Abstract
Background
Tigilanol tiglate (TT) is a novel small molecule for intratumoral treatment of nonmetastatic mast cell tumors (MCTs) in dogs. In a randomized controlled clinical study, 75% of dogs that received a single TT treatment achieved complete resolution of the MCT by 28 days, with no recurrence in 93% of dogs at 84 days. Critical to TT's efficacy was the area of the wound (tissue deficit) after slough of the necrotic tumor relative to pretreatment tumor volume.
Objectives
To analyze data collected during the previous study to (a) describe wounds after slough of treated MCTs and (b) identify determinants of wound area and speed of wound healing.
Methods
Wound presence, condition, and area were determined from clinical records of 117 dogs over 84 days after a single intratumoral TT treatment.
Results
Tumor slough occurred 3 to 14 days after treatment, exposing granulation tissue in the wound bed. Wound area after tumor slough in general was related to pretreatment tumor volume, with maximal recorded wound area fully evident in 89% of dogs by day 7. In dogs achieving complete tumor resolution, all wounds were left to heal by secondary intention. Bandaging and other wound management interventions only were required in 5 dogs. Time to healing (ie, full re‐epithelialization of treatment site) depended on wound area and location on the body, with most wounds being fully healed between 28 and 42 days after treatment.
Conclusions
Wound area and healing after slough of TT‐treated tumors follow a consistent clinical pattern for most dogs.
Keywords: diterpene ester, dog, EBC‐46, intratumoral, MCT, wound healing
Abbreviations
- CI
confidence interval
- CR
complete response
- FNA
fine needle aspirate
- LN
lymph node
- MCT
mast cell tumor
- RECIST
response evaluation criteria in solid tumors
- TT
tigilanol tiglate (also known as EBC‐46)
- Tvol
- VCOG
Veterinary Cooperative Oncology Group
- WTT wound area
wound surface area formed by tigilanol tiglate treatment (cm2) calculated by ellipse formula )
1. INTRODUCTION
Tigilanol tiglate (TT) is a novel small molecule recently approved as a veterinary pharmaceutical for intratumoral treatment of nonmetastatic mast cell tumors (MCTs) in Europe, United Kingdom, and United States. The drug is also in broader clinical development for a range of other cutaneous and subcutaneous cancers in humans, 1 , 2 companion animals, and horses. 3 A previous report 4 described a randomized, controlled, and blinded clinical study involving 123 dogs conducted at 11 veterinary clinics in the United States to evaluate the safety and efficacy to 84 days of intratumorally administered TT in the treatment of nonmetastatic MCTs in dogs. In that study, 75% of dogs that received a single intratumoral injection of TT achieved complete response (CR, ie, complete resolution of the target tumor) by 28 days using the modified Veterinary Comparative Oncology Group (VCOG) response evaluation criteria in solid tumors (RECIST 1.1) 5 compared to only 5% of dogs in the control treatment group. No local recurrence was seen in 93% of TT‐treated CR dogs after a further 8 weeks. The study also identified that (a) the formation of a wound at the treatment site and (b) the size of the wound relative to the volume of the target tumor were strongly associated with complete tumor resolution and are thus critical indicators of the drug's efficacy. This relationship between wound formation, and especially wound size relative to target tumor volume, and the efficacy of TT is an expected result of an intratumorally administered treatment where the drug's mode of action causes very rapid hemorrhagic necrosis of the target tumor, followed by slough of the necrotic tumor mass to expose an underlying wound bed of healthy granulation tissue up to 14 days after treatment. 4 , 6
Here we present further results from that clinical study. We describe and analyze in more detail the wounds that developed at the site of the tumor treated with TT and assess potential determinants of (a) the area of the wound present after tumor slough and (b) subsequent rates of wound healing.
2. MATERIALS AND METHODS
2.1. Study design
This article reports the second series of analyses from a randomized, controlled, and blinded clinical study to evaluate the efficacy and safety of TT as a local treatment for nonmetastatic MCTs in dogs. The study was conducted at 11 veterinary clinics in the United States between November 2016 and March 2018. Details of the objectives, design, protocols, and efficacy and safety results for the study have been described previously. 4 In brief, the primary purpose of the study was to compare a group of dogs that received a single dose of TT injected into a target tumor with a control (sham treatment) group. The study population consisted of 123 dogs with MCTs between 0.1 and 10 cm3 in volume with only a single tumor selected for treatment on each dog. Dogs with cutaneous MCTs located anywhere on the body were eligible for enrollment whereas dogs with SC MCTs were eligible if the MCT was present at or distal to the elbow or hock. Mast cell tumor disease was confirmed by cytological grading of fine needle aspirates (FNAs) that were taken from the target tumors using the Scarpa method 7 , 8 and assessed independently by pathologists at IDEXX laboratories. Cytological grading was chosen over histological grading to minimize potential leakage of intratumoral TT through the tissue sampling site. 4 Fine needle aspirates also were taken at time of patient screening from any enlarged locoregional lymph nodes (LNs) to assess for the presence of metastatic MCT disease. Dogs with metastatic disease in LNs confirmed by FNA were ineligible for enrollment and were excluded from the study. For dogs with enlarged LNs where FNA did not confirm the presence of metastatic disease at screening, palpable changes in size of LNs were noted by the investigators at each assessment point during the study, but there was no protocol‐mandated requirement for subsequent postscreening assessment of LNs by FNA. Volume of the target tumor was calculated from measurements of tumor length, width, and depth using a modified ellipsoid formula (tumor volume [T vol] in cm3 = ]). 9 , 10 At the time of treatment, dogs were assigned to either the TT or control group using random allocation. Treatment was administered by an unblinded veterinarian, and all subsequent evaluation of patients and their responses was performed by ≥2 veterinarians or veterinary clinical staff blinded to treatment. Owners also were blinded to the treatment their dogs had received. Eighty‐one dogs were allocated to receive TT during this first phase of the study, whereas 42 dogs were allocated to the control group. For the 81 TT treatment group dogs, 1 mg/mL of the drug (in acetate buffered 40% propylene glycol; QBiotics Group, Yungaburra, Australia) was injected using a “fanning” technique into the target tumor to distribute the drug as evenly as possible throughout the tumor mass. Dosing was based on the volume of the target tumor at a rate of 0.5 mg (0.5 mL) TT per cm3 tumor volume. All enrolled dogs in both groups received concomitant medications (corticosteroids, H1 and H2 antagonists) 4 to decrease the potential for degranulation of the MCT. In the second phase of the study, dogs in both groups that did not achieve complete resolution of their target tumors by 28 days after first assessment were eligible to receive either a first treatment (control group “cross‐over”) or a second treatment (the original TT group) 2 days later. In the second phase, all dogs treated with TT were managed using the same protocol as those dogs treated with TT in the first phase, including concomitant medications. 4 Other than excluding administration of any other cancer treatment, no restrictions were imposed in the study protocol on patients undergoing other veterinary interventions or on any normal daily activities (including bathing) immediately after treatment or during the wound healing period.
After treatment, dogs were assessed at 2, 4, 8, and 24 hours and at 4, 7, 14, 28, 42, and 84 days (±2 days). Treatment response was evaluated by physical examination, measurement and assessment of the size and condition of the target tumor and of any wound that subsequently formed, and laboratory analyses of blood and urine samples. Digital photographs also were taken of the treatment site at each of these assessment times. Efficacy was determined using the modified VCOG RECIST criteria 5 , with CR equating to complete resolution of the target tumor. Safety and tolerability were assessed through reporting of adverse events by the investigators using VCOG categories 11 and by owners completing a health‐related quality of life survey. 12
2.2. Assessment of wound formation, wound area, and healing
At each assessment time, investigators recorded whether a wound had formed at the treatment site, whether the tumor had fully sloughed, and the condition and area of the exposed wound bed. Wound area was determined by measuring the length of longest wound axis (length) and width at the widest point (width). During these assessments, investigators also recorded whether the wound had healed, with healing defined as complete re‐epithelialization of the wound site. The area of each wound at each assessment time was estimated using the ellipse formula (W TT wound area = ).
Data on formation and area of wounds were available for 117 dogs (81 dogs in phase 1, 36 dogs in phase 2) in the study that had received a single intratumoral TT treatment.
Four potential determinants of maximal wound area (largest observed wound area) after tumor slough were assessed: (a) tumor location on the body, (b) volume of the target tumor at treatment, (c) tumor cytological grade, and (d) locoregional LN enlargement (without confirmed MCT metastasis on FNA) at time of pretreatment screening. In addition, a separate analysis was performed using only the subset of tumors on lower limbs to determine whether any difference existed in wound area between cutaneous and SC tumors on lower limbs.
Progression of wound healing, including potential determinants of the time for healing, was assessed for the 77 dogs that (a) formed wounds, (b) were assessed at all assessment times until day 84 (this group included 8 dogs that had enlarged locoregional LNs at pretreatment screening), and (c) had achieved complete resolution of the treated tumor by day 84 after a single TT treatment. Two potential determinants of the rates of wound healing after treatment in these dogs were evaluated: (a) maximal wound area and (b) tumor (and hence wound) location on the body.
2.3. Statistical analyses
Statistical analyses were performed using Stata (version 15, StataCorp, College Station, Texas). Exact binomial 95% confidence intervals (CIs) for proportions were calculated using Stata's ‐cii‐ command.
Multivariable analyses of determinants of wound area were performed using generalized linear regression models fitted using Stata's ‐glm‐ command with the Gaussian error distribution and log link. Estimated ratios of arithmetic means of wound areas were calculated by exponentiating the coefficients (ie, by raising Euler's number to the power of the coefficient). The best‐fitting shape for the relationship between tumor volume and maximal wound area was assessed by fractional polynomial regression using Stata's ‐fp‐ command; tumor volume−2 (ie, 1/[tumor volume2]) was used for all analyses. Two‐way interactions were not assessed because there was no prior evidence for those hypotheses.
Multivariable analyses were performed to evaluate potential determinants of rates of wound healing, described by the binary variables, wound healed or not healed by each of day 28 and day 42. Logistic regression models were used, fitted with Stata's ‐logistic‐ command. Maximal wound area was fitted on the linear scale based on analyses using fractional polynomial regression. To assess whether any effect of tumor location was partly mediated by wound area, effects of tumor location were assessed, adjusted, and not adjusted for maximal wound area fitted on the linear scale, based on analyses using fractional polynomial regression. The 2‐way interaction was not assessed because there was no prior evidence for this hypothesis.
3. RESULTS
3.1. Wound formation and wound area
Slough of necrotic tumor mass after TT treatment to expose a granulating underlying wound bed occurred in 95% (111) of the 117 dogs that had received a single intratumoral treatment of TT. In 6 dogs, no tumor slough occurred and no wound formed. In all 6 of these dogs, the target MCTs showed no signs of hemorrhagic necrosis associated with the action of TT in tumor destruction and all of these tumors were still present 28 days after treatment.
In the 111 dogs in which slough of the treated tumor did occur, this slough was first observed in some dogs as early as 3 days after TT injection, with wounds in most dogs (89%; 99/111) fully evident and reaching their maximal recorded area by day 7. For the remaining 12 dogs, the necrotic tumor mass had not completely sloughed by day 7 and in these cases the maximal documented wound area was only fully evident at the day 14 assessment. The median value for the maximal areas of wounds was 3.5 cm2 (25th and 75th percentiles of maximal wound area were 1.5 cm2 and 9.1 cm2, respectively), with 76% (84) of wounds being <10 cm2 in area. A further 19% of dogs had wounds between 10 and 40 cm2 (21), whereas 5% (6) developed large wounds >40 cm2, including 2 substantial outliers in which maximal wound area exceeded 100 cm2. Both of the 2 outlier patients with the largest wounds were later found to have clinical relevant comorbidities that were undiagnosed at the time of treatment; 1 had more extensive disease including primary bone neoplasia, the second had hypothyroidism. The mean value of maximal wound area in the study was 11.1 cm2, which was substantially higher than the median because of the influence of the small group of 6 dogs with wounds >40 cm2.
3.2. Determinants of maximal wound area
Figure 1 shows the crude relationship between maximal wound area and pretreatment tumor volume, unadjusted for other potential determinants of wound area, for each of the 111 dogs in the study that formed wounds after receiving a single TT treatment.
FIGURE 1.
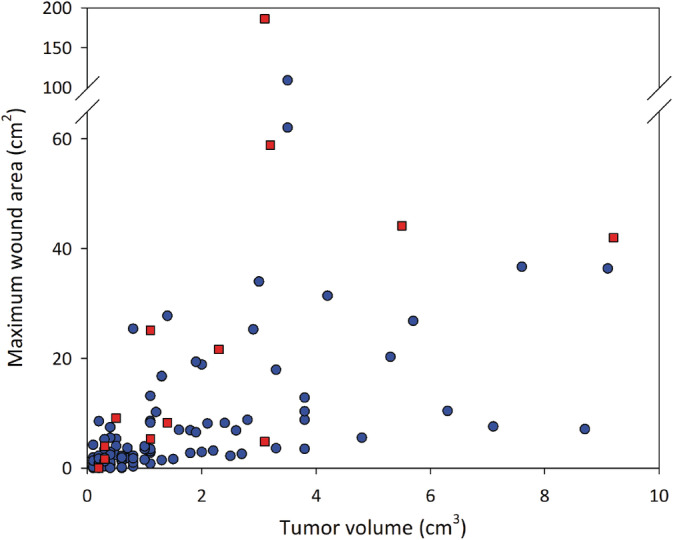
Relationship between tumor volume at time of treatment and the maximal area of the wound that formed after slough of MCTs treated with TT. Data are for 111 dogs that formed wounds after intratumoral injection of TT. Blue circles represent data for 99 individual dogs where locoregional LNs were not enlarged at pretreatment screening; red squares are for 12 dogs where locoregional LNs were enlarged at screening. MCT, mast cell tumor; TT, tigilanol tiglate
Multivariable analyses identified 3 variables associated with wound area after tumor slough in these 111 dogs (Table 1). These were (a) volume of the target tumor at treatment (P < .001), (b) locoregional LN enlargement at screening (P < .001), and (c) tumor cytological grade (P < .001). Wound area increased with increasing tumor volume and generally was larger when locoregional LNs were enlarged at screening (Table 1). Wound area also was much larger (mean estimated to be 3.4 times larger) when cytological grade of the tumor was high or suspected high compared to low or suspected low (Table 1). However, this result was almost entirely because of the dog with the largest wound in the study that had a suspected high grade MCT. Estimated effects of tumor location on wound area were statistically imprecise and hence uninformative, as were estimated effects for SC tumors relative to cutaneous tumors on lower limbs (Table 1).
TABLE 1.
Descriptive statistics and results of multivariable analyses of estimated ratios of means of maximal wound area after treatment of MCT with TT in 111 dogs that subsequently developed wounds, and in the subset of 48 dogs with cutaneous and subcutaneous mast cell tumors on the lower limb
| (Potential) determinant and categories | No. dogs | Mean of maximum wound areas (25th, 75th percentiles; cm2) | Adjusted estimated ratio of means a | 95% CI | P value b |
|---|---|---|---|---|---|
| Tumor location | .09 | ||||
| Body | 45 | 6.3 (1.3, 7.4) | 0.8 | 0.3‐1.7 | .5 |
| Upper limb | 18 | 7.1 (1.0, 6.5) | 0.4 | 0.1‐1.5 | .17 |
| Lower limb | 48 | 17.1 (1.9, 18.6) | Reference category | ||
| Tumor volume at time of first treatment (cm 3 ) | <.001 | ||||
| 111 | 1.7 (0.3, 2.5) | 0.2 c | 0.1‐0.9 | .03 | |
| Cytological grade of tumor | <.001 | ||||
| Low or low suspected | 98 | 9.8 (1.5, 9.1) | Reference category | ||
| High or high suspected | 9 | 25.2 (2.2, 8.8) | 3.4 | 2.5‐4.6 | <.001 |
| Grade not available d | 4 | 10.1 (1.3, 19.0) | |||
| Regional lymph node(s) enlarged at screening | <.001 | ||||
| No | 99 | 8.3 (1.4, 8.5) | Reference category | ||
| Yes | 12 | 34.3 (5.1, 43.1) | 4.8 e | 3.0‐7.5 | <.001 |
| Tumor type (lower limb) | .38 | ||||
| Cutaneous | 34 | 19.1 (1.8, 19.3) | Reference category | ||
| Subcutaneous | 14 | 12.3 (5.3, 17.9) | 0.6 f | 0.1‐2.4 |
Abbreviations: CI, confidence interval; FNA, fine needle aspirate; MCT, mast cell tumor; TT, tigilanol tiglate.
Adjusted for all other variables in table (ie, all four variables were simultaneously fitted in the same model; n = 107 dogs; tumor volumes was first transformed to tumor volumes−2).
Bolded P values are overall likelihood ratio test P values for the variables; unbolded P values are Wald P values for assessing the respective category relative to the reference category.
Estimated ratio of mean wound area for a 1 unit increase in tumor volume−2; predicted wound areas for tumor volumes of 0.3 and 2.5 (the 25th and 75th percentile values) were 0.0 cm2 and 26.4 cm2, respectively.
For the 4 dogs where tumor cytological grading results were not available, this was due to an inadequate FNA sample or lack of cellularity noted by the IDEXX veterinary pathologist so the Scarpa cytological grading methodology could not be applied.
For example, the arithmetic mean wound area where regional lymph nodes were enlarged was estimated as being 4.8 times that where regional lymph nodes were not enlarged after adjusting for tumor location, tumor volume, and cytological grade of tumor.
Adjusted for tumor volume, cytological grade, and regional lymph node enlargement.
To further explore the effect of these 3 variables as determinants of wound area, data for the 12 dogs with enlarged pretreatment locoregional LNs were separated from dogs without LN enlargement. The maximal wound area for each of these 2 groups then was plotted in relation to 3 tumor volume classes (Figure 2).
FIGURE 2.
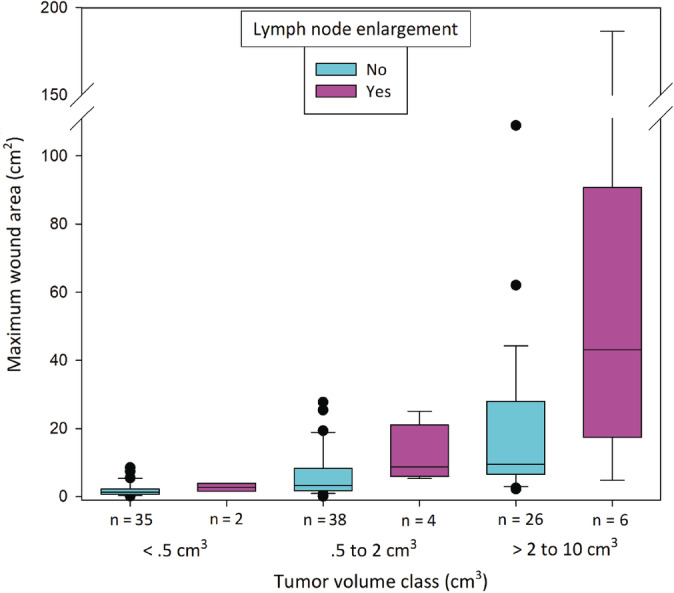
Boxplot showing maximal wound areas after slough of individual MCTs for 99 dogs treated with TT where there was no enlargement of locoregional LNs at pretreatment screening and for 12 dogs where regional LNs were enlarged at screening. Boxes in the plot represent the 25th, 50th, and 75th percentiles; whiskers show the 10th and 90th percentiles, black dots are individual outlying wounds. MCT, mast cell tumor; TT, tigilanol tiglate
For the dogs with pretreatment LN enlargement, wound size tended to be larger and more variable than for dogs that did not have enlarged locoregional LNs (Figure 2) and this difference was most evident in the 2 larger wound size classes. However, the numbers of cases in each of these classes with enlarged LNs (n = 4 and n = 6, respectively) were too low for definitive comparison. After re‐running the multivariable analyses excluding the 12 dogs with locoregional LN enlargement, only tumor volume remained a strong determinant of wound size (P < .001; Table 2).
TABLE 2.
Descriptive statistics and results of multivariable analyses of estimated ratios of means of maximal wound area after treatment of a mast cell tumor with TT in 99 dogs in which locoregional LNs were not enlarged that subsequently developed wounds, and in the subset of 39 dogs with cutaneous and SC MCTs on the lower limb
| (Potential) determinant and categories | No. dogs | Mean of maximum wound areas (25th, 75th percentiles; cm2) | Adjusted estimated ratio of means a | 95% CI | P value b |
|---|---|---|---|---|---|
| Tumor location | .92 | ||||
| Body | 43 | 6.3 (1.2, 7.4) | 0.8 | 0.5‐1.4 | .48 |
| Upper limb | 17 | 7.2 (1.0, 6.5) | 0.8 | 0.4‐1.5 | .43 |
| Lower limb | 39 | 10.9 (1.8, 12.8) | Reference category | ||
| Tumor volume at time of first treatment (cm 3 ) | <.001 | ||||
| 99 | 1.6 (0.3, 2.2) | 0.2 c | 0.0‐1.0 | .04 | |
| Cytological grade of tumor | .08 | ||||
| Low or low suspected | 88 | 8.4 (1.5, 8.4) | Reference category | ||
| High or high suspected | 7 | 5.6 (2.2, 8.8) | 0.4 | Undefined to 1.1 d | |
| Grade not available | 4 | 10.1 (1.3, 19.0) | |||
| Tumor type (lower limb) | .09 | ||||
| Cutaneous | 25 | 10.1 (1.7, 7.5) | Reference category | ||
| Subcutaneous | 14 | 12.3 (5.0, 20.1) | 0.5 e | 0.2‐1.3 | .16 |
Abbreviations: CI, confidence interval; MCT, mast cell tumor; TT, tigilanol tiglate.
Adjusted for all other variables in table (ie, all three variables were simultaneously fitted in the same model; n = 95 dogs; tumor volumes were first transformed to tumor volumes−2).
Bolded P values are overall likelihood ratio test P values for the variables; unbolded P values are Wald P values for assessing the respective category relative to the reference category.
Estimated ratio of mean wound area for a 1 unit increase in tumor volume−2.
Likelihood‐based confidence interval (rather than Wald confidence intervals as elsewhere); lower limit could not be defined.
Adjusting for tumor volume and cytological grade.
The estimated effect of tumor cytological grade now was not a determining factor (Table 2), indicating that grade interacts with locoregional LN enlargement in its effect on wound area. For the subset of tumors on lower limbs, estimated effects for SC relative to cutaneous tumors were still imprecise (Table 2).
From the above described multivariable models, it was evident that enlarged locoregional LNs at screening was a source of variability in the areas of the wounds that formed after TT treatment. Although no MCT disease was confirmed by FNA at pretreatment screening in these enlarged nodes, pathologist comments made it clear in all 12 cases that sampling was inadequate or uninterpretable and that clear suspicion of MCT was present in 4 cases. No resampling of the LNs was performed and thus localized metastasis cannot be ruled out. On this basis, we restricted our subsequent evaluation of the predictability of wound area to the 95 dogs in which locoregional LNs were not enlarged and tumor cytological grade was available (for the 4 dogs in which tumor cytological grading results were not available, it was a consequence of inadequate FNA sample or lack of cellularity noted by the pathologist such that the Scarpa cytological grading methodology could not be applied). 7 Using a generalized linear regression model, tumor volume, location, and cytological grade were fitted and predicted wound areas obtained for each dog. When these predictions were compared to the actual wound areas for each dog, actual wound areas were either smaller, or ≤10 cm2 larger, than those predicted from the model in 92% (87/95) of cases (Figure S1). The remaining 8 dogs had wounds between 10.7 and 86.3 cm2 larger than predicted from the model (median, 16.3 cm2; mean, 27.7 cm2).
3.3. Wound management and healing
The wounds that were exposed after slough of the necrotic tumor tissue were left to heal by secondary intention. Other than antibiotics prescribed prophylactically for 47 patients, only 5 dogs received wound interventions during the study. One of these interventions was antibiotic treatment for a bacterial infection and cellulitis that developed at the wound site 7 days after treatment, 1 was bandaged, 1 involved irrigation of the wound with saline to prevent odor, and the remaining 2 interventions (Elizabethan collar, light wound cover) were used to prevent self‐trauma of the wound.
Among the 77 dogs that had CR at day 84, 6 small wounds had healed by 14 days after treatment (maximal wound areas: median, 0.96 cm2; range, 0.2‐3.1 cm2), with 57% (n = 44; 95% CI = 45%‐68%), 78% (n = 60; 95% CI = 67%‐87%), and 96% (n = 74; 95% CI = 89%‐99%) of wounds healed by days 28, 42, and 84, respectively. Of the 3 dogs with wounds that were not fully healed by day 84, the residual wound areas were 0.2 cm2, 0.8 cm2, and 3.7 cm2, respectively, and these all were <3% of their original maximal area. The largest of these residual wounds was from the largest wound (186 cm2) that formed in the study and occurred on a dog with a suspected high grade MCT on the metacarpus where the locoregional LN also was noted to be enlarged at screening. This dog also was diagnosed with hypothyroidism at screening and did not achieve stable management of this condition until 72 days later.
The time taken for wounds to heal in the 77 dogs that had CR at day 84 was related to both wound area (Figure 3A) and location (Figure 3B). Univariable modeling of effects of wound area and location showed that smaller wounds and wounds on the body and upper limbs were more likely to be healed by days 28 and 42 than were larger wounds and wounds on lower limbs, respectively (Table S1 and Figure S2 for day 28; Table S2 and Figure S3 for day 42).
FIGURE 3.
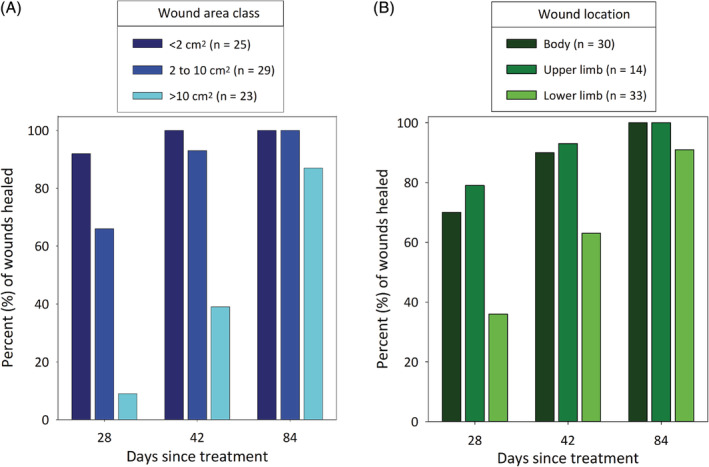
Rate of wound healing as measured by percentages of wounds that had healed by days 28, 42, and 84 after a single treatment with TT (A) in each of 3 maximal wound area classes and (B) at 3 locations (body, upper limb, and lower limb). Data are from the 77 dogs that formed a wound, were assessed at all assessment times through to day 84 after a single treatment with TT, and had complete resolution of the tumor at that time. Data include those dogs with enlarged locoregional LNs detected (metastatic MCT disease could not be confirmed on FNA) at screening. FNA, fine needle aspirate; MCT, mast cell tumor; TT, tigilanol tiglate
Typical examples of the clinical progression of healing of the wounds after slough of TT‐treated tumors are illustrated for different wound locations in Figures 4, 5, 6 where locoregional LNs were not enlarged at screening. Figure 7 illustrates 2 tumors on lower limbs where locoregional LNs were enlarged at the time of screening, whereas Figure 8 shows the progression of healing of the wound on a dog with locoregional LN enlargement and other comorbidities that resulted in the largest wound formed in the study.
FIGURE 4.
Examples of the progression of wound healing after slough of MCTs on the body (which include those on the head, trunk, and in perineal locations) that had received a single treatment with TT. Case 11‐003 (A‐D) treated in phase 1 of the study had a 1.1 cm3 MCT at time of treatment and a maximal wound of 8.2 cm2 after tumor slough. Case 11‐002 (E‐H) treated in phase 2 of the study had a 0.4 cm3 MCT at time of treatment and a maximal wound of 7.4 cm2 after tumor slough. MCT, mast cell tumor; TT, tigilanol tiglate
FIGURE 5.
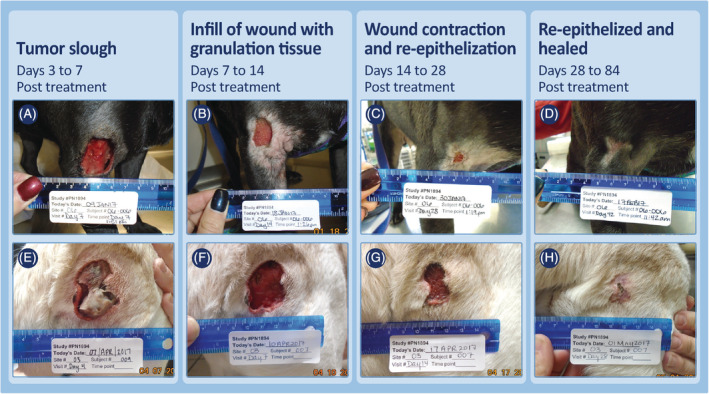
Examples of the progression of wound healing after slough of MCTs on upper limbs (above the elbow and hock) that had received a single treatment with TT. Case 06‐006 (A‐D) was treated in phase 1 of the study had a 2.8 cm3 MCT at time of treatment and a maximal wound at time of tumor slough of 8.8 cm2. Case 03‐007 (E‐H, note figure E on day 4 post‐treatment has incorrect subject number written) also was treated in phase 1 of the study and had a 3.8 cm3 MCT at time of treatment. A maximal wound of 10.3 cm2 was recorded after tumor slough. MCT, mast cell tumor; TT, tigilanol tiglate
FIGURE 6.
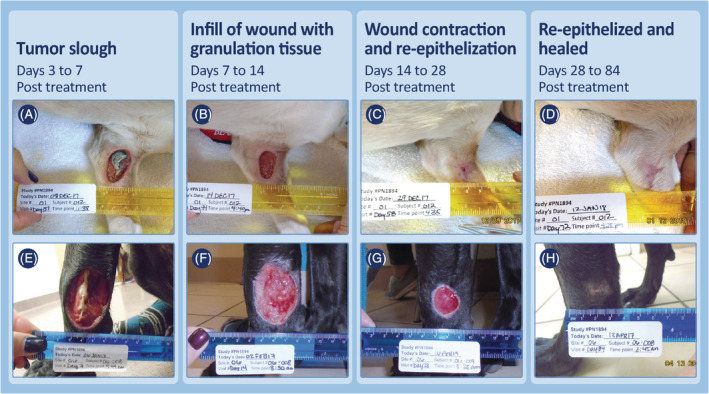
Examples of the progression of wound healing after slough of MCTs on lower limbs (at or below the elbow and hock) that had received a single treatment with TT. Case 01‐012 (A‐D) was treated in phase 2 of the study with a 0.5 cm3 MCT at time of treatment and a maximal wound of 5.3 cm2 was recorded after tumor slough. Case 06‐008 (E‐H) was treated in phase 1 of the study with a 1.9 cm3 MCT and after tumor slough had a maximal wound of 19.3 cm2. MCT, mast cell tumor; TT, tigilanol tiglate
FIGURE 7.
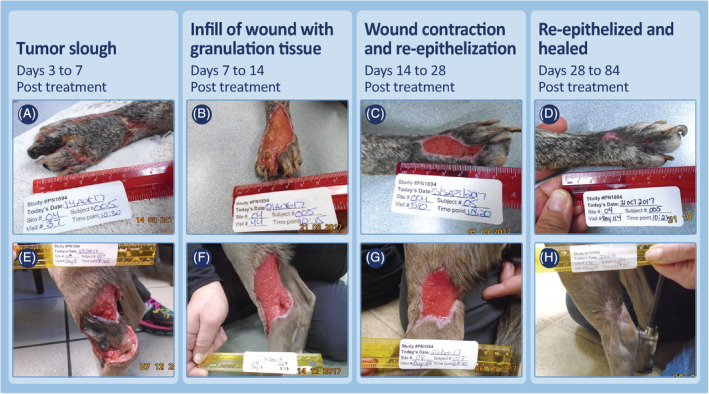
Examples of the progression of wound healing after slough of MCTs on lower limbs that had received a single treatment with TT and locoregional LNs were enlarged at time of pretreatment screening (MCT metastasis not confimed on FNA). Case 04‐005 (A‐D) was treated in phase 2 of the study with a 1.1 cm3 MCT at time of treatment. After tumor slough, a maximal wound of 25.1 cm2 was recorded. Case 08‐017 (E‐H) was treated in phase 1 of the study and had a 3.2 cm3 MCT at time of treatment. After tumor slough, a maximal wound of 58.9 cm2 was recorded. FNA, fine needle aspirate; MCT, mast cell tumor; TT, tigilanol tiglate
FIGURE 8.
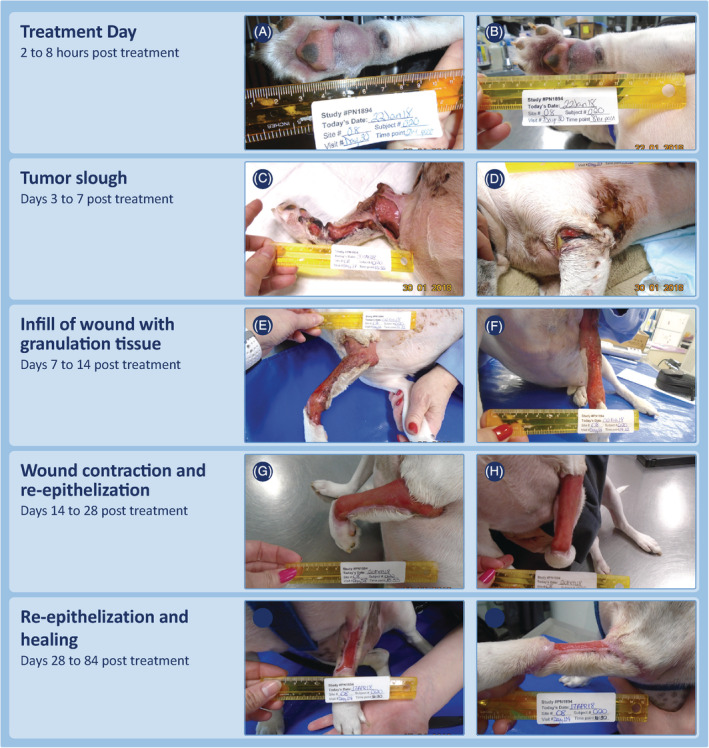
Progression of wound formation and wound healing for the dog with the largest wound that occurred after treatment of a target MCT with TT. This dog was treated in phase 2 of the study for a suspected high grade MCT on the metacarpus and had enlargement of the locoregional draining lymph node at time of pretreatment screening. The wound that formed after treatment of this MCT caused extensive localized slough beyond the immediate margins of the treated tumor. Substantial inflammation and edema had been noted to extend to the axilla between treatment and day 7 (study day 37). This wound was managed with bandaging. By the last assessment time at 84 days post‐TT treatment (study day 114), the residual wound was 3.7 cm2 in area, approximately 2% of its maximal size of 186 cm2 at day 7. MCT, mast cell tumor; TT, tigilanol tiglate
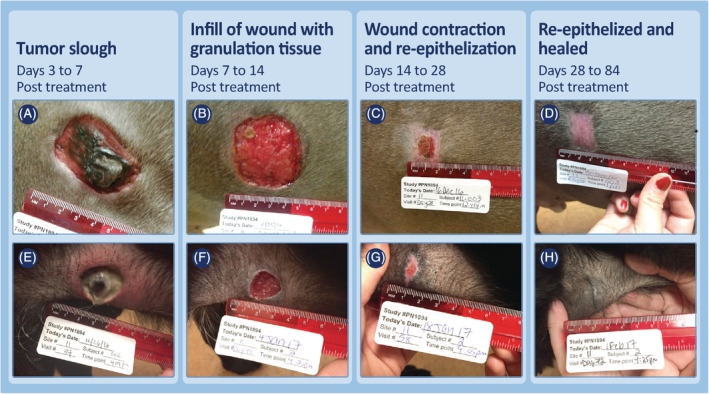
Regardless of location or whether locoregional LNs were enlarged at screening, wound healing progressed in a clinically predictable manner. Tumor slough exposed healthy granulation tissue that had formed in the underlying wound bed as the tumor was undergoing necrosis (panels A and E, Figures 4, 5, 6, 7; panels C and D, Figure 8). Within 7 days, this granulation tissue had filled in much of the tissue deficit that occurred after tumor slough (panels B and F, Figures 4, 5, 6, 7). Subsequently, contraction contributed most to wound closure on the body and upper limbs (panels C, D, G, and H, Figures 4 and 5), whereas on lower limbs, healing generally was slower and relied primarily on re‐epithelization for closure (panels C, D, G, and H, Figure 6), including cases in which LNs were enlarged (panels C, D, G, and H, Figure 7; panels G to J, Figure 8). The healed wound sites consistently had good cosmetic outcome, with no evidence of the original tissue deficit or of excessive fibrosis and contraction of the scar.
4. DISCUSSION
The area of the wound, or tissue deficit, present after slough of MCTs treated intratumorally with TT relative to the initial volume of that target tumor previously has been shown to be strongly associated with complete tumor resolution and hence is a critical predictor of the drug's efficacy. 4 Here we have characterized in more detail the formation and resolution of such wounds and found that:
For most dogs, excluding those with enlarged locoregional LNs, wound area after slough of TT‐treated tumors primarily was related to tumor volume; and,
Healing of these wounds in dogs that achieve complete tumor resolution progresses in a consistent, clinically predictable manner with time to healing dependent on both size and location of the wound.
Regardless of tumor size, 89% (99/111) of MCTs that received a single treatment of TT in our study became necrotic and sloughed within 7 days to expose underlying wound beds of healthy granulation tissue. The remaining 12 tumors subsequently sloughed ≤14 days post‐treatment. The primary determinant of the area of these postslough wounds was tumor volume and this finding is consistent with the majority of the wound occurring in the area immediately surrounding the treated tumor mass. Enlargement of the locoregional LNs at the time of pretreatment screening also was an important determinant of wound area and was associated with (a) generally more extensive wounds, (b) wound occurrence on lower limbs (8 of 12), and (c) a higher proportion of high or suspected high grade MCTs (25% [3/12]) compared to 7% (7/105) in dogs without LN enlargement. Although MCT metastasis to locoregional LNs was not confirmed by FNA at the time of screening for these dogs, pathologists reported that only 6 of the 12 FNAs were of moderate to good cellularity for diagnostic interpretation and that 4 of these were suspicious, but not confirmed, for MCT metastasis. For all 12 FNAs, repeat sampling and interpretation was requested by the pathologist, but resampling was not performed. Recognizing the intrinsic challenges of cytological evaluation of LN spread of MCTs, the fact that LNs normally contain low numbers of mast cells, and that neoplastic mast cells can recruit non‐neoplastic mast cells via the lymphatics, it is possible, that for these 12 dogs, MCT metastasis was present and a contributing factor for the more extensive wounds seen in these cases. 8 Where LNs were enlarged, and for a small number of large MCTs on lower limbs, larger than expected wounds were associated with more extensive tissue slough developing well beyond the immediate margins of the treated tumor. This situation invariably was associated with clinically relevant edema and swelling surrounding the necrotic tumor and extending to the local draining locoregional LN within the first 7 days after treatment. This clinical presentation suggests that impeded drainage of edema fluid from the treatment site, because of the pre‐existing reactive state of the local lymphatics or possible unconfirmed metastatic MCT disease, may have played a role in disruption of lymphatics and slough of larger areas of surrounding tissue. 13
In the 75% of dogs that achieved complete resolution of the target tumor after TT treatment, wounds exposed after tumor slough healed in a consistent and clinically predictable manner by secondary intention with minimal or no veterinary intervention. Unsurprisingly, rate of wound healing was related to wound area and location of the wound. Larger wounds healed more slowly than did smaller wounds, and wounds on the lower limbs, where closure relies predominantly on re‐epithelialization, healed more slowly than did wounds on the body and upper limbs, where wound contraction contributes more to closure. Even for the more extensive wounds where tissue slough occurred beyond the immediate tumor margins, most were healed by day 84.
The presence of well‐developed granulation tissue in the exposed wound bed after tumor slough and rapid filling of the resulting tissue deficit were consistent features of TT wounds. These clinical observations indicate that wound healing processes were already underway before tumor slough and may relate to effects of TT on healing. In vitro studies using adult human fibroblasts have shown that TT modifies fibroblast gene expression, differentiation, and functioning, especially in relation to growth factor signaling, proteolytic remodeling, and composition of the extracellular matrix. 14 Similarly, the rapid re‐epithelialization that occurred around the edges of lower limb wounds is consistent with in vitro effects of TT on keratinocyte migration. 15
Interventions to manage wounds were minimal and only required in 5 cases. Bandaging, particularly in the days immediately after TT treatment, was discouraged in the study for 2 main reasons. First, bandaging has the potential to interfere with the resolution of any local edema resulting from the drug's mode of action in initiating an acute, transient, and localized inflammatory response at the treatment site. If drainage and resolution of any such edema is impaired, and further restricted or aggravated by bandaging, it has potential to increase wound size at the treatment site by damaging surrounding normal tissue beyond the immediate vicinity of the tumor margin. Second, increasing evidence indicates that leaving wounds open to heal in ambient oxygen may improve and enhance wound closure. 16 , 17 , 18
Clearly, the wounds that form after tumor destruction by TT are somewhat alarming when first encountered by clinicians and owners. However, as we have shown in our study and the previous report 4 , these wounds heal relatively rapidly by secondary intention after tumor slough and generally require minimal intervention. For a clinician using TT, discussion with owners before TT treatment is essential to explain and prepare them for the process of tumor destruction and healing and to emphasize the importance of allowing the wounds to heal with minimal intervention and without bandaging. Owners also should be made aware that despite the open wound their dogs will be able to undertake normal daily activities, including bathing, throughout the treatment and healing period.
The clinical importance of our study is that we have described in detail the formation and subsequent resolution of wounds that occurred after slough of MCTs treated intratumorally with TT. Because of the unique nature of the drug as a local intratumoral treatment and its mode of action, these results are highly relevant to clinicians contemplating its use. Our results indicate that wound area after slough of the necrotic tumor is related to pretreatment tumor volume. However, also in some cases, often those associated with pre‐existing enlargement of the locoregional LN, wound area was larger than expected. It is likely that metastatic MCT disease, impeded drainage of edema from the treatment site, or both may contribute to more extensive wound development. This is an important consideration for the treating clinician and further in‐depth staging is recommended before considering treatment when enlarged LNs are found or metastasis is suspected. After TT treatment, most wounds heal uneventfully by secondary intention with time to complete healing related to tumor size and location. Clinical observations of the progression of wound resolution were consistent with evidence from in vitro studies that TT has direct positive effects on fibroblasts and keratinocytes in promoting wound healing responses. Longitudinal studies currently are in progress to monitor local recurrence rates after TT treatment in this patient group. With these considerations in mind, TT provides a new approach to consider for the local treatment of MCTs in dogs in many clinical situations.
CONFLICT OF INTEREST DECLARATION
Drs Paul Reddell, Thomas De Ridder, Justine E. Campbell, Graham Brown, Pamela D. Jones, Peter F. Schmidt, and Victoria Gordon are employed by QBiotics Group Limited. QBiotics Group Limited owns the intellectual property and patents associated with tigilanol tiglate. Drs Chad M. Johannes and John M. Morton receive payments as independent consultants and service providers to QBiotics Group Limited.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Institutional animal ethics was not required for this study as it was under a US Center for Veterinary Medicine ‐ Food and Drug Administration Protocol ‐ Investigational New Animal Drug (INAD) No. I‐012436 (July 25, 2016).
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
Funding for this study was provided by QBiotics Group Limited. The authors gratefully acknowledge the clinicians and other veterinary staff who gathered the case data at each study site. Triveritas Limited provided independent VICH GCP study management for the clinical efficacy study. Sheryl Pacchiardi formatted and proof‐read the manuscript. The data sets generated and analyzed for this study are not publicly available due to being part of a data package to support the evaluation of tigilanol tiglate with regulatory authorities for the purpose of registration and commercialization. They may be available from QBiotics Group upon reasonable requests following an assessment process.
Reddell P, De Ridder TR, Morton JM, et al. Wound formation, wound size, and progression of wound healing after intratumoral treatment of mast cell tumors in dogs with tigilanol tiglate. J Vet Intern Med. 2021;35:430–441. 10.1111/jvim.16009
Funding information QBiotics Group Limited
REFERENCES
- 1. Barnett CME, Broit N, Yap P‐Y, et al. Optimising intratumoral treatment of head and neck squamous cell carcinoma models with the diterpene ester Tigilanol tiglate. Invest New Drugs. 2019;37(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 2. Panizza BJ, de Souza P, Cooper A, Roohullah A, Karapetis CS, Lickliter JD. Phase I dose‐escalation study to determine the safety, tolerability, preliminary efficacy and pharmacokinetics of an intratumoral injection of tigilanol tiglate (EBC‐46). EBioMedicine. 2019;50:433‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Ridder TR, Ruppin M, Wheeless M, Williams S, Reddell P. Use of the intratumoural anticancer drug tigilanol tiglate in two horses. Front Vet Sci. 2020;7:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Ridder TR, Campbell JE, Burke‐Schwarz C, et al. Randomized controlled clinical study evaluating the efficacy and safety of intratumoral treatment of canine mast cell tumors with tigilanol tiglate (EBC‐46). J Vet Intern Med. 2020;1‐15. 10.1111/jvim.15806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2015;13(3):176‐183. [DOI] [PubMed] [Google Scholar]
- 6. Boyle GM, D'Souza MMA, Pierce CJ, et al. Intra‐lesional injection of the novel PKC activator EBC‐46 rapidly ablates tumors in mouse models. PLoS One. 2014;9(10):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scarpa F, Sabattini S, Bettini G. Cytological grading of canine cutaneous mast cell tumours. Vet Comp Oncol. 2016;14(3):245‐251. [DOI] [PubMed] [Google Scholar]
- 8. Kiupel M, Camus M. Diagnosis and prognosis of canine cutaneous mast cell tumors. Vet Clin North Am Small Anim Pract. 2019;49(5):819‐836. [DOI] [PubMed] [Google Scholar]
- 9. Monga SP, Wadleigh R, Sharma A, et al. Intratumoral therapy of cisplatin/epinephrine injectable gel for palliation in patients with obstructive esophageal cancer. Am J Clin Oncol. 2000;23(4):386‐392. [DOI] [PubMed] [Google Scholar]
- 10. Celikoglu F, Celikoglu SI, Goldberg EP. Intratumoural chemotherapy of lung cancer for diagnosis and treatment of draining lymph node metastasis. J Pharm Pharmacol. 2010;62(3):287‐295. [DOI] [PubMed] [Google Scholar]
- 11. VCOG‐CTCAE . Veterinary cooperative oncology group ‐ common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2016;14(4):417‐446. [DOI] [PubMed] [Google Scholar]
- 12. Lynch S, Savary‐Bataille K, Leeuw B, Argyle DJ. Development of a questionnaire assessing health‐related quality‐of‐life in dogs and cats with cancer. Vet Comp Oncol. 2011;9(3):172‐182. [DOI] [PubMed] [Google Scholar]
- 13. Scallan J, Huxley VH, Korthuis RJ. Chapter 4 ‐ Pathophysiology of edema formation, 4.4 Permeability Edema and Inflammation Capillary Fluid Exchange: Regulation, Functions and Pathology. San Rafael, CA: Morgan & Claypool Life Sciences; 2010. https://www.ncbi.nlm.nih.gov/books/NBK53445/. Assessed December 24, 2020. [PubMed] [Google Scholar]
- 14. Dally J. Evaluation of Novel Epoxy‐Tigliane Compounds as Modulators of Dermal Fibroblast‐Myofibroblast Differentiation, Scar Tissue Resolution and Fibrosis; and Elucidation of Their Underlying Mechanisms of Action [PhD thesis]. Cardiff University; 2018. Retrieved from http://orca.cf.ac.uk/id/eprint/120278. Assessed December 24, 2020.
- 15. Moses RL, Boyle GM, Howard‐Jones RA, et al. Novel epoxy‐tiglianes stimulate skin keratinocyte wound healing responses and re‐epithelialization via protein kinase C activation. Biochem Pharmacol. 2020;178:114048. [DOI] [PubMed] [Google Scholar]
- 16. Ladizinsky D, Roe D. New insights into oxygen therapy for wound healing. Wounds Res. 2010;22(12):294‐300. [PubMed] [Google Scholar]
- 17. Yip WL. Influence of oxygen on wound healing. Int Wound J. 2015;12(6):620‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17(1):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


