46 (0.07‐3.25) | 1 | 0.52 (0.13‐2.09) | 7 | 1.23 (0.58‐2.60) |
| 9.2–9.5 | 49 | 0 | 0.33 (0.05–2.25) | 0 | 0.18 (0.03‐1.25) | 2 | 0.27 (0.09‐0.84) |
| 9.6–9.9 | 53 | 0 | 0.30 (0.04‐2.08) | 1 | 0.33 (0.08‐1.32) | 4 | 0.43 (0.18‐1.04) |
| 10.0–10.3 | 51 | 0 | 0.31 (0.05‐2.16) | 0 | 0.17 (0.02‐1.20) | 0 | 0.08 (0.01‐0.59) |
| ≥10.4 | 132 | 0 | 0.12 (0.02‐0.84) | 0 | 0.07 (0.01‐0.46) | 1 | 0.07 (0.02–0.26) |
| Total | 398 | 16 | 34 | 70 | |||
Abbreviations: DBRIX, digital Brix refractometer; DSTP, digital serum total protein refractometer; IgG, immunoglobulin G; LHR, likelihood ratio; OSTP, optical serum total protein refractometer; RID, radial immunodiffusion assay; STP, serum total protein.
IgG obtained by RID.
Diagnostic test characteristics for these selected thresholds are presented in Table 2. The J for detection of IgG <10, <16, and <24 g/L at the proposed thresholds ranged from 0.82 to 0.94 for DSTP and OSTP and from 0.73 to 0.79 for DBRIX. The PPV ranged from 32.7% to 75.3%, depending on the refractometer used in this study population with prevalences of IgG <10 g/L of 4%, IgG <16 g/L of 8.5%, and IgG <24 g/L of 17.6%. Notably, the PPV for identification of IgG <10 g/L was very low for all 3 refractometers, potentially limiting refractometry usefulness for this application for herds similar to our study herds. In herds with a higher expected prevalence of IgG <10 g/L of 14%, IgG <16 g/L of 16%, and IgG <24 g/L of 33%, such as reported in or extrapolated from other studies 2 , 4 , 9 , the PPV would range from 65% to 87.5% using the selected thresholds of our study. The NPV was high for all 3 refractometers for detection of IgG <10, <16, and <24 g/L at all examined prevalences. The accuracy at the suggested STP and Brix thresholds ranged from 90.7% to 96.0%.
TABLE 2.
Test characteristics of DSTP, OSTP, and DBRIX for detecting various IgG concentrations in serum samples of 398 beef calves age 1 to 7 days using optimal thresholds as determined by LHR compared with results obtained by RID assay
| IgG a | Refractometer | Threshold | Test characteristics (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Se (95% CI) | Sp (95% CI) | J | PPV b | NPV b | Accuracy | PPV c | NPV c | |||
| <10 g/L | DSTP (g/dL) | ≤5.1 | 100 (79.4‐100) | 91.4 (88.1‐94.0) | 0.91 | 32.7 | 100 | 91.7 | 65.3 | 100 |
| OSTP (g/dL) | ≤5.1 | 100 (79.4–100) | 93.7 (90.8‐95.9) | 0.94 | 40 | 100 | 94.0 | 72.2 | 100 | |
| DBRIX (% Brix) | ≤7.9 | 81.2 (54.4‐96.0) | 94.8 (92.0‐96.8) | 0.76 | 39.4 | 99.2 | 94.2 | 71.6 | 96.9 | |
| <16 g/L | DSTP (g/dL) | ≤5.1 | 94.1 (80.3‐99.3) | 95.3 (92.6‐97.3) | 0.89 | 65.3 | 99.4 | 95.2 | 79.3 | 98.8 |
| OSTP (g/dL) | ≤5.1 | 85.3 (68.9‐95.0) | 97.0 (94.7‐98.5) | 0.82 | 72.5 | 98.6 | 96.0 | 84.3 | 97.2 | |
| DBRIX (% Brix) | ≤8.3 | 88.2 (72.5‐96.7) | 90.9 (87.5‐93.7) | 0.79 | 47.6 | 98.8 | 90.7 | 65.0 | 97.6 | |
| <24 g/L | DSTP (g/dL) | ≤5.7 | 95.7 (88.0‐99.1) | 93.3 (90.0‐95.7) | 0.89 | 75.3 | 99.0 | 93.7 | 87.5 | 97.8 |
| OSTP (g/dL) | ≤5.7 | 91.4 (82.3‐96.8) | 91.2 (87.5‐94.0) | 0.83 | 68.8 | 98.0 | 91.2 | 83.6 | 95.6 | |
| DBRIX (% Brix) | ≤8.7 | 80.0 (68.7‐88.6) | 93.0 (89.6‐95.5) | 0.73 | 70.9 | 95.6 | 90.7 | 84.9 | 90.4 | |
Note: The PPV and NPV have been calculated for the prevalence in this study population as well as for higher prevalences reported in the literature. 1 , 2 , 3
Abbreviations: DBRIX, digital Brix refractometer; DSTP, digital serum total protein refractometer; IgG, immunoglobulin G; J, Youden index; NPV, negative predictive value; PPV, positive predictive value; OSTP, optical serum total protein refractometer; RID, radial immunodiffusion assay; Se, sensitivity; Sp, specificity.
IgG concentration obtained by RID.
Predictive values based on prevalence of calves with IgG <10 g/L (4%), <16 g/L (8.5%) and 24 g/L (17.6%) reported in this study population.
3.1.4. Agreement between tests at selected thresholds
The agreement between refractometry results and results obtained by the reference test RID is presented in Table 3. Cohen's kappa was moderate for agreement between refractometers and RID for detection of IgG <10 g/L, whereas it was mostly substantial for detection of IgG <16 and IgG <24 g/L. Notably, κ was only moderate (κ = 0.57) for DBRIX for determination of IgG <16 g/L but substantial for DSTP and OSTP (κ = 0.75 and 0.76, respectively). The agreement among results of the different refractometers for detection of serum IgG <10, <16, and <24 g/L at the suggested thresholds was substantial (κ = 0.62‐0.89; Table 3). The agreement between DSTP and OSTP ranged from 0.81 to 0.89 for detection of IgG <10, <16, and <24 g/L and was further evaluated by Bland‐Altman analysis, which showed no systematic bias with a small mean difference of 0.1 g/dL between DSTP and OSTP (Figure 4). In addition, the Bland‐Altman plot showed no trend of differences between the 2 refractometers across the STP concentrations measured in this study, indicating that performance is similar at low, moderate, and high STP concentrations.
TABLE 3.
Level of agreement between STP concentrations and Brix percentages obtained by refractometry and IgG concentration obtained by RID assay at the thresholds selected by LHR, as well as agreement between DSTP, OSTP, and DBRIX based on 398 serum samples of beef calves age 1 to 7 days
| Test 1 (unit) | Threshold Test 1 | Test 2 (unit) | Threshold Test 2 | Agreement | κ | P value |
|---|---|---|---|---|---|---|
| Detection of IgG < 10 g/L | ||||||
| DSTP (g/dL) | ≤5.1 | RID (g/L) | <10 | 91.7 | 0.46 | <.0001 |
| OSTP (g/dL) | ≤5.1 | RID (g/L) | <10 | 94.0 | 0.55 | <.0001 |
| DBRIX (%) | ≤7.9 | RID (g/L) | <10 | 94.2 | 0.50 | <.0001 |
| DSTP (g/dL) | ≤5.1 | OSTP (g/dL) | ≤5.1 | 96.2 | 0.81 | <.0001 |
| DBRIX (%) | ≤7.9 | DSTP (g/dL) | ≤5.1 | 93.0 | 0.62 | <.0001 |
| DBRIX (%) | ≤7.9 | OSTP (g/dL) | ≤5.1 | 96.2 | 0.77 | <.0001 |
| Detection of IgG < 16 g/L | ||||||
| DSTP (g/dL) | ≤5.1 | RID (g/L) | <16 | 95.2 | 0.75 | <.0001 |
| OSTP (g/dL) | ≤5.1 | RID (g/L) | <16 | 96.0 | 0.76 | <.0001 |
| DBRIX (%) | ≤8.3 | RID (g/L) | <16 | 90.7 | 0.57 | <.0001 |
| DSTP (g/dL) | ≤5.1 | OSTP (g/dL) | ≤5.1 | 96.2 | 0.81 | <.0001 |
| DBRIX (%) | ≤8.3 | DSTP (g/dL) | ≤5.1 | 95.0 | 0.78 | <.0001 |
| DBRIX (%) | ≤8.3 | OSTP (g/dL) | ≤5.1 | 95.2 | 0.77 | <.0001 |
| Detection of IgG < 24 g/L | ||||||
| DSTP (g/dL) | ≤5.7 | RID (g/L) | <24 | 93.7 | 0.80 | <.0001 |
| OSTP (g/dL) | ≤5.7 | RID (g/L) | <24 | 91.2 | 0.73 | <.0001 |
| DBRIX (%) | ≤8.7 | RID (g/L) | <24 | 90.7 | 0.69 | <.0001 |
| DSTP (g/dL) | ≤5.7 | OSTP (g/dL) | ≤5.7 | 96.0 | 0.89 | <.0001 |
| DBRIX (%) | ≤8.7 | DSTP (g/dL) | ≤5.7 | 92.0 | 0.76 | <.0001 |
| DBRIX (%) | ≤8.7 | OSTP (g/dL) | ≤5.7 | 94.0 | 0.82 | <.0001 |
Abbreviations: DBRIX, digital Brix refractometer; DSTP, digital serum total protein refractometer; IgG, immunoglobulin G; LHR, likelihood ratio; OSTP, optical serum total protein refractometer; RID, radial immunodiffusion; STP, serum total protein; κ, Cohen's kappa.
FIGURE 4.
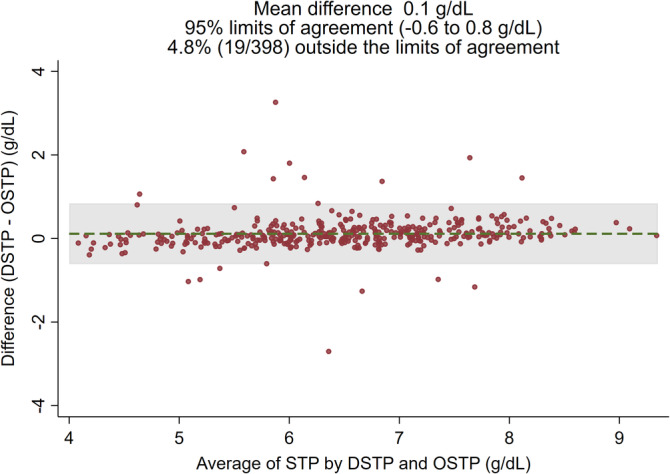
Bland‐Altman plot illustrating the agreement between STP results measured by DSTP versus OSTP. DSTP, digital serum total protein refractometer; OSTP; optical serum total protein refractometer; STP, serum total protein
4. DISCUSSION
Our study demonstrates the clinical utility of 3 different refractometers (DSTP, OSTP, BRIX) as monitoring tools for TPI in beef calves. All 3 refractometers showed good correlation and moderate to substantial agreement with the reference test RID, and high overall test accuracy. We determined that STP concentrations of ≤5.1, ≤5.1, and ≤5.7 g/dL are indicative of serum IgG <10, <16, and <24 g/L, respectively, when using DSTP and OSTP. When using the Brix refractometer, percentages of <7.9%, <8.3%, and <8.7% Brix are indicative of serum IgG <10, <16, and <24 g/L, respectively. Using these proposed thresholds, >90% of serum samples were correctly classified by all 3 refractometers evaluated in our study.
The correlation between STP concentrations obtained by refractometry and IgG concentrations in serum obtained by the reference RID assay in our study (r = 0.91 and 0.85 for DSTP and OSTP, respectively) was higher than the 0.64 reported in a recent study 10 , but lower than the 0.95 to 0.96 reported elsewhere. 9 The correlation between Brix % and IgG concentrations in our study (r = 0.82) was slightly higher than the correlation coefficient (r = 0.77) found in a recent study. 10 It is important to note, however, that the reference tests varied between studies (ELISA 10 or Biuret method 9 ). Correlation among the different refractometers was high in our study (r = 0.91‐0.95), which is in agreement with previous reports. 9 , 24 To our knowledge, ours is the only study to date establishing correlation and agreement between RID and refractometer results for beef calves.
Although recent research focuses increasingly on the validation of diagnostic tests based on a Bayesian framework 25 and outcome‐oriented approaches (morbidity, mortality) 10 , general herd recommendations usually still are made based on IgG concentrations 1 , 2 , 3 , 4 , 26 , for which RID remains the reference test. It generally is accepted that IgG <10 g/L should be considered as failed TPI for any calf. 1 , 4 , 8 , 26 Very recently, a group of experts recommended use of the following 4 IgG categories in dairy calves: excellent (≥25 g/L), good (18‐24.9 g/L), fair (10‐17.9 g/L), and poor (<10 g/L) 26 , along with consensus recommendations for proportions of calves in each category in a given herd. Unfortunately, no such consensus has been reached for beef calves, likely because of smaller numbers of studies investigating passive immunity. However, IgG concentrations of ≥16 4 and ≥24 2 , 4 g/L have been discussed as adequate or optimal based on decreased morbidity and mortality in beef calves. Yet, it is questionable if a “one‐size‐fits‐all” model would be appropriate for beef operations, given the variability in herd‐level (eg, pathogen load, extensive versus intensive management, dam vaccination strategies) and individual‐animal (eg, breed, colostrum management, calving assistance) factors. These considerations led to our aim to establish STP concentrations and Brix % identifying several levels of TPI (<10, <16, and <24 g/L) in this study, so that our thresholds could be adapted to various scenarios.
Refractometry appeared to be less useful for determination of IgG <10 g/L in our study population compared to other applications, demonstrated by the same thresholds for determination of IgG <10 and <16 g/L and low PPV for this threshold. However, Se, Sp, and accuracy as well as correlation with the reference RID test were excellent even for this application. These findings highlight the importance of appropriate study population selection, because prevalence of the assessed condition is a major contributing factor to any diagnostic test evaluation. A population with lower average serum IgG concentration and a higher prevalence of calves with failed TPI (IgG <10 g/L) would likely be better suited to determine appropriate STP and Brix thresholds for detection of IgG <10 g/L. Even in dairy calves, no agreement on the most appropriate STP thresholds has been reached, with recently suggested optimal thresholds ranging from 5.1 to 5.8 g/L. 9 , 26 , 27 , 28 , 29 , 30 We acknowledge the limited usefulness of our proposed thresholds for detection of IgG <10 g/L in populations similar to ours. As shown in Table 2, however, in herds with a higher prevalence of IgG <10 g/L, performance of all 3 refractometers was improved, with PPV ranging from 65.3% to 72.2%.
Serum total protein concentrations ≤5.1 g/dL were identified as optimal for identification of serum IgG <16 g/L using DSTP and OSTP. Calves with STP of 4.6 to 5.1 g/dL were 10 to 19 times more likely to have serum IgG concentrations <16 g/L compared to calves with STP >5.1 g/dL. This threshold is lower than the 5.4 to 5.8 g/dL suggested previously 9 , but again, the reference methods used were different. The observed differences also may be explained by differences in study population, study design, and statistical methods.
Ours is the first study to establish a STP threshold (≤5.7 g/dL) for identification of serum IgG <24 g/L, which has been suggested previously as indicative of inadequate TPI in beef calves. 2 , 4 This finding is in agreement with a recent study based on Irish suckler calves 10 , in which STP concentrations of 5.3 to 6.3 g/L were selected as optimal test cut‐offs predictive of morbidity and mortality.
The Se and Sp for thresholds established for the 3 refractometers in our study ranged from 80.0% to 100% and 91.2% to 97.0%, respectively. The test characteristics of DSTP and OSTP were comparable to those previously described in a study comparing 4 refractometers against the Biuret method of estimating serum IgG concentrations 9 (Se = 100%, Sp = 90.0%‐93.3%), but higher than those described in another study (Se = 55.6%, Sp = 59.2%) for predicting morbidity in beef calves. 10 A recent meta‐analysis evaluating STP refractometry for assessment of failed TPI (<10 g/L) established a summary Se of 76.1% to 88.2% and a summary Sp of 77.9% to 89.3%, depending on the threshold used. 8 However, only 3 of the studies evaluated in this meta‐analysis included serum from beef calves.
To date, no reported studies have assessed Brix % indicative of serum IgG <16 g/L or <24 g/L, but calves with serum Brix % ≤8.4% had significantly higher odds of morbidity and mortality. 10 This result aligns with findings of our study, where optimal thresholds were ≤8.3% and ≤8.7% for prediction of IgG <16 and <24 g/L, respectively. No studies to date have reported test characteristics for Brix refractometer performance in beef calf serum when comparing results to RID. However, a previous study reported a Se and Sp of 44.8% and 65.4%, receptively, for Brix refractometry predicting morbidity in beef calves. 10
The small mean difference of 0.1 g/dL between DSTP and OSTP indicates that, on average, DSTP measured 0.1 units (g/dL) more than OSTP. This small difference can be explained by the different precisions of the 2 refractometers (0.1 g/dL for DSTP and 0.2 g/dL for OSTP). Inevitably, whenever the “true” STP concentration was an uneven number (eg, 5.7 g/dL), the sample would have been misclassified (eg, 5.6 or 5.8 g/dL) using OSTP, whereas it would have been correctly classified using DSTP. Therefore, we concluded that optical and digital STP refractometers may be used interchangeably. Although RID and DSTP were performed concurrently in a single laboratory, OSTP and DBRIX were performed concurrently in another laboratory. Minor differences in sample handling, including different sample storage and processing, numbers of freeze‐thaw cycles, and different personnel could have led to some of the observed differences among refractometers and should be considered a limitation of our study. Finally, refractometry was performed only once per sample and therefore, analytical variation within the same refractometer was not assessed. However, this approach is likely reflective of how these tests would be used on farm or in clinical practice.
The high average serum IgG concentration in our study population is in agreement with other recent reports in this geographic region 31 , 32 , and also reflects the generally high average colostral IgG concentration in western Canadian beef cows. 33 Although relevant to the local production system, these findings may have limited external validity for farms with lower overall TPI. An attempt was made to make the results of our study clinically applicable to herds with a slightly higher prevalence of failed or inadequate TPI by calculating PPV and NPV for prevalences reported elsewhere. 2 , 4 , 9 In such herd settings, PPV would be substantially increased whereas NPV would only be mildly decreased.
Caution is warranted when interpreting refractometry results in certain circumstances. High STP or total solids concentrations could be associated with ongoing illness of the calf, leading to concentration of solids in the blood and subsequent falsely increased refractometry results. 8 Serum total protein evaluation therefore cannot be recommended for reliable assessment of TPI in calves that are clinically dehydrated, have increased hematocrit, or both. Although subclinical disease cannot be ruled out for the calves enrolled in our study, calves with overt signs of dehydration or illness were not included. Furthermore, administration of colostrum products may change the refractive index of neonatal calf serum and thus interpretation of refractometry results, because the protein profile of commercially available colostrum supplement and replacement products differs from that of maternal colostrum. 33 This difference has been shown to be particularly true after administration of plasma‐derived colostrum products to dairy calves 20 , 21 , whereas optimal STP cut‐points for calves fed colostrum‐derived replacement product or maternal colostrum were similar. 21 No calves in our study received plasma‐derived colostrum product, and a very small number (n = 23) received a single dose of a commercially available colostrum‐derived product.
The refractometers in our study were chosen because DSTP is routinely used at the laboratory at which the RID assays were performed, the OSTP is commonly available in veterinary practices, and the BRIX has been studied previously for use in colostrum 34 , 35 , 36 and dairy calf serum. 25 , 27 , 28 Although some significant differences existed in test performance among the evaluated refractometers, with DBRIX performing slightly inferior overall compared to DSTP and OSTP, the clinical relevance of these differences is questionable. Particularly, producers already using Brix refractometry for colostrum IgG assessment 36 , 37 may elect to use the Brix refractometer for serum IgG evaluation as well. On the other hand, many veterinarians have DSTP and OSTP available in their veterinary practice because of their multipurpose utility (eg, urine specific gravity, STP in adults and neonates), and their use should be encouraged for assessment of TPI in beef calves.
In conclusion, DSTP, OSTP, and DBRIX all showed good utility for assessment of TPI status in neonatal beef calves, particularly for identification of calves with IgG concentrations below the target concentrations recommended recently for beef calves (IgG <16 or < 24 g/L). Our results can be used for the individual animal or at the herd level. Veterinarians and producers may elect to monitor individual calves with STP <5.7 g/dL and Brix <8.7% more closely for signs of morbidity. Herds with a high percentage of tested calves with low STP concentrations or Brix % may elect to test maternal colostrum IgG 36 , adjust precalving management practices, or implement early colostrum intervention strategies for at‐risk calves. The optimal target IgG concentration depends on the individual farm environment, which is why our study examined various thresholds that can be used by veterinarians and producers to monitor beef calves and subsequently improve colostrum management strategies as needed.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of Calgary Veterinary Sciences Animal Care Committee (AC16‐0209).
ACKNOWLEDGMENTS
Funding provided by University of Calgary Faculty of Veterinary Medicine Simpson Ranch Research Grant and Alberta Agriculture and Forestry. Dr Gamsjӓger's stipend was paid in part by the American College of Veterinary Internal Medicine Fellowship in Advanced Research Training. The authors thank the Saskatoon Colostrum Company Ltd. Quality Assurance Laboratory for their in‐kind contribution of RID testing. Additionally, the authors thank Jackie Annis, Jackie Elgert, Zeanna Janmohamed, and Nicole Hawe for their technical assistance with refractometry.
Gamsjäger L, Elsohaby I, Pearson JM, Levy M, Pajor EA, Windeyer MC. Evaluation of 3 refractometers to determine transfer of passive immunity in neonatal beef calves. J Vet Intern Med. 2021;35:632–643. 10.1111/jvim.16016
Funding information Alberta Agriculture and Forestry; American College of Veterinary Internal Medicine, Grant/Award Number: Fellowship in Advanced Research Training; University of Calgary Faculty of Veterinary Medicine Simpson Ranch Research Grant
REFERENCES
- 1. Godden SM, Lombard JE, Woolums AR. Colostrum management for dairy calves. Vet Clin North Am Food Anim Pract. 2019;35(3):535‐556. 10.1016/j.cvfa.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dewell RD, Hungerford LL, Keen JE, et al. Association of neonatal serum immunoglobulin G1 concentration with health and performance in beef calves. J Am Vet Med Assoc. 2006;228(6):914‐921. 10.2460/javma.228.6.914. [DOI] [PubMed] [Google Scholar]
- 3. Chigerwe M, Hagey JV, Aly SS. Determination of neonatal serum immunoglobulin G concentrations associated with mortality during the first 4 months of life in dairy heifer calves. J Dairy Res. 2015;82(4):400‐406. 10.1017/S0022029915000503. [DOI] [PubMed] [Google Scholar]
- 4. Waldner CL, Rosengren LB. Factors associated with serum immunoglobulin levels in beef calves from Alberta and Saskatchewan and association between passive transfer and health outcomes. Can Vet J. 2009;50:275‐281. [PMC free article] [PubMed] [Google Scholar]
- 5. Lee S‐H, Jaekal J, Bae C‐S, et al. Enzyme‐linked immunosorbent assay, single radial immunodiffusion, and indirect methods for the detection of failure of transfer of passive immunity in dairy calves. J Vet Intern Med. 2008;22(1):212‐218. 10.1111/j.1939-1676.2007.0013.x. [DOI] [PubMed] [Google Scholar]
- 6. Elsohaby I, Riley CB, Hou S, McClure JT, Shaw RA, Keefe GP. Measurement of serum immunoglobulin G in dairy cattle using Fourier‐transform infrared spectroscopy: a reagent free approach. Vet J. 2014;202(3):510‐515. 10.1016/j.tvjl.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 7. Alley ML, Haines DM, Smith GW. Short communication: evaluation of serum immunoglobulin G concentrations using an automated turbidimetric immunoassay in dairy calves. J Dairy Sci. 2012;95(8):4596‐4599. 10.3168/jds.2012-5420. [DOI] [PubMed] [Google Scholar]
- 8. Buczinski S, Gicquel E, Fecteau G, Takwoingi Y, Chigerwe M, Vandeweerd JM. Systematic review and meta‐analysis of diagnostic accuracy of serum refractometry and Brix refractometry for the diagnosis of inadequate transfer of passive immunity in calves. J Vet Intern Med. 2018;32(1):474‐483. 10.1111/jvim.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vandeputte S, Detilleux J, Rollin F. Comparison of four refractometers for the investigation of the passive transfer in beef calves. J Vet Intern Med. 2011;25:1465‐1469. 10.15537/smj.2019.12.24643. [DOI] [PubMed] [Google Scholar]
- 10. Todd CG, McGee M, Tiernan K, et al. An observational study on passive immunity in Irish suckler beef and dairy calves: tests for failure of passive transfer of immunity and associations with health and performance. Prev Vet Med. 2018;159:182‐195. 10.1016/j.prevetmed.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 11. Chelack BJ, Morley PS, Haines DM. Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can Vet J. 1993;34(7):407‐412. [PMC free article] [PubMed] [Google Scholar]
- 12. Shivley CB, Lombard JE, Urie NJ, et al. Preweaned heifer management on US dairy operations: part V. Factors associated with morbidity and mortality in preweaned dairy heifer calves. J Dairy Sci. 2018;101(10):9229‐9244. 10.3168/jds.2017-14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diedenhofen B, Musch J. Cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One. 2015;10(4):1‐12. 10.1371/journal.pone.0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 15. Gardner IA, Greiner M. Receiver‐operating characteristic curves and likelihood ratios: improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Vet Clin Pathol. 2006;35(1):8‐17. 10.1111/j.1939-165X.2006.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 16. Robin X, Turck N, Hainard A, et al. pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinf. 2011;12:77 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown MD, Reeves MJ. Interval likelihood ratios: another advantage for the evidence‐based diagnostician. Ann Emerg Med. 2003;42(2):292‐297. 10.1067/mem.2003.274. [DOI] [PubMed] [Google Scholar]
- 18. Hayden SR, Brown MD. Likelihood ratio: a powerful tool for incorporating the results of a diagnostic test into clinical decisionmaking. Ann Emerg Med. 1999;33(5):575‐580. 10.1016/S0196-0644(99)70346-X. [DOI] [PubMed] [Google Scholar]
- 19. Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44(8):763‐770. 10.1016/0895-4356(91)90128-V. [DOI] [PubMed] [Google Scholar]
- 20. Quigley JD, Kost CJ, Wolfe TM. Absorption of protein and IgG in calves fed a colostrum supplement or replacer. J Dairy Sci. 2002;85(5):1243‐1248. 10.3168/jds.S0022-0302(02)74188-X. [DOI] [PubMed] [Google Scholar]
- 21. Priestley D, Bittar JH, Ibarbia L, Risco CA, Galvão KN. Effect of feeding maternal colostrum or plasma‐derived or colostrum‐derived colostrum replacer on passive transfer of immunity, health, and performance of preweaning heifer calves. J Dairy Sci. 2013;96(5):3247‐3256. 10.3168/jds.2012-6339. [DOI] [PubMed] [Google Scholar]
- 22. Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Stat. 1983;32(3):307 10.2307/2987937. [DOI] [Google Scholar]
- 23. Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver‐operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23‐41. 10.1016/0009-2797(70)90001-3. [DOI] [PubMed] [Google Scholar]
- 24. Wallace MM, Jarvie BD, Perkins NR, Leslie KE. A comparison of serum harvesting methods and type of refractometer for determining total solids to estimate failure of passive transfer in calves. Can Vet J. 2006;47(6):573‐575. [PMC free article] [PubMed] [Google Scholar]
- 25. Elsohaby I, Mweu MM, Mahmmod YS, McClure JT, Keefe GP. Diagnostic performance of direct and indirect methods for assessing failure of transfer of passive immunity in dairy calves using latent class analysis. Prev Vet Med. 2019;164:72‐77. 10.1016/j.prevetmed.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 26. Lombard J, Urie N, Garry F, et al. Consensus recommendations on calf‐ and herd‐level passive immunity in dairy calves in the United States. J Dairy Sci. 2020;103(8):7611‐7624. 10.3168/jds.2019-17955. [DOI] [PubMed] [Google Scholar]
- 27. Elsohaby I, Mcclure JT, Keefe GP. Evaluation of digital and optical refractometers for assessing failure of transfer of passive immunity in dairy calves. J Vet Intern Med. 2015;29(2):721‐726. 10.1111/jvim.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elsohaby I, McClure JT, Waite LA, Cameron M, Heider LC, Keefe GP. Using serum and plasma samples to assess failure of transfer of passive immunity in dairy calves. J Dairy Sci. 2019;102(1):567‐577. 10.3168/jds.2018-15070. [DOI] [PubMed] [Google Scholar]
- 29. Hernandez D, Nydam DV, Godden SM, et al. Brix refractometry in serum as a measure of failure of passive transfer compared to measured immunoglobulin G and total protein by refractometry in serum from dairy calves. Vet J. 2016;211:82‐87. 10.1016/j.tvjl.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 30. Cuttance EL, Mason WA, Denholm KS, Laven RA. Comparison of diagnostic tests for determining the prevalence of failure of passive transfer in New Zealand dairy calves. N Z Vet J. 2017;65(1):6‐13. 10.1080/00480169.2016.1230525. [DOI] [PubMed] [Google Scholar]
- 31. Homerosky ER, Timsit E, Pajor EA, Kastelic JP, Windeyer MC. Predictors and impacts of colostrum consumption by 4 h after birth in newborn beef calves. Vet J. 2017;228:1‐6. 10.1016/j.tvjl.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 32. Pearson JM, Pajor E, Campbell J, Levy M, Caulkett N, Windeyer MC. A randomised controlled trial investigating the effects of administering a non‐steroidal anti‐inflammatory drug to beef calves assisted at birth and risk factors associated with passive immunity, health, and growth. Vet Rec Open. 2019;6(1):1‐10. 10.1136/vetreco-2019-000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lago A, Socha M, Geiger A, et al. Efficacy of colostrum replacer versus maternal colostrum on immunological status, health, and growth of preweaned dairy calves. J Dairy Sci. 2018;101(2):1344‐1354. 10.3168/jds.2017-13032. [DOI] [PubMed] [Google Scholar]
- 34. Chigerwe M, Tyler JW, Middleton JR, Spain JN, Dill JS, Steevens BJ. Comparison of four methods to assess colostral IgG concentration in dairy cows. J Am Vet Med Assoc. 2008;233(5):761‐766. 10.2460/javma.233.5.761. [DOI] [PubMed] [Google Scholar]
- 35. Elsohaby I, McClure JT, Cameron M, Heider LC, Keefe GP. Rapid assessment of bovine colostrum quality: how reliable are transmission infrared spectroscopy and digital and optical refractometers? J Dairy Sci. 2017;100(2):1427‐1435. 10.3168/jds.2016-11824. [DOI] [PubMed] [Google Scholar]
- 36. Gamsjäger L, Elsohaby I, Pearson JM, et al. Assessment of Brix refractometry to estimate immunoglobulin G concentration in beef cow colostrum. J Vet Intern Med. 2020;34:1662‐1673. 10.1111/jvim.15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vandeputte S, Detilleux J, Rollin F. Investigation of colostrum quality in beef cattle by radial immunodiffusion and brix refractometry. Vet Rec. 2014;175(14):353 10.1136/vr.101590. [DOI] [PubMed] [Google Scholar]


