Abstract
Over the past decade, natural compounds have been proven to be effective against many human diseases, including cancers. Triptolide (TPL), a diterpenoid triepoxide from the Chinese herb Tripterygium wilfordii Hook F, has exhibited attractive cytotoxic activity on several cancer cells. An increasing number of studies have emphasized the antitumor effects of TPL on non-small cell lung cancer (NSCLC). Here we mainly focused on the key molecular signaling pathways that lead to the inhibitory effects of TPL on human NSCLC, such as mitogen-activated protein kinases (MAPKs) modulation, inhibition of NF-κB activation, suppression of miRNA expression, etc. In addition, the effect of TIG on immune response in cancer patients is summarized for improved immune modulation utilization. However, the clinical use of TPL is often limited by its severe toxicity and water insolubility. Future clinical trials and drug delivery strategies that will evaluate the security and validate the underlying tumor-killing properties of TPL in human NSCLC are also to be discussed.
Key words: Triptolide (TPL), Cancer cells, Molecular signaling pathways, Antitumor effects
INTRODUCTION
In recent years, more natural compounds obtained from plants have received considerable attention from the scientific community, owing to their increasing merit in the prevention and treatment of cancer1,2. Growing evidence indicates that multiple natural compounds, such as alkaloids, polyphenols, and flavonoids, are capable of fighting a variety of diseases, including cancer, and their many preparations have been used clinically3. For instance, Taxotere, a semisynthetic terpenoid antineoplastic agent, has recently demonstrated high efficacy in the treatment of a wide variety of solid tumors4. Irinotecan is a semisynthetic derivative of camptothecin used clinically for the treatment of adult metastatic colorectal cancer5. In addition to their nontoxic nature and fewer side effects compared to chemotherapeutic drugs, natural compounds are used in clinical practice due to advantages such as multitargeting properties, immediate availability, and low cost6.
Since the turn of the century, research volume has been increasing in the prevention and treatment of cancer by traditional Chinese medicine. It has been reported that Danggui Buxue Tang induced autophagic death of colorectal cancer cells by upregulating Atg7 and regulating the mTOR/p70s6k signaling pathway7. A pseudonatural product inhibited cancer cell growth by selectively inhibiting glucose transporters GLUT-1 and GLUT-38. Diosmetin, the aglycone of the lavonoid glycoside from medicinal herbs, enhanced the radiosensitivity of radioresistant non-small cell lung cancer (NSCLC) cells A549/IR9. Therefore, there is great potential for the development of new anticancer drugs extracted from Chinese medicine.
Triptolide (TPL), a diterpenoid triepoxide, is the major active component of extracts derived from the Chinese herb thunder god vine (Tripterygium wilfordii Hook F), which possesses potent anticancer, anti-inflammatory, and immunosuppressive properties 10. To date, many studies have reported that TPL inhibits multiple human solid tumors in vivo and in vitro11,12, and revealed various anticancer mechanisms, such as inhibiting heat shock factor-1 (HSF-1)13, suppression of DNA damage response14,15, and regulating mRNA stability16,17. Taken together, these results suggest that TPL has a broad anticancer effect and can be used to treat NSCLC. In the present report, we review the activities and mechanisms of TPL and its prodrug Minnelide in inhibiting human NSCLC cells, providing ideas for further research.
CUMULATIVE ANTITUMOR EVIDENCE SUPPORTING TPL ACTIVITY AGAINST NSCLC
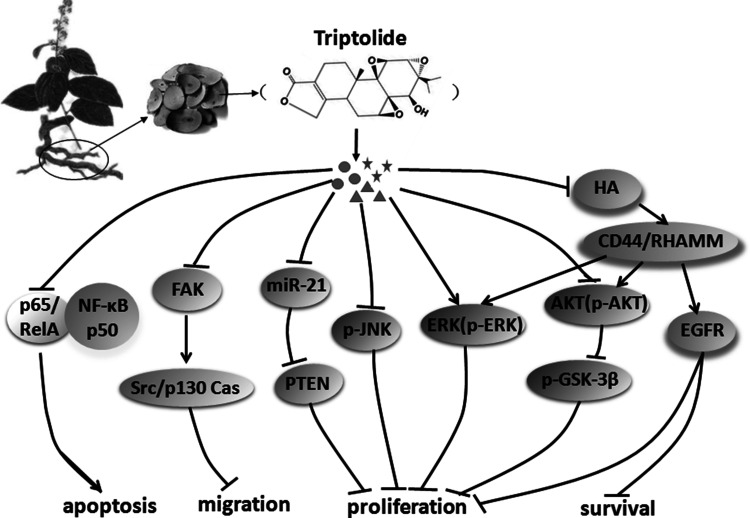
NSCLC is one of the malignant tumors with the highest rate of growth of morbidity and mortality and is the greatest threat to human health and life. At present, the clinical treatment of NSCLC includes chemotherapy, radiotherapy, surgical treatment, and traditional Chinese medicine treatment, which have achieved some clinical effects18,19. Recently, several studies have emphasized the antitumor effects of TPL on NSCLC cells (Table 1). These inhibitory effects of TPL, along with the unique clinical features, provide the possibility that TPL could act as a promising agent against NSCLC. To provide an exhaustive description of the rapid development in this field, the molecular mechanisms and potential functions of TPL in NSCLC will be discussed in the following sections (Fig. 1).
Table 1.
The Targets and Mechanism of Triptolide (TPL) in Human Non-Small Cell Lung Cancer (NSCLC)
| Target | Action | Outcome | Model Used | Reference(s) |
|---|---|---|---|---|
| MAPKs | p-JNK↓; p-ERK↑; p-P38↑ | Apoptosis↑; proliferation↓; | A549; A549/Taxol | 22, 23 |
| MKP-1↓ | Reversing antimetastatic effect of PPARγ agonist rosiglitazone | H441 | 24 | |
| ERK2↑; caspase 3 and 8↑ | Apo2L/TRAIL-induced apoptosis sensitization | A549; NCI-H358; Calu1; SkLu1 | 27 | |
| NF-κB | p65 transactivation↓ | TRAIL-induced apoptosis↑ | A549 | 26 |
| IKBα phosphorylation and degradation↓ NF-κB nuclear translocation↓ | Incidence↓ | H460; H460; PC3; FEN1 E160D mice; LLC-grafted mice | 29 | |
| TRAIL-induced NF-κB transcriptional activity↓ | Sensitive to TRAIL-induced apoptosis↑ | A549; NCl-H1299 | 30 | |
| NF-κB↓; NF-κB-regulated drug-resistant gene expression↓ | Reversal of the Taxol resistance | A549; A549/Taxol | 51 | |
| HA-CD44/RHAMM | ERK↓; Akt↓; EGFR↓ | Growth and survival↓ | A549; H520; H1299; H1650; H1975; A549 xenograft in male nude mice | 34 |
| Potential EGFR antagonist | Proliferation↓ | Molecular dynamics simulation; H2347 | 35 | |
| miRNA | 126 miRNAs↑; 101 miRNAs↓ | Altering miRNA expression profile | H358 | 43 |
| miR-21↓; PTEN protein↑; caspase 3 and 9↑ | Proliferation↓; apoptosis↑ | PC-9 | 45, 46 | |
| FAK | p130Cas↓; Src↓; less metastatic colonization compared to control mice | Migration, invasion, and metastasis↓ | H460; A549; H358; H358 xenograft in NOD SCID γ mice | 43 |
| PI3K/Akt | p-Akt↓; p-GSK-3β↑; Bax↑; caspases↑; Bcl-2↓ | Proliferation↓; S-phase arrest | A549; A549/Taxol | 23 |
| p-Akt↓ | Cytotoxicity | H1299, H460 | 36 | |
| Tumor-related protein expression | Dysregulation of tumor-related protein | Apoptosis↑; G2/M phase arrest | A549 (ITRAQ-based proteomics analysis) | 52 |
| Keap1/Nrf2 | Nrf2 transcriptional activity↓ | Chemoresistance to antitumor drugs↓ | LLC; Nrf2-KD; 3LL and Nrf2-KD xenograft in C57BL/6 mice | 53 |
| Wnt | Epigenetic modifications to histone H3; WIF1 | Growth↓ | A549; H460; H358; H1299; E160D mice; A549 and H460 xenograft in NOD SCID γ mice | 55 |
| WIF1 demethylation | Apoptosis↑; migration↓ | A549; H460 | 57 |
MAPKs, mitogen-activated protein kinases; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; MKP-1, kinase phosphatase-1; Apo2L/TRAIL, Apo2L/tumor necrosis factor-related apoptosis-inducing ligand; Akt, protein kinase B; LLC, the Lewis lung cancer; EGFR, epidermal growth factor receptor, PTEN, phosphatase and tensin homolog; FAK, focal adhesion kinase; MMP14, matrix metalloproteinase 14, iTRAQ, isobaric tags for relative and absolute quantitation; Nrf2, nuclear factor erythroid 2-related factor 2; WIF1, Wnt inhibitory factor 1; ↑, activation/upregulation; ↓, suppression/downregulation.
Figure 1.
Overview of the natural compound triptolide (TPL) on aberrant molecular signaling pathways for non-small cell lung cancer (NSCLC) research and therapy. TPL exerts its anticancer effects by inhibiting proliferation, suppressing survival, and inducing apoptosis of NSCLC cells via modulating several key signaling pathways, including MAPK, NF-κB, and FAK.
TPL MODULATION ON THE MAPK SIGNALING PATHWAYS
Mitogen-activated protein kinases (MAPKs) consist of three serine/threonine kinase cascades. These are important cell signaling pathways in vivo and play important roles in regulating cellular functional activities, especially in cancer cells, which offer prospective benefits for cancer therapy20,21. Four types of MAPK cascades have been found in mammalian cells: extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), P38 MAPK, and ERK5.
Studies have shown that multidrug resistance cells can resist cell apoptosis induced by anticancer drugs through upregulation of survival signaling pathways, like p38 and MAPK, and preliminary observations have demonstrated that TPL can enhance apoptosis by regulating MAPK signaling pathways22. Indeed, Xie et al. performed an MTT assay and showed that SP600125, a JNK inhibitor, and U0126, an ERK inhibitor, significantly reinforced the inhibitory effects of TPL on human Taxol-resistant lung adenocarcinoma cell lines A549/Taxol23. Western blot results showed that TPL exerts its antiproliferative effect via downregulation of p-JNK and upregulation of p-ERK and p-P38. These findings suggest that the antiproliferative activity of TPL in multidrug resistance cancer cell lines is mainly related to the modulation of MAPKs and its downstream pathways. However, an opposite finding from Tai and colleagues showed that, functioning as inhibitor of MAPK phosphatase-1 (MKP-1), TPL pretreatment could reverse the antimetastatic effect of peroxisome proliferator-activated receptor-γ (PPARγ) agonist in NSCLC NCI-H441GL cells24. These inconsistent findings might be due to the different cellular environment and phenotype, suggesting that the precise roles of TPL-mediated MAPK signaling pathway in cancer biology need to be carefully revised.
Apo2L/tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which belongs to the tumor necrosis factor (TNF) superfamily and is closely connected with TNF-α and FasL, induces apoptosis of various tumor cells25. However, many cancer cells develop resistance to Apo2L/TRAIL-induced apoptosis. An increase in TNF-α-induced apoptosis by TPL has been suggested previously in A549, HT180, and MCF-7 cell lines26, and Apo2L/TRAIL-induced apoptosis was enhanced when combined with chemotherapeutic drugs to treat NSCLC cells27. Studies have reported that combination treatment with TPL and Apo2L/TRAIL sensitized several cancer cells (A549, NCI-H358, Calu1, and SkLu) for Apo2L/TRAIL-induced apoptosis by phosphorylating ERK2 dually at Thr202 and Tyr20427.
TPL SENSITIZES NSCLC CELLS BY INHIBITION OF NF-κB ACTIVATION
NF-κB is involved in a wide variety of physiological processes in cells through a group of important transcriptional factors. The NF-κB family consists of five members: p50, p52, RelA/p65, c-Rel, and RelB28. Lee et al. demonstrated that TPL almost blocked TNF-mediated induction of NF-κB activation by impairing p65 transactivation in NSCLC cells A54926, but further exploration is still required to determine how p65 transactivation is inhibited by TPL. Further studies from Zheng and colleagues group revealed that TPL effectively inhibited IKBα phosphorylation and degradation, and then blocked TNF-α-induced nuclear translocation of NF-κB, leading to reduction of NSCLC incidence with no obvious side effects29. In addition, TRAIL activates NF-κB via an interaction with TRAIL receptors. Lee et al. showed that TPL-sensitized TRAIL-induced apoptosis by anti-NF-κB activity is achieved by another process in A549 and NCl-H1299 cells because TRAIL-induced apoptosis was enhanced when MG132 was added after TRAIL, but after the process was reversed this effect weakened30. However, the exact mechanism is still to be elucidated. Based on a report that downstream effectors of NF-κB activation and members of the inhibitor-of-apoptosis (IAP) family were required to suppress TNF-induced apoptosis31, the team above assumed that TPL deactivated c-IAP1 and c-IAP2 by TRAIL. Although they failed to prove this speculation in A549 and NCI-H1299 cell lines, they found differences in NF-κB activation induced by TRAIL and TNF potency, which might explain this finding.
TPL SUPPRESSES NSCLC CELL GROWTH BY TARGETING HA-CD44/RHAMM SIGNALING
Hyaluronan (HA) is a nonsulfated linear glycosaminoglycan polymer comprised of d-glucuronic acid and N-acetyl-d-glucosamine. In tumor tissues, markedly elevated levels of HA can be observed, leading to increased development and progression of cancer cells7. In cancer cells, the receptor for hyaluronic acid-mediated motility (RHAMM) and clusters of differentiation 44 (CD44), a transmembrane glycoprotein and expressing as multiple isoforms in many cells, are the best characterized cell surface receptors for HA. CD44 promotes cancer cell movement and is often used as a marker for cancer stem cells in human tumors. Studies indicated that HA-CD44 binding leads to the interaction of CD44 and its signaling receptors like epidermal growth factor receptor (EGFR), which affects various downstream signaling pathways, the PI3k/Akt and MAPK pathways in particular32.
Previous studies have demonstrated that HA and its receptors CD44 and RHAMM are at elevated levels in NSCLC patients, and this increased expression can influence its poor prognosis and metastasis to some extent33. Song et al. separately inoculated A549-expressing cells and liposomal TPL 4 weeks apart into the lung of male nude rats via intranasal instillation and observed growth suppression of orthotopically xenografted NSCLC cells34. This study also demonstrated that the antiproliferative activity of TPL is associated with the inhibition of HA-CD44/RHAMM signaling axis in NSCLC, inhibiting the level of downstream effector EGFR, Akt, and ERK. Moreover, using molecular docking analysis, Zhao et al. also identified TPL as a potential EGFR antagonist and its antiproliferative potency similar to the well-known EGFR-targeted drugs35. In addition, TPL exerts obvious cytotoxicity in NSCLC cells by docking into the activation pocket of Akt-1 protein structure and then downregulating Akt phosphorylation in NSCLC cell lines H1299 and H46036. These results are consistent with previous reports32,37, which demonstrated that the phosphorylation of EGFR and Akt increased when the interaction of HA and RHAMM was blocked by the EGFR signaling inhibitors. These results indicated that HA-RHAMM signaling promotes cell survival via EGFR in a mouse model of islet cell tumorigenesis38. Studies also indicated that apart from being a ligand of CD44 and RHAMM, HA also acts as HA synthase 2 (HAS2) siRNA to downregulate CD44 and RHAMM expression in NSCLC cells34. Although this phenomenon is also observed in other tumors, the mechanisms by which CD44 and RHAMM regulate each other are unclear and require further study.
TPL REDUCES NSCLC CELL GROWTH BY TARGETING miRNAs
MicroRNAs (miRNAs) are a type of noncoding small RNAs approximately 20–25 nucleotide in length with regulatory functions, and they usually deregulate gene expression during the onset and progression of cancer39,40. Based on the findings that TPL can regulate miRNA expression by binding to xeroderma pigmentosum type B, a subunit of the human transcription factor II41, or by inducing proteasome degradation of RNA polymerase II42, Reno et al. demonstrated 126 miRNAs that were upregulated and 101 miRNAs that were downregulated after TPL treatment in NSCLC cells H358 and determined the top molecular and cellular functions regulated by these genes with the use of ingenuity pathway analysis, which showed that TPL alters miRNA expression profile in NSCLC cells43.
miR-21 is one of the earliest miRNA molecules, of which there are now over 700 identified types. The expression of miR-21 is increased in NSCLC. Yang et al. indicated that miR-21 downregulation results in the inhibition of proliferation and migration of NSCLC A549 cells by inducing programmed cell death44. Li et al. showed that TPL decreased miR-21 expression in NSCLC cells PC-945. Phosphatase and tensin homolog (PTEN) is a cancer-suppressor gene that is involved in cancer progression. Previous studies have reported the association between PTEN and NSCLC46. Li et al. further demonstrated that in PC-9 cells, TPL inhibited miR-21 expression but increased the level of PTEN protein expression further to promoting cell apoptosis45. They also found that the effect of TPL on the expression of PTEN protein and cell viability was inhibited when upregulating the expression of miR-21. In a word, TPL influenced NSCLC cell proliferation and apoptosis through PTEN by downregulating miR-21.
TPL DECREASES FAK PROTEIN EXPRESSION AND IMPAIRS DOWNSTREAM SIGNALING
The anticancer effects of TPL described in this article are mostly achieved by regulating the proliferation and apoptosis of cancer cells. In fact, the TPL anticancer mechanism is also involved in cellular movement. Cell migration, a collection of physiological processes, is also a common form of exercise in living cells. Metastasis of cancer cells depends on abnormal activity of the mechanisms of cell migration. Likewise, it is required for cancer cell metastasis to invade and degrade the surrounding tissues and surrounding the extracellular matrix of the basement membrane47. Studies demonstrated that TPL treatment decreased metastatic colonization of the lungs and reduced metastatic lesions in the liver, thus suppressing NSCLC cell migration, invasion, and metastasis in vivo. Tail vein injections of H358 cells in NOD SCID γ mice before intraperitoneal injection of TPL also showed a significantly reduced growth of NSCLC43.
Focal adhesion kinase (FAK) is an important member of integrin-mediated signal transduction, which plays an important role in tumor cell invasion and is one of the factors that affect cell migration48. Reno et al. also found that the activation and expression of FAK were decreased after TPL treatment, which leads to impairment of downstream signaling, including the phosphorylation level of p130Cas and Src43. A result showed that PYK2, an ortholog of FAK, could make up for the inhibitory effects of FAK losses. Furthermore, an increase in PYK2 activity can be observed after treatment with FAK inhibitors49. Further research is needed to more fully understand this mechanism in the future.
TPL INHIBITS NSCLC BY THE REGULATION OF OTHER MECHANISMS
Previous studies have shown that TPL enhances cell apoptosis and cell cycle arrest activities and contributes to its antitumor effect through the modulation PI3K/Akt and JAK/STAT, which subsequently modulates Bcl-2 family expression22,50. On the basis of these studies, the antiproliferative activity exerted by TPL on A549/Taxol cells was due to the modulation of MAPK pathways23 or inhibition of NF-κB-regulated drug-resistant genes51, thereby improving the Taxol sensitivity and clinical outcomes. Li et al. used an ITRAQ-based proteomics analysis to investigate TPL antitumor activity on NSCLC A549 lung adenocarcinoma cells, revealing that the antitumor effect is achieved by regulating tumor-related protein expression and that TPL blocked A549 cells at the G2/M phase, promoting cell apoptosis52.
In addition to the mechanisms described above, there are probably many other TPL anticancer mechanisms that have yet to be discovered. Moreover, for all anticancer mechanisms of TPL, further research and validation of clinical trials are needed. Recent studies indicated that nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated oxidative stress response pathway is associated with tumor cell resistance to chemotherapy. Zhu et al. found that TPL could make NSCLC cells more sensitive toward chemotherapeutics in vitro and in vivo by effectively inhibiting Nrf2 transcriptional activity53.
In the last decade, it has become clear that epigenetic changes in genome play key roles in promoting every stage of tumorigenesis and cancer progression54. This knowledge has triggered pharmacologists to look for “epigenetic drugs” that could be developed into new cancer therapies. TPL administration could decrease the methylation status of H3 lysine residues in A549 and H460 NSCLC cell lines along with the xenograft model. These downregulated epigenetic changes in H3 promoted the expression of Wnt inhibitory factors, leading to cell growth depression55. TPL derivative MRx102 exerts potent antitumor effects by significantly inhibiting the Wnt pathway in NSCLC patient tumors56. In addition, using methylation-specific PCR assays, Mao and colleagues found that TPL treatment could directly demethylate Wnt inhibitory factor 1 (WIF1), subsequently inducing apoptosis and inhibiting migration of NSCLC cells A549 and H46057. These studies establish that TPL could serve as a prospective novel epigenetic agent in NSCLC and is a prospective novel NSCLC therapeutic.
THE EFFECT OF TPL WATER-SOLUBLE PRODRUG MINNELIDE ON NSCLC CELLS
TPL is a diterpenoid triepoxide from the Chinese herb T. wilfordii, possessing anticancer, antifertility, anti-inflammatory, and immunosuppressive properties. However, it cannot be well used in the clinic due to its hydrophobic nature. Therefore, Chugh and colleagues synthetized a water-soluble TPL prodrug called Minnelide58, and the findings demonstrated that Minnelide could target pancreatic cancer cells and inhibit the function of HSP70 that help tumor growth, thereby blocking the necessary factors for growing and disintegrating pancreatic cancer cells58. Subsequent studies further explored the antipancreatic cancer mechanism of Minnelide. One study showed that Minnelide effectively depleted the stromal architecture in pancreatic cancer by inhibiting collagen stabilization and HA synthesis, which eventually resulted in improved vascular function, promoted drug delivery, and enhanced survival of treated KPC (KRasG12D; Trp53R172HPdx-1Cre) mice59. In an orthotopic murine model of pancreatic cancer, another study showed combined therapy of low doses of Minnelide and oxaliplatin were superior to either drug alone in the inhibition of tumor growth60. In addition, there are studies that show that Minnelide effectively reduced not only the bulk tumor but also CD133+ stem cell population in the KPC mouse model of pancreatic cancer61, and subsequent studies further confirmed that tumors derived from CD133+ cells in pancreatic ductal adenocarcinoma cell lines responded to Minnelide62. Several phase I clinical trials have been completed, and phase II clinical trials are currently ongoing, with promising initial responses (Table 2).
Table 2.
Clinical Trials of TLP and its Derivatives in Anticancer Research
| Studies | Ref. 79 | NCT01927965* | NCT03129139* | NCT03117920* |
|---|---|---|---|---|
| Type | Phase I (completed) | Phase I (completed) | Phase I (recruiting) | Phase II (recruiting) |
| Targeted enrolment | 20 | 45 | 54 | 35 |
| Tumor type | Advanced solid tumors | Advanced gastrointestinal tumors | Advanced cancer, gastric cancer, breast cancer, pancreatic cancer, prostate cancer metastatic, colorectal cancer, solid tumor, solid carcinoma, solid carcinoma of stomach, cancer of stomach | Pancreatic cancer |
| Interventions | Drug: F60008 | Drug: Minnelide™ 001 | Drug: Minnelide™ capsules | Drug: Minnelide |
| Major findings/purpose | F60008 cannot be considered the optimal derivative of triptolide | Determined the maximum tolerated dose (MTD), the dose-limiting toxicities (DLT), and the recommended dose for future phase 2 protocol | Research on safety, pharmacokinetics, and pharmacodynamics | To investigate whether it can slow tumor growth in patients with unresponsive pancreatic cancer |
| Year of registration | – | 2013 | 2017 | 2017 |
Further information can be found at http://clinicaltrials.gov
These reports provided promising evidence for studying Minnelide in NSCLC treatment. Most patients diagnosed with NSCLC are already in advanced stages of disease. Hence, chemotherapy becomes a crucial treatment strategy when compared with surgery. Rousalova et al. investigated the therapeutic effect of Minnelide on a murine model of human NSCLC tumors and demonstrated that Minnelide inhibits tumor growth in NSCLC cells and xenograft mouse models63. Recent studies have also provided evidence that Minnelide significantly reduced the expression of genes associated with prosurvival and antiapoptosis in NSCLC tumors64. Although the precise mechanism of how Minnelide kills NSCLC cells remains ambiguous, Minnelide has been evaluated as a potent chemopreventive drug against pancreatic cancer65, osteosarcoma66, prostate cancer67, and gastric cancer68 in current preclinical studies. The in vitro and in vivo antitumor properties of TPL described above provide favorable evidence for the therapeutic potential of Minnelide as an anticancer drug for NSCLC.
IMMUNE MODULATION BY TPL IN CANCER RESEARCH
Nowadays, due to its low toxicity and inducing the life-long immune response, immune therapy is changing the current treatment pattern for NSCLC patients, especially with the novel drug development that could modulate immune checkpoint pathways69. TPL has multiple promising pharmacological activities including anti-inflammatory, immune modulation, and antiproliferative activity70. In an array study, TPL administration has been proven to regulate the expression of 22.5% of 195 immune signaling genes by inhibiting TNF-α-induced NF-κB activation in RAW 264.7 mouse macrophage cells71. Recently, TPL could be used to target senescent malignant cells that escape immunosurveillance by restraining the cytoprotective Nrf2 pathway further to enhance the radiochemotherapy sensitivity in human cancers72. In the presence of lipopolysaccharide (LPS), TPL significantly promoted T- and B-cell proliferation and increased macrophage phagocytosis, inducing apoptosis and autophagic cell death of murine leukemia cells WEHI-3 in vivo73. Liang et al. found that TPL could promote the reactive immune responses in human breast cancer cells by downregulating programmed death-1 ligand 1 (PD-L1), an important modulator for tumor immune evasion74. Though there is no direct evidence, further research should be focused on the detailed immune modulation of TPL in NSCLC patients, which might provide a new understanding of the strategy to improve anti-NSCLC therapeutic effect.
CLINICAL PERSPECTIVE OF TPL
The extensive pharmacological effects of TPL are particularly prominent and widespread in its anticancer activities. Up to now, based on the attractive preclinical pharmacokinetics data75, several clinical trials about TPL administration for cancer patients are still in progress at present76 (Table 2). Preclinic study showed that a water-soluble derivative of TPL, PG490-88, could act in synergy with DNA-damaging chemotherapeutic agents, acting as a potential chemosensitizer for the treatment of patients with solid tumors76. Wu et al. performed an open clinical study of 103 patients with psoriasis vulgaris who received TPL tablet, and obtained an exciting therapeutic effect with little adverse reactions, apart from slight white blood cell (WBC) decrease in a few patients77. Another prospective and controlled study demonstrated the favorable short-term disease remission of TPL in children with severe Henoch–Schonlein purpura nephritis78. However, a recent phase I and pharmacological study indicated that intravenous injection of F60008, a semisynthetic derivate of TPL, results in some severe adverse reactions in patients with advanced solid tumors, like lethal immune cell death79. Thus, further knowledge should provide more opportunities to evaluate the detailed influence of TPL in cancer patients, and might enable us to improve prognosis and ameliorate side effects. If clinical tests show safety and tolerability, TPL may be approved for a much greater range of applications.
Even though TPL administration could effectively kill cancer cells in vitro and in vivo, their biological effects are easily influenced by many factors, such as the delivery strategy. Studies have shown that development of drug delivery systems (DDSs) can greatly increase the therapeutic effect of TPL, paving the way for TPL toward clinical applications. Recently, a novel intravenous TPL-loaded delivery system, TP-loaded lipid emulsion (TP-LE), has been developed by Li and colleagues. The pharmacokinetic study showed that utility of TP-LE conferred improvements in biodistribution and therapeutic efficacy in pancreatic cancer cells, with reduced toxicity to the normal tissues80. Lin et al. developed an anti-carbonic anhydrase IX (CA IX) antibody and CPP33 dual-ligand-modified TPL-loaded liposomes (dl-TPL-lips), and demonstrated that dl-TPL-lip use significantly enhanced tumor cell penetration of TPL and improved TPL anti-NSCLC effect specifically in NSCLC cells A549-Red-FLuc, without apparent systemic toxicity81. CA IX-TPL-Lips ameliorated the cellular uptake efficiency, then restrained cell growth of NSCLC A549 cells in vitro and in vivo82. Therefore, with the development of improved DDSs, the cytotoxicity effects of TPL in cancer cells could be enhanced, with minimum side effects. Although the development of TPL as drug is still in its infancy, this agent may become economical and practical anticancer therapies in the near future.
CONCLUSION
NSCLC has become one of the most serious diseases that threaten human health, and TPL has shown great potential in the treatment of human NSCLC. Some TPL derivatives are undergoing clinical trials for the treatment of cancer and have broad application prospects. There is a preliminary understanding of the mechanism of the anti-NSCLC activity of TPL, but its exact mechanism of action has not yet been fully elucidated, and further studies are needed, especially in vivo. In conclusion, with TPL cancer prevention and anticancer mechanisms continuing to be studied and recognized in depth, TPL is extremely promising as a new drug in the clinical treatment of NSCLC.
ACKNOWLEDGMENTS
This work is supported by the National Natural Science Foundation of China (Nos. 81703036, 81803035, 81572946), the China Postdoctoral Science Foundation (No. 2017M610510), the Changsha Science and Technology Project (No. k1508024-31), the Youth Fund of Xiangya Hospital (No. 2017Q17), and the Postdoctoral Science Foundation of Central South University (185702). We thank Elsevier’s English Language Editing Service for assistance with language editing. Dr. Zhijie Xu is currently a Postdoctoral Fellow in the Department of Pharmacy, Xiangya Hospital, Central South University, and thanks all members in the Institute of Hospital Pharmacy for their critical comments. J.W., Z.X., and Z.G. were the main authors of the manuscript; L.Q., S.Z., and X.C. contributed to the design and format of figures and tables; Y.Y. and Z.L. revised the manuscript for important intellectual content and corrected the language form; J.W. and Z.X. were responsible for the manuscript writing. All authors read and approved the final manuscript.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Braicu C, Mehterov N, Vladimirov B, Sarafian V, Nabavi SM, Atanasov AG, Berindan-Neagoe I. Nutrigenomics in cancer: Revisiting the effects of natural compounds. Semin Cancer Biol. 2017;46:84–106. [DOI] [PubMed] [Google Scholar]
- 2. Shanmugam MK, Lee JH, Chai EZ, Kanchi MM, Kar S, Arfuso F, Dharmarajan A, Kumar AP, Ramar PS, Looi CY, Mustafa MR, Tergaonkar V, Bishayee A, Ahn KS, Sethi G. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin Cancer Biol. 2016;40–41:35–47. [DOI] [PubMed] [Google Scholar]
- 3. Mukhtar E, Adhami VM, Khan N, Mukhtar H. Apoptosis and autophagy induction as mechanism of cancer prevention by naturally occurring dietary agents. Curr Drug Targets 2012;13(14):1831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andriguetti NB, Raymundo S, Antunes MV, Perassolo MS, Verza SG, Suyenaga ES, Linden R. Pharmacogenetic and pharmacokinetic dose individualization of the taxane chemotherapeutic drugs paclitaxel and docetaxel. Curr Med Chem. 2017;24(33):3559–82. [DOI] [PubMed] [Google Scholar]
- 5. Tomasello G, Petrelli F, Ghidini M, Russo A, Passalacqua R, Barni S. FOLFOXIRI Plus Bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: A systematic review and pooled analysis. JAMA Oncol. 2017;3(7):e170278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hussain SS, Kumar AP, Ghosh R. Food-based natural products for cancer management: Is the whole greater than the sum of the parts? Semin Cancer Biol. 2016;40–41:233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen ST, Lee TY, Tsai TH, Huang YC, Lin YC, Lin CP, Shieh HR, Hsu ML, Chi CW, Lee MC, Chang HH, Chen YJ. Traditional Chinese medicine Danggui Buxue Tang inhibits colorectal cancer growth through induction of autophagic cell death. Oncotarget 2017;8(51):88563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karageorgis G, Reckzeh ES, Ceballos J, Schwalfenberg M, Sievers S, Ostermann C, Pahl A, Ziegler S, Waldmann H. Chromopynones are pseudo natural product glucose uptake inhibitors targeting glucose transporters GLUT-1 and -3. Nat Chem. 2018;10(11):1103–11. [DOI] [PubMed] [Google Scholar]
- 9. Xu Z, Yan Y, Xiao L, Dai S, Zeng S, Qian L, Wang L, Yang X, Xiao Y, Gong Z. Radiosensitizing effect of diosmetin on radioresistant lung cancer cells via Akt signaling pathway. PLoS One 2017;12(4):e0175977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan P, Sun X. Triptolide: A new star for treating human malignancies. J Cancer Res Ther. 2018;14(Suppl):S271–5. [DOI] [PubMed] [Google Scholar]
- 11. He QL, Minn I, Wang Q, Xu P, Head SA, Datan E, Yu B, Pomper MG, Liu JO. Targeted delivery and sustained antitumor activity of triptolide through glucose conjugation. Angew Chem Int Ed Engl. 2016;55(39):12035–9. [DOI] [PubMed] [Google Scholar]
- 12. Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, Yang H, Samadi AK, Russo GL, Spagnuolo C, Ray SK, Chakrabarti M, Morre JD, Coley HM, Honoki K, Fujii H, Georgakilas AG, Amedei A, Niccolai E, Amin A, Ashraf SS, Helferich WG, Yang X, Boosani CS, Guha G, Bhakta D, Ciriolo MR, Aquilano K, Chen S, Mohammed SI, Keith WN, Bilsland A, Halicka D, Nowsheen S, Azmi AS. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 2015;35(Suppl):S78–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Velayutham M, Cardounel AJ, Liu Z, Ilangovan G. Discovering a reliable heat-shock factor-1 inhibitor to treat human cancers: Potential opportunity for phytochemists. Front Oncol. 2018;8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng Y, Li F, He P, Yang Y, Yang J, Zhang Y, Liu J, Tong Y, Li Q, Mei X, Shu Z, Zhao Q. Triptolide sensitizes breast cancer cells to doxorubicin through the DNA damage response inhibition. Mol Carcinog. 2018;57(6):807–14. [DOI] [PubMed] [Google Scholar]
- 15. Wang G, Wang X, Xu X. Triptolide potentiates lung cancer cells to cisplatin-induced apoptosis by selectively inhibiting the NER activity. Biomark Res. 2015;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun L, Zhang S, Jiang Z, Huang X, Wang T, Huang X, Li H, Zhang L. Triptolide inhibits COX-2 expression by regulating mRNA stability in TNF-alpha-treated A549 cells. Biochem Biophys Res Commun. 2011;416(1–2):99–105. [DOI] [PubMed] [Google Scholar]
- 17. Vispe S, DeVries L, Creancier L, Besse J, Breand S, Hobson DJ, Svejstrup JQ, Annereau JP, Cussac D, Dumontet C, Guilbaud N, Barret JM, Bailly C. Triptolide is an inhibitor of RNA polymerase I and II-dependent transcription leading predominantly to down-regulation of short-lived mRNA. Mol Cancer Ther. 2009;8(10):2780–90. [DOI] [PubMed] [Google Scholar]
- 18. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 19. Yan Y, Su W, Zeng S, Qian L, Chen X, Wei J, Chen N, Gong Z, Xu Z. Effect and mechanism of Tanshinone I on the radio-sensitivity of lung cancer cells. Mol Pharm. 2018;15(11):4843–53. [DOI] [PubMed] [Google Scholar]
- 20. Li L, Guo L, Tao Y, Zhou S, Wang Z, Luo W, Hu D, Li Z, Xiao L, Tang M, Yi W, Tsao SW, Cao Y. Latent membrane protein 1 of Epstein-Barr virus regulates p53 phosphorylation through MAP kinases. Cancer Lett. 2007;255(2):219–31. [DOI] [PubMed] [Google Scholar]
- 21. Dai Z, Lei P, Xie J, Hu Y. Antitumor effect of resveratrol on chondrosarcoma cells via phosphoinositide 3-kinase/AKT and p38 mitogen-activated protein kinase pathways. Mol Med Rep. 2015;12(2):3151–5. [DOI] [PubMed] [Google Scholar]
- 22. Meng G, Wang W, Chai K, Yang S, Li F, Jiang K. Combination treatment with triptolide and hydroxycamptothecin synergistically enhances apoptosis in A549 lung adenocarcinoma cells through PP2A-regulated ERK, p38 MAPKs and Akt signaling pathways. Int J Oncol. 2015;46(3):1007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie CQ, Zhou P, Zuo J, Li X, Chen Y, Chen JW. Triptolide exerts pro-apoptotic and cell cycle arrest activity on drug-resistant human lung cancer A549/Taxol cells via modulation of MAPK and PI3K/Akt signaling pathways. Oncol Lett. 2016;12(5):3586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tai CJ, Wu AT, Chiou JF, Jan HJ, Wei HJ, Hsu CH, Lin CT, Chiu WT, Wu CW, Lee HM, Deng WP. The investigation of mitogen-activated protein kinase phosphatase-1 as a potential pharmacological target in non-small cell lung carcinomas, assisted by non-invasive molecular imaging. BMC Cancer 2010;10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Contassot E, Kerl K, Roques S, Shane R, Gaide O, Dupuis M, Rook AH, French LE. Resistance to FasL and tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in Sezary syndrome T-cells associated with impaired death receptor and FLICE-inhibitory protein expression. Blood 2008;111(9):4780–7. [DOI] [PubMed] [Google Scholar]
- 26. Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem. 1999;274(19):13451–5. [DOI] [PubMed] [Google Scholar]
- 27. Frese S, Pirnia F, Miescher D, Krajewski S, Borner MM, Reed JC, Schmid RA. PG490-mediated sensitization of lung cancer cells to Apo2L/TRAIL-induced apoptosis requires activation of ERK2. Oncogene 2003;22(35):5427–35. [DOI] [PubMed] [Google Scholar]
- 28. Xie B, Cao K, Li J, Chen J, Tang J, Chen X, Xia K, Zhou X, Cheng Y, Zhou J, Xie H. Hmgb1 inhibits Klotho expression and malignant phenotype in melanoma cells by activating NF-kappaB. Oncotarget 2016;7(49):80765–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng L, Jia J, Dai H, Wan L, Liu J, Hu L, Zhou M, Qiu M, Chen X, Chang L, Kim JY, Reckamp K, Raz DJ, Xia Z, Shen B. Triptolide-assisted phosphorylation of p53 suppresses inflammation-induced NF-kappaB survival pathways in cancer cells. Mol Cell Biol. 2017;37(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee KY, Park JS, Jee YK, Rosen GD. Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappaB activation. Exp Mol Med. 2002;34(6):462–8. [DOI] [PubMed] [Google Scholar]
- 31. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998;281(5383):1680–3. [DOI] [PubMed] [Google Scholar]
- 32. Lan T, Pang J, Wu Y, Zhu M, Yao X, Wu M, Qian H, Zhang Z, Gao J, Chen Y. Cross-linked hyaluronic acid gel inhibits metastasis and growth of gastric and hepatic cancer cells: In vitro and in vivo studies. Oncotarget 2016;7(40):65418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang D, Narula N, Azzopardi S, Smith RS, Nasar A, Altorki NK, Mittal V, Somwar R, Stiles BM, Du YN. Expression of the receptor for hyaluronic acid mediated motility (RHAMM) is associated with poor prognosis and metastasis in non-small cell lung carcinoma. Oncotarget 2016;7(26):39957–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song JM, Molla K, Anandharaj A, Cornax I, MG OS, Kirtane AR, Panyam J, Kassie F. Triptolide suppresses the in vitro and in vivo growth of lung cancer cells by targeting hyaluronan-CD44/RHAMM signaling. Oncotarget 2017;8(16):26927–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao GF, Huang ZA, Du XK, Yang ML, Huang DD, Zhang S. Molecular docking studies of traditional Chinese medicinal compounds against known protein targets to treat non-small cell lung carcinomas. Mol Med Rep. 2016;14(2):1132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamdi AM, Jiang ZZ, Guerram M, Yousef BA, Hassan HM, Ling JW, Zhang LY. Biochemical and computational evaluation of triptolide-induced cytotoxicity against NSCLC. Biomed Pharmacother. 2018;103:1557–66. [DOI] [PubMed] [Google Scholar]
- 37. Hatano H, Shigeishi H, Kudo Y, Higashikawa K, Tobiume K, Takata T, Kamata N. RHAMM/ERK interaction induces proliferative activities of cementifying fibroma cells through a mechanism based on the CD44-EGFR. Lab Invest. 2011;91(3):379–91. [DOI] [PubMed] [Google Scholar]
- 38. Du YC, Chou CK, Klimstra DS, Varmus H. Receptor for hyaluronan-mediated motility isoform B promotes liver metastasis in a mouse model of multistep tumorigenesis and a tail vein assay for metastasis. Proc Natl Acad Sci USA 2011;108(40):16753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu Z, Yan Y, Qian L, Gong Z. Long non-coding RNAs act as regulators of cell autophagy in diseases (Review). Oncol Rep. 2017;37(3):1359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu Z, Yan Y, Zeng S, Dai S, Chen X, Wei J, Gong Z. Circular RNAs: Clinical relevance in cancer. Oncotarget 2018;9(1):1444–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang Y, Smeaton M, Demain AL, Miller PS, Kugel JF, Goodrich JA, Liu JO. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol. 2011;7(3):182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manzo SG, Zhou ZL, Wang YQ, Marinello J, He JX, Li YC, Ding J, Capranico G, Miao ZH. Natural product triptolide mediates cancer cell death by triggering CDK7-dependent degradation of RNA polymerase II. Cancer Res. 2012;72(20):5363–73. [DOI] [PubMed] [Google Scholar]
- 43. Reno TA, Kim JY, Raz DJ. Triptolide inhibits lung cancer cell migration, invasion, and metastasis. Ann Thorac Surg. 2015;100(5):1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang Y, Meng H, Peng Q, Yang X, Gan R, Zhao L, Chen Z, Lu J, Meng QH. Downregulation of microRNA-21 expression restrains non-small cell lung cancer cell proliferation and migration through upregulation of programmed cell death 4. Cancer Gene Ther. 2015;22(1):23–9. [DOI] [PubMed] [Google Scholar]
- 45. Li X, Zang A, Jia Y, Zhang J, Fan W, Feng J, Duan M, Zhang L, Huo R, Jiao J, Zhu X. Triptolide reduces proliferation and enhances apoptosis of human non-small cell lung cancer cells through PTEN by targeting miR-21. Mol Med Rep. 2016;13(3):2763–8. [DOI] [PubMed] [Google Scholar]
- 46. Poliseno L, Pandolfi PP. PTEN ceRNA networks in human cancer. Methods 2015;77–78:41–50. [DOI] [PubMed] [Google Scholar]
- 47. Hamidi H, Ivaska J. Every step of the way: Integrins in cancer progression and metastasis. Nat Rev Cancer 2018;18(9):533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tan S, Zhao S, Chen Z, Ma Q, Wang W, Cheng S, Wen Q, Tan S, Xie J. Altered biological properties in Dp71 over-expressing HBE cells. Cell Physiol Biochem. 2017;43(5):2022–36. [DOI] [PubMed] [Google Scholar]
- 49. Cabrita MA, Jones LM, Quizi JL, Sabourin LA, McKay BC, Addison CL. Focal adhesion kinase inhibitors are potent anti-angiogenic agents. Mol Oncol. 2011;5(6):517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Z, Jin H, Xu R, Mei Q, Fan D. Triptolide downregulates Rac1 and the JAK/STAT3 pathway and inhibits colitis-related colon cancer progression. Exp Mol Med. 2009;41(10):717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jiang N, Dong XP, Zhang SL, You QY, Jiang XT, Zhao XG. Triptolide reverses the Taxol resistance of lung adenocarcinoma by inhibiting the NF-kappaB signaling pathway and the expression of NF-kappaB-regulated drug-resistant genes. Mol Med Rep. 2016;13(1):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li F, Zhao D, Yang S, Wang J, Liu Q, Jin X, Wang W. ITRAQ-based proteomics analysis of triptolide on human A549 lung adenocarcinoma cells. Cell Physiol Biochem. 2018;45(3):917–34. [DOI] [PubMed] [Google Scholar]
- 53. Zhu J, Wang H, Chen F, Lv H, Xu Z, Fu J, Hou Y, Xu Y, Pi J. Triptolide enhances chemotherapeutic efficacy of antitumor drugs in non-small-cell lung cancer cells by inhibiting Nrf2-ARE activity. Toxicol Appl Pharmacol. 2018;358:1–9. [DOI] [PubMed] [Google Scholar]
- 54. Tomkova M, Schuster-Bockler B. DNA modifications: Naturally more error prone? Trends Genet. 2018;34(8):627–38. [DOI] [PubMed] [Google Scholar]
- 55. Nardi I, Reno T, Yun X, Sztain T, Wang J, Dai H, Zheng L, Shen B, Kim J, Raz D. Triptolide inhibits Wnt signaling in NSCLC through upregulation of multiple Wnt inhibitory factors via epigenetic modifications to Histone H3. Int J Cancer 2018;143(10):2470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reno TA, Tong SW, Wu J, Fidler JM, Nelson R, Kim JY, Raz DJ. The triptolide derivative MRx102 inhibits Wnt pathway activation and has potent anti-tumor effects in lung cancer. BMC Cancer 2016;16:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mao X, Tong J, Wang Y, Zhu Z, Yin Y, Wang Y. Triptolide exhibits antitumor effects by reversing hypermethylation of WIF1 in lung cancer cells. Mol Med Rep. 2018;18(3):3041–9. [DOI] [PubMed] [Google Scholar]
- 58. Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4(156):156ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Banerjee S, Modi S, McGinn O, Zhao X, Dudeja V, Ramakrishnan S, Saluja AK. Impaired synthesis of stromal components in response to minnelide improves vascular function, drug delivery, and survival in pancreatic cancer. Clin Cancer Res. 2016;22(2):415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Modi S, Kir D, Giri B, Majumder K, Arora N, Dudeja V, Banerjee S, Saluja AK. Minnelide overcomes oxaliplatin resistance by downregulating the DNA repair pathway in pancreatic cancer. J Gastrointest Surg. 2016;20(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Banerjee S, Nomura A, Sangwan V, Chugh R, Dudeja V, Vickers SM, Saluja A. CD133+ tumor initiating cells in a syngenic murine model of pancreatic cancer respond to minnelide. Clin Cancer Res. 2014;20(9):2388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nomura A, McGinn O, Dudeja V, Sangwan V, Saluja AK, Banerjee S. Minnelide effectively eliminates CD133(+) side population in pancreatic cancer. Mol Cancer 2015;14:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rousalova I, Banerjee S, Sangwan V, Evenson K, McCauley JA, Kratzke R, Vickers SM, Saluja A, D’Cunha J. Minnelide: A novel therapeutic that promotes apoptosis in non-small cell lung carcinoma in vivo. PLoS One 2013;8(10):e77411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kumar A, Corey C, Scott I, Shiva S, D’Cunha J. Minnelide/triptolide impairs mitochondrial function by regulating SIRT3 in P53-dependent manner in non-small cell lung cancer. PLoS One 2016;11(8):e0160783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Garg B, Giri B, Majumder K, Dudeja V, Banerjee S, Saluja A. Modulation of post-translational modifications in beta-catenin and LRP6 inhibits Wnt signaling pathway in pancreatic cancer. Cancer Lett. 2017;388:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Banerjee S, Thayanithy V, Sangwan V, Mackenzie TN, Saluja AK, Subramanian S. Minnelide reduces tumor burden in preclinical models of osteosarcoma. Cancer Lett. 2013;335(2):412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Isharwal S, Modi S, Arora N, Uhlrich C 3rd, Giri B, Barlass U, Soubra A, Chugh R, Dehm SM, Dudeja V, Saluja A, Banerjee S, Konety B. Minnelide inhibits androgen dependent, castration resistant prostate cancer growth by decreasing expression of androgen receptor full length and splice variants. Prostate 2017;77(6):584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Arora N, Alsaied O, Dauer P, Majumder K, Modi S, Giri B, Dudeja V, Banerjee S, Von Hoff D, Saluja A. Downregulation of Sp1 by minnelide leads to decrease in HSP70 and decrease in tumor burden of gastric cancer. PLoS One 2017;12(2):e0171827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hirsch FR, Suda K, Wiens J, Bunn PA Jr. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016;388(10048):1012–24. [DOI] [PubMed] [Google Scholar]
- 70. Liu Q. Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol. 2011;11(3):377–83. [DOI] [PubMed] [Google Scholar]
- 71. Premkumar V, Dey M, Dorn R, Raskin I. MyD88-dependent and independent pathways of Toll-Like receptors are engaged in biological activity of triptolide in ligand-stimulated macrophages. BMC Chem Biol. 2010;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Malavolta M, Bracci M, Santarelli L, Sayeed MA, Pierpaoli E, Giacconi R, Costarelli L, Piacenza F, Basso A, Cardelli M, Provinciali M. Inducers of senescence, toxic compounds, and senolytics: The multiple faces of Nrf2-activating phytochemicals in cancer adjuvant therapy. Mediators Inflamm. 2018;2018:4159013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chan SF, Chen YY, Lin JJ, Liao CL, Ko YC, Tang NY, Kuo CL, Liu KC, Chung JG. Triptolide induced cell death through apoptosis and autophagy in murine leukemia WEHI-3 cells in vitro and promoting immune responses in WEHI-3 generated leukemia mice in vivo. Environ Toxicol. 2017;32(2):550–68. [DOI] [PubMed] [Google Scholar]
- 74. Liang M, Fu J. Triptolide inhibits interferon-gamma-induced programmed death-1-ligand 1 surface expression in breast cancer cells. Cancer Lett. 2008;270(2):337–41. [DOI] [PubMed] [Google Scholar]
- 75. Song W, Liu M, Wu J, Zhai H, Chen Y, Peng Z. Preclinical pharmacokinetics of triptolide: A potential antitumor drug. Curr Drug Metab. 2018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 76. Sampath D, Discafani CM, Loganzo F, Beyer C, Liu H, Tan X, Musto S, Annable T, Gallagher P, Rios C, Greenberger LM. MAC-321, a novel taxane with greater efficacy than paclitaxel and docetaxel in vitro and in vivo. Mol Cancer Ther. 2003;2(9):873–84. [PubMed] [Google Scholar]
- 77. Wu SX, Guo NR. Clinical observation on effect of triptolide tablet in treating patients with psoriasis vulgaris. Chin J Integr Med. 2005;11(2):147–8. [DOI] [PubMed] [Google Scholar]
- 78. Wu L, Mao J, Jin X, Fu H, Shen H, Wang J, Liu A, Shu Q, Du L. Efficacy of triptolide for children with moderately severe Henoch-Schonlein purpura nephritis presenting with nephrotic range proteinuria: A prospective and controlled study in China. Biomed Res Int. 2013;2013:292865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kitzen JJ, de Jonge MJ, Lamers CH, Eskens FA, van der Biessen D, van Doorn L, Ter Steeg J, Brandely M, Puozzo C, Verweij J. Phase I dose-escalation study of F60008, a novel apoptosis inducer, in patients with advanced solid tumours. Eur J Cancer 2009;45(10):1764–72. [DOI] [PubMed] [Google Scholar]
- 80. Li X, Mao Y, Li K, Shi T, Yao H, Yao J, Wang S. Pharmacokinetics and tissue distribution study in mice of triptolide-loaded lipid emulsion and accumulation effect on pancreas. Drug Deliv. 2016;23(4):1344–54. [DOI] [PubMed] [Google Scholar]
- 81. Lin C, Zhang X, Chen H, Bian Z, Zhang G, Riaz MK, Tyagi D, Lin G, Zhang Y, Wang J, Lu A, Yang Z. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv. 2018;25(1):256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lin C, Wong BCK, Chen H, Bian Z, Zhang G, Zhang X, Kashif Riaz M, Tyagi D, Lin G, Zhang Y, Wang J, Lu A, Yang Z. Pulmonary delivery of triptolide-loaded liposomes decorated with anti-carbonic anhydrase IX antibody for lung cancer therapy. Sci Rep. 2017;7(1):1097. [DOI] [PMC free article] [PubMed] [Google Scholar]