Abstract
The aim of this study was to develop a rabbit neurosphere culture to characterize differences in basic processes of neurogenesis induced by intrauterine growth restriction (IUGR). A novel in vitro neurosphere culture has been established using fresh or frozen neural progenitor cells from newborn (PND0) rabbit brains. After surgical IUGR induction in pregnant rabbits and cesarean section 5 days later, neural progenitor cells from both control and IUGR groups were isolated and directly cultured or frozen at −80°C. These neural progenitor cells spontaneously formed neurospheres after 7 days in culture. The ability of control and IUGR neurospheres to migrate, proliferate, differentiate to neurons, astrocytes, or oligodendrocytes was compared and the possibility to modulate their responses was tested by exposure to several positive and negative controls. Neurospheres obtained from IUGR brains have a significant impairment in oligodendrocyte differentiation, whereas no significant differences are observed in other basic processes of neurogenesis. This impairment can be reverted by in vitro exposure of IUGR neurospheres to thyroid hormone, which is known to play an essential role in white matter maturation in vivo. Our new rabbit neurosphere model and the results of this study open the possibility to test several substances in vitro as neuroprotective candidates against IUGR induced neurodevelopmental damage while decreasing the number of animals and resources and allowing a more mechanistic approach at a cellular functional level.
Keywords: cell culture, differentiation, experimental models, growth inhibition, nervous system, oligodendrocytes, progenitor cells
Rabbit “Neurosphere Assay.” PND0 rabbit brains are dissected and either frozen or directly cultured until neurosphere formation. “Neurosphere Assay” starts when neurospheres are plated in 8‐chamber slides and maintained under differentiating conditions to evaluate migration and differentiation into neurons, into astrocytes or into oligodendrocytes. Neurospheres were also plated in 96‐well plates under proliferating conditions and evaluated for diameter increase.

Significance statement.
To the best of the authors' knowledge, this article describes for the first time a valuable method for generation of 3D rabbit neurospheres that can be differentiated to neurons, astrocytes, and oligodendrocytes. This method is applied for modeling the neurodevelopmental effects of intrauterine growth restriction (IUGR), for toxicity testing, and for efficacy testing of possible new pharmaceuticals. In this study, the authors found differences in basic processes of neurogenesis induced by IUGR, showed that neurospheres obtained from IUGR brains have a significant impairment in oligodendrocyte differentiation, and demontrated that this impairment can be reverted by in vitro exposure of IUGR neurospheres to thyroid hormone.
1. INTRODUCTION
Intrauterine growth restriction (IUGR) is defined as a significant reduction of the fetal growth rate leading to a birth weight below the 10th centile for the corresponding gestational age. 1 The prevalence in developing countries accounts for 5% to 10% of all pregnancies, being a global health issue that is associated to short‐ and long‐term neurodevelopmental damage, cognitive dysfunctions and cardiovascular adverse outcomes. 2 , 3 Placental insufficiency, the primary cause of IUGR, reduces the quantity of nutrients reaching the fetus and leads to fetal development under chronic hypoxia followed by fetal acidosis. Placental insufficiency affects up to 7% of all gestations 4 , 5 and in approximately 50% of these cases it derives in clinically evident middle and long‐term neurological consequences defined as subtle cognitive and behavioral disabilities. 6 , 7 , 8 , 9 Different animal models and advanced imaging techniques have been used to better understand the mechanisms underlying neuronal impairments and perinatal brain maturation alterations induced by IUGR. 10 However, there is still a large knowledge gap on the mechanisms underlying these alterations 2 and a lack of research models to better characterize the IUGR‐associated brain injury (reviewed by Fleiss et al. 2019 11 ).
In order to have a human‐relevant experimental model of neurodevelopmental damage induced by IUGR, the BCNatal research group developed an IUGR model in pregnant rabbits. 12 This animal model reproduces the neurodevelopmental manifestations of IUGR occurring in clinical cases, including postnatal functional and structural deficits. 12 , 13 The selection of the rabbit species was based on the higher similarity to humans in terms of placentation and gestational circulatory changes, 12 , 14 as well as the resemblance regarding white matter maturation, which in both species happens during the postnatal period. 13 , 15 , 16 According to the model developed by Workman et al., 16 to predict the “precocial score” for neurodevelopment, rabbit species presents a precocial score at birth (0.537) more similar to humans (0.654) than other species, including rats (0.445) or mice (0.408). Likewise, at the neonatal period, diffusion MRI in whole organ preparations from a rabbit model showed differences on diffusion related parameters either at gray and white matter, revealing a pattern of microstructural brain changes produced by IUGR already at birth. 13 Interestingly, decreased fractional anisotropy in white matter structures has been suggested to reflect oligodendrocyte injury, 17 , 18 whereas in the gray matter areas it seems to reflect changes in neuronal arborisation. 19 Furthermore, follow‐up studies of IUGR rabbits have unraveled changes in their cerebral connections, 3 , 20 which correlate very well with the clinical observation that IUGR‐affected children also present cerebral connection alterations in connectomic‐studies. 21 Linking this clinical adverse outcome with the neurodevelopmental alterations observed in rabbits gives evidence that the rabbit IUGR model is a good model to study IUGR‐induced structural changes in humans. 12 , 22 , 23
However, in this in vivo model, it is difficult to identify the origin of the functional and structural deficits induced by IUGR, and so far, it was not possible to understand which basic cellular processes are altered during brain development under IUGR. To solve this limitation, we established an in vitro model based on primary rabbit neuronal progenitor cells (NPCs) allowing the investigation of the basic neurodevelopmental processes affected by IUGR. In this model, rabbit NPCs obtained from control and IUGR pups are cultured as three‐dimensional (3D) cell aggregates called neurospheres. Neurosphere cultures from other species have previously been established and have been utilized for mimicking basic processes of fetal brain development like NPC proliferation, migration and differentiation into neurons, oligodendrocytes and astrocytes. 24 , 25 , 26 , 27 , 28 , 29 In this study, for the very first time, a rabbit neurosphere culture was developed and characterized. In addition, we successfully developed a freezing and thawing protocol, which allows storing and prolonging the use of rabbit NPCs obtained from one litter and enables interlaboratory transfer of material. The ability to freeze and thaw rabbit neurospheres reduces the number of animals used and is thus much more time‐ and cost‐efficient. Due to the multicellular nature of the neurosphere method and the possibility to study a variety of neurodevelopmental processes with it, neurospheres are a valuable test system for studying a plethora of cellular effects initiated by a variety of different modesofaction. 24 , 28 , 30 , 31 , 32 , 33 Therefore, they seemed well suited for characterizing so far unknown neurodevelopmental effects on the cellular level induced by IUGR. With this method, we identified that rabbit NPCs derived from IUGR pups have a significant impairment in oligodendrocyte differentiation. Moreover, we discovered that exposure to thyroid hormone L‐triiodothyronine (T3) can revert this significant impairment unraveling a possible future therapeutic strategy. This new methods thus opens the door to easy and cost‐efficient testing of possible neuroprotective therapies for IUGR.
2. MATERIALS AND METHODS
2.1. Rabbit neurosphere generation
Rabbit tissue obtention for this study was approved by the Ethic Committee for Animal Experimentation of the University of Barcelona. Protocols were accepted with the license number: OB 392/19 SJD. Rabbit neural progenitor cells were isolated from nine newborn postnatal day (PND) 0 or 1 New Zealand rabbits whole brains by dissection, mechanical dissociation, digestion (20 minutes incubation with papain 20 U/mL at 37°C), mechanical homogenization into a cell suspension, and centrifugation (5 minutes at 800 rpm). The cell pellet obtained was cultured as a cell suspension for 1 week at 37°C and 7.5% CO2 in polyhydroxyethylmethacrylate (Poly‐HEMA) coated dishes with “proliferation medium” [consisting in DMEM and Hams F12 3:1 supplemented with 2% B27, and 20 ng/mL epidermal growth factor (EGF) and recombinant human fibroblast growth factor (FGF), 100 U/mL penicillin and 100 μg/mL streptomycin], half volume of which was exchanged every 2 to 3 days.
2.2. Neurosphere freezing and thawing protocol
During the neural progenitor cells isolation from 8 out of the 9 whole rabbit pup brains described above, half of the volume of the cell suspension obtained after resuspension in proliferation medium was centrifuged again (5 minutes at 800 rpm), the supernatant discarded and the pellet gently resuspended in freezing media instead (1:1; volume of pellet: volume of “freezing medium” [consisting in 70% (v/v) proliferation medium, 20% (v/v) fetal calf serum, and 10% (v/v) dimethyl sulfoxide (DMSO)]). Cell suspension in freezing medium was distributed in 1 mL per cryo‐vial and immediately placed in a cryo‐device filled with propanol to ensure a freezing rate of −1°C/min, and stored at −80°C.
Each cryo‐vial was thawed approximately 1 month after freezing by brief immersion in a 37°C water bath, transference of cells to 15 mL of “proliferation medium” preconditioned at 37°C and 7.5% CO2 for 2 hours, and gentle resuspension. Cell suspension was centrifuged (5 minutes, 800 rpm), supernatant discarded and cells transferred to Poly‐HEMA coated dishes filled with “proliferation medium” supplemented with Rho kinase (ROCK) inhibitor Y‐276322 at a final concentration of 10 μM, to enhance recovery and growth of cryopreserved cells. 34 Half of the volume of “proliferation medium” per petri dish was exchanged every 2 to 3 days by “proliferation medium” without ROCK inhibitor.
2.3. Rabbit “Neurosphere Assay”
Fresh or thawed neurospheres formed in the “proliferation medium” culture, were mechanically passaged before starting experiments with a chopper to 0.2 mm2 squares to ensure homogeneous neurosphere size, and allowed to recover spherical shape in “proliferation medium” in Poly‐HEMA coated dishes. On the experiment plating day (considered experiment day 0), 0.3 mm diameter neurospheres were selected and plated in one of the following conditions depending on the assay to perform.
2.3.1. Proliferation assay
One neurosphere per well was plated in 96 well‐round bottom‐plates coated with Poly‐HEMA and filled with 100 μL of “proliferation medium.” Plate was cultured at 37°C and 7.5% CO2 for 7 days. One bright‐field picture per neurosphere was taken on days 0, 2, 4, 6, and 7. Every 2 days, 50 μL of “proliferation medium” per well were renewed. Neurosphere diameter was measured in each picture with ImageJ.
2.3.2. Migration assays
Five neurospheres per chamber were plated in PDL/Laminin coated 8‐chamber slides filled with 500 μL of “differentiation medium” [consisting in DMEM and Hams F12 3:1 supplemented with N2 (Invitrogen), penicillin and streptomycin (100 U/mL and 100 μg/mL)]. After 1 and 3 days of culture, bright‐field pictures were taken to monitor migration progression by measuring the distance from the neurosphere core to the furthest migrated cells in four points per neurosphere using ImageJ.
2.3.3. Neuronal differentiation assay
At day 3, after taking pictures for migration, neurospheres were fixed with paraformaldehyde (PFA) 4% (1 hour, 37°C), washed twice with phosphate‐buffered saline (PBS) and stored in PBS until immunostained. For immunostaining, slides were incubated at room temperature with a blocking solution with 10% goat serum in PBS‐T (PBS containing 0.1% Triton X‐100) for 5 minutes. Neurospheres were incubated with a primary antibody solution containing 10% goat serum and 1:100 rabbit IgG anti‐βIII‐tubulin antibody in PBS‐T for 1 hour at 37°C. After three washing steps with PBS, slides were incubated with secondary antibody solution containing 2% goat serum, 1:100 Hoechst 33258 and 1:200 Alexa 546 anti‐rabbit IgG in PBS for 30 minutes at 37°C. After three washing steps with PBS and one with distilled water, slides were mounted with Acqua Poly/Mount (Polysciences, Inc.) and stored at 4°C until image acquisition. After immunostaining, 200× fluorescent images of two extracts of the migration area were taken per sphere in five spheres per condition in at least three independent experiments. The percentage of βIII‐tubulin positive cells was quantified manually using ImageJ software 34 cell count tool.
2.3.4. Astrocyte immunostaining
To immunostain astrocytes, the same protocol used to immunostain neurons was applied but the primary antibody solution contained 10% goat serum and 1:100 rabbit IgG anti GFAP antibody in PBS and the secondary antibody solution contained 2% goat serum, 1:100 Hoechst 33258, and 1:200 Alexa 546 anti‐rabbit IgG in PBS 1:200.
2.3.5. Oligodendrocyte differentiation assay
Five neurospheres per chamber were plated following the same procedure described for the migration assay. After 5 days of culture, neurospheres were fixed (4% PFA, 1 hour, 37°C) washed twice with PBS and stored in PBS until immunostained. Slides were incubated with a primary antibody solution containing 10% goat serum, 1:200 mouse IgM anti‐O4 antibody in PBS for 1 hour at 37°C. After three washing steps with PBS, slides were incubated with secondary antibody solution containing 2% goat serum, 1:100 Hoechst 33258, and 1:200 Alexa 488 anti‐mouse IgM in PBS for 30 minutes at 37°C. After three washing steps with PBS and one with distilled water, slides were mounted with Acqua Poly/Mount (Polysciences, Inc.) and stored at 4°C until image acquisition. After immunostaining, 200× fluorescent images of two extracts of the migration area were taken per sphere in five spheres per condition in at least three independent experiments. The percentage of O4 positive cells was quantified manually using ImageJ software 35 cell count tool.
2.3.6. Viability assay
At the end of migration/neuronal differentiation and oligodendrocyte differentiation assays (on days 3 and 5, respectively), and prior to fixation, a viability assay was performed by removing 200 μL medium per chamber, adding 100 μL of CellTiter‐Blue Reagent (Promega) diluted 1:3 (v/v) in “differentiation medium” per chamber and incubating for 2 hours at 37°C and 7.5% CO2 to measure the metabolic activity of the culture. Fluorescence of the supernatant was measured at 544/590 nm in two replicates of 100 μL per chamber transferred to two wells of a 96 well‐plate and expressed as relative fluorescence units.
2.4. IUGR induction in rabbits
Animal experimentation of this study was approved by the Ethic Committee for Animal Experimentation of the University of Barcelona. Protocols were accepted by the Department of Environment and Housing of the Generalitat de Catalunya with the license number: 03/17. New Zealand pregnant rabbits provided by a certified breeder were housed in separate cages with a 12/12 hours light/dark cycle, with free access to water and standard chow.
After at least 72 hours of acclimatization, IUGR was surgically induced following a previously described technique in four pregnant rabbits at 25 days of gestation. 12 Briefly, 40% to 50% of uteroplacental vessels that irrigate each gestational sac of one uterine horn (left or right randomly selected) were ligated obtaining the IUGR fetuses, while non‐ligated gestational sacs from the contralateral horn provided normally‐grown fetuses (controls). Postoperative analgesia with Buprenorphine 0.05 mg/kg was administered subcutaneously and animals were again housed with free access to water and standard chow ad libitum and were monitored daily for general health. Cesarean section was performed at 30 days of gestation. All living PND0 pups were identified and classified in control or IUGR groups depending on the uterine horn, weighted and sacrificed. Brains were immediately dissected and neural progenitor cells obtained as described in the “rabbit neurosphere generation” section.
All neural progenitor cells obtained from control and IUGR siblings were frozen at −80°C and thawed in parallel before performing any Neurosphere Assay.
2.5. Inclusion criteria of IUGR PND0 rabbit pups
Strong IUGR cases are defined by a body weight lower than the 10th percentile (32.7 g; Table 1). To cover a broad range of IUGR cases, neurospheres were prepared from mild and strong IUGR pups. In this study, IUGR rabbit pups were included for neurosphere preparation if their body weight was lower than the 90th percentile (57.8 g).
TABLE 1.
Percentile and body weight of rabbit pups on day PND0
| Percentile | 5 | 10 | 25 | 50 | 75 | 90 | 95 |
| PND0 body weight (g) | 29.5 | 32.7 | 39.7 | 46.3 | 52.2 | 57.8 | 62.1 |
2.6. Statistics
Statistical analysis was performed using GraphPad Prism v6 and v7 (GraphPad Software, La Jolla, California). Concentration‐dependent effects were assessed performing a one‐way ANOVA analysis followed by Bonferroni post hoc test for multiple comparisons. Comparisons of two groups were performed with two‐tailed t test. Significance threshold was established at P < .05.
3. RESULTS
3.1. Establishment of a rabbit neurosphere model
To establish a rabbit developmental neurosphere model, we first started isolating NPCs from control rabbit PND0 or PND1 brains, testing their ability to spontaneously form neurospheres and evaluating the competence of these freshly formed neurospheres to perform basic processes of neurogenesis like proliferation, migration, and differentiation. Freshly isolated rabbit NPCs spontaneously formed recognizable floating neurospheres after 4 days in culture, kept proliferating further and were big enough to be mechanically passaged (chopped) after 7 days in culture (Figure 1). Chopped neurospheres were used to start the “Neurosphere Assay” in control conditions, always compared to a positive control condition which was specific for each endpoint tested (endpoint‐specific control known to alter the measured variable): the src‐kinase inhibitor PP2 for migration, Epidermal Growth Factor (EGF) for neuronal differentiation and viability, bone morphogenic protein 7 (BMP7) for oligodendrocyte differentiation and withdrawal of all growth factors for proliferation. Freshly prepared rabbit NPCs growing as neurospheres migrated (mean ± SEM = 806 ± 29 μm, Figure 2) and proliferated (mean ± SEM = 18 ± 2 μm/day, Figure 2) in culture. Among cells in the migration area, we identified cells differentiating to astrocytes (GFAP+ cells), to oligodendrocytes (O4+ cells) and to neurons (βIII‐tubulin+ cells) (Figure 1). We also quantified the percentage of migrating cells differentiating to O4+ cells after 5 days (mean ± SEM = 12 ± 1%, Figure 2) and to βIII‐tubulin+ cells after 3 days (mean ± SEM = 3 ± 1, Figure 2). For all endpoints, results obtained with rabbit neurospheres allowed to observe alterations of the endpoint in both directions, positive and negative as recommended for the development of new alternative methods. 36 Besides, all positive controls included to each endpoint and tested in parallel to control, proved to be able to significantly modify the response obtained similar to human NPCs, indicating that the system is dynamic and can be influenced by known substances interfering with neurogenic processes. However, neuronal differentiation was relatively low compared to the values established for other species (human neurospheres = 5% 37 ). Therefore, other culture conditions were tested, mainly the addition of fetal calf serum (FCS) either in differentiation medium or in both proliferation and differentiation medium (Figure 2). Both conditions led to an increased percentage of cells differentiating into neurons and exceeding 5% of differentiation. The longer the cells were exposed to FCS, the higher the percentage of neurons was (Figure 2).
FIGURE 1.

Graphical summary of the rabbit “Neurosphere Assay.” Culture establishment: PND0 rabbit brains are dissected and either frozen or directly cultured until neurosphere formation (A, 7 days in culture after dissection; B, 7 days in culture after thawing). “Neurosphere Assay” starts when neurospheres of a diameter of 300 μm are plated in 8‐chamber slides and maintained under differentiating conditions to evaluate migration (C, 0 hours; D, 48 hours; E, 72 hours after plating) and differentiation into neurons (F, 72 hours after plating, βIII‐tubulin in red and Hoechst 33258 in blue), into astrocytes (G, 72 hours after plating, GFAP in red and Hoechst 33258 in blue) or into oligodendrocytes (H, 120 hours after plating, O4 in green and Hoechst 33258 in blue). Scale bars = 100 μm. Neurospheres were also plated in 96‐well plates under proliferating conditions and evaluated for diameter increase up to 7 days (I, representative image of a proliferating neurosphere from day 0 to day 7)
FIGURE 2.

Results of the “Neurosphere Assay” establishment with freshly prepared rabbit neurospheres. Rabbit neurospheres were cultured for 3, 5, or 7 days under control or specific positive control conditions for each endpoint tested (PP2 10 μM; EGF 10 ng/mL; BMP7 100 ng/mL). A, 3 days migration; B, 3 days neuronal differentiation without supplementary FCS in the medium (wo FCS/wo FCS); C, 3 days neuronal differentiation with addition of 1% FCS in the differentiation medium (wo FCS/+FCS); D, 3 days neuronal differentiation with addition of 1% FCS in the proliferation and differentiation medium (+FCS/+FCS); E, 5 days oligodendrocyte differentiation; F, 7 days proliferation. All endpoints were evaluated in five neurospheres/condition in at least three independent experiments. Results presented as boxes and whiskers according to Tukey method. *P < .05 vs control
After establishing all endpoints of the “Neurosphere Assay” for fresh neurospheres, a freezing protocol was tested, either after first passaging of neurospheres (passage 1) or directly after isolation of neural progenitor cells from rabbit brain (passage 0).
When the freezing protocol was applied after the first passaging of neurospheres (passage 1), NPCs lost their capabilities to develop in all endpoints of the “Neurosphere Assay” (mainly due to too low neuronal differentiation and too low proliferation; see Supporting Information Figure S1), and therefore, this option was directly discarded. Nevertheless, the application of the freezing protocol on freshly isolated NPCs, before the formation of neurospheres (passage 0), led to results in the “Neurosphere Assay” comparable to fresh neurospheres (see Material and methods section for more details about freezing and thawing protocol). Thawed NPCs spontaneously formed recognizable neurospheres after 4 days in vitro and were big enough to be chopped after 7 days in vitro (Figure 1). Results of proliferation, migration and differentiation assays met quality criteria as shown in Figure 3. Interestingly, thawed neurospheres also achieved a 5% mean differentiation to neurons without adding any FCS to the differentiation or proliferation medium, probably because the freezing medium already contained FCS. Even so, as previously assayed with fresh spheres, FCS addition to differentiation and/or proliferating medium was also tested. In this case, FCS addition also induced an increase in neuronal differentiation, and as previously observed for fresh neurospheres, the longer the cells were exposed to FCS, the higher the percentage of neurons was. However, in this case, the percentage of neurons was so high in some wells (Figure 3; mean > 15%) that neurons were growing in clumps and overlapping one another making it very difficult to correctly quantify them. As thawed neurospheres without FCS addition in culture produced a percentage of neurons that could be precisely quantified, and also with the aim to not introduce more factors to the medium which could mask adverse effects induced by physical or chemical agents in future tests, this culture condition (without FCS) was chosen for further experiments.
FIGURE 3.

Results of the “Neurosphere Assay” establishment with thawed rabbit neurospheres. Thawed rabbit neurospheres were cultured for 3, 5, or 7 days under control conditions or exposed to specific positive controls for each endpoint tested (PP2 10 μM; EGF 10 ng/mL; BMP7 100 ng/mL). A, 3 days migration; B, 3 days neuronal differentiation without supplementary FCS in the medium (wo FCS/wo FCS); C, 3 days neuronal differentiation with addition of 1% FCS in the differentiation medium (wo FCS/+FCS); D, 3 days neuronal differentiation with addition of 1% FCS in the proliferation and differentiation medium (+FCS/+FCS); E, 5 days oligodendrocyte differentiation; F, 7 days proliferation. All endpoints were evaluated in five neurospheres/condition in at least three independent experiments. Results presented as boxes and whiskers according to Tukey's method. *P < .05 vs control
Thawed neurospheres were also tested in parallel in control and positive control conditions, using the same compounds than for fresh neurospheres. In all cases, positive controls decreased the result obtained in control conditions. In view of these results, all further experiments were performed with neurospheres submitted to the freezing protocol at passage 0.
To prove that rabbit neurospheres are able to detect alterations induced during the course of neurodevelopment without being oversensitive, we exposed them to two compounds in a concentration‐range mode, a positive and a negative control, following the recommendations of Crofton et al. 36 One is a known neurodevelopmental toxicant in humans and animals, methylmercury chloride (MeHgCl; positive control) and the other one a compound known to have no effects on neurodevelopment in humans and animals, saccharine (negative control; reviewed by Aschner et al. 38 ). MeHgCl induced a significant and concentration‐dependent decrease in neural progenitor cell migration, with an IC50 of 2 μM (Figure 4), while saccharine did not significantly decrease migration at any concentration tested (maximum concentration tested = 100 μM).
FIGURE 4.
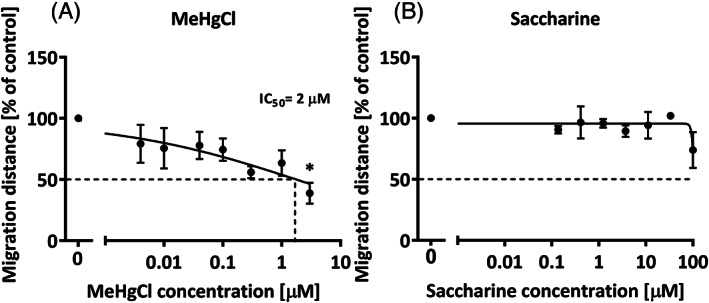
Rabbit neurospheres sensitivity and specificity evaluation. Thawed rabbit neurospheres were cultured for 1 day with increasing concentrations of the known neurodevelopmental toxicant: MeHgCl, A, or a compound known to have no neurodevelopmental adverse effects: Saccharine, B. Results presented as mean ± SEM of at least three independent experiments including five neurospheres/concentration and analyzed by ANOVA and Bonferroni post hoc test. *P < .05 vs control
3.2. Evaluation of the effects of IUGR in neurodevelopmental processes
The aim of establishing a rabbit neurosphere culture was to develop a model to study the effects of IUGR on brain development in a more time‐ and cost‐efficient way with using fewer numbers of animals. Therefore, we used the rabbit “Neurosphere Assay” to investigate the specific consequences of IUGR induction by ligation of 40% to 50% of uteroplacental vessels irrigating the gestational sac between GD25 and GD30. Neurospheres from four control and four IUGR pups with mean birth weight ± SEM = 48.4 ± 1.4 g and 30.3 ± 2.7 g respectively (P = .06; Figure 5), and belonging to three different litters were frozen and thawed in pairs and submitted to exactly the same conditions in vitro. The neurospheres of these four pups per experimental group were used to perform all following experiments. Neurospheres from IUGR animals did not present any significant difference in NPC proliferation after 7 days in culture or in migration after 1 or 3 days in culture (Figure 5). A non‐significant increase in neuronal differentiation was observed in IUGR neurospheres (Figures 5), while oligodendrocyte differentiation was significantly decreased in IUGR neurospheres compared to control spheres (Figures 5). Viability of these NPCs, measured by their metabolic activity, was not altered in any of these cases (3 or 5 days in culture; Figure 5), indicating that the decreased number of differentiated oligodendrocytes is a specific effect not induced by general cytotoxicity. By contrast, there were no differences between control and IUGR neurospheres proliferating capabilities (Figure 5). In summary, the results of the “Neurosphere Assay” indicated that IUGR impairs NPCs' differentiation ability into oligodendrocytes, while their proliferation and migration potential is not affected. To contextualize these in vitro results, it is important to remark that both, mild and strong IUGR cases were included (Figure 5) in the preparation of neurospheres for these tests and therefore, the total body weight of IUGR pups was not significantly reduced compared to the controls (P = .06). This approach was chosen to cover the broad range of cases that IUGR can display, and to exclude the possibility of detecting effects that are only present in the most severe IUGR cases.
FIGURE 5.

Comparison of control and IUGR neurospheres on basic processes of neurogenesis. Thawed rabbit neurospheres obtained from control and IUGR animals were cultured for 3, 5, or 7 days and comparatively tested for each endpoint of the “Neurosphere Assay.” A, Migration distance after 1 day; or B, 3 days; C, neuronal differentiation after 3 days; D, metabolic activity after 3 days; E, oligodendrocyte differentiation after 5 days; F, metabolic activity after 5 days; G, diameter increase after 7 days; H, body weight of control and IUGR pups on PND0; I and J, representative pictures of oligodendrocyte differentiation in control and IUGR neurospheres, respectively. Scale bars = 100 μm. All endpoints (except body weight, H) were evaluated in five neurospheres/condition in at least three independent experiments. Results presented as boxes and whiskers according to Tukey method. *P < .05 IUGR vs control
Furthermore, the response of control and IUGR neurospheres to positive controls known to modulate the response of each endpoint was also compared (Figure 6). As previously shown in Figure 3, positive controls significantly reduce the respective readouts of all endpoints in control spheres. However, in IUGR spheres, the exposure to BMP7 did not significantly reduce oligodendrocyte differentiation, probably because in IUGR neurospheres oligodendrocyte differentiation was already reduced per se (Figure 6). Even if the effect of BMP7 was not significant, it reduced the mean oligodendrocyte differentiation in IUGR neurospheres. These data show that the process is also dynamic in IUGR neurospheres as compounds in vitro can still modulate the impairment of oligodendrocyte differentiation. In the next step,we tested if this decreased differentiation can also be modulated in the opposite direction to increase the percentage of oligodendrocytes and revert the observed adverse effect. Thyroid hormone (TH) plays an essential role in white matter maturation and myelin formation. 39 , 40 , 41 , 42 For this reason, we exposed control and IUGR neurospheres to L‐triiodothyronine (T3; CAS: 55‐06‐1; Sigma‐Aldrich), the active form of TH, at a concentration similar to the total T3 concentration in human serum, 3 nM. 43 , 44 , 45 , 46 T3 exposure significantly induced the percentage of oligodendrocyte differentiation of IUGR neurospheres to control levels, while it did not affect the percentage of differentiation in control neurospheres (Figure 6). Hence, although the cellular damage was set while cells were still in the in vivo context, in vitro exposure of ex vivo NPCs to T3 can revert the deleterious effect of IUGR in oligodendrocyte differentiation.
FIGURE 6.

Comparison of the response of IUGR and control neurospheres to known modulators of basic neurogenesis processes. Thawed rabbit neurospheres obtained from control and IUGR animals were cultured for 3 or 5 days and comparatively tested for each endpoint. Neuronal differentiation was analyzed after 3 days under control conditions or exposed to either PP2 10 μM, A, or EGF 20 ng/mL, B, oligodendrocyte differentiation was analyzed after 5 days under control conditions or exposed to either BMP7 100 ng/mL, C, or T3 3 nM, D. All endpoints were evaluated in five neurospheres/condition in at least three independent experiments. Results presented as boxes and whiskers according to Tukey method. *P < .05 vs control condition; n.s.: not significant
4. DISCUSSION
The neurosphere model based on NPCs is a valuable tool to study the progress of basic neurodevelopmental processes. 47 Therefore, we have previously established “Neurosphere Assays” based on rat, mouse or human NPCs as methods to evaluate the adverse effects of chemical or physical agents on neurodevelopment. 24 , 25 , 26 , 27 , 28 , 30 , 37 , 48 Due to its nature as a primary cell culture, the neurosphere model retains the original characteristics of the neuro‐ and gliogenic populations of the brain, and reflects their physiological and physiopathological characteristics. To evaluate the effects of IUGR in neurodevelopment, the ideal situation would be to use a human NPC based “Neurosphere Assay” to avoid species translation limitations. 49 Yet commercially available hNPCs are derived from healthy individuals, and hNPCs from IUGR individuals are such an extremely scarce material that they are unviable to establish a permanent research model for mechanistic investigations. Therefore, and considering that the rabbit in vivo model previously developed in BCNatal mimics the neurodevelopmental features of human IUGR individuals better than other rat in vivo models, and that rabbits are perinatal brain developers 50 similar to humans and unlike rodents, 51 we decided to establish a rabbit neurosphere culture for the first time. The combination of a 3D in vitro neurodevelopmental testing strategy, with the clinically relevant IUGR experimental animal model allowed us to study IUGR neurodevelopmental consequences by decomposing them into basic neurodevelopmental processes, which are evaluated separately. This methodology offers a research approach that allows studying a complex clinical problem on the cellular, and eventually also molecular level. By employing this newly established in vitro model we identified that rabbit NPCs from IUGR individuals have a significantly reduced ability to form oligodendrocytes. Moreover, we were able to revert this damage by T3 in vitro exposure.
In the present study, we established the in vitro rabbit neurosphere model with cells obtained from freshly dissected rabbit brains (Figure 2) and we tested different freezing protocols, choosing the best one (based on P0 cells) for the use of NPCs in the “Neurosphere Assay” (Figure 3). Neurospheres obtained from both protocols (from fresh and frozen cells) spontaneously formed after 7 days in culture and were able to perform all basic neurodevelopmental processes (proliferation, migration, and differentiation) similar to neurospheres from other species, for example, rats, mice and humans. 24 , 25 , 26 , 27 , 28 , 30 , 37 , 48 Among the differentiated cells, glial cells outnumbered neuronal cells, according to the situation described in vivo for other species indicating that a massive number of glial cells is generated during postnatal neurodevelopment. 52 Specific data in vivo for rabbits during the postnatal period could not be found, but the glia neuron ratio described in adult rabbits also shows that in all brain areas except in the cerebellum, glial cells outnumber neurons. 53 According to the 3R principle of Russel and Burch (1959) for the reduction, replacement and refinement of animal experimentation the established freezing and thawing protocol for rabbit NPCs constitutes a durable technique to reduce and refine the use of animals. Neurospheres obtained from both protocols are dynamic systems since their ability to proliferate, migrate or differentiate was modified by exposure to known positive control compounds (PP2, EGF, BMP7, or growth factor removal in Figures 2 and 3). With respect to the modulation of the performance of these neurospheres, we also proved that a known developmental neurotoxic compound, MeHgCl, induces a significant decrease in cell migration (Figure 4), showing that this rabbit neurosphere model is sensitive to known noxious agents. The IC50 concentration detected by the model is in line with previously detected IC50 concentrations in human primary or human iPSC‐derived neurosphere models for migration disturbance 26 , 30 , 54 and correlates well with concentrations achieved in vivo after the administration of MeHgCl doses affecting neurodevelopment. 55 Understanding the sensitivity of a model is a key step for its establishment, that is, it is also essential to ensure that the system is not overreactive to any stimulus, which means that known innocuous compounds for neurodevelopment should not alter the response of neurospheres in the assay. For this purpose, saccharine was chosen as negative control, because it was already selected as a harmless compound for neurodevelopment by a panel of experts. 38 When saccharine was added to the neurosphere culture in concentrations up to 100 μM no adverse effect in migration was observed, indicating the specificity of the assay. This tiered approach for the establishment of the rabbit neurosphere culture, besides being a necessary preliminary group of steps for our subsequent study of IUGR effects, is an essential procedure for the establishment of the method as an alternative model for developmental neurotoxicity detection and the screening and prioritization of chemicals (following the recommendations from Crofton et al. 36 ). Current neurodevelopmental studies on the effects of exposure to substances during the pre‐ and early postnatal period are based on the in vivo OECD guidelines 426 (OECD, 2007), 414 (OECD, 2018), and FDA guideline S5 (R3) (FDA 2017) which define rat as the preferred rodent and rabbit as the preferred nonrodent animal. A rat neurosphere model for compound evaluation has already been available since 2014, 37 and now the addition of the neurosphere model of the preferred nonrodent animal opens the door to combine these two models for in vitro DNT evaluation in case the use of human material is not possible or for understanding species differences in response to exogenous insults.
After performing all establishment steps with control neurospheres: establishment of the (a) fresh culture, (b) freezing and thawing protocol, and analyzing the system's, (c) dynamism, (d) sensitivity, and (e) specificity, we compared the performance of control and IUGR NPCs in the “Neurosphere Assay.” Here, we identified a significant reduction in oligodendrocyte formation in IUGR NPCs. This observation in vitro leads to the possible correlation with a poor myelination in vivo as a cause of neurological damage secondary to IUGR. Our finding is in line with previous studies in vivo reporting that IUGR leads to white matter injury with a deficiency of mature oligodendrocytes 56 , 57 and adversely affected myelination processes in brain histology studies. 13 It is important to remark that the adverse effect on oligodendrogenesis in this study was detected in neurospheres obtained both from severe and mild IUGR cases, and therefore the effect was not only observed in the most severe IUGR cases. These findings are in line with clinical and neuroimaging data supporting the existence of fetal abnormal neurodevelopment across all stages of severity in IUGR. In comparison of control and IUGR neurospheres, we also observed an increase in neuronal differentiation after 3 days in culture but this effect was not significant. Future investigations should focus on the prolongation of the period in culture to obtain more stable results (decrease deviation of IUGR results) and to clarify if this incipient increase is significant after 5, 7, 14, or more days in culture and it is consequently another alteration underlying IUGR neural damage. Besides, after a longer culture period, further neurodevelopmental endpoints could also be added to the assay, like neurite outgrowth, neurite branching, synaptogenesis and probably neuronal network activity. In future work it would also be of high interest to assess alterations in astrocyte differentiation, using the protocol established here.
We finally tested a possible future neurotherapy for IUGR: in vitro exposure to 3 nM of T3 completely reverted the damage induced by IUGR by inducing oligodendrocyte differentiation, while it did not increase the percentage of oligodendrocytes in control neurospheres (Figure 6). This TH effect in rabbit control NPCs is similar to the one observed in human neurospheres in vitro, where 3 nM T3 exposure does not induce an increase in the percentage of oligodendrocytes in control neurospheres, but induces oligodendrocyte maturation. 32 If T3 also induces rabbit oligodendrocyte maturation in vitro will be subject to future studies. The transferability of this possible therapy to the clinical field, needs to be carefully assessed for this particular condition, but in support of its transferability it is important to mention that preclinical studies and clinical studies using Tetrac, the TH analog 3,5,3′,5′‐tetraiodothyroacetic acid, have already been conducted or are conducted at the moment with the goal of supplementing hypothyroid developing brains with TH (ClinicalTrials.gov identifier: NCT04143295). Preclinical studies in mice show that postnatal administration of this potent TH receptor agonist offers the opportunity to reduce the neurological damage associated to the lack of MCT8, a transporter playing a critical role in the uptake of thyroid hormones. 58 The results of the clinical trials conducted in MCT8 deficiency patients (also known as Allan‐Herndon‐Dudley syndrome) will be of high importance to evaluate the future transferability of the proposed therapeutic strategy in IUGR cases.
In summary, we established an in vitro model for the study of IUGR‐induced neural damage which is faster, more economic and more ethical than the current in vivo approaches in rabbits. Using our new model we detected that oligodendrogenesis is reduced in IUGR brains and that this effect is reversed by exposure to T3. This work opens the door to the use of the rabbit “Neurosphere Assay” (a) for testing developmental neurotoxic compounds as a complementary method to the rat “Neurosphere Assay,” (b) for studying IUGR‐induced neural damage on the cellular and molecular level, (c) for a future identification of possible disease biomarkers, and (d) for the evaluation and selection of specific neuroprotective therapies.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
M.B.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; M.I.: conception and design, provision of study material, data analysis and interpretation, manuscript critical revision; M.H.: collection and assembly of data, data analysis and interpretation; C.L., L.P., J.K.: collection and assembly of data; B.A.K.: data analysis and interpretation, manuscript writing; J.G.‐C.: provision of study material, manuscript critical revision; J.M.B.: administrative support, provision of study material; F.C.: conception and design, data analysis and interpretation, financial support; E.G.: conception and design, financial support, manuscript critical revision; E.F.: conception and design, data analysis and interpretation, manuscript critical revision. All authors have read and approved the final manuscript.
Supporting information
Figure S1: Comparison of results of neurospheres which were frozen at passage 0 (P0) or at passage 1 (P1). Results of passage 0 are the same results presented in Figure 3 as control group.
ACKNOWLEDGMENTS
We thank Dr. Catrin Albrecht for her logistical support and valuable advice. Graphical abstract and Figure 1 were created with BioRender.com. This study has been funded by Instituto de Salud Carlos III through the project “PI18/01763” (co‐funded by European Regional Development Fund/European Social Fund, “Investing in your future”), from “LaCaixa” Foundation under grant agreements LCF/PR/GN14/10270005 and LCF/PR/GN18/10310003, and from AGAUR under grant 2017 SGR n° 1531. B.A.K. received a scholarship from Fundació Bosch i Gimpera (project number: 300155) and C.L. received the support the Health Departament of the Catalan Government (grant n° SLT006/17/00325). Open access funding enabled and organized by Projekt DEAL.
Barenys M, Illa M, Hofrichter M, et al. Rabbit neurospheres as a novel in vitro tool for studying neurodevelopmental effects induced by intrauterine growth restriction. STEM CELLS Transl Med. 2021;10:209–221. 10.1002/sctm.20-0223
[Correction added on 14 December 2020, after first online publication: Projekt Deal funding statement has been added.]
Funding information Health Departament of the Catalan Government, Grant/Award Number: SLT006/17/00325; Fundació Bosch i Gimpera, Grant/Award Number: 300155; AGAUR, Grant/Award Number: 2017 SGR n° 1531; “LaCaixa” Foundation, Grant/Award Numbers: LCF/PR/GN18/10310003, LCF/PR/GN14/10270005; European Regional Development Fund/European Social Fund; Instituto de Salud Carlos III, Grant/Award Number: PI18/01763
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr. 2016;10:CMPed.S40070 10.4137/CMPed.S40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eixarch E, Muñoz‐Moreno E, Bargallo N, Batalle D, Gratacos E. Motor and cortico‐striatal‐thalamic connectivity alterations in intrauterine growth restriction. Am J Obstet Gynecol. 2016;214(6):725.e1‐725.e9. 10.1016/j.ajog.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 3. Batalle D, Muñoz‐Moreno E, Arbat‐Plana A, et al. Long‐term reorganization of structural brain networks in a rabbit model of intrauterine growth restriction. Neuroimage. 2014;100:24‐38. 10.1016/j.neuroimage.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 4. Kady SM, Gardosi J. Perinatal mortality and fetal growth restriction. Best Pract Res Clin Obstet Gynaecol. 2004;18(3):397‐410. 10.1016/j.bpobgyn.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 5. Marsal K. Intrauterine growth restriction. Curr Opin Obstet Gynecol. 2002;14(2):127‐135. [DOI] [PubMed] [Google Scholar]
- 6. Geva R, Eshel R, Leitner Y, Valevski AF, Harel S. Neuropsychological outcome of children with intrauterine growth restriction: a 9‐year prospective study. Pediatrics. 2006;118(1):91‐100. 10.1542/peds.2005-2343. [DOI] [PubMed] [Google Scholar]
- 7. Morsing E, Åsard M, Ley D, Stjernqvist K, Maršál K. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics. 2011;127(4):e874‐e882. 10.1542/peds.2010-1821. [DOI] [PubMed] [Google Scholar]
- 8. Tideman E, Maršál K, Ley D. Cognitive function in young adults following intrauterine growth restriction with abnormal fetal aortic blood flow. Ultrasound Obstet Gynecol. 2007;29(6):614‐618. 10.1002/uog.4042. [DOI] [PubMed] [Google Scholar]
- 9. Geva R, Eshel R, Leitner Y, Fattal‐Valevski A, Harel S. Memory functions of children born with asymmetric intrauterine growth restriction. Brain Res. 2006;1117(1):186‐194. 10.1016/j.brainres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 10. Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. 2011;29(6):551‐563. 10.1016/j.ijdevneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleiss B, Wong F, Brownfoot F, et al. Knowledge gaps and emerging research areas in intrauterine growth restriction‐associated brain injury. Front Endocrinol (Lausanne). 2019;10:1‐24. 10.3389/fendo.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eixarch E, Figueras F, Hernández‐Andrade E, et al. An experimental model of fetal growth restriction based on selective ligature of uteroplacental vessels in the pregnant rabbit. Fetal Diagn Ther. 2009;26(4):203‐211. 10.1159/000264063. [DOI] [PubMed] [Google Scholar]
- 13. Eixarch E, Batalle D, Illa M, et al. Neonatal neurobehavior and diffusion MRI changes in brain reorganization due to intrauterine growth restriction in a rabbit model. PLoS One. 2012;7(2):1‐12. 10.1371/journal.pone.0031497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carter AM. Animal models of human placentation—a review. Placenta. 2007;28(Suppl):S41‐S47. 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 15. Derrick M, Luo NL, Bregman JC, et al. Preterm fetal hypoxia‐ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci. 2004;24(1):24‐34. 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33(17):7368‐7383. 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drobyshevsky A, Derrick M, Wyrwicz AM, et al. White matter injury correlates with hypertonia in an animal model of cerebral palsy. J Cereb Blood Flow Metabol 2007:27;270–281. 10.1038/sj.jcbfm.9600333 [DOI] [PubMed] [Google Scholar]
- 18. van de Looij Y, Lodygensky GA, Dean J, et al. High‐field diffusion tensor imaging characterization of cerebral white matter injury in lipopolysaccharide‐exposed fetal sheep. Pediatr Res. 2012;72(3):285‐292. 10.1038/pr.2012.72. [DOI] [PubMed] [Google Scholar]
- 19. McKinstry RC, Mathur A, Miller JH, et al. Radial organization of developing preterm human cerebral cortex revealed by non‐invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12(12):1237‐1243. 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- 20. Illa M, Eixarch E, Batalle D, et al. Long‐term functional outcomes and correlation with regional brain connectivity by MRI diffusion tractography metrics in a near‐term rabbit model of intrauterine growth restriction. PLoS One. 2013;8(10):e76453 10.1371/journal.pone.0076453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Batalle D, Eixarch E, Figueras F, et al. Altered small‐world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage. 2012;60(2):1352‐1366. 10.1016/j.neuroimage.2012.01.059. [DOI] [PubMed] [Google Scholar]
- 22. Bassan H, Leider Trejo L, Kariv N, et al. Experimental intrauterine growth retardation alters renal development. Pediatr Nephrol. 2000;15(3–4):192‐195. 10.1007/s004670000457. [DOI] [PubMed] [Google Scholar]
- 23. Eixarch E, Hernandez‐Andrade E, Crispi F, et al. Impact on fetal mortality and cardiovascular Doppler of selective ligature of uteroplacental vessels compared with undernutrition in a rabbit model of intrauterine growth restriction. Placenta. 2011;32(4):304‐309. 10.1016/j.placenta.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 24. Barenys M, Gassmann K, Baksmeier C, et al. Epigallocatechin gallate (EGCG) inhibits adhesion and migration of neural progenitor cells in vitro. Arch Toxicol. 2017;91:827‐837. 10.1007/s00204-016-1709-8. [DOI] [PubMed] [Google Scholar]
- 25. Moors M, Cline JE, Abel J, Fritsche E. ERK‐dependent and ‐independent pathways trigger human neural progenitor cell migration. Toxicol Appl Pharmacol. 2007;221(1):57‐67. 10.1016/j.taap.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 26. Moors M, Rockel TD, Abel J, et al. Human neurospheres as three‐dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect. 2009;117(7):1131‐1138. 10.1289/ehp.0800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Breier JM, Gassmann K, Kayser R, et al. Neural progenitor cells as models for high‐throughput screens of developmental neurotoxicity: state of the science. Neurotoxicol Teratol. 2010;32(1):4‐15. 10.1016/j.ntt.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 28. Gassmann K, Abel J, Bothe H, et al. Species‐specific differential AhR expression protects human neural progenitor cells against developmental neurotoxicity of PAHs. Environ Health Perspect. 2010;118(11):1571‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schreiber T, Gassmann K, Götz C, et al. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ Health Perspect. 2010;118(4):572‐578. 10.1289/ehp.0901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baumann J, Gassmann K, Masjosthusmann S, et al. Comparative human and rat neurospheres reveal species differences in chemical effects on neurodevelopmental key events. Arch Toxicol. 2016;90(6):1415‐1427. 10.1007/s00204-015-1568-8. [DOI] [PubMed] [Google Scholar]
- 31. Gassmann K, Schreiber T, Dingemans MML, et al. BDE‐47 and 6‐OH‐BDE‐47 modulate calcium homeostasis in primary fetal human neural progenitor cells via ryanodine receptor‐independent mechanisms. Arch Toxicol. 2014;88(8):1537‐1548. 10.1007/s00204-014-1217-7. [DOI] [PubMed] [Google Scholar]
- 32. Dach K, Bendt F, Huebenthal U, et al. BDE‐99 impairs differentiation of human and mouse NPCs into the oligodendroglial lineage by species‐specific modes of action. Sci Rep. 2017;7(March):1‐11. 10.1038/srep44861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masjosthusmann S, Siebert C, Hübenthal U, Bendt F, Baumann J, Fritsche E. Arsenite interrupts neurodevelopmental processes of human and rat neural progenitor cells: the role of reactive oxygen species and species‐specific antioxidative defense. Chemosphere. 2019;235:447‐456. 10.1016/j.chemosphere.2019.06.123. [DOI] [PubMed] [Google Scholar]
- 34. Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76(8):722‐732. 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671‐675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crofton KM, Mundy WR, Lein PJ, et al. Developmental neurotoxicity testing: recommendations for developing alternative methods for the screening and prioritization of chemicals. ALTEX. 2011;28(1):9‐15. 10.14573/altex.2011.1.009. [DOI] [PubMed] [Google Scholar]
- 37. Baumann J, Barenys M, Gassmann K, Fritsche E. Comparative human and rat “neurosphere assay” for developmental neurotoxicity testing. Curr Protoc Toxicol. 2014;1(SUPPL.59):1‐24. 10.1002/0471140856.tx1221s59. [DOI] [PubMed] [Google Scholar]
- 38. Aschner M, Ceccatelli S, Daneshian M, et al. Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: example lists and criteria for their selection and use. ALTEX. 2017;34(1):49‐74. 10.14573/altex.1604201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Namba N, Etani Y, Kitaoka T, et al. Clinical phenotype and endocrinological investigations in a patient with a mutation in the MCT8 thyroid hormone transporter. Eur J Pediatr. 2008;167(7):785‐791. 10.1007/s00431-007-0589-6. [DOI] [PubMed] [Google Scholar]
- 40. Rodrigues F, Grenha J, Ortez C, et al. Hypotonic male infant and MCT8 deficiency—a diagnosis to think about. BMC Pediatr. 2014;14(38 cm):252 10.1186/1471-2431-14-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marta CB, Adamo AM, Soto EF, Pasquini JM. Sustained neonatal hyperthyroidism in the rat affects myelination in the central nervous system. J Neurosci Res. 1998;53(2):251‐259. . [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez‐Pena A, Ibarrola N, Iniguez MA, Munoz A, Bernal J. Neonatal hypothyroidism affects the timely expression of myelin‐associated glycoprotein in the rat brain. J Clin Invest. 1993;91(3):812‐818. 10.1172/JCI116301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anckarsäter R, Zetterberg H, Blennow K, Anckarsäter H. Association between thyroid hormone levels and monoaminergic neurotransmission during surgery. Psychoneuroendocrinology. 2007;32(8–10):1138‐1143. 10.1016/j.psyneuen.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 44. Johansson P, Almqvist EG, Johansson J‐O, et al. Reduced cerebrospinal fluid level of thyroxine in patients with Alzheimer's disease. Psychoneuroendocrinology. 2013;38(7):1058‐1066. 10.1016/j.psyneuen.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 45. Hagen GA, Elliott WJ. Transport of thyroid hormones in serum and cerebrospinal fluid. J Clin Endocrinol Metab. 1973;37(3):415‐422. 10.1210/jcem-37-3-415. [DOI] [PubMed] [Google Scholar]
- 46. Nishikawa M, Inada M, Naito K, et al. 3,3′,5′‐Triiodothyronine (reverse T3) in human cerebrospinal fluid. J Clin Endocrinol Metab. 1981;53(5):1030‐1035. 10.1210/jcem-53-5-1030. [DOI] [PubMed] [Google Scholar]
- 47. Svendsen CN, ter Borg MG, Armstrong RJ, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85(2):141‐152. 10.1016/S0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 48. Fritsche E, Cline JE, Nguyen N‐H, Scanlan TS, Abel J. Polychlorinated biphenyls disturb differentiation of normal human neural progenitor cells: clue for involvement of thyroid hormone receptors. Environ Health Perspect. 2005;113(7):871‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leist M, Hartung T. Inflammatory findings on species extrapolations: humans are definitely no 70‐kg mice. Arch Toxicol. 2013;87(4):563‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harel S, Shapira Y, Tomer A, Donahue MJ, Quilligan E. Vascular‐induced intrauterine growth retardation: relations between birth weight and the development of biochemical parameters in young rabbits. Isr J Med Sci. 1985;21(10):829‐832. [PubMed] [Google Scholar]
- 51. Drobyshevsky A, Jiang R, Derrick M, Luo K, Tan S. Functional correlates of central white matter maturation in perinatal period in rabbits. Exp Neurol. 2014;261:76‐86. 10.1016/j.expneurol.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herculano‐Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62(9):1377‐1391. 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- 53. Herculano‐Houzel S, Ribeiro P, Campos L, et al. Updated neuronal scaling rules for the brains of Glires (rodents/lagomorphs). Brain Behav Evol. 2011;78(4):302‐314. 10.1159/000330825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hofrichter M, Nimtz L, Tigges J, et al. Comparative performance analysis of human iPSC‐derived and primary neural progenitor cells (NPC) grown as neurospheres in vitro. Stem Cell Res. 2017;25:72‐82. 10.1016/j.scr.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 55. Lewandowski TA, Ponce RA, Charleston JS, Hong S, Faustman EM. Effect of methylmercury on midbrain cell proliferation during organogenesis: potential cross‐species differences and implications for risk assessment. Toxicol Sci. 2003;75(1):124‐133. [DOI] [PubMed] [Google Scholar]
- 56. Tolcos M, Petratos S, Hirst JJ, et al. Blocked, delayed, or obstructed: what causes poor white matter development in intrauterine growth restricted infants? Prog Neurobiol. 2017;154:62‐77. 10.1016/j.pneurobio.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 57. Reid MV, Murray KA, Marsh ED, Golden JA, Simmons RA, Grinspan JB. Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenetic protein 4. J Neuropathol Exp Neurol. 2012;71(7):640‐653. 10.1097/NEN.0b013e31825cfa81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Horn S, Kersseboom S, Mayerl S, et al. Tetrac can replace thyroid hormone during brain development in mouse mutants deficient in the thyroid hormone transporter Mct8. Endocrinology. 2013;154(2):968‐979. 10.1210/en.2012-1628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Comparison of results of neurospheres which were frozen at passage 0 (P0) or at passage 1 (P1). Results of passage 0 are the same results presented in Figure 3 as control group.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


