Abstract
The purpose of this study was to determine the effects of resection coupled with standard chemotherapy on the survival prognosis of patients with early stage small cell lung carcinoma (SCLC). Patients (n = 110) with mediastinal lymph node-negative SCLC were enrolled in this study. The baseline clinical data of patients with surgery were retrospectively reviewed. Overall survival (OS) and progression-free survival (PFS) were measured by Kaplan–Meier and log-rank test analyses. Ninety-eight patients received mediastinoscopy biopsy, and pulmonary lobectomy or sublobar resection, and 67 patients underwent adjuvant chemotherapy after pulmonary lobectomy. Adjuvant chemotherapy after surgical intervention was associated with longer OS (median OS: 42.14 vs. 33.53 months, p = 0.01) and PFS (median PFS: 25.20 vs. 13.48 months, p = 0.000) compared to resection alone for all patients. Adjuvant chemotherapy was associated with improvement of survival for N1 patients with stage II (median OS: 36.42 vs. 26.68 months, p = 0.021). The median PFS was 19.02 m (16.08, 21.96) and 13.25 m (10.19, 16.30) (p = 0.031), respectively, for patients of N1 stage who received chemotherapy and those who did not. Cox regression analysis demonstrated that age, TNM stage (N stage, not T stage), and chemotherapy were independent risk factors that might affect overall survival in patients with mediastinal lymph node-negative SCLC. These findings suggest that the application of adjuvant chemotherapy following pulmonary lobectomy is associated with improvements of survival prognoses for patients with SCLC. The combination of surgical intervention with conventional therapy should be taken into consideration as a prospective multidisciplinary regimen for early stage SCLC.
Key words: Small cell lung carcinoma (SCLC), Chemotherapy, Lymph node, Survival prognosis, Surgical resection
INTRODUCTION
Lung carcinoma accounts for a large proportion of cancer deaths in both men and women. Small cell lung carcinoma (SCLC) is one of the main pathological classes of lung cancer, and patients with SCLC encompass 13–15% of all patients with lung cancer and is characterized by rapid progression and early development of lymph node metastases1,2. Chemoradiotherapy is recommended as primary therapy for patients with SCLC, especially those with extensive disease, while a multitherapy regimen for patients with mediastinal lymph node-negative disease remains controversial3,4. Although patients often experience high sensitivity to chemoradiotherapy during initial therapy, most experience resistance to subsequent therapies and shortened survival5. Recently, in several retrospective surveys, surgical resections have been regarded as multimodality therapies that improve limited disease SCLC prognoses6–8. Wakeam et al. demonstrated that surgical resection was associated with improved survival prognoses for patients with early stage SCLC9. The application of TNM stage in limited stage SCLC promotes the selection of patients who underwent pulmonary lobectomy, which could be a major factor contributing to the increase in the number of patients receiving surgical intervention10. It remains unclear whether surgical intervention for patients with early stage disease is the major therapeutic approach.
Small-dose spiral CT scanning is recommended for detecting early stage lung carcinomas, and the therapeutic strategy for treating early stage SCLC, including diseases presenting no lymph node metastasis (N0) or only the hilar lymph node metastasis that do not traffic to mediastinal lymph nodes (N1), should be further elucidated to improve outcomes of patients with early stage SCLC11. Conventionally, it was believed that surgical therapy provides no therapeutic benefit for SCLC, which can be attributed to the incomplete tumor stage assignments. The subtypes of SCLC, defined as “limited disease (LD-SCLC)” and “extensive disease (ED-SCLC),” do not distinguish mediastinal lymph node-negative from LD-SCLC patients with N2 disease, even those who present supraclavicular region and contralateral mediastinal lymph node metastases12. Surgical resection plays a key role in removing limited early lesions. Conventional chemotherapy combined with surgical intervention for patients with N0 and N1 SCLC could provide greater benefits than resection alone or conventional chemoradiotherapy because this approach takes into consideration the sensitivity to chemotherapy and elimination of tumor load.
In this study, we reviewed data from patients with early stage SCLC, which we divided into resection and resection combined with chemotherapy cohorts based on retrospective analyses. Subsequently, we analyzed the data and compared the survival prognosis between the two groups.
METHODS AND MATERIALS
Patient and Data Collection
The retrospective study included 110 patients with early stage and postoperative histologically proven SCLC, who underwent surgical intervention in our hospital. The patients presented without mediastinal lymph node metastasis, by mediastinoscopy and chest CT scanning. Patients who refused chemotherapy because of preference or the intolerance of side effects were enrolled in the surgical intervention alone group. No patients received thoracic irradiation before and after surgical intervention. Clinical and pathological tumor stages were defined according to the TNM staging approach (seventh edition). We reviewed the medical records of all patients who underwent, or did not undergo, adjuvant chemotherapy after pulmonary lobectomy. The proposed follow-up period lasted 5 years after treatment. We excluded patients with contralateral pulmonary disease, separate nodules in different lobes, and those diagnosed with T3NxM0 disease. Information about (i) demographic features of patients: age, sex, and ECOG PS score before performing chemotherapy, presurgery TNM stage, and postsurgery histological pathological diagnosis; (ii) chemotherapeutic regimen, including agents used, dosage, and cycle of treatment; (iii) duration of relapse-free survival (RFS), and overall survival (OS); and (iv) combination of SCLC with other pathological types were collected. All the patients gave informed written consent for this analysis, based on their medical records. This study was approved by the ethics committee of Ningbo No. 2 Hospital.
Chemotherapy Regimen
The chemotherapy regimen was either cisplatin plus etoposide (EP) or carboplatin and etoposide (CE) with/without paclitaxel. The doses of agents were as follows: cisplatin (80 mg/m2, day 1) and etoposide (80 mg/m2, days 1–3), which were repeated once every 3 weeks; carboplatin (400 mg/m2, day 1) and etoposide (80 mg/m2, days 1–3) plus paclitaxel (180 mg/m2, day 2) or not in a repetition cycle every 3 weeks. Each treatment method lasts for four or six cycles in total. Patients who did not complete all chemotherapy cycles were excluded. Adverse events (AEs) and side effects were graded based on the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical Analysis
We assessed OS and progress-free survival (PFS) through Kaplan–Meier analysis and determined survival with 95% confidence intervals (CIs). Log-rank analyses were applied to determine survival variances among groups, and we used Cox proportional hazards regression models to assess the hazard ratios (HRs) for overall survival. Covariates included in the model included age, type of surgical procedure, TNM stage (N and T stage), prophylactic cranial irradiation (PCI), and postoperative adjuvant chemotherapy. All statistical analyses were carried out using SPSS 23.0 software.
RESULTS
Patient Traits
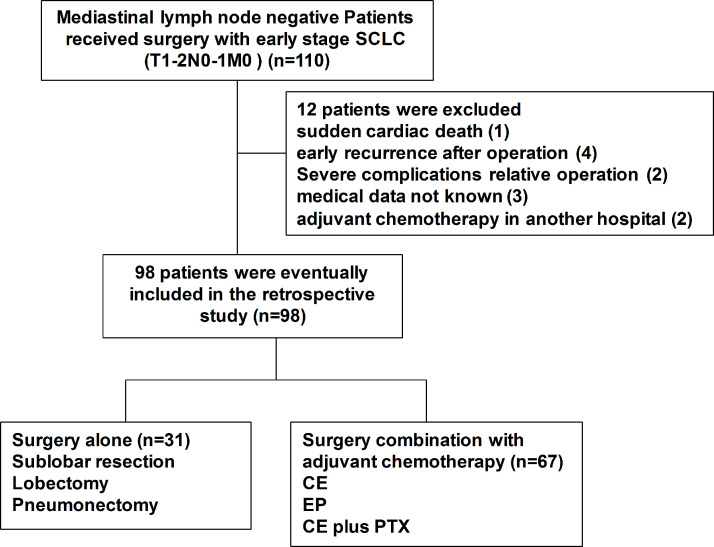
There were 110 patients with SCLC who were recruited from the medical record system of our hospital, and basic patient features are recorded in Table 1. All patients underwent an initial treatment of surgery without chemotherapy or radiotherapy before operation, and 98 patients who met the inclusion criteria were included in this retrospective study. Twelve patients were excluded because of reasons such as sudden cardiac death (1), early recurrence within 3 months after operation (4), severe complications related to therapy (3), medical data not known (3), and adjuvant chemotherapy in another hospital (2) (Fig. 1).
Table 1.
Clinical Characteristics of 98 Mediastinal Lymph Node-Negative Patients With Small Cell Lung Cancer (SCLC)
| Clinical Data | Surgery and Chemotherapy | Surgery Alone | p Value |
|---|---|---|---|
| Age | 0.129 | ||
| >70 | 25 (25.51%) | 15 (15.31%) | |
| <70 | 42 (42.86%) | 16 (16.32%) | |
| Sex | 0.081 | ||
| Male | 52 (53.06%) | 24 (24.49%) | |
| Female | 15 (15.31%) | 7 (7.14%) | |
| ECOG PS score | 0.053 | ||
| 0 | 43 (43.87%) | 16 (16.33%) | |
| 1 | 21 (21.43%) | 11 (11.22%) | |
| 2 | 3 (3.00%) | 4 (4.10%) | |
| T stage | 0.132 | ||
| T1 | 32 (32.66%) | 11 (11.22%) | |
| T2 | 35 (35.71%) | 20 (20.41%) | |
| N stage | 0.113 | ||
| N0 | 27 (27.55%) | 15 (15.31%) | |
| N1 | 35 (35.71%) | 21 (21.43%) | |
| Pathological histology | 0.431 | ||
| Small cell carcinoma | 38 (38.77%) | 19 (19.39%) | |
| Combined small cell carcinoma | 29 (29.59%) | 12 (12.25%) | |
| Prophylactic cranial irradiation | 0.065 | ||
| Yes | 18 (18.37%) | 9 (9.18%) | |
| No | 49 (50.00%) | 22 (21.45%) |
Figure 1.
Study design (clinical T1-2N0-1M0 patients).
Treatment Scheme of the Groups
Among the 98 patients who were enrolled in this analysis, 41 received lobectomy, 8 sublobar resection, and 49 pneumonectomy. With respect to chemotherapy-defined groups, 67 patients who initially received complete adjuvant chemotherapy (corresponding to four chemotherapeutic cycles and at least 80% relative dose intensity) were evaluated in our study, of whom 33 received EP, 28 received CE, and 6 received CE combined with paclitaxel (Table 2). Patients who were treated with EP had a range of ages from 61 to 75 years, with a median age of 67.3 years, whereas for those treated with CE and CE paclitaxel, the median was 69.8 (range of 62–77) years. A majority of elderly patients over 70 years of age received CE (21, 84.0%). Furthermore, 27 patients received PCI in stable status after operation or chemotherapy. Eighteen of the 67 patients subjected to surgery plus chemotherapy and 9 of the 31 without chemotherapy after surgery received PCI (Table 2).
Table 2.
Therapeutic Scheme of 98 Mediastinal Lymph Node-Negative Patients With Small Cell Lung Cancer (SCLC)
| Clinical Data | All | TNM I | TNM II | p Value |
|---|---|---|---|---|
| Chemotherapy | 0.071 | |||
| EP | 33 (33.67%) | 12 (12.25%) | 21 (21.43%) | |
| CE | 28 (28.58%) | 10 (10.20%) | 18 (18.37%) | |
| CE plus PTX | 6 (6.12%) | 2 (2.02%) | 4 (4.1%) | |
| No chemotherapy | 31 (31.63%) | 10 (10.20%) | 21 (21.43%) | |
| Prophylactic cranial irradiation | 0.070 | |||
| Yes | 27 (27.55%) | 11 (11.22%) | 16 (16.33%) | |
| No | 71 (72.45%) | 23 (23.47%) | 48 (48.98%) | |
| Surgery procedure | 0.062 | |||
| Sublobar resection | 8 (8.16%) | 8 (8.16%) | 0 (0%) | |
| Lobectomy | 41 (41.84%) | 13 (13.27%) | 28 (28.57%) | |
| Pneumonectomy | 49 (50.00%) | 13 (13.27%) | 36 (36.73%) |
Chemotherapy-Related Adverse Events (AEs)
Chemotherapy-related AEs are described in Table 3. AEs were observed in 43 (64%) patients who received complete chemotherapeutic cycles: neutropenia in 20 subjects, thrombocytopenia in 10, anemia in 15, fatigue in 10, nausea in 18, renal failure in 6, and hepatic dysfunction in 4. Chemotherapy treatments were forced to stop because of severe side effects in 12 patients, who were then categorized in the surgery-only group. Severe side effects included cerebral brain hemorrhage (3), gastrointestinal bleeding (2), severe marrow depression (5), and acute hepatic failure (2). Two patients who were over 70 years of age died from chemotherapy-related AEs due to fatal brain hemorrhage and acute hepatic failure, respectively. The two patients were excluded in the present retrospective study.
Table 3.
Chemotherapy-Related Toxicity by CTCAE Version 4.0
| Toxicity | Grade | 3/4 (%) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Anemia | 7 | 2 | 3 | 3 | 40.0 |
| Neutropenia | 3 | 7 | 8 | 2 | 50.0 |
| Thrombocytopenia | 5 | 3 | 0 | 2 | 20.0 |
| Nausea | 10 | 4 | 2 | 2 | 22.2 |
| Fatigue | 7 | 2 | 1 | 0 | 10.0 |
| Hepatic dysfunction | 2 | 2 | 0 | 0 | 0 |
| Renal failure | 4 | 1 | 0 | 1 | 16.7 |
Survival Assessment
For patients with SCLC who were mediastinal lymph node metastasis-negative, Kaplan–Meier overall survival analyses are shown in Figure 2A–C. Surgery combined with adjuvant chemotherapy was associated with longer survival for all cohorts [median OS: 42.14 vs. 33.53 months, p = 0.01], although the difference was not statistically significant for patients diagnosed with N0 and stage I disease (median OS: 38.74 vs. 32.35 months, p = 0.211). Adjuvant chemotherapy was associated with improvements in survival for patients with N2 and stage II disease (median OS: 36.42 vs. 26.68 months, p = 0.021). Additionally, a significantly longer PFS was observed for patients who were node-negative and any N stage (median PFS: 25.20 vs. 13.48 months, p = 0.000), N0 (median PFS: 28.05 vs. 15.21 months, p = 0.001), and in N1 (median PFS: 19.02 vs.13.25 months, p = 0.031) (Fig. 3A–C).
Figure 2.
Overall survival (OS) curves for early stage small cell lung carcinoma (SCLC) patients. (A) OS of all patients, stratified by surgery alone versus surgery in combination with adjuvant chemotherapy (median OS: 42.14 vs. 33.53 months, p = 0.01). (B) OS of cT1–2N0M0 SCLC patients, stratified by surgery alone versus surgery in combination with adjuvant chemotherapy (median OS: 38.74 vs. 32.35 months, p = 0.211). (C) OS of cT1–2N1M0 SCLC patients, stratified by surgery alone versus surgery combination with adjuvant chemotherapy (median OS: 36.42 vs. 26.68 months, p = 0.021).
Figure 3.
Progress-free survival (PFS) curves for early stage SCLC patients. (A) PFS of all patients, stratified by surgery alone versus surgery combination with adjuvant chemotherapy (median PFS: 25.20 vs. 13.48 months, p = 0.000). (B) PFS of cT1–2N0M0 SCLC patients, stratified by surgery alone versus surgery in combination with adjuvant chemotherapy (median PFS: 28.05 vs. 15.21 months, p = 0.001). (C) PFS of cT1–2N1M0 SCLC patients, stratified by surgery alone versus surgery combination with adjuvant chemotherapy (median PFS: 19.02 vs. 13.25 months, p = 0.031).
Table 4 displays the results of Cox multivariate regression analysis, which we used to evaluate the survival rate of all patients with SCLC. Multivariate analyses demonstrated that among all factors analyzed, only age, TNM stage (N), PCI, and chemotherapy correlated with improved survival. N stage was an independent indicator related to OS (OR = 3.079; 95% CI = 1.721, 5.508; p < 0.001), and age (OR = 1.628; 95% CI = 1.005, 5.508; p = 0.048), PCI (OR = 0.468; 95% CI = 0.270, 0.813; p = 0.007), and adjuvant chemotherapy after surgery (OR = 0.570; 95% CI = 0.356, 0.912; p = 0.019) correlated with higher overall survival.
Table 4.
Independent Predictors of Overall Survival After Cox Proportional Hazards Regression Model for all Patients With Early Stage SCLC
| Variable | Hazard Ratio | 95% CI | p Value |
|---|---|---|---|
| Age | 1.628 | 1.005, 2.639 | 0.048 |
| N stage | 3.079 | 1.721, 5.508 | 0.000 |
| T stage | 0.913 | 0.570, 1.462 | 0.704 |
| Chemotherapy | 0.570 | 0.356, 0.912 | 0.019 |
| PCI | 0.468 | 0.270, 0.813 | 0.007 |
| Surgery procedure | |||
| Sublobar resection | Reference | ||
| Lobectomy | 1.283 | 0.580, 2.835 | 0.539 |
| Pneumonectomy | 1.938 | 0.847, 4.644 | 0.115 |
DISCUSSION
As the dominant reason for cancer deaths worldwide, lung cancer causes a considerably larger number of mortalities than that by breast, prostate, pancreatic, and colon cancers together1,2. SCLC encompasses around 13–20% of various types of lung cancers, making it the fifth primary cause of cancer-related deaths13. The initial SCLC staging system was established by the Veterans Administration Lung Cancer Study Group and distinguishes patients based on “limited stage” (LS) disorders within the hemithorax, as well as “extensive stage” (ES) disorders that disseminated from the hemithorax site14. Under this staging system, screening patients who may benefit from surgery became very difficult, due to the lack of consideration for local lymph node metastases. On the other hand, the conventional concept accepts that patients with SCLC of LS classification ought to be treated with chemotherapy followed by radiation therapy. Modern TNM stage systems are not viewed as practical for SCLC, due to the shortage of surgical confirmations15, although the TNM stage system could be used for predictions of SCLC prognosis. The IASLC reviewed 12,620 SCLC cases during the revision of the TNM stage system, among which merely 349 cases were associated with surgery (2.8%), and 56% of these cases displayed a 5-year survival rate in stage I, 38%–40% in stage II, and below 12% in stage III16.
The therapeutic strategy for treating early stage SCLC has advanced over recent decades because of reevaluations of the role of surgery in patients with T1-2N0-1M0 SCLC. There has been a gradual shift toward thoracic radiotherapy, based on the results of a comparative investigation of surgery and radiotherapy, which found higher survival rates for combined therapy compared with surgery alone17. A recent population study analysis of data from the American Surveillance, Epidemiology, and End Results (SEER) collected and reviewed information regarding cancer stages of patients with SCLC who were under treatment18. OS was measured for patients categorized by surgery type and the status of either having had irradiation or being irradiation free. Patients who underwent surgery achieved an ∼40% overall 5-year survival, which was greater than those without surgery: median, 36 versus 18 months (stage I) and 25 vs. 14 months (stage II). This study reevaluated the role of surgical intervention in improving overall survival and helped surgery regain attention as a multidisciplinary therapy for patients with early stage SCLC. The guidelines of the European Society for Medical Oncology (EMSO) suggest that 5% of patients with “very limited” SCLC (T1, 2 N0, 1 M0) have more favorable results, with 5-year OS of approximately 50%, and surgical intervention is applicable for such patients after excluding lymph node metastasis13. Current instructions from the National Comprehensive Cancer Network (NCCN) are not entirely identical, and only patients with stage I (T1-T2 N0) are generally advised to undergo surgery2.
Austin et al. performed a multicenter research study of 48,037 individuals who were at risk for lung cancer and underwent voluntary CT scans19. In comparison with the normal distribution of cancer stages published by IASLC in 200716,20, patients who underwent CT screenings had a considerably greater percentage of stage I lung cancer. This raises a novel question: What is the optimal treatment strategy for early stage SCLC? Meanwhile, the incidence of AEs is higher than anticipated for the elderly population of our retrospective study, and the high rate of chemotherapy-related AEs in elderly patients at our institution suggests that we should reconsider the value of surgical intervention alone toward survival improvements, especially in patients with complications and other systematic diseases. In our study, we found that age correlates with intolerability and therapy-related side effects and AEs. In our current study, surgery, as an initial treatment, was carried out in 98 patients with SCLC, among whom adjuvant chemotherapy was aborted for various reasons in 31 cases. This suggests that patients who underwent surgery and chemotherapy in our study were not chosen randomly. Twenty-seven subjects who underwent surgery only were inclined to have poor OS, compared with those who received adjuvant chemotherapy, among all patients with SCLC. However, we did not find differences between groups who received adjuvant chemotherapy or those who did not among patients with N0 stage disease. Our findings demonstrate that in our institution, surgery is an alternative strategy for elderly patients with N0 SCLC without lymph node involvement, especially for those with complications and other systemic diseases.
For patients with SCLC, chemotherapy is a crucial element of optimized treatment schemes, and EP is the most regular standard chemotherapy regimen. Carboplatin is often used instead of cisplatin to help suppress vomit, nephrosis, and neuropathy. Nevertheless, carboplatin also has myelosuppression side effects18, and adjuvant chemotherapy is recommended for patients who undergo resection. However, there have been no prospective random control trials of adjuvant chemotherapy combined with surgery for early stage patients nor of the optimal regimen for early stage and elderly patients with SCLC. Hence, the optimal treatment regimen remains controversial with respect to therapeutic strategy selections and chemotherapeutic regimens.
Based on early trials of patients with ED-SCLC, EP, IP (irinotecan plus cis-platinum), and IP combined with PTX (paclitaxel) were chosen in adjuvant chemotherapy schemes6,22. In our current evaluation, the therapy selection scheme depended on the doctor’s experience of previous cases. CE was widely applied in cases where patients presented severe SCLC and were older and more vulnerable23. However, from our research, CE also displays intolerant toxicity for some elderly patients, which presents as severe marrow repression and hemorrhages due to thrombocytopenia. For patients enrolled in the retrospective study, those who received PCI displayed longer OS than those without PCI therapy. PCI has proven effective as supplementary therapy in reducing the incidence of cerebral metastases in the stable period after chemotherapy for patients who respond24,25. A meta-analysis of randomized PCI trials showed a 25% decrease in 3-year incidence of cerebral metastasis, from 58.6% in the control group to 33.3% in the PCI group26. Therefore, PCI should be considered as a key part of multidisciplinary therapy for patients with SCLC in N0 and N1 stages. However, according to the multivariate analysis, we found that the type of surgical procedure and the N stage have no influence on clinical outcomes. The selection of surgical intervention could be based on the TNM staging rather than actual anatomical tumor sites because the patients in stage I display no statistical differences in OS, and in this stage, surgical procedures do not affect survival. These observations could guide the therapeutic strategies for patients with stage I SCLC who have the opportunity to receive surgery.
In conclusion, we found that surgical intervention with sequential adjuvant chemotherapy is the fundamental therapy for patients with mediastinal lymph node-negative SCLC. For patients with N0 disease and those who are elderly, the selection and regimen of chemotherapy might be reconsidered in planning a treatment scheme because of poor tolerability at elderly ages and early stages. In the future, more prospective clinical trials should be carried out to help guide therapeutic strategies for the treatment of early stage SCLC. Meanwhile, safer chemotherapy regimens and novel drugs should be given more attention as therapeutics for older patients with lung cancer.
ACKNOWLEDGMENT
This study was funded by Zhejiang Medical Association study on the detection of lung carcinomas using circulating plasma DNA integrity and multiple genes methylation combined with serum tumor markers (2017ZYC-B5).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Hong QY, Wu GM, Qian GS, Hu CP, Zhou JY, Chen LA, Li WM, Li SY, Wang K, Wang Q, Zhang XJ, Li J, Gong X, Bai CX, Lung Cancer Group of Chinese Thoracic Society, Chinese Alliance Against Lung Cancer. Prevention and management of lung cancer in China. Cancer 2015;121(Suppl 17):3080–8. [DOI] [PubMed] [Google Scholar]
- 2. Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ, Gandhi L, Ganti AK, Govindan R, Grecula JC, Hayman J, Heist RS, Horn L, Jahan T, Koczywas M, Loo BW Jr, Merritt RE, Moran CA, Niell HB, O’Malley J, Patel JD, Ready N, Rudin CM, Williams CC Jr, Gregory K, Hughes M, National Comprehensive Cancer Network. Small cell lung cancer. J Natl Compr Canc Netw. 2013;11(1):78–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hou SZ, Cheng ZM, Wu YB, Sun Y, Liu B, Yuan MX, Wang XD. Evaluation of short-term and long-term efficacy of surgical and non-surgical treatment in patients with early-stage small cell lung cancer: A comparative study. Cancer Biomark. 2017;19(3):249–56. [DOI] [PubMed] [Google Scholar]
- 4. Jones CD, Cummings IG, Shipolini AR, McCormack DJ. Does surgery improve prognosis in patients with small-cell lung carcinoma? Interact Cardiovasc Thorac Surg. 2013;16(3):375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim ES. Chemotherapy resistance in lung cancer. Adv Exp Med Biol. 2016;893:189–209. [DOI] [PubMed] [Google Scholar]
- 6. Reck M, Jagos U, Grunwald F, Kaukel E, Koschel G, von Pawel J, Hessler S, Gatzemeier U. Long-term survival in SCLC after treatment with paclitaxel, carboplatin and etoposide—A phase II study. Lung Cancer 2003;39(1):63–9. [DOI] [PubMed] [Google Scholar]
- 7. Yang CJ, Chan DY, Shah SA, Yerokun BA, Wang XF, D’Amico TA, Berry MF, Harpole DH Jr. Long-term survival after surgery compared with concurrent chemoradiation for node-negative small cell lung cancer. Ann Surg. 2017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8. Yang CJ, Chan DY, Speicher PJ, Gulack BC, Tong BC, Hartwig MG, Kelsey CR, D’Amico TA, Berry MF, Harpole DH. Surgery versus optimal medical management for N1 small cell lung cancer. Ann Thorac Surg. 2017;103(6):1767–72. [DOI] [PubMed] [Google Scholar]
- 9. Wakeam E, Acuna SA, Leighl NB, Giuliani ME, Finlayson SRG, Varghese TK, Darling GE. Surgery versus chemotherapy and radiotherapy for early and locally advanced small cell lung cancer: A propensity-matched analysis of survival. Lung Cancer 2017;109:78–88. [DOI] [PubMed] [Google Scholar]
- 10. de Hoyos A, DeCamp MM. Surgery for small cell lung cancer. Thorac Surg Clin. 2014;24(4):399–409. [DOI] [PubMed] [Google Scholar]
- 11. Saghir Z, Dirksen A, Ashraf H, Bach KS, Brodersen J, Clementsen PF, Dossing M, Hansen H, Kofoed KF, Larsen KR, Mortensen J, Rasmussen JF, Seersholm N, Skov BG, Thorsen H, Tonnesen P, Pedersen JH. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: Status after five annual screening rounds with low-dose CT. Thorax 2012;67(4):296–301. [DOI] [PubMed] [Google Scholar]
- 12. Yokouchi H, Ishida T, Yamazaki S, Kikuchi H, Oizumi S, Uramoto H, Tanaka F, Harada M, Akie K, Sugaya F, Fujita Y, Fukuhara T, Takamura K, Kojima T, Harada T, Higuchi M, Matsuura Y, Honjo O, Minami Y, Watanabe N, Nishihara H, Suzuki H, Dosaka-Akita H, Isobe H, Nishimura M, Munakata M. Prognostic impact of clinical variables on surgically resected small-cell lung cancer: Results of a retrospective multicenter analysis (FIGHT002A and HOT1301A). Lung Cancer 2015;90(3):548–53. [DOI] [PubMed] [Google Scholar]
- 13. Fruh M, De Ruysscher D, Popat S, Crino L, Peters S, Felip E, Group EGW. Small-cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi99–105. [DOI] [PubMed] [Google Scholar]
- 14. Stinchcombe TE, Gore EM. Limited-stage small cell lung cancer: Current chemoradiotherapy treatment paradigms. Oncologist 2010;15(2):187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi HS, Jeong BK, Jeong H, Lee YH, Ha IB, Song JH, Kang KM. Application of the new 8th TNM staging system for non-small cell lung cancer: Treated with curative concurrent chemoradiotherapy. Radiat Oncol. 2017;12(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z, Goldstraw P, International Association for the Study of Lung Cancer International Staging C, Participating I. The International Association for the Study of Lung Cancer lung cancer staging project: Proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2(12):1067–77. [DOI] [PubMed] [Google Scholar]
- 17. Zhang A, Li J, Wang W, Wang Y, Mu D, Chen Z, Shao Q, Li F. A comparison study between gross tumor volumes defined by preoperative magnetic resonance imaging, postoperative specimens, and tumor bed for radiotherapy after breast-conserving surgery. Medicine 2017;96(2):e5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu JB, Decker RH, Detterbeck FC, Wilson LD. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol. 2010;5(2):215–9. [DOI] [PubMed] [Google Scholar]
- 19. Austin JH, Yip R, D’Souza BM, Yankelevitz DF, Henschke CI, International Early Lung Cancer Action Program Investigators. Small-cell carcinoma of the lung detected by CT screening: Stage distribution and curability. Lung Cancer 2012;76(3):339–43. [DOI] [PubMed] [Google Scholar]
- 20. Vallieres E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z, Goldstraw P, International Association for the Study of Lung Cancer International Staging C, Participating I. The IASLC Lung Cancer Staging Project: Proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4(9):1049–59. [DOI] [PubMed] [Google Scholar]
- 21. Karim SM, Zekri J. Chemotherapy for small cell lung cancer: A comprehensive review. Oncol Rev. 2012;6(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kakolyris S, Mavroudis D, Tsavaris N, Souglakos J, Tsiafaki P, Kalbakis K, Agelaki S, Androulakis N, Georgoulias V. Paclitaxel in combination with carboplatin as salvage treatment in refractory small-cell lung cancer (SCLC): A multicenter phase II study. Ann Oncol. 2001;12(2):193–7. [DOI] [PubMed] [Google Scholar]
- 23. Matsui K, Masuda N, Yana T, Takada Y, Kobayashi M, Nitta T, Hirashima T, Fukuoka M. Carboplatin calculated with Chatelut’s formula plus etoposide for elderly patients with small-cell lung cancer. Intern Med. 2001;40(7):603–6. [DOI] [PubMed] [Google Scholar]
- 24. Eaton BR, Kim S, Marcus DM, Prabhu R, Chen Z, Ramalingam SS, Curran WJ Jr, Higgins KA. Effect of prophylactic cranial irradiation on survival in elderly patients with limited-stage small cell lung cancer. Cancer 2013;119(21):3753–60. [DOI] [PubMed] [Google Scholar]
- 25. Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, Werner-Wasik M, Videtic GM, Garces YI, Choy H. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pechoux CL, Sun A, Slotman BJ, De Ruysscher D, Belderbos J, Gore EM. Prophylactic cranial irradiation for patients with lung cancer. Lancet Oncol. 2016;17(7):e277–93. [DOI] [PubMed] [Google Scholar]