Abstract
Background
Since the discovery of the Mik antigen, several studies have described blood incompatibilities unrelated to the AB system in cats.
Objective
To estimate the prevalence of cats with non‐AB incompatibilities associated with naturally occurring alloantibodies (NOAb), and to begin mapping the corresponding feline erythrocyte antigens (FEA).
Animals
Two hundred and fifty‐eight type A cats.
Methods
Prospectively, cats were evaluated for the presence of NOAb by crossmatching in groups of 4‐6 cats. When NOAb were detected in a cat, its plasma was used as reagent to assess for the presence of the corresponding FEA in all cats included thereafter, and agreement observed between results of this extensive blood typing was evaluated.
Results
The chance of detecting incompatibilities by randomly crossmatching 2 cats was 3.9%, which resulted in at least 7% of type A cats having NOAb. Blood typing and agreement analyses performed with 7 newly detected NOAb allowed the identification of 5 presumably distinct FEA. Feline erythrocyte antigens 1 and 5 were most frequent with prevalence of 84% and 96%, respectively. Only FEA 1‐negative status was associated with a higher risk of presenting NOAb; with 16.7% of 42 FEA 1‐negative cats having NOAb compared to 5.1% of 216 FEA 1‐positive cats.
Conclusions and Clinical Importance
This study represents a first step of FEA identification outside the AB system. Because of its prevalence and association with NOAb, FEA 1 might correspond to the Mik antigen.
Keywords: alloimmunisation, blood compatibility, blood typing, crossmatch, Mik antigen, transfusion
Abbreviations
- CHUV
Centre Hospitalier Universitaire Vétérinaire
- CI
confidence intervals
- EDTA
ethylenediaminetetraacetic
- FEA
feline eryhtorcyte antigens
- FMV
Faculty of Veterinary Medicine
- NOAb
naturally occurring alloantibodies
- OR
odds ratio
- RBC
red blood cells
1. INTRODUCTION
Blood types are antigens on the red blood cells (RBC) surface identified after the discovery of antibodies that have reacted to them in a certain population subset. 1 In cats, the only blood group system currently defined is the AB system, consisting of types A, B, and AB. 2 The prevalence of these blood types varies among breeds and geographic locations, with type A being the most common. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 The AB system is also characterized by the presence of naturally occurring anti‐A and anti‐B alloantibodies (NOAb anti‐A and anti‐B). 11 The clinical relevance of these alloantibodies manifests by severe, acute hemolytic transfusion reactions in type B cats receiving type A blood as well as neonatal isoerythrolysis, whereas shortened survival of transfused RBC in type A cats receiving type B blood is reported. 12 , 13 , 14 Therefore, AB‐typing of feline blood donors and recipients is critical before a first transfusion.
In 2007, a novel feline RBC antigen named Mik was recognized in a group of domestic cats and appeared to be common with only 4 Mik‐negative cats identified among 66 type A cats. Some Mik‐negative cats presented NOAb anti‐Mik that could mediate a clinically relevant transfusion reaction despite blood donor and recipient being AB‐matched. 15 This study raised the question of the existence and clinical relevance of feline RBC antigens independent of the AB system. Several other crossmatch studies documented the presence of NOAb outside the AB system with 0%‐29% of transfusion‐naive cats showing at least 1 non‐AB related incompatibility. Most of these studies focused on the possible clinical impact of the NOAb on efficacy and safety of transfusion and did not describe the RBC antigens reacting to those NOAb. 16 , 17 , 18 , 19 , 20 , 21 , 22
The first aims of this study were to evaluate the chance of detecting non‐AB incompatibilities by randomly crossmatching 2 cats, to estimate the prevalence of cats with non‐AB incompatibilities, to investigate whether the presence of NOAb was associated with sex, age, purebred or health status, and to evaluate the possible interference of sample's hemolysis with NOAb detection. The second aim of this study was to use the newly detected NOAb as reagent for an extensive and systematic blood typing investigation, and then to assess the agreement between reactions obtained with the different NOAb in order to begin mapping the corresponding feline erythrocyte antigens (FEA).
2. MATERIALS AND METHODS
This study was conducted prospectively at the Centre Hospitalier Universitaire Vétérinaire (CHUV) of the Faculty of Veterinary Medicine (FMV) of the Université de Montréal between November 2017 and June 2019 and was approved by the Animal Care and Use Committee of Université de Montréal (approval number: Rech 18‐1912).
2.1. Animals
Cats were recruited from 4 different sources (Figure 1):
Adult cats that belonged to the CHUV blood donor colony (n = 11), which were recruited overtime from various animal shelters. All blood donor cats were healthy based on an annual physical examination and complete blood test. They were screened for feline immunodeficiency virus, feline leukemia virus (Snap FIV/FeLV Combo, IDEXX Laboratories Inc), Mycoplasma haemofelis, Bartonella species, Candidatus Mycoplasma haemominutum, Candidatus Mycoplasma turicenis, Cytauxzoon felis, Anaplasma, and Ehrlichia species (PCR testing, IDEXX Reference Laboratories, Canada) and were up‐to‐date on vaccinations and deworming.
Adult cats that belonged to the research colony of the FMV (n = 24) and also came from different animal shelters. Cats were clinically healthy based on an annual physical examination. They were screened for FIV/FeLV and were up to date on vaccinations and deworming.
Client‐owned cats referred to the CHUV or employed‐owned cats that could have been healthy or sick (n = 102). Written owner consent was obtained before enrollment of the cat in the study.
Surplus feline ethylenediaminetetraacetic (EDTA) blood samples submitted to the FMV's Diagnostic Laboratory from client‐owned cats, that could have been healthy or sick (n = 134).
FIGURE 1.
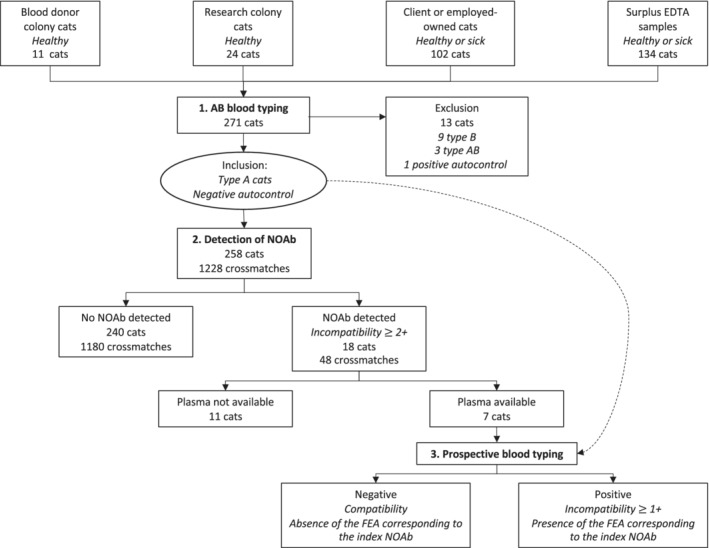
Flow diagram documenting case enrollment and the 3 steps of the study (1. AB blood typing, 2. Detection of naturally occurring alloantibodies, and 3. Prospective blood typing for novel antigens). NOAb: naturally occurring alloantibodies; FEA: feline erythrocyte antigens; dotted arrow: When NOAb were detected in a cat during the detection of NOAb step, its plasma was tested during the blood typing step against the red blood cells of all other cats included thereafter in the study
For client or employed‐owned cats and surplus EDTA samples, the following information were recorded when available: health and FIV/FeLV status, reason for presentation, transfusion history, and hematocrit. Sex, age and breed were recorded for all cats, when available. Cats with a history of previous transfusion were excluded. History regarding transfusions was unknown for 39 cats, which were still included in the study because previous transfusion was considered unlikely.
2.2. Samples
Ethylenediaminetetraacetic‐anticoagulated blood samples (<7 days old stored at 4°C) were available for all cats in the study. The samples were centrifuged at 3000g for 5 minutes to collect packed RBC as well as plasma from each cat. When the hematocrit was not available, the PCV of the EDTA sample was determined. The plasma was visually inspected for the degree of hemolysis which was scored from 0 to 4 by 1 observer (Marie Binvel). RBC were washed 3 times in isotonic saline (0.9% NaCl), and then 15 μL were suspended in 1500 μL of low ionic strength saline (Ortho Clinical Diagnostics, Raritan, New Jersey) to make a resultant 0.8% RBC suspension.
2.3. AB blood typing (step 1)
AB‐blood type was determined for all cats using only a gel column method performed by 2 veterinarians (Marie Binvel and Boris Depré) at different points in the study. This technique uses cards of 6 columns filled with gel beads and no added reagent (ID‐Micro Typing System Cards, Ortho Clinical Diagnostics, Pompano Beach, Florida). Fifty microliters of the 0.8% RBC suspension were mixed with 25 μL of Triticum vulgaris lectin (anti‐B reagent, 8 μg/mL, stored at −24°C) in 1 column, and with 25 μL of type B serum (anti‐A reagent) in another column. Autocontrols, referring to RBC incubated with plasma from the same animal, were also performed. The gel cards were incubated at 37°C for 15 minutes (ID‐Incubator, DiaMed Microtyping System, Switzerland), followed by centrifugation (ID‐centrifuge, DiaMed Microtyping System, Switzerland) at 80g for 10 minutes. AB and B cats, and 1 cat with positive autocontrol (autoagglutination) were excluded from the next steps of the study (Figure 1).
2.4. Detection of naturally occurring alloantibodies by crossmatch gel column test (step 2)
Type A cats were evaluated for the presence of non‐AB alloantibodies through the use of extensive crossmatching with each other, in groups of 4‐6 cats formed in chronological order. More specifically, each cat's plasma (25 μL) was mixed with 0.8% RBC suspension (50 μL) of 3‐5 other individuals within its group, in addition to its own RBC (autocontrol). The gel cards were then incubated and centrifuged according to the description above. The RBCs' migration through the gel was scored according to the following scale: 4+: all RBCs were agglutinated and formed a red line on the top of the gel; 3+: most RBCs were agglutinated on the top half of the gel with some retained on the surface of the gel; 2+: RBC agglutinates were predominantly observed in the lower half or were dispersed throughout the gel; 1+: few RBC agglutinates were dispersed in the lower half of gel, with most of the RBCs found at the bottom of the gel; 0: all RBCs were at the bottom of the tube (none agglutinated). For this step, reactions ≥2+ were considered incompatible (ie, presence of NOAb). A threshold of 2+ was chosen to select plasmas containing NOAb that led to easily visible agglutination reactions for the following prospective blood typing step (Figure 1).
2.5. Prospective blood typing for novel antigens (step 3)
When NOAb were detected in a cat (index cat) and according on the cat's availability, additional EDTA‐anticoagulant blood samples (5‐10 mL) were collected and its plasma was frozen and stored at −20°C for later testing. Plasma from the index cat was then tested against RBC of all other cats included thereafter in the study (ie, blood typing). The blood typing was performed using the same gel column method described above. The results were graded using the same scale, and for this step a result ≥1+ was considered positive (ie, presence of the FEA corresponding to the index NOAb) (Figure 1). Corresponding FEA were numbered in the chronological order that the index cats were identified.
2.6. Agglutinin titer
Strength of NOAb was evaluated through agglutination titers. The agglutinin titer is the highest dilution of serum or plasma at which agglutination is still detected (≥1+). This was determined by making serial 2‐fold dilutions of the index cat's plasma in phosphate‐buffered saline solution crossmatched against RBCs of 2 previously typed‐positive cats, randomly selected, as previously described. 11 , 15
2.7. Statistical analyses
Statistical analyses were performed using SAS Statistical Software, Version 9.3 (SAS Institute Cary, North Carolina). Descriptive data are presented as median, range, and percentage. Prevalence of cats with non‐AB incompatibilities with 95% confidence intervals (CI) was estimated. Univariable logistic regressions were used to model the presence of NOAb (reaction ≥2+) according to cat's characteristics (source, sex, age, purebred status, health status, hemolysis) and extensive blood typing results. To identify factors independently associated with the presence of NOAb, a multivariate logistic regression analysis was performed including all variables with P < .2 (likelihood ratio test) in univariate analyses, followed by a backward selection procedure with P > .05 as the criteria for rejection. Odds ratios (OR) were used to present the results with a significance level of .05. To determine whether there was agreement between reactions obtained for each pairwise combination of 2 different NOAb (ie, between 2 FEA blood typing), McNemar's test, the percentage of agreement, kappa statistic, and Gwet's coefficient were estimated. For the McNemar's test, the null hypothesis was that the proportion of cats with a positive reaction for 1 NOAb was the same as the proportion of cats with a positive reaction for a second NOAb. The statistical significance was set at .01 to account for multiple testing. The percentage of agreement between 2 NOAb was calculated as the proportion of cats that had the same FEA typing results (defined as positive or negative) divided by the total of cats tested for the 2 given NOAb. Cohen's Kappa statistics and Gwet's coefficient provide chance‐corrected agreement coefficients, with the Gwet's coefficient being less sensitive to prevalence and marginal probabilities. Kappa values and Gwet's coefficient were interpreted to indicate strength of agreement as follows: <0.20 = poor; 0.21‐0.40 = fair; 0.41‐0.6 = moderate; 0.61‐0.80 = good, and 0.81‐1.00 = very good. 23 , 24
3. RESULTS
3.1. Description of cats and AB blood typing results
A total of 271 cats were included in the study with 126 females, 138 males and 7 cats for which sex was not recorded. The median age of the 212 cats for which birth date was available was 7.7 years (range, 4 months to 19.5 years). The majority were domestic cats (n = 217), in addition to the following breeds: Bengal (n = 4), Cornish Rex (n = 4), Himalayan (n = 4), Persian (n = 4), Siamese (n = 4), Chartreux (n = 3), Maine Coon (n = 3), Sphynx (n = 3), Savannah (n = 1), Tonkinese (n = 1), Munchkin (n = 1), Abyssinian (n = 1), British Shorthair (n = 1), Highlander (n = 1). Breed was not recorded for 19 cats. A total of 101 cats were tested for FIV/FeLV, of which 96 were FIV/FeLV negative, 4 were FIV‐positive and 1 was FIV and FeLV‐positive. One hundred thirty‐two cats were clinically unhealthy, 110 cats were considered healthy, and the reason of presentation was not available for 29 cats. Red blood cell volume percentage evaluated by hematocrit or PCV ranged from 19% to 49% (median of 34%). The blood‐type proportions in cats were 95.6% type A (n = 259), 3.3% type B (n = 9), and 1.1% type AB (n = 3). Of the 271 cats, 12 cats were excluded from the rest of the study because of their B or AB blood type and 1 type A cat was excluded because of a positive autocontrol.
3.2. Detection of naturally occurring alloantibodies results
A total of 1228 crossmatches were performed with 258 type A cats divided into 46 groups; therefore, each cat was crossmatched against a median of 4.8 other cats: 3 other cats (n = 4), 4 other cats (n = 54), or 5 other cats (n = 200). A total of 48 incompatible crossmatches (3.9%) were observed. Overall, 18 cats had at least 1 incompatible reaction (≥ 2+), for a prevalence of 7% of cat with incompatibilities (95% CI: 4.2%‐10.8%). Six cats (2.3%) showed incompatibility to only 1 other cat, 3 (1.2%) showed incompatibilities to 2 cats, 4 (1.6%) showed incompatibilities to 3 other cats, and 5 cats (1.9%) showed incompatibilities to all individuals to which they were tested (between 4 and 5 cats) (Table 1 and Figure 2). Out of these 18 cats, there were 14 domestic cats, 1 Bengal, 1 Siamese, 1 Himalayan, and 1 British Shorthair.
TABLE 1.
Crossmatches (n = 1228) and cats (n = 258) distribution according to compatible or incompatible results in Quebec, Canada (2017‐2019)
| Reaction | Crossmatch results | Number (%) of crossmatches | Number (%) of cats a |
|---|---|---|---|
| Compatible | 0 | 1174 (95.6) | 236 (91.5) |
| 1+ | 6 (0.5) | 4 (1.5) | |
| Total | 1180 (96.1) | 240 (93) | |
| Incompatible | 2+ | 35 (2.9) | 11 (4.3) |
| 3+ | 12 (1.0) | 6 (2.3) | |
| 4+ | 1 (0.1) | 1 (0.4) | |
| Total | 48 (3.9) | 18 (7) |
If a cat had >1 incompatible result, the cat was classified according to the strongest reaction he presented.
FIGURE 2.
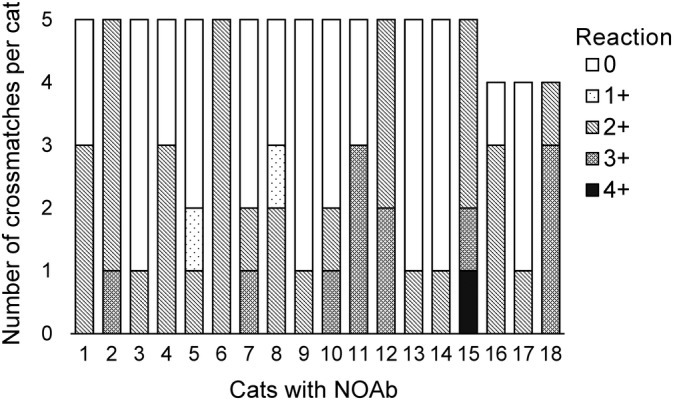
Distribution of cats with naturally occurring alloantibodies (NOAb) according to the number and the grade of incompatibilities. Results ≥2+ are considered as incompatible (ie, presence of NOAb)
Table 2 shows the clinical and demographic characteristics of cats included in the study, and their distribution according to the presence of NOAb. Feline erythrocyte antigens 1 status was the only variable kept in the final model (discussed in the next section).
TABLE 2.
Distribution of cats with naturally occurring alloantibodies (NOAb) according to their clinical and demographic characteristics in Quebec, Canada (2017‐2019)
| Characteristics a | Total of cats | Presence of NOAb b | P value c | ||
|---|---|---|---|---|---|
| Number of cats | Percentage (%) | ||||
| Source | Blood donor colony | 11 | 0 | .0 | .22 |
| Teaching colony | 23 | 0 | .0 | ||
| Client and employed‐owned cats | 98 | 10 | 10.2 | ||
| Surplus EDTA sample | 126 | 8 | 6.4 | ||
| Sex | Male | 134 | 9 | 6.7 | .81 |
| Female | 119 | 9 | 7.6 | ||
| Age | < 2 years | 19 | 3 | 15.8 | .13 |
| 2 ≤ age < 8 years | 82 | 3 | 3.7 | ||
| ≥ 8 years | 100 | 11 | 11.0 | ||
| Breed | Mixed | 204 | 14 | 6.9 | .49 |
| Purebred | 35 | 4 | 11.4 | ||
| Health status | Unhealthy | 126 | 10 | 7.9 | 1 |
| Healthy | 105 | 8 | 7.6 | ||
| Hemolysis | Yes | 167 | 13 | 7.8 | .48 |
| No | 91 | 5 | 5.5 | ||
| FEA 1 d | Negative | 42 | 7 | 16.7 | .01 |
| Positive | 216 | 11 | 5.1 | ||
| FEA 2 | Negative | 141 | 6 | 4.3 | 1 |
| Positive | 15 | 1 | 6.7 | ||
| FEA 3 | Negative | 103 | 5 | 4.9 | .61 |
| Positive | 23 | 2 | 8.7 | ||
| FEA 4 | Negative | 23 | 2 | 8.7 | .64 |
| Positive | 70 | 4 | 5.7 | ||
| FEA 5 | Negative | 3 | 0 | .0 | 1 |
| Positive | 74 | 2 | 2.6 | ||
| FEA 6 | Negative | 52 | 1 | 1.9 | .24 |
| Positive | 71 | 5 | 7.0 | ||
| FEA 7 | Negative | 32 | 0 | .0 | .5 |
| Positive | 42 | 2 | 4.8 | ||
Abbreviation: FEA, feline erythrocyte antigen.
One type B cat was excluded from the teaching colony, one type AB and 3 type B cats were excluded from the client or employed‐owned cats, 2 type AB and 6 type B cats were excluded from the surplus EDTA samples. Sex, age, breed, and health status were not recorded for 5, 56, 19, and 27 cats, respectively.
Results ≥2+ are considered as incompatible (ie, presence of NOAb)—these results derived from the second step of the study (detection of NOAb).
P value from univariable logistic regression modeling the presence of NOAb (likelihood ratio test).
The results of FEA blood typing derived from the third step of the study (prospective blood typing for novel antigens). Negative: absence of the FEA corresponding to the index NOAb used as reagent; positive: presence of the FEA corresponding to the index NOAb used as reagent. NOAb present in a FEA‐positive cat cannot be the same as the index NOAb used as reagent. For example, NOAb detected in a FEA 1 positive cat cannot be NOAb 1. In contrast, NOAb detected in a FEA‐negative cat can be the same as the index NOAb or another NOAb.
3.3. Blood typing for novel antigens results
Of the 18 cats with NOAb, 7 were included for the prospective blood typing starting at different moments from day 0 (group 1) to day 480 (group 32). Nine cats could not be included because their owners were not reachable or were unable to return with their cat for the second blood sample. One cat was not included because it was 1 of the cats from the second to last group to be crossmatched. One cat was not included because he died before a second sample of blood could be taken. As indicated above, a number from 1 to 7 was attributed to the index cats and their NOAb, as well as the corresponding FEA. None of the 7 index cats belonged to the same family and therefore they did not live in the same environment. Six were mixed cats and 1 was purebred, and they were 3‐10 years old.
The prospective blood typing results are summarized in Table 2. Feline erythrocyte antigens 1 and 5 were most frequent with 83.7% and 96% of positive cats, respectively. Feline erythrocyte antigens 2 and FEA 3 were the least common, with 9.6% and 18.2% of positive cats. All cats negative for 1 FEA could potentially have NOAb, but FEA 1‐negative status was the only 1 significantly associated with the presence of NOAb (Table 2). Indeed, FEA 1‐negative cats were more likely to present NOAb (OR = 3.90, 95% CI, 1.37‐11.1; P = .01) than FEA 1 positive cats. During step 2 of the study, the 7 FEA 1‐negative cats with NOAb showed incompatibility to a median of 4 other cats (range, 3‐5) within their own group.
The results of the comparisons between reactions obtained with 2 different NOAb (ie, between 2 FEA blood typing) are presented in Table 3. Whenever possible, extensive blood typing was also performed for the 7 index cats in which NOAb were discovered, and the results are presented in Table 4. McNemar's test was significant for each pairwise combination of FEA typing results, except for FEA 1 and FEA 4 (P = .06), FEA 2 and FEA 3 (P = .13), FEA 4 and FEA 5 (P = .03), FEA 4 and FEA 7 (P = .03). Agreement between pairs of FEA were variable, ranging from poor to very good. For example, the median percentage of agreement was considered acceptable (>75%) and Gwet's coefficient was good (0.61‐0.8) to very good (>0.8) between FEA 1 and FEA 5 (88.6%; 0.86), FEA 2 and FEA 3 (81.6%; 0.76), FEA 4 and FEA 6 (87.7%; 0.78), FEA 4 and FEA 7 (84.4%; 0.73). Median Cohen's kappa was good between FEA 4 and FEA 6 (0.73), and between FEA 4 and FEA 7 (0.64). The agreement was perfect between reactions obtained with NOAb 6 and NOAb 7, which most likely indicates that they both reacted to the same antigen, that is, [FEA 6.7] (Figure 3). It also appeared that NOAb 4 often led to the same results as NOAb 6 and 7. Despite a good but imperfect agreement, it seems likely that NOAb 4 reacted against the same antigen as NOAb 6 and 7. Feline erythrocyte antigens 4, FEA 6 and FEA 7 could therefore be considered as 1 antigen, that is, [FEA 4.6.7] or more simply FEA 4.
TABLE 3.
Agreement assessment between blood typing results obtained with different pairs of naturally occurring alloantibodies (NOAb) [reagent] determined by McNemar's test, percentage of agreement, Cohen's kappa agreement, and Gwet's coefficient in Quebec, Canada (2017‐2019)
| Reagent 1 | Reagent 2 | Blood typing results | P‐value McNemar | % agreement | Kappa | Gwet | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| neg/neg | neg/pos | pos/neg | pos/pos | Estimate | 95% CI | Estimate | 95% CI | ||||
| NOAb1 | NOAb2 | 11 | 13 | 130 | 2 | <.001 | 8.3 | −.18 | (−.27, −.08) | −.83 | (−.92, −.73) |
| NOAb1 | NOAb3 | 12 | 9 | 91 | 14 | <.001 | 20.6 | −.12 | (−.21, −.02) | −.59 | (−.73, −.45) |
| NOAb1 | NOAb4 | 0 | 12 | 23 | 58 | .06 | 62.4 | −.2 | (−.29, −.12) | .46 | (.27, .65) |
| NOAb1 | NOAb5 | 3 | 9 | 0 | 67 | <.01 | 88.6 | .36 | (.06, .66) | .86 | (.77, .96) |
| NOAb1 | NOAb6 | 0 | 18 | 52 | 53 | <.001 | 43.1 | −.28 | (−.39, −.17) | .04 | (−.17, .25) |
| NOAb1 | NOAb7 | 0 | 11 | 32 | 31 | <.01 | 41.9 | −.28 | (−.43, −.14) | .01 | (−.26, .28) |
| NOAb2 | NOAb3 | 87 | 14 | 7 | 6 | .13 | 81.6 | .26 | (.03, .49) | .76 | (.64, .87) |
| NOAb2 | NOAb4 | 22 | 51 | 0 | 8 | <.001 | 37 | .08 | (.02, .14) | −.22 | (−.45, .01) |
| NOAb2 | NOAb5 | 2 | 69 | 1 | 7 | <.001 | 11.4 | −.02 | (−.07, .03) | −.77 | (−.91, −.62) |
| NOAb2 | NOAb6 | 50 | 61 | 2 | 10 | <.001 | 48.8 | .09 | (.00, .17) | .07 | (−.12, .27) |
| NOAb2 | NOAb7 | 31 | 36 | 1 | 6 | <.001 | 50 | .1 | (−.01, .21) | .1 | (−.15, .36) |
| NOAb3 | NOAb4 | 18 | 41 | 0 | 13 | <.001 | 43.1 | .14 | (.05, .22) | −.13 | (−.37, .10) |
| NOAb3 | NOAb5 | 1 | 49 | 2 | 6 | <.001 | 12.1 | −.07 | (−.17, .03) | −.75 | (−.93, −.56) |
| NOAb3 | NOAb6 | 40 | 45 | 3 | 14 | <.001 | 52.9 | .15 | (.03, .27) | .12 | (−.09, .32) |
| NOAb3 | NOAb7 | 20 | 26 | 3 | 4 | <.001 | 45.3 | 0 | (−.16, .17) | 0 | (−.31, .30) |
| NOAb4 | NOAb5 | 0 | 9 | 2 | 26 | .03 | 70.3 | −.1 | (−.21, .02) | .6 | (.35, .85) |
| NOAb4 | NOAb6 | 22 | 0 | 10 | 49 | <.01 | 87.7 | .73 | (.57, .88) | .78 | (.64, .91) |
| NOAb4 | NOAb7 | 7 | 0 | 5 | 20 | .03 | 84.4 | .64 | (.36, .91) | .73 | (.50, .96) |
| NOAb5 | NOAb7 | 0 | 2 | 32 | 40 | <.001 | 54.1 | −.05 | (−.13, .02) | .29 | (.05, .53) |
| NOAb5 | NOAb6 | 0 | 35 | 3 | 41 | <.001 | 51.9 | −.08 | (−.16, .01) | .24 | (.00, .48) |
| NOAb6 | NOAb7 | 32 | 0 | 0 | 42 | NE | 100 | 1 | NE | 1 | NE |
Abbreviations: FEA, feline erythrocyte antigens; NE, not estimated; neg, negative; pos, positive.
TABLE 4.
Extensive blood typing results of each index cat with naturally occurring alloantibodies (NOAb) in Quebec, Canada (2017–2019)
| Index cat with NOAb | NOAb discovered | Extensive feline erythrocyte antigen (FEA) blood typing | ||||||
|---|---|---|---|---|---|---|---|---|
| FEA 1 | FEA 2 | FEA 3 | FEA 4 | FEA 5 | FEA 6 | FEA 7 | ||
| Cat 1 | NOAb 1 | − | − | − | + | + | + | + |
| Cat 2 | NOAb 2 | + | − | + | + | + | − | − |
| Cat 3 | NOAb 3 | + | − | − | + | NE | NE | NE |
| Cat 4 | NOAb 4 | + | − | − | − | NE | NE | NE |
| Cat 5 | NOAb 5 | − | − | + | + | − | + | + |
| Cat 6 | NOAb 6 | + | − | − | − | NE | − | NE |
| Cat 7 | NOAb 7 | + | − | − | − | + | − | − |
Abbreviations: NE, not evaluated; +, positive = presence of the FEA on the surface of the index cat's red blood cells; −: negative, absence of the FEA on the surface of the index cat's red blood cells.
FIGURE 3.
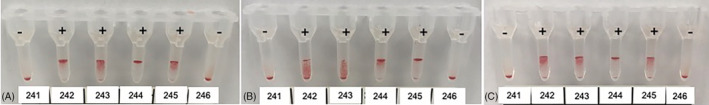
Gel column results showing feline erythrocyte antigen (FEA) 4 (A), 6 (B) and 7 (C) blood typing against the same 6 cats (number 241‐246). +: positive = presence of the FEA on the surface of the index cat's red blood cells; −: negative = absence of the FEA on the surface of the index cat's red blood cells. All autocontrols were negative
3.4. Agglutinin titers results
The NOAb agglutinin titers was measured for 2 cats in each NOAb groups, except for NOAb 4 because of the limited amount of the index plasma available (the cat died in the first weeks of the study). The titers varied from 1 : 4 to 1 : 8 for NOAb 1, 3, 5, and 6. The NOAb 2 agglutinin titers were 1 : 1 and the NOAb 7 agglutinin titers were 1 : 16 and 1 : 32.
4. DISCUSSION
In our study, we identified the presence of naturally occurring non‐AB alloantibodies and we used them to begin mapping unidentified corresponding FEA. Comparison of results obtained from an extensive blood typing supports the existence of 5, presumably different, novel FEA. We described the frequency, the distribution, and the association of these newly identified FEA with NOAb, although more studies are warranted to clarify those findings and to determine their clinical importance.
Previous reports have already documented crossmatch incompatibilities and transfusion reactions in AB‐matched transfusion naïve cats, highlighting the presence of NOAb outside of the AB system. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 Our study supports the presence of non‐AB NOAb with 3.9% chance of detecting incompatibilities by randomly crossmatching 2 cats, which resulted in at least 7% of 258 type A cats having NOAb. The history of transfusion was unknown for 39 studied cats, but none of them presented incompatibilities. Incompatibilities observed in our study were therefore all related to NOAb. Our study population was not a random sample, but a convenience sample of cats belonging to 2 colonies, employed‐owned cats and cats seen at our hospital, and more than half of them were unhealthy. However, since the presence of NOAb was not statistically associated with health status, nor with cat's source, sex, age or purebred status, our sample is likely representative of the feline population in the great area of Montreal.
Previous studies reported variable prevalence of non‐AB blood type incompatibilities. Two European studies failed to detect any non‐AB NOAb in transfusion naïve cats (in 112 cats and less than 20 cats, respectively). 17 , 18 In a study in the United States, 24 cats had a pretransfusion crossmatch performed with 29% having NOAb for a total of 10 positive reactions of 52 crossmatches (19%). 20 Another study in the US documented 23 of 154 (14.9%) transfusion naïve cats with major crossmatch incompatibilities for a total of 39 positive reactions of 462 crossmatches (8%). 19 Geographical variation and number of crossmatch tested by cat might influence the prevalence of and the ability to detect non‐AB incompatibilities, respectively. This large variability of prevalence among studies could also be explained by difference in crossmatch methods.
Currently, the reference method of crossmatching in the United States, which is the method used in studies presented above, is the laboratory tube agglutination assay. 17 , 18 , 19 , 20 Problems with this technique include low reproducibility, interobserver variation in interpretation, labor‐intensiveness, requirements for technical expertise, and lack of standardization. 25 Several other methods are available including slide assay, saline gel column technique, antiglobulin‐enhanced gel column test and commercial gel‐tube assay. 1 We chose to use the saline gel column technique because this method has been proven to be highly accurate in human medicine with a sensitivity between 97.58% and 100% and a specificity close to 100%. 26 It can be better standardized, is simple to perform and easy to interpret with grading being independent of the skill of the reader; as other studies have previously shown for dogs, cats, and horses. 15 , 21 , 27 , 28 , 29 , 30 Two studies compared crossmatch results of tube and column gel methods for detecting NOAb in a limited number of cats (66 and 10) and found overall agreement between both methods. 15 , 19 However, a prospective observational study of 101 cats showed marked difference in the proportion of crossmatch incompatibility between the laboratory method (27%) and a commercial gel tube test (4%). 22 In a study comparing a saline gel column test and an antiglobulin‐enhanced gel column test in 446 plasma to RBC pairings, both methods showed the same compatibility results for all pairings, except for 15 pairings for which incompatibility was only detected with the antiglobulin‐enhanced gel column test (including 14 incompatibilities outside the expected AB mismatches). 21 These comparison studies emphasized that not all crossmatch methods are interchangeable and it remains difficult to compare results obtained from 2 different techniques.
It should also be noted that there is a lack of standardization of grade at which gel column crossmatch results are considered incompatible in veterinary medicine, with some studies using the grade 1+ and others the grade 2+. 15 , 21 , 28 , 29 , 31 The interpretation of low‐grade agglutination (1+) can sometimes be confused with grade 0. As mentioned earlier, a threshold of 2+ was chosen during the step of NOAb detection to increase the specificity. The selection of plasma that led to an easily visible agglutination reaction was important for the extensive blood typing step to enable repeatable and interpretable results. However, even if we had considered incompatibility from grade 1+, our results would have been similar because only 4 cats showed 1+ reactions in step 2 of the study, which would have led to a total of 22 cats with NOAb (8.5% vs 7%) and 54 positive reactions (4.4% vs 3.9%). A better standardization of crossmatch protocol appears to be of growing importance in feline transfusion medicine in order to correctly interpret and compare prevalence of incompatibilities.
During the prospective blood typing, 7 NOAb were used to start mapping the FEA to which they bind (NOAb 1 to FEA 1, NOAb 2 to FEA 2, etc). As highlighted in the result section, our results strongly suggest that FEA 4, FEA 6 and FEA 7 correspond to the same antigen, that is, [FEA 4.6.7] or more simply FEA 4. Indeed, a perfect agreement was identified between reactions obtained with NOAb 6 and NOAb 7, most likely indicating that they both reacted to the same antigen, that is, [FEA 6.7]. Similarly, despite a good but imperfect agreement, it seems likely that NOAb 4 reacted to the same antigen as NOAb 6 and 7. The first argument in favor of this conclusion is that index cats 6 and 7 were both FEA 4‐negative. Since cats can only have NOAb against FEA they do not express, NOAb 6 and 7 were likely directed against FEA 4, which would suggest, given the good agreement of their corresponding NOAb, that [FEA 6.7] is the same antigen as FEA 4. In comparison, index cats 6 and 7 were also FEA 2 and FEA 3‐negative, but a poor agreement was detected between reactions obtained with NOAb 6‐7, NOAb 2, and NOAb 3. Therefore, it seems unlikely that [FEA 6.7] is the same antigen as FEA 2 or FEA 3. The second argument is that discrepancies between reactions obtained with NOAb 4 and NOAb 6‐7 had a similar trend: positive reactions to NOAb 4 were observed in 11 cats that had negative reactions to NOAb 6 and NOAb 7, which could suggest that NOAb 4 is stronger (greater affinity, higher titer, or both) than NOAb 6 and NOAb 7. A variability in the antibody titer (NOAb 4 titer was not determined but could be higher), in antigenic expression, and in the affinity of polyclonal antibodies to the FEA could have contributed to those differences. Additionally, the reagent being plasma from an index cat, it could have contained several antibodies directed against other erythrocyte antigens that might have crossreacted during blood typing and contributed to those discrepancies. Unfortunately, the agreement between NOAb 4/FEA 4 and NOAb 6‐7/FEA 6‐7 cannot be studied furthermore because the index NOAb 4/FEA 4‐negative cat has died and no residual reagent remains.
Gwet's coefficient showed good agreement between reactions obtained with NOAb 1 and NOAb 5. However, NOAb 1 and 5 could not react to the very same FEA because the index cat 1 was FEA 5‐positive; therefore, it could not express anti‐FEA5 NOAb. In opposite, the index cat 5 being FEA 1‐negative, it is possible that its plasma contained antibodies anti‐FEA 1 and NOAb 5 (ie, anti‐FEA 5), which could participate to the observed agreement. The same reasoning applies for the agreement observed between FEA 2 and FEA 3.
Considering that cats can present NOAb against RBC antigen they do not express, FEA‐negative cats were examined for the presence of NOAb. Unfortunately, only 7 index cats out of 18 with NOAb were included for the extensive blood typing. NOAb were found in a low percentage of FEA 2 and FEA 3‐negative cats (4.3% and 4.9%, respectively). It was not possible to determine if there were anti‐FEA 2 and anti‐FEA 3 alloantibodies because most of FEA 2 and FEA 3‐negative cats with NOAb were also negative for 1 or 2 others studied FEA. The only 2 FEA 4‐negative cats (in addition to the index cat 4) with NOAb were the index cats 6 and 7, and the only FEA 6‐negative cat (in addition to the index cat 6) with NOAb was the index cat 7; therefore, we concluded that a low percentage of FEA 4‐negative cats (between 0% and 8.7%) showed natural anti‐FEA 4 alloantibodies in cats of our study. Only FEA 1‐negative cats were shown to be more at risk of presenting NOAb, with 16.7% of FEA 1‐negative cats having NOAb compared to 5.1% of FEA 1‐positive cats. All but 1 FEA 1‐negative cats with NOAb were incompatible to all FEA 1‐positive cats and compatible to all FEA 1‐negative cats in their respective group. Thus, NOAb found in FEA 1‐negative cats contained likely anti‐FEA 1 antibodies (NOAb 1). However, the presence of other NOAb in FEA 1‐negative cats could not be excluded because the last FEA 1‐negative cats was compatible to 2 FEA 1‐positive cats in its group (and incompatible to 3 remaining FEA 1‐positive cats). Unfortunately, all these cats except the index cat 1 were unavailable for the extensive blood typing (step 3) preventing the investigation of their respective NOAb specificity.
The strength of almost all NOAb was weak with titers of ≤1 : 8, but was moderate for NOAb 7 with titer of 1 : 16 to 1 : 32. In comparison, previous reports demonstrate type A cats anti‐B alloantibodies are weak to moderate with a titer of <1 : 32 whereas type B cats anti‐A titers are stronger and range from 1 : 64 to 1 : 2048. 11 The titer strength of each NOAb slightly varied depending on which FEA‐positive RBCs were used, which might reflect variable expression of FEA on erythrocytes. From a practical perspective, polyclonal antiserum with NOAb are not optimal reagents for use in serologic testing because they are heterogeneous, can vary in concentration, serologic properties, epitope recognition, and can contain nonspecific antibodies. The development of uniformly reactive and highly specific monoclonal antibodies could improve our ability to detect positive reactions for screening cats for these novel FEA and, thus, could facilitate future studies.
From a clinical perspective, the importance of non‐AB NOAb still needs to be determined. Recent studies have conflicting results with some of them supporting the use of crossmatched compatible units to increase the efficacy and safety of transfusion, while others show no significant difference in the incidence of transfusion reactions or increase in the posttransfusion PCV between cats with or without pretransfusion crossmatches. 19 , 20 , 32 , 33 Firm conclusions concerning the clinical relevance of NOAb cannot be drawn from these studies because they compare efficacy and safety of transfusion between cats with pretransfusion compatible crossmatch and cats with unknown compatibility status (ie, can be compatible or incompatible). Only 1 study compared transfusion events between cats with pretransfusion compatible crossmatch and cats with pretransfusion incompatible crossmatch. In this study, detection of incompatibilities by a bedside commercial kit, but not by a laboratory method, appeared to be associated with the development of hemolytic transfusion reactions. 22 With at least 7% of cats presenting NOAb, almost 2% of them having incompatibilities to all RBC to which they were tested, conflicting results in recent studies, and previous documentation of acute hemolytic transfusion reactions caused by non‐AB NOAb (anti‐Mik), crossmatching cats before a first transfusion remains valid cautionary recommendation as long as the clinical importance of NOAb and crossmatch incompatibility is not better characterized. 15
Another important clinical aspect concerns the immunogenicity of the newly discovered FEA, that is, their ability to stimulate the production of clinically relevant acquired alloantibodies that might result in ineffective additional transfusions and even acute life‐threatening hemolytic transfusion in case of multiple transfusions. Blood group antigen immunogenicity is a crucial factor in alloimmunization, which is a common posttransfusion sequelae described in dogs and recently in cats. 18 , 19 , 34 Development of alloantibodies against erythrocyte antigens outside the AB system has been observed in 25% of an anemic cat population within a median of 5 days after the first transfusion event. 18 In another study, 27% of previously transfused cats had major crossmatch incompatibilities. Furthermore, 16.3% of cats showed some degree of incompatibility after an initial transfusion, even though they had no incompatibility on their first crossmatch to 2‐3 donors tested. 19
It is uncertain if any of the discovered FEA is actually the Mik antigen because typing reagents for Mik are no longer available and Mik‐typed cats could not be found to analyze their extensive blood typing. Previous data on Mik antigen documented a frequency of 94% in a population of 66 cats, the presence of NOAb anti‐Mik in Mik‐negative cats, and agglutinin titers between 1 : 1 and 1 : 64. 15 Given its high frequency and its association with NOAb, FEA 1 might correspond to the lost Mik antigen. The lack of Mik, and now NOAb 4, reagents highlights the problematic of relying solely on natural alloantibodies for blood typing. Although not a long‐term solution, banking NOAb is essential to maintain our ability to compare novel FEA with already reported antigens, and to identify rapidly a compatible donor in case of incompatibility outside the AB system. In this way, this study led to the banking of NOAb against all 5 FEA. A more sustainable solution, such as the production of monoclonal antibodies, remains necessary.
Finally, naming a novel blood antigen is not standardized in human blood banking, and even less in veterinary transfusion medicine. 35 Using a part (3 letters) of the name of the first reactive donor was chosen in 2007 for the Mik antigen. 15 We chose the label FEA (feline erythrocyte antigen) to mimic the label of most canine blood types and to facilitate our discussion. This points out how nomenclature could become an issue as more alloantibodies and corresponding antigens are found. A discussion group should again be considered to standardize canine and feline blood group system nomenclature with standards made for the appropriate designation of newly found antigens. 36 , 37
It is important to note that unhealthy cats and cats with unknown health or FeLV status were accepted in our study to facilitate animal recruitment. Blood typing results can be affected by disease status in humans and animals. For example, FeLV infections and immune diseases can cause anemia and autoagglutination, which might contribute to inaccuracies in crossmatching and blood typing. 38 In our study, the preparation of a fixed‐concentration RBC suspension alleviates a possible effect of hematocrit on crossmatching and blood typing results. In addition, autocontrols were systematically performed, and RBC washed 3 times, making unlikely an impact of autoagglutination on our results. Although rarely described in human medicine, we cannot rule out the presence of acquired antigen or altered antigen expression on RBC surface in unhealthy cats. 39 , 40 , 41 , 42 , 43 Furthermore, while statistical association was not found between the presence of NOAb and health status in our study, the effect of some diseases on NOAb (titer, specificity, etc) is unknown. That said, even if health status had an influence on FEA and NOAb expression, which remains to be investigated, our main conclusion would remain unchanged (ie, 5 presumably distinct novel antigens were identified).
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Animal Care and Use Committee of Université de Montréal (approval number: Rech 18‐1912), and written owner consent was obtained before enrollment of cats into the study.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
Funding provided by Companion Animals Health Fund from the Faculty of Veterinary Medicine of the Université de Montréal, supported by Zoetis. Abstract was presented at the 2020 American College of Veterinary Internal Medicine (ACVIM) Forum On Demand.
Binvel M, Arsenault J, Depré B, Blais M‐C. Identification of 5 novel feline erythrocyte antigens based on the presence of naturally occurring alloantibodies. J Vet Intern Med. 2021;35:234–244. 10.1111/jvim.16010
Funding information Companion Animals Health Fund from the Faculty of Veterinary Medicine of the Université de Montréal, supported by Zoetis
REFERENCES
- 1. Zaremba R, Brooks A, Thomovsky E. Transfusion medicine: an update on antigens, antibodies and serologic testing in dogs and cats. Top Companion Anim Med. 2019;34:36‐46. [DOI] [PubMed] [Google Scholar]
- 2. Auer L, Bell K. The AB blood group system of cats. Anim Blood Groups Biochem Genet. 1981;12:287‐297. [DOI] [PubMed] [Google Scholar]
- 3. Knottenbelt CM. The feline AB blood group system and its importance in transfusion medicine. J Feline Med Surg. 2002;4:69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arikan S, Gurkan M, Ozaytekin E, Dodurka T, Giger U. Frequencies of blood type a, B and AB in non‐pedigree domestic cats in Turkey. J Small Anim Pract. 2006;47:10‐13. [DOI] [PubMed] [Google Scholar]
- 5. Marques C, Ferreira M, Gomes JF, et al. Frequency of blood type a, B, and AB in 515 domestic shorthair cats from the Lisbon area. Vet Clin Pathol. 2011;40:185‐187. [DOI] [PubMed] [Google Scholar]
- 6. Zheng L, Zhong Y, Shi Z, Giger U. Frequencies of blood types a, B, and AB in non‐pedigree domestic cats in Beijing. Vet Clin Pathol. 2011;40:513‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fosset FT, Blais MC. Prevalence of feline blood groups in the Montreal area of Quebec, Canada. Can Vet J. 2014;55:1225‐1228. [PMC free article] [PubMed] [Google Scholar]
- 8. Spada E, Miglio A, Proverbio D, et al. Signalment and blood types in cats being evaluated as blood donors at two italian university blood banks. Vet Med Int. 2014;2014:704836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cattin RP. Distribution of blood types in a sample of 245 New Zealand non‐purebred cats. N Z Vet J. 2016;64:154‐157. [DOI] [PubMed] [Google Scholar]
- 10. Vieira SM, Ferreira RRF, de Matos AJ, et al. Distribution of feline AB blood types: a review of frequencies and its implications in the Iberian Peninsula. JFMS Open Rep. 2017;3:2055116917727693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bucheler J, Giger U. Alloantibodies against a and B blood types in cats. Vet Immunol Immunopathol. 1993;38:283‐295. [DOI] [PubMed] [Google Scholar]
- 12. Auer L, Bell K. Transfusion reactions in cats due to AB blood group incompatibility. Res Vet Sci. 1983;35:145‐152. [PubMed] [Google Scholar]
- 13. Giger U, Bucheler J. Transfusion of type‐a and type‐B blood to cats. J Am Vet Med Assoc. 1991;198:411‐418. [PubMed] [Google Scholar]
- 14. Cain GR, Suzuki Y. Presumptive neonatal isoerythrolysis in cats. J Am Vet Med Assoc. 1985;187:46‐48. [PubMed] [Google Scholar]
- 15. Weinstein NM, Blais MC, Harris K, Oakley DA, Aronson LR, Giger U. A newly recognized blood group in domestic shorthair cats: the Mik red cell antigen. J Vet Intern Med. 2007;21:287‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weingart C, Giger U, Kohn B. Whole blood transfusions in 91 cats: a clinical evaluation. J Feline Med Surg. 2004;6:139‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tasker S, Barker EN, Day MJ, Helps CR. Feline blood genotyping versus phenotyping, and detection of non‐AB blood type incompatibilities in UKcats. J Small Anim Pract. 2014;55:185‐189. [DOI] [PubMed] [Google Scholar]
- 18. Hourani L, Weingart C, Kohn B. Alloimmunisation in transfused patients: serial cross‐matching in a population of hospitalised cats. J Feline Med Surg. 2017;19:1231‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McClosky ME, Cimino Brown D, Weinstein NM, et al. Prevalence of naturally occurring non‐AB blood type incompatibilities in cats and influence of crossmatch on transfusion outcomes. J Vet Intern Med. 2018;32:1934‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sylvane B, Prittie J, Hohenhaus AE, Tozier E. Effect of cross‐match on packed cell volume after transfusion of packed red blood cells in transfusion‐naive anemic cats. J Vet Intern Med. 2018;32:1077‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goy‐Thollot I, Nectoux A, Guidetti M, et al. Detection of naturally occurring alloantibody by an in‐clinic antiglobulin‐enhanced and standard crossmatch gel column test in non‐transfused domestic shorthair cats. J Vet Intern Med. 2019;33:588‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Humm KR, Chan DL. Prospective evaluation of the utility of cross‐matching prior to first transfusion in cats: 101 cases. J Small Anim Pract. 2020;61:285‐291. [DOI] [PubMed] [Google Scholar]
- 23. Gwet KL. Computing inter‐rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61:29‐48. [DOI] [PubMed] [Google Scholar]
- 24. Zec S, Soriani N, Comoretto R, Baldi I. High agreement and high prevalence: the paradox of Cohen's kappa. Open Nurs J. 2017;11:211‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tocci LJ, Ewing PJ. Increasing patient safety in veterinary transfusion medicine: an overview of pretransfusion testing. J Vet Emerg Crit Care (San Antonio). 2009;19:66‐73. [DOI] [PubMed] [Google Scholar]
- 26. Cid J, Nogues N, Montero R, Hurtado M, Briega A, Parra R. Comparison of three microtube column agglutination systems for antibody screening: DG gel, DiaMed‐ID and Ortho BioVue. Transfus Med. 2006;16:131‐136. [DOI] [PubMed] [Google Scholar]
- 27. Euler CC, Lee JH, Kim HY, Raj K, Mizukami K, Giger U. Survey of two new (Kai 1 and Kai 2) and other blood groups in dogs of North America. J Vet Intern Med. 2016;30:1642‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luethy D, Owens SD, Stefanovski D, Nolen‐Walston R, Giger U. Comparison of tube, gel, and Immunochromatographic strip methods for evaluation of blood transfusion compatibility in horses. J Vet Intern Med. 2016;30:1864‐1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casenave P, Leclere M, Beauchamp G, Blais MC. Modified stall‐side crossmatch for transfusions in horses. J Vet Intern Med. 2019;33:1775‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goulet S, Blais MC. Characterization of anti‐ dal alloantibodies following sensitization of two dal‐negative dogs. Vet Pathol. 2018;55:108‐115. [DOI] [PubMed] [Google Scholar]
- 31. Kessler RJ, Reese J, Chang D, Seth M, Hale AS, Giger U. Dog erythrocyte antigens 1.1, 1.2, 3, 4, 7, and dal blood typing and cross‐matching by gel column technique. Vet Clin Pathol. 2010;39:306‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weltman JG, Fletcher DJ, Rogers C. Influence of cross‐match on posttransfusion packed cell volume in feline packed red blood cell transfusion. J Vet Emerg Crit Care (San Antonio). 2014;24:429‐436. [DOI] [PubMed] [Google Scholar]
- 33. Martinez‐Sogues L, Blois SL, Manzanilla EG, Abrams‐Ogg AO, Cosentino P. Exploration of risk factors for non‐survival and for transfusion‐associated complications in cats receiving red cell transfusions: 450 cases (2009 to 2017). J Small Anim Pract. 2020;61:177‐184. [DOI] [PubMed] [Google Scholar]
- 34. Goy‐Thollot I, Giger U, Boisvineau C, et al. Pre‐ and post‐transfusion Alloimmunization in dogs characterized by 2 Antiglobulin‐enhanced cross‐match tests. J Vet Intern Med. 2017;31:1420‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garratty G, Dzik W, Issitt PD, Lublin DM, Reid ME, Zelinski T. Terminology for blood group antigens and genes‐historical origins and guidelines in the new millennium. Transfusion. 2000;40:477‐489. [DOI] [PubMed] [Google Scholar]
- 36. Vriesendorp HM, Westbroek DL, D'Amaro J, et al. Joint report of 1st international workshop on canine Immunogenetics. Tissue Antigens. 1973;3:145‐163. [PubMed] [Google Scholar]
- 37. Vriesendorp HM, Albert ED, Templeton JW, et al. Joint report of the second international workshop on canine Immunogenetics. Transplant Proc. 1976;8:289‐314. [PubMed] [Google Scholar]
- 38. Seth M, Jackson KV, Giger U. Comparison of five blood‐typing methods for the feline AB blood group system. Am J Vet Res. 2011;72:203‐209. 10.2460/ajvr.72.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roath S, Todd CE, Shaw D. Transient acquired blood group B antigen associated with diverticular bowel disease. Acta Haematol. 1987;77:188‐190. 10.1159/000205990. [DOI] [PubMed] [Google Scholar]
- 40. Matsushita S, Imamura T, Mizuta T, et al. Acquired B antigen and polyagglutination in a patient with gastric cancer. Jpn J Surg. 1983;13:540‐542. 10.1007/BF02469500. [DOI] [PubMed] [Google Scholar]
- 41. Judd WJ, Annesley TM. The acquired‐B phenomenon. Transfus Med Rev. 1996;10:111‐117. 10.1016/s0887-7963(96)80087-3. [DOI] [PubMed] [Google Scholar]
- 42. Garratty G, Arndt P, Co A, et al. Fatal hemolytic transfusion reaction resulting from ABO mistyping of a patient with acquired B antigen detectable only by some monoclonal anti‐B reagents. Transfusion. 1996;36:351‐357. 10.1046/j.1537-2995.1996.36496226152.x. [DOI] [PubMed] [Google Scholar]
- 43. Kaur A, Jain A, Marwaha N, et al. Acquired‐B phenomenon in a neonate presenting with necrotizing enterocolitis. Transfus Apher Sci. 2019;58:30‐31. 10.1016/j.transci.2018.10.021. [DOI] [PubMed] [Google Scholar]


