Abstract
Objective
To evaluate the efficacy and safety of tigilanol tiglate (TT) for local intratumoral treatment of mast cell tumors (MCTs) in dogs.
Methods
A randomized controlled clinical study in 2 phases involving 123 dogs with cytologically diagnosed MCT. Phase 1 compared 81 TT‐treated dogs with 42 control dogs; phase 2 allowed TT treatment of control dogs and retreatment of dogs that failed to achieve tumor resolution after TT treatment in phase 1. Tigilanol tiglate (1 mg/mL) was injected intratumorally with dose based on tumor volume. Concomitant medications were used to minimize potential for MCT degranulation. Modified response evaluation criteria in solid tumors were used to evaluate treatment response at 28 and 84 days. Adverse events and quality of life were also assessed.
Results
A single TT treatment resulted in 75% complete response (CR) (95% confidence interval [CI] = 61‐86) by 28 days, with no recurrence in 93% (95% CI = 82‐97) of dogs by 84 days. Eight TT‐treated dogs that did not achieve CR in phase 1 achieved CR after retreatment, increasing the overall CR to 88% (95% CI = 77‐93). Control dogs had 5% CR (95% CI = 1‐17) at 28 days. Wound formation after tumor slough and wound size relative to tumor volume were strongly associated with efficacy. Adverse events typically were low grade, transient, and directly associated with TT's mode of action.
Conclusions
Tigilanol tiglate is efficacious and well tolerated, providing a new option for the local treatment of MCTs in dogs.
Keywords: cutaneous, diterpene ester, dog, local treatment, MCT, subcutaneous
Abbreviations
- AE
adverse event
- CI
confidence interval
- CR
complete response
- FNA
fine needle aspirate
- MCT
mast cell tumor
- RECIST
response evaluation criteria in solid tumors
- TT
tigilanol tiglate (also known as EBC‐46)
- VCOG
Veterinary Cooperative Oncology Group
1. INTRODUCTION
Mast cell tumors (MCTs) account for approximately 7% to 21% of all skin tumors in dogs, 1 , 2 , 3 occur in all regions of the body and usually present as solitary tumors. Their clinical appearance is highly variable and they are notoriously unpredictable in behavior. Initial diagnosis relies primarily on cytological assessment of fine needle aspirates (FNAs). 1 , 2 , 3
The first‐line standard of care for local treatment of MCTs is surgery, often involving wide margins (up to 3 cm) and 1 fascial plane deep with the aim of achieving clean margins to minimize risk of local recurrence. 4 , 5 Concomitant supportive medications (H1 and H2 antagonists, corticosteroids) are recommended to decrease the effects of potential local or systemic degranulation that can occur when MCTs are disturbed. 1 , 2 Whenever surgical resection with clean margins is not possible, treatment relies on surgical debulking combined with chemotherapy or radiotherapy, or both. 2 , 6 , 7 , 8 , 9 , 10 , 11 , 12 However, these treatments often are too costly for some pet owners and not readily available in geographic locations distant from specialist centers. Additionally, dogs with comorbidities have a higher anesthetic death risk, leaving some owners with a considerable dilemma. 13 , 14 Consequently, an opportunity exists for a new efficacious alternative or adjunctive treatments to surgery to manage MCTs, especially in primary care veterinary practice.
Tigilanol tiglate (TT, also known as EBC‐46) is a novel diterpene ester under clinical evaluation as a simple‐to‐administer, intratumoral treatment for a range of cancers in humans and companion animals, including MCTs in dogs. 15 , 16 , 17 , 18 , 19 It is a potent cellular signaling molecule with activation of protein kinase C responsible, in part, for its efficacy. 15 Intratumoral injection of TT elicits a rapid but highly localized inflammatory response, disruption of tumor vasculature, and induction of tumor cell death by oncosis. 15 These processes lead to hemorrhagic necrosis and destruction of the tumor mass within 2 to 7 days followed by resolution of the resulting wound with good cosmetic and functional outcomes between 28 and 84 days after treatment. 18 , 19 Herein we report a randomized, blinded, controlled study evaluating the efficacy, safety, and tolerability of TT for intratumoral treatment of nonmetastatic MCT disease in dogs.
2. MATERIALS AND METHODS
2.1. Study objectives
Our primary objective was to evaluate efficacy (full resolution of a treated target tumor) at 28 days after administration of a single intratumoral dose of TT compared to a control (sham) treatment. Efficacy was assessed using modified Response Evaluation Criteria in Solid Tumors (RECIST; Veterinary Cooperative Oncology Group [VCOG]), 20 with complete response (CR) equating to complete resolution of the target tumor.
Secondary objectives were to assess:
Durable efficacy in dogs receiving a single TT treatment by recording any incident of local recurrence of the treated MCTs at 84 days after treatment;
Responsiveness to a second TT treatment for any dogs that did not achieve CR after initial treatment;
Safety and tolerability profile (occurrence, frequency, and severity of adverse events [AEs]) from exposure to TT over 84 days after treatment;
Owners' opinions of their dogs' quality of life (QoL) over the first 28 days after TT treatment;
Wounds that formed after effective tumor resolution at the treatment site and the subsequent progression of their healing; and,
Whether selected patient and tumor features are determinants of TT efficacy.
2.2. Study design
A randomized, controlled, investigator‐ and owner‐blinded study reviewed by the Center of Veterinary Medicine, Food and Drug Authority (CVM‐FDA) was conducted at 11 veterinary clinics in the United States between November 2016 and March 2018. Study sample size was based on the assumption of a 20% CR in the control group and a 50% CR in the TT treatment group. To detect a significant difference (2‐sided α = .05), the minimum sample size for at least 80% power with a 2 : 1 ratio of treated to control dogs was 60 TT treated and 30 control dogs. A minimum enrollment was set at 120 dogs. On successful screening, each dog was enrolled and assigned to either the TT treated or control group using a random allocation table based on a blocked (n = 6) randomization design, with 4 TT treated and 2 control dogs in each block, and each block completed based on sequential dates of enrollment before commencing the next block. Each study site had at least 1 nonblinded veterinarian responsible for administering treatment and ≥2 veterinarians or clinical staff undertaking blinded evaluations for the entire study.
The study had 2 phases (Figure 1). Phase 1 addressed the primary efficacy objective comparing TT treated to control dogs after 28 days. Phase 1 treatment day was defined as day 0 for both TT treated and control (sham) dogs. Dogs in both groups that showed CR at 28 days in this phase then were followed for an additional 2 months (to 84 days post‐treatment). Phase 2 involved dogs from both the TT treated group from phase 1 that did not achieve CR at 28 days and dogs receiving their first treatment with TT (original control group dogs that were Not CR at 28 days). Blinding of the TT and control (sham) group dogs continued throughout phase 2, including maintaining the anonymity of which dogs were TT treated or control (sham) in phase 1.
FIGURE 1.
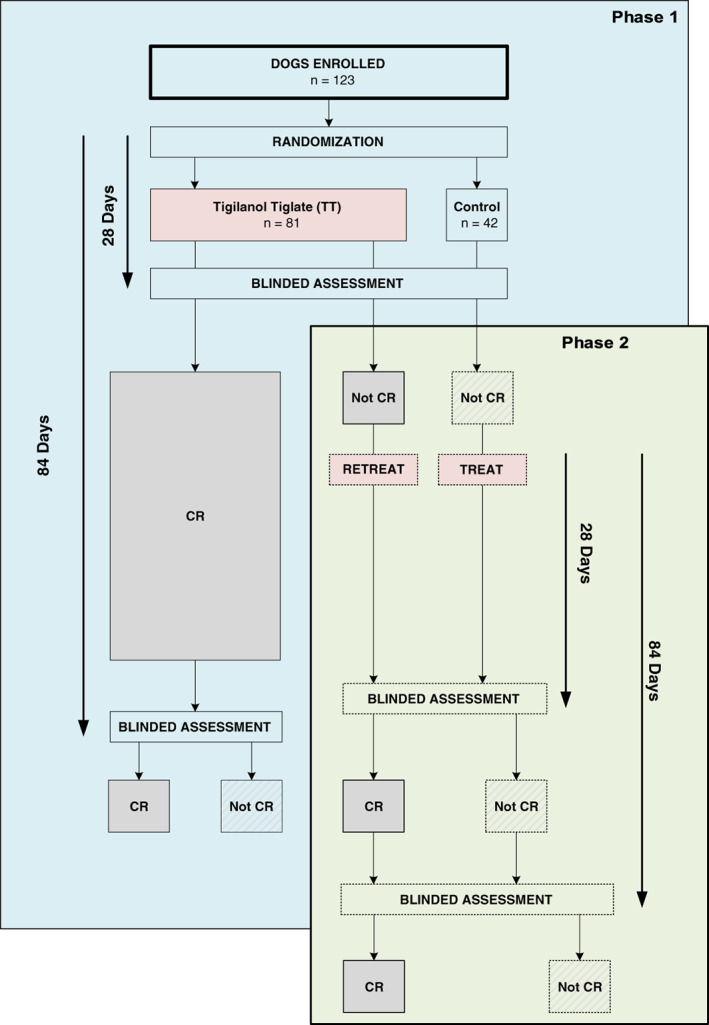
Enrolled dogs, randomization, and treatment outcome categories for phase 1 and 2
2.3. Study protocol
At initiation of the study (start of phase 1), potential study dogs were screened and eligible dogs enrolled 2 to 7 days before treatment, with owners of eligible dogs providing written informed consent before enrollment. For enrollment, dogs needed to be ≥1 year of age with stage Ia or IIIa MCT according to the World Health Organization (WHO) staging criteria for MCTs 1 , but, complete clinical staging was not undertaken. 2 Instead, investigators identified dogs that were stage Ia or IIIa at the time of screening based on the following: (1) absence of systemic signs of MCT metastasis on systemic health assessment, and (2) absence of palpably enlarged locoregional lymph nodes (LN) and, if enlarged LNs were detected, absence of confirmed metastasis to LNs based on FNA. Additionally, grading of target MCTs was determined using the Scarpa system of cytological grading, with FNAs performed at time of screening. Fine needle aspirates of both the target MCTs and enlarged LNs (when present) were examined by veterinary pathologists at IDEXX BioResearch at West Sacramento, California. 21 In the case of stage IIIa dogs, in which multiple MCTs were present, only a single target MCT was to be treated. Mast cell tumors could be cutaneous anywhere on the body, or subcutaneous if located at or distal to the elbow or hock.
Systemic health assessments included physical examination, modified Karnovsky's performance score for dogs, 22 CBC and serum biochemistry, and urinalysis. These assessments occurred at screening and 7, 28, 42, and 84 days after treatment in each phase of the study. Tumor evaluation was performed on screening and enrollment day. The target MCT was measured using digital calipers to estimate length, width, and thickness and tumor volume then was calculated using a modified ellipsoid formula (tumor volume [T vol] in cm3 = ½ [length × width × thickness]). 23 , 24 All enrolled dogs received concomitant medications (corticosteroids, H1 and H2 antagonists) to decrease the potential for degranulation as described in Table 1, and to maintain consistency of treatments (other than TT) between TT treated and control (sham) dogs. The only medications specifically excluded in the study were nonsteroidal anti‐inflammatory (NSAIDs, no NASIDs were administered up to 7 days before enrollment) and immunosuppressive medications (including cyclosporine and long‐acting corticosteroids), with a medication‐free period of up to 14 days before enrollment. Additionally, no other treatments for cancer were permitted during the study.
TABLE 1.
Dosing schedule for protocol mandated concomitant medication to minimize risk of degranulation reactions in the treated MCT. All dogs in both the tigilanol tiglate and control groups received concomitant medications: Prednisolone/prednisone (0.5 mg/kg PO q12h), diphenhydramine (2 mg/kg PO q12h), and famotidine (0.5 mg/kg PO q12h)
| Protocol mandated concomitant medications | Day 2 | Day 1 | Day 0 (Tx Day) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| am | pm | am | pm | am | pm | am | pm | am | pm | am | pm | am | pm | am | pm | am | pm | am | pm | |
| Corticosteroid (prednisolone/prednisone) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| H1 antagonist (diphenhydramine) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| H2 antagonist (famotidine) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
Abbreviation: MCT, mast cell tumor.
Exclusion criteria were: (1) locoregional LN metastasis confirmed on FNA or signs of systemic MCT disease, (2) tumor ulceration (because it could lead to partial loss of the TT dose during administration), (3) tumor recurrence at a previous biopsy or surgical site, and (4) radiotherapy, chemotherapy, or other anticancer treatment within the previous 2 months. Patients also were excluded if: (1) they had a performance score of 2, 3, or 4 (equivalent to compromised activity, ambulatory only, and completely recumbent or imminent death), (2) calculated dose of TT for treatment was >0.25 mg TT/kg body weight, (3) tumor volume was >10 cm3, or (4) tumor diameter was <1 cm (≥1 cm diameter was required to use RECIST).
In both phases of the study, on the day of treatment (day 0), the area around the target tumor was shaved, the target tumor remeasured and tumor volume recalculated using the same method as at screening. In phase 1, dogs randomized to the control group received no additional treatment other than concomitant medications. Dogs in the TT group received a single intratumoral injection of 1 mg/mL TT (in acetate buffered 40% propylene glycol; QBiotics Group Limited, Yungaburra, Australia) with the dose dependent on tumor volume. The dose rate was 0.5 mg (0.5 mL) TT per cm3 of tumor volume (ie, 50%v/v TT/tumor), except when the estimated tumor volume was <0.2 cm3 in which instance a minimum fixed dose of 0.1 mL was injected. Tigilanol tiglate was administered intratumorally using a 23‐gauge needle on a Luer‐lock syringe at a single injection point (where possible) and the solution “fanned” throughout the tumor to evenly distribute the drug. Sedation could be used if considered appropriate by the treating veterinarian. As part of the safety assessment protocol for the study, all dogs (TT treated and control) were hospitalized overnight at the veterinary facility on the day of treatment, with cage‐side monitoring of vital signs (heart rate, respiratory rate, and temperature) performed at 2, 4, and 8 hours after treatment. For phase 2, all dogs that were treated with TT followed the same protocol as dogs in the TT group of phase 1 as outlined above, with dogs receiving concomitant medications beginning on day 28 (Table 1), and treatment or retreatment with TT occurring on day 30.
After treatment, dogs were permited to receive analgesics, antibiotics, and antiemetics at the veterinarian's discretion. It was recommended that wounds formed at the treatment site after tumor slough be left to heal by secondary intention without bandaging or other interventions. If excessive licking or self‐trauma to the site occurred, an Elizabethan collar or a dry gauze bandage could be used.
For 17 dogs where the IDEXX clinical pathologist was unable to categorically grade the MCT according to study protocol, grading subsequently was assigned as suspected high or suspected low based on the pathologist's professional judgment of possible grade as recorded in the comments section of the laboratory report.
2.4. Assessment of treatment response
Dogs were assessed by treatment‐blinded investigators at 2, 4, and 8 hours and at 1, 4, 7, 14, 21, 28, 42, and 84 days after treatment. Digital photographs of the target tumor were taken at all assessment times to record the size and condition of the target tumor and of the wound resulting from tumor slough in the TT treated dogs. Wound length and width (cm) were measured at 7, 14, 28, 42, and 84 days after treatment. Blood and urine samples for CBC, serum biochemistry, and urinalysis were taken at screening and at 7, 28, and 42 days after treatment.
Tumor response was evaluated 28 days after treatment and categorized as either CR or not complete response (Not CR). Other RECIST subcategories: objective response, partial response, stable disease, or progressive disease were not considered relevant to analyzing efficacy of TT because it is a local treatment and the aim was complete resolution of the target tumor. 20 For dogs with CR at 28 days, further blinded assessments were made at 42 and 84 days.
Wounds that formed around the tissue defect at the treatment site after tumor necrosis and slough were measured to estimate maximum wound surface area. This estimate of wound size (W SA) was derived from the product of the length of longest wound axis (a) by breadth (b) at widest point of the wound and then applying the formula for an ellipse: WSA = π × a × b.
Possible determinants of enduring efficacy of TT treatment were examined using multivariable analyses and comparing the prevalence of CR at 84 days for dogs that had received a single treatment across both phases of the study. For these analyses, we made a conservative assumption, based on TT's mode of action, that whenever phase 1 TT treated dogs were Not CR at 28 days, they would have remained Not CR at 84 days (Figure S1). Potential determinants considered were: (1) tumor location, (2) tumor volume at treatment, (3) tumor cytological grade, (4) locoregional LN enlargement (without confirmed MCT disease on FNA at screening), (5) wound formation at the treatment site after tumor slough (yes/no), and (6) maximum size of the wound that developed after tumor slough relative to initial volume of the treated tumor (W SA/T vol). A separate analysis used a subset of the dogs to determine whether any difference in CR existed between cutaneous and subcutaneous tumors on lower limbs.
Safety and tolerability of TT were assessed by reporting AEs using VCOG categories, 25 physical examination findings, as well as serum biochemistry, hematology, and urinalysis results. We adopted the US FDA definition of an AE 26 as “any undesired event, expected or not, occurring to a study animal, regardless if the event was considered to have a causal relationship to the study treatment.” Adverse events were classified as any change from baseline values at screening, whether or not veterinary intervention had occurred, and were ranked by the investigators on their severity 26 and likelihood that TT treatment contributed to the event. Adverse events were recorded in both TT treated and control groups during phase 1 at all assessment points in the study to allow comparison of possible effects associated with concomitant medications or characteristics of the general patient population.
Owner assessments of their dogs, health‐related QoL over the first 28 days (specifically at screening, and 0, 7, 14 and 28 days) after treatment were gathered using a questionnaire developed specifically for veterinary oncology patients. 27
2.5. Statistical analysis
Statistical analyses were performed using Stata (version 15, StataCorp, College Station, Texas). Exact binomial 95% confidence intervals (CIs) for proportions were calculated using Stata's ‐cii‐ command. Probabilities of CR at 28 days were compared between TT treated and control dogs using a generalized linear model with binomial error term and log link fitted using Stata's ‐binreg‐ command and odds were compared using exact logistic regression models fitted using Stata's ‐exlogistic‐ command. Potential determinants of efficacy (CR at 84 days) were assessed using exact and maximum likelihood logistic regression models fitted using Stata's ‐exlogistic‐ and ‐logistic‐ commands, respectively.
Quality of life data were analyzed in Minitab (version 19, Minitab, State College, Pennsylvania) using Kruskal‐Wallis comparison of rank medians tests.
3. RESULTS
3.1. Patient demographics and tumor characteristics
Of 155 dogs screened, 123 satisfied the eligibility criteria and were enrolled.
In phase 1, 81 dogs were randomized to the TT treatment group and 42 dogs to the control group. No marked differences in demographics or tumor characteristics were identified between these 2 groups (Table 2), with these characteristics reflective of MCT patient populations reported in the literature. 1 , 2 , 3 , 28 , 29
TABLE 2.
Demographics and tumor characteristics of dogs in phase 1 of the study (tigilanol tiglate‐treatment group n = 81, control group n = 42)
| Variable | Tigilanol tiglate treated group | Control group |
|---|---|---|
| Demographics | ||
| Age (y) at screening | ||
| Mean (range) | 8.8 (3.5‐16) | 8.7 (4‐15) |
| Sex | ||
| Female | 49 (60%) | 26 (62%) |
| Male | 32 (40%) | 16 (38%) |
| Weight on treatment day (kg) | ||
| Mean (range) | 25.1 (3.2‐55.4) | 23.1 (5.2‐64) |
| Breed | ||
| High MCT‐risk breeds a | 44 (54%) | 25 (60%) |
| Other breeds | 37 (46%) | 17 (40%) |
| Tumor characteristics | ||
| Tumor location | ||
| Body | 34 (42%) | 15 (36%) |
| Upper limb | 17 (21%) | 3 (7%) |
| Lower limb | 30 (37%) | 24 (57%) |
| Tumor volume on day 0 (cm 3 ) | ||
| <0.5 | 25 (31%) | 17 (40%) |
| 0.5 to <2 | 31 (38%) | 14 (33%) |
| 2‐10 | 25 (31%) | 11 (26%) |
| Median (25th and 75th percentiles) | 1.1 (0.4, 2.6) | 0.6 (0.3, 2.0) |
| Cytological grade of tumor | ||
| High | 5 (6%) | 4 (10%) |
| High suspected | 2 (3%) | 2 (5%) |
| Low | 57 (74%) | 28 (70%) |
| Low suspected | 13 (17%) | 6 (15%) |
| Grade not available | 4 (5%) | 2 (5%) |
| Regional lymph node(s) enlarged at screening | ||
| No | 74 (91%) | 35 (83%) |
| Yes b | 7 (9%) | 7 (17%) |
| Tumor type | ||
| Cutaneous | 74 (91%) | 35 (83%) |
| Subcutaneous | 7 (9%) | 7 (17%) |
Abbreviation: MCT, mast cell tumor.
Dog breeds with known high risk of MCT; consisted of boxers and other brachycephalic breeds, Staffordshire bull terriers, Labradors, Golden retrievers, Rhodesian ridgebacks, Beagles, and Mastiffs.
Regional lymph nodes that were enlarged on palpation at screening but no MCT metastases was found on fine needle aspiration, allowing the dog to be eligible for enrolment in the study; no aspirates were collected where lymph nodes were not enlarged.
3.2. Efficacy
For analysis of the study's primary efficacy objective in comparing response after 28 days for dogs that had received a single intratumoral TT injection with that of the control group, 4 control dogs and 1 TT treated dog were removed from the data set for minor protocol deviations on agreement from the CVM‐FDA (1 dog for administration of NSAIDs and 2 dogs for extended use of corticosteroids, 1 dog had its 28 day assessment undertaken by an unblinded treating veterinarian which could have introduced misclassification bias, and 1 dog had an incidental mass diagnosed in its stomach and was removed from the study on day 7 of phase 1), whereas a second TT treated dog was reassigned from CR to Not CR because of an ambiguous data record. Based on this decreased data set, 75% of TT treated dogs (60/80) achieved CR compared with only 5% (2/38) of the control dogs (Table 3). The probability of CR by 28 days in TT treated dogs was estimated to be 14.3 times higher (95% CI = 3.7‐55.2; P < .001) relative to control dogs. Similarly, a very strong association was evident from odds ratios (Table S1).
TABLE 3.
Summary of primary efficacy data after treatment with tigilanol tiglate. The primary efficacy objective is a comparison of the percent of dogs achieving CR at 28 days after either treatment with tigilanol tiglate or an untreated control group
| Treatment group | Day 28 | |
|---|---|---|
| No. dogsCR/total no. dogs | %CR (95% CI) | |
| TT treated (based on full data set) a | 62/81 | 77 (66‐85) |
| TT treated (exclusions for protocol deviations) b | 60/80 | 75 (61‐86) |
| Control | 2/42 | 5 (1‐17) |
| Control (exclusions for protocol deviations) b | 2/38 | 5 (1‐19) |
Abbreviations: CR, complete response; FNA, fine needle aspirate; MCT, mast cell tumor; TT, tigilanol tiglate.
Note that in the TT group, 28 of the 81 dogs were sedated for administration of the treatment, of the 28 sedated dogs 71% were CR (20/28).
In calculating this primary efficacy outcome for the study comparing TT treated and control groups, 4 control dogs and 1 TT treated dog were excluded from the data set for minor protocol deviations and a second TT treated dog that was CR at day 28 was reassigned to the Not CR category due to MCT being detected on FNA at Day 84 on request from the regulatory agency. Day 28 CR based on analysis with these changes was 75% (60/80) and this is the CR value that is reported as the primary efficacy outcome in the abstract and body of this article.
For analyses of the study's secondary objectives related to AEs, determination of incidence of local recurrence of treated tumors after 28 days, identifying determinants of efficacy, and describing wound formation and wound healing, all dogs available for each assessment time to 84 days were used. Details of the number of dogs in each treatment group during each phase of the study, their treatment response, and the numbers and reasons they were lost to follow‐up, are summarized in Figure S1.
Two secondary objectives of the study related to efficacy. These were to evaluate: (1) enduring efficacy of a single treatment to 84 days and (2) responsiveness to retreatment. For the first of these, phase 1 TT treated dogs that had achieved CR at 28 days were reassessed at 84 days with 93% (55; 95% CI = 84‐98) of 59 dogs available for assessment having no local recurrence. The 28‐day data from phase 1 TT treated dogs were combined with that for control dogs from phase 1 that had been treated with TT in phase 2. From this combined data, the overall CR at 28 days after a single TT treatment was 73% with 94% of the CR dogs having no local recurrence of the target tumor by 84 days (Table 4).
TABLE 4.
Summary of secondary efficacy data after treatment with tigilanol tiglate. The secondary efficacy objectives relate to CR at 28 days and the percent of dogs with no local recurrence of the target tumor by 84 days. The first group is for dogs receiving a single treatment either in phase 1 or phase 2, the second group is for the original phase 1 group after either 1 or 2 treatments
| Treatment group | Day 28 | Day 84 | ||
|---|---|---|---|---|
| No. dogs CR/total no. dogs | %CR (95% CI) | No. dogs CR/total no. dogs | %CR (95% CI) | |
| Single treatment with TT (phase 1 and 2) | 85/116 a | 73 (64‐81) | 77/82 c | 94 (85‐97) |
| Original phase 1 TT treated dogs (after 1 or 2 treatments) | 70/80 b | 88 (77‐93) | 62/67 b | 93 (82‐97) |
Abbreviations: CR, complete response; FNA, fine needle aspirate; MCT, mast cell tumor; TT, tigilanol tiglate.
Sixty‐two of the 81 dogs in phase 1 and 23 of the 35 dogs in phase 2 at 28 days post treatment (Figure S1). The TT treated dog that was CR at day 28 and reassigned to the Not CR category due to MCT being detected on FNA at Day 84 on request from the regulatory agency was reverted back to CR at Day 28 for this analysis on the assumption that local recurrence occurred after Day 28. Of the 18 phase 1 TT treated dogs that were not CR at day 28, we assumed for this analysis that, had they not been retreated, none would have been CR on day 84.
Efficacy outcomes for dogs treated in phase 1 that did not achieve CR and were retreated in phase 2; 62 of the 81 dogs in phase 1 and 8 out of the 18 phase 1 Not CR dogs retreated in phase 2; of 18 dogs treated in phase 2, 44% (8; 95% CI = 22‐69) were CR at Day 28 and 88% had no local recurrence at Day 84 (7; 95% CI = 35‐97) (see Figure S1 for further details).
55 out of 59 dogs in phase 1 and 22 out of 23 dogs in phase 2 at 84 days post treatment (see Figure S1 for further details).
With respect to responsiveness to retreatment, 18 dogs that did not achieve CR after their first TT treatment received a second TT treatment. Twenty‐eight days later, 44% of these retreated dogs achieved CR (Table 4). Combining data for retreated dogs with that of dogs from phase 1 that achieved CR after a single TT treatment, overall CR for this group at 28 days was 88%, with 93% of the CR dogs having no local recurrence 84 days after their last treatment (Table 4).
In all dogs that achieved CR after TT treatment, a consistent pattern of clinical response at the treatment site was observed. This response involved the development of bruising and edema at the treatment site within 15 minutes to 24 hours post‐treatment, the onset of hemorrhagic necrosis of the mass followed by tumor slough within 3 to 10 days resulting in exposure of a well granulated underlying wound bed that healed by secondary intention. Typical examples of the progression of clinical response to TT treatment are shown in Figure 2.
FIGURE 2.
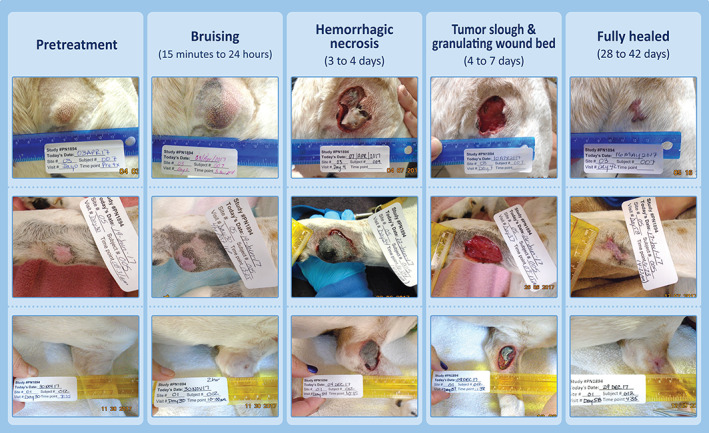
Photo series showing examples of the typical progression of clinical response after treatment of a single MCT with tigilanol tiglate. MCTs, mast cell tumors
3.3. Determinants of efficacy
Multivariable analyses identified 3 determinants of CR for the 109 dogs that had received a single TT treatment and were available for assessment at 84 days (Table 5). These determinants were: (1) the formation of a wound after slough of the treated tumor (P = .002), (2) the maximum surface area of that wound relative to initial tumor volume (W SA/T vol; P = .02), and (3) tumor cytological grade (P = .03). In relation to wound formation, of 6 TT treated dogs that did not develop wounds by 14 days after first treatment, none subsequently achieved CR at either 28 or 84 days after this treatment. The odds of CR by 84 days if a wound formed within 14 days of treatment were estimated to be 16.1 times higher than if no wound formed. Furthermore, when a wound formed, CR at 84 days was much more likely for wounds with larger surface areas relative to initial tumor volumes (Table 5, Figure 3). Tumors with a W SA/T vol ratio of ≥4 cm2/cm3 were much more likely to experience CR at 84 days (CR, 85%; 44/52) compared to tumors with small W SA/T vol ratios of >0 to <2 cm2/cm3 (CR, 52%; 12/23; Table 5). Cytological grade also was important. Although efficacy was demonstrated for both low and high‐grade MCTs, CR at 84 days was more likely when cytological grade was low or suspected low (72%) rather than high or suspected high (38%). For other possible determinants (tumor location, tumor volume, locoregional LN enlargement), estimated odds ratios were imprecise and not informative of their effects on CR at 84 days. For the subset of tumors on lower limbs, no significant difference in CR was found at 84 days between cutaneous and subcutaneous tumors (Table 5).
TABLE 5.
Descriptive statistics and results of multivariable analysis with adjusted odds ratios for complete response (CR) of mast cell tumors at day 84 after first treatment with tigilanol tiglate in 109 dogs a and for a subset of 45 dogs with cutaneous or subcutaneous MCT on the lower limb
| (Potential) determinant and categories b | No. dogs | % (no.) that were CR at day 84 after first treatment | Adjusted odds ratio | 95% CI | P value c |
|---|---|---|---|---|---|
| Tumor location | .11 | ||||
| Body | 45 | 67% (30) | 0.83 | 0.3‐2.4 | .74 |
| Upper limb | 19 | 74% (14) | 4.23 | 0.8‐21.1 | .09 |
| Lower limb | 45 | 73% (33) | Reference category | ||
| Tumor volume at time of first treatment (cm 3 ) | .62 | ||||
| <0.5 | 39 | 72% (28) | Reference category | ||
| 0.5 to <2 | 39 | 67% (26) | 0.73 | 0.2‐2.1 | .49 |
| 2‐10 | 31 | 74% (23) | 1.13 | 0.3‐4.0 | .85 |
| Cytological grade of tumor d | .03 | ||||
| Low or low suspected | 97 | 72% (70) | 5.03 | 0.9‐30.3 | .03 |
| High or high suspected | 8 | 38% (3) | Reference category | ||
| Grade not available | 4 | 100% (4) | |||
| Regional lymph node(s) enlarged at screening | .15 | ||||
| No | 96 | 72% (69) | Reference category | ||
| Yes | 13 | 62% (8) | 0.43 | 0.1‐1.9 | .15 |
| Wound formation | .002 | ||||
| No | 5 | 0% (0) | Reference category | ||
| Yes | 104 | 74% (77) | 16.14 | 2.8 to +∞ | .002 |
| Ratio of wound area to tumor volume (WSA /Tvol ) (cm 2 /cm 3 ) | .02 | ||||
| 0 | 5 | 0% (0) | |||
| >0 to <2 | 23 | 52% (12) | Reference category | ||
| 2 to <4 | 29 | 72% (21) | 2.03 | 0.6‐6.9 | .30 |
| 4 to <6 | 15 | 87% (13) | 7.13 | 1.4‐55.8 | .01 |
| ≥6 | 37 | 84% (31) | 5.13 | 1.5‐18.8 | .005 |
| Tumor type on lower limb e | .34 | ||||
| Cutaneous | 32 | 69% (22) | Reference category | ||
| Subcutaneous | 13 | 58% (11) | 2.40 | 0.4‐21.8 | .34 |
Abbreviations: CR, complete response; MCT, mast cell tumor.
See Figure S1.
Model included cytological grade, ratio of wound area to tumor volume, and the potential determinant; only dogs where cytological grade was available and that developed a wound were included (n = 100); exact logistic models were used other than for tumor location and tumor volume where maximum likelihood logistic models were used.
Bolded P values are overall P values for the variables; nonbolded P values are P values for assessing the respective category relative to the reference category.
Adjusted for cytological grade; only dogs where cytological grade was available were included (n = 105); an exact logistic model was used.
Subcutaneous MCTs were able to be treated below the elbow and hock in the study protocol so analysis of this subset was important to rule out possible effects on secondary efficacy.
FIGURE 3.
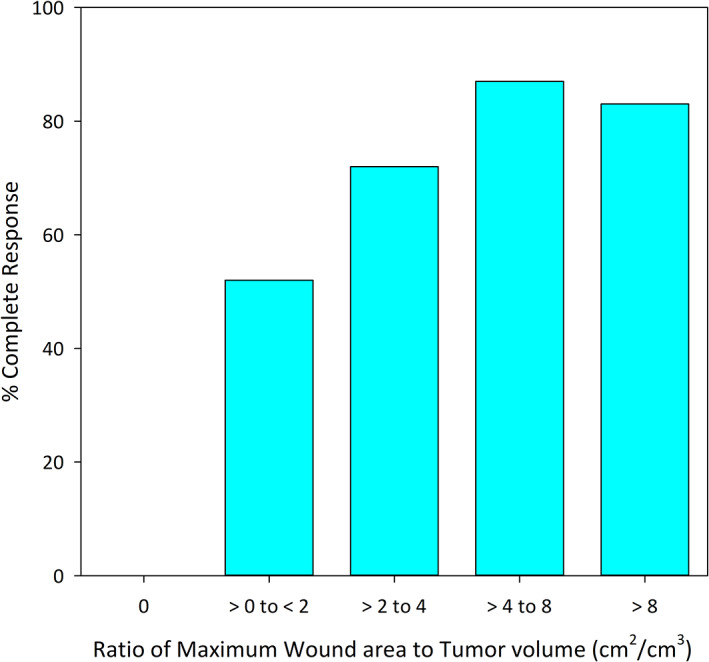
Relationship between the ratio of maximum wound area after tumor slough to initial volume of the target tumor (W SA/T vol) and the percent of dogs achieving complete response (CR) after a single treatment with tigilanol tiglate. Data are for the 109 dogs availablefor assessment 84 days after treatment. CR, complete response
3.4. Safety and tolerability
A total of 587 AEs in 21 VCOG categories were recorded by the investigators over the course of the study in the 117 dogs that received a single TT treatment (phases 1 and 2). Most AEs (94%; 549/587) were grade 1 or 2 with 61% (356) of these low‐grade events considered by the investigators to be definitely, probably, or possibly related to TT treatment. No or minimal veterinary intervention was required in these cases. Thirty‐eight grade 3 and 4 AEs were recorded in the study, 25 of which were considered related to TT treatment (Table 6). The investigators classified 7 AEs as serious (using the FDA definition), 26 with 2 of these possibly related to treatment (see Section 3.4.3 below). The most frequent AE was formation of a wound at the treatment site after tumor slough, a process directly related to the efficacy of the drug in full or partial tumor destruction.
TABLE 6.
The number of grade 3 and 4 AEs that were reported by investigators for all naïve single tigilanol tiglate treated dogs in phase 1 and 2. Adverse Events are classified according to VCOG category and subcategory (AE type)
| VCOG AE category | AE type | No. grade 3 AEs | No. grade 4 AEs | Related to TT (Y = Yes, P = Possible, N = No) a | Time of occurrence posttreatment (d) |
|---|---|---|---|---|---|
| Administration site conditions | Pain at Tx site | 2 | 1 | Y | 0 and 5 |
| Wound at Tx site | 3 | Y | 5, 7, and 12 | ||
| Wound infection | 1 | P | 9 | ||
| Scar contracture | 2 | Y | 33 and 47 | ||
| Blood/bone marrow | Leukocytosis and neutrophilia | 2 | Y | 12 and 26 | |
| Thrombocytopenia | 1 | P | 26 | ||
| Cardiac arrhythmia | Sinus tachycardia | 1 | Y | 0 | |
| Constitutional clinical signs | Lethargy/fatigue/general performance | 1 | P | 12 | |
| Dermatologic/skin | Pruritus | 1 | P | 14 | |
| Gastrointestinal | Constipation | 1 | N | 39 | |
| Inappetence | 1 | P | 21 | ||
| Hemorrhage/bleeding | Ingestion of toxic level of NSAID's | 1 | N | 84 | |
| Metabolic/laboratory | ALP elevation | 1 | N | 8 | |
| ALT elevation | 1 | N | 6 | ||
| CPK elevation | 1 | N | 54 | ||
| GGT elevation | 1 | N | 8 | ||
| Triglyceridemia | 1 | N | 8 | ||
| Musculoskeietal/soft tissue | Extremity (gait/ambulation) lameness at Tx site | 5 | 1 | Y | 0, 1, and 9 |
| Neoplasms benign, malignant, etc | Primary pelvic mass b | 1 | N | 33 | |
| Primary bone neoplasia | 1 | N | 53 | ||
| Neurology | Somnolence/depressed/dullness | 1 | P | 0 | |
| Neuropathy: cranial nerve CNVII | 1 | N | — c | ||
| Neuropathy: motor | 1 | P | 8 | ||
| Renal/genitourinary | Proteinuria | 2 | N | 9 or 29 | |
| Obstruction, urinary | 1 | N | 39 | ||
| Vascular | Thrombosis/thrombus/embolism b | 1 | N | 63 |
Abbreviations: AE, adverse event; MCT, mast cell tumor; TT, tigilanol tiglate; VCOG, Veterinary Cooperative Oncology Group.
Investigator opinions of the potential causes of AEs as whether they were related to TT treatment or not. Yes (Y) are the combined VCOG AE Attribution Standards of Definite/Probable, defined as AEs that is clearly or likely to be related to TT treatment; Possible (P) is the VCOG AE Attribution Standard that is defined as an AE that may or may not be related to TT treatment; No (N) are the combined VCOG AE Attribution Standards of Unrelated/Unlikely, these are AEs that are doubtful or not related to TT treatment.
Death due to thrombus was found on post mortem to be attributable to the development of a primary pelvic mass not related to the treated MCT.
Occurred 16 days prior to TT treatment in a control dog treated in phase 2 of the study.
3.4.1. Wound formation and healing
Ninety‐five percent of the 117 dogs that received a single TT treatment in either phase 1 or 2 developed a wound at the treatment site by 14 days, with 3 of these considered a grade 3 or 4 AE. Wounds resulting from slough of the target tumor were first observed as early as 3 days after treatment, with maximum wound areas in 89% of cases (99/111) recorded 7 days after treatment and at 14 days for the remaining 12 cases. Wounds were less likely to form with small tumor volumes. Of tumors with volumes <0.5 cm3, 12% (5/42) formed no wound compared to 2% (1/43) for tumors of 0.5 to <2.0 cm3 and 0% (0/32) for tumors ≥2.0 cm3.
The average surface area of wounds after tumor slough increased with increasing tumor volume (Figure 4). The mean and the median of maximum wound surface areas for the 117 dogs were 10.5 cm2 and 3.4 cm2, respectively; the size class distribution of these wounds is shown in Figure 5.
FIGURE 4.
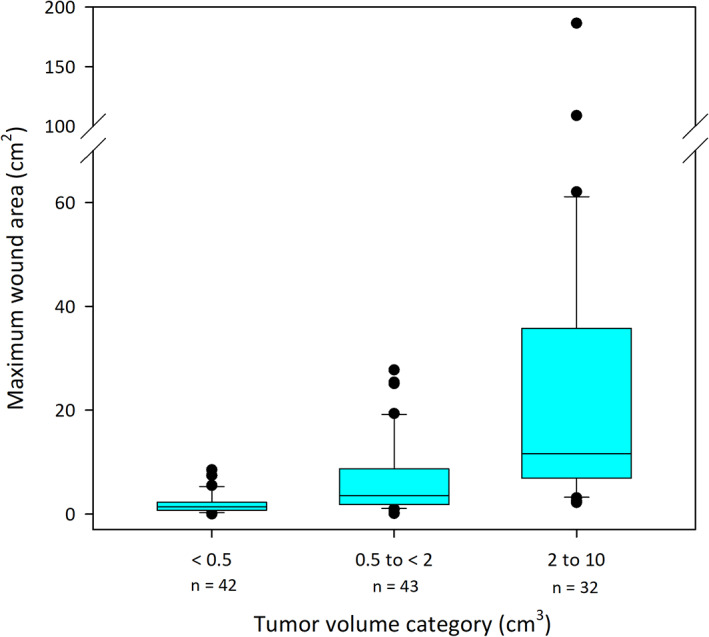
Boxplot of maximum surface area of individual wounds formed after slough of tumors in 117 dogs treated with tigilanol tiglate. Boxes on plot represent the 25th, 50th, and 75th percentiles, whiskers on plot represent the 10th and 90th percentiles. Data are plotted by category of initial tumor volume (<0.5 cm3, 0.5 to <2 cm3, and 2‐10 cm3). Median tumor volumes in each of these categories were 0.3 cm3 (range 0.1‐0.4), 1.1 cm3 (range 0.5‐2.0), and 3.5 cm3 (range 2.1‐9.2), respectively, whereas median maximum wound surface area in each tumor volume category was 1.2 cm2 (range 0‐8.5), 3.4 cm2 (range 0‐27.7), and 11.6 cm2 (range 2.2‐186.4), respectively. Percentages of dogs with CR at 28 days in these 3 tumor volume categories were 67%, 77%, and 75%, respectively. CR, complete response
FIGURE 5.
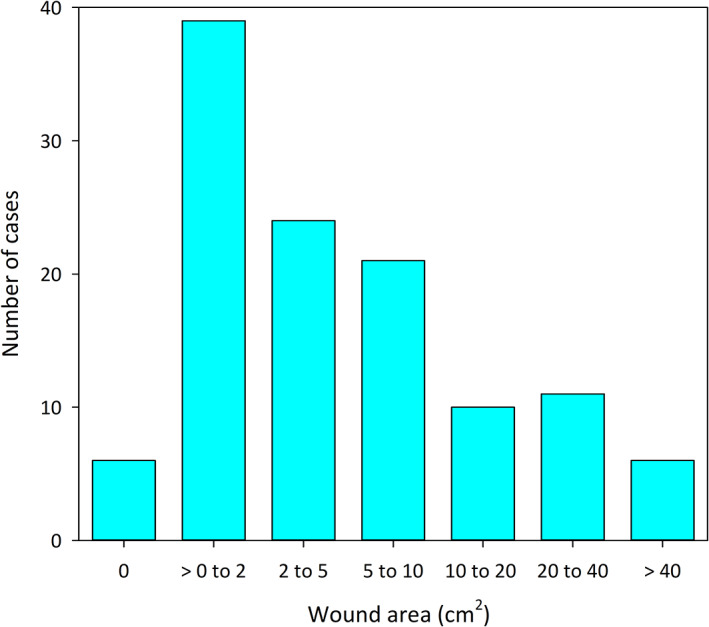
The distribution of maximum wound areas that developed after a single treatment with tigilanol tiglate arranged by number of cases in each of 7 wound size classes
Most wounds were managed by the investigators and owners without bandaging or other interventions and were left to heal by secondary intention. Other than antibiotics prescribed prophylactically in 47 cases, only 5 dogs received active wound management during the study: 1 was treated with antibiotics for cellulitis and bacterial infection (the serious AE case outlined below), 1 was bandaged, 2 wore Elizabethan collars to prevent self‐trauma of the wound, and 1 was irrigated with saline to prevent odor.
The speed of wound healing for the 77 dogs achieving CR after a single TT treatment was related to wound area, with smaller wounds more likely to be healed by 28 and 42 days than larger wounds. Importantly, of the 77 wounds in dogs that were CR at day 84, only 1 required bandaging, 99% of wounds healed uneventfully by secondary intention.
3.4.2. Other frequent AEs
Other than wound formation at the treatment site, 17 other types of frequent AEs (ie, those occurring in >5% of TT treatments) were frequently recorded in the study; these are shown in Figure 6 in comparison to the corresponding frequency of events in the phase 1 control group. Four of these other frequent AEs were expected outcomes of TT treatment related directly to the drug's mode of action in: (1) inducing an acute and transient inflammatory response at the treatment site (injection site or tumor pain, lameness in the treated limb, injection site bruising, erythema or edema, locoregional LN enlargement) and (2) causing hemorrhagic necrosis of the tumor mass resulting in wound formation after tumor slough (Figures 6 and 7).
FIGURE 6.
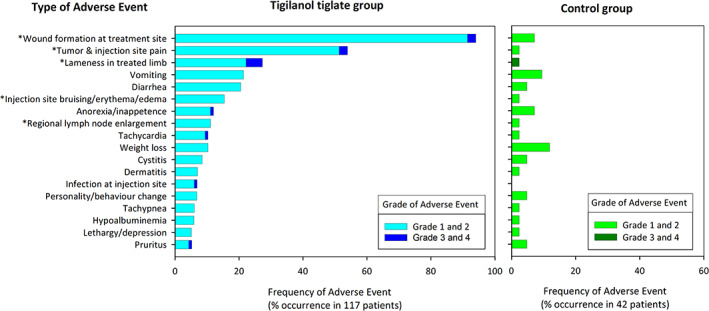
Occurrence and category of adverse events occurring at frequency of >5% in 117 dogs receiving a single treatment of tigilanol tiglate (phase 1 and 2 of the study) and the percentages of their occurrence in 42 control dogs (from phase 1 of the study). Asterisk (*) highlights adverse events that were ranked according to investigator opinion as definite or probably associated with the tigilanol tiglate treatment
FIGURE 7.
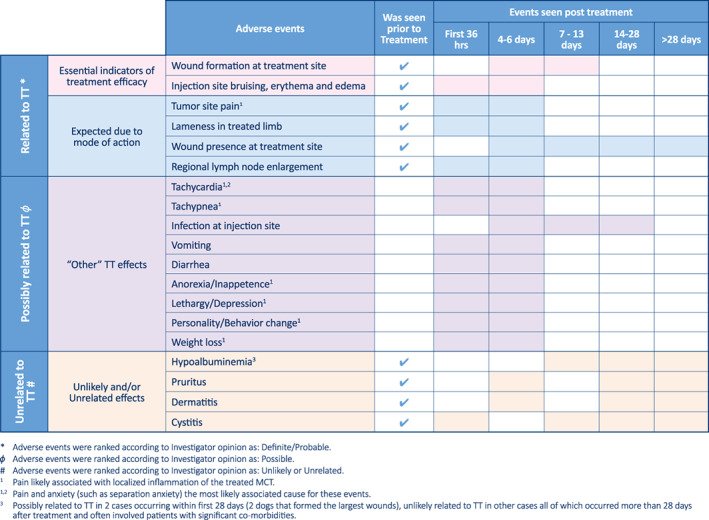
Most frequently recorded adverse events in relation to expected time of onset, and their likely duration, after treatment with tigilanol tiglate. Shading in each row indicates timing at which events were observed
Of the remaining 13 frequent AEs, all occurred in ≤20% of treatments (Figure 6). Investigators considered 9 of these possibly related to TT treatment, with the majority (eg, tachycardia, tachypnea, lethargy, inappetence, weight loss) probably associated with pain associated with the inflammatory response at the tumor site and the tumor destruction process during the first 7 days after treatment (Figure 7). Pain was managed by the investigators by the prescription PO analgesics. For the 117 dogs receiving a single TT treatment, 63% (74/117) received analgesics, with a median course duration of 6 days and an average of 9 days. Sixty‐nine percent of these dogs (51/74) received pain relief within the first 7 days after treatment. Tramadol HCl was the pain medication most often prescribed (78%; 80/103 prescribed courses), with buprenorphine (12 courses) and gabapentin (6 courses) being less commonly used along with Tramadol HCl. Three of the remaining frequent AEs were considered unlikely related to TT treatment. The final frequent AE, low‐grade hypoalbuminemia, had a probable association with TT treatment in 2 dogs that formed the 2 largest wounds (outlier values >100 cm2 in Figure 4). In these 2 dogs, low‐grade hypoalbuminemia (2.2 g/dL and 2.4 g/dL, respectively) compared to the reference range (2.7‐3.9 g/dL) was first recorded at 7 days after treatment; serum albumin concentrations in both dogs returned to normal after 28 days as wound healing progressed. Other occurrences of hypoalbuminemia were considered unlikely to be associated with TT because these occurred 28 days or later after treatment and typically were found in dogs with other comorbidities (eg, urinary tract infection, nephropathy).
3.4.3. Serious AEs
Of the 38 grade 3 and 4 AEs reported after TT treatment (Table 6), investigators considered 7 to be serious, 26 2 of which were considered likely related to treatment. One of these likely TT related AEs involved development of a bacterial infection (cultured mixed aerobes and anaerobes) and cellulitis at the wound site 7 days after treatment. After a night in hospital, IV fluids and antibiotics (enrofloxacin 4 mg/kg PO q24h for 10 days concurrently with a course of amoxicillin/clavulanic acid 15.5 mg/kg PO q12h for 28 days), the cellulitis and swelling resolved by day 14 and the wound was well granulated by day 19. The second AE considered possibly TT related occurred in a 15‐year old poodle with clinically relevant comorbidities which was euthanized at the owner's request 82 days after treatment because of deteriorating QoL and the owner's assessment of pain because of tumor recurrence at the treatment site. The other 5 serious AEs, considered unrelated to TT treatment, were severe thrombocytopenia (likely associated with previously undiagnosed concurrent hepatic neoplasia, euthanasia was elected 25 days after treatment), primary bone neoplasia (euthanasia was elected 83 days after treatment), 1 case of hemorrhage after ingestion of toxic amounts of the owner's personal NSAIDs, 1 case of neuropathy caused by traumatic injury in a control (sham) dog from playing with another dog, and 1 case with a primary pelvic mass resulting in death because of thrombosis 71 days after treatment.
3.4.4. Quality of life assessment
In the QoL survey completed by owners, differences (P < .05) between TT treated and control dogs were found in only 6 questions at specific times after treatment (Table S2). Compared to control dogs, TT treated dogs were assessed as enjoying life slightly less and being in slightly more pain at day 7, and being slightly less active and less mobile at 7 and 14 days, than control dogs. In contrast, at 14 and 28 days, owners of TT treated dogs considered their dogs' health to have improved compared to owners of control dogs both since the previous visit and since the initial diagnosis of MCT.
4. DISCUSSION
We have demonstrated that, when used with concomitant medications to minimize potential degranulation events, TT administered intratumorally was efficacious as a local treatment for MCTs, either cutaneous anywhere on the body or subcutaneous at or distal to the elbow or hock. This effect included at sites where surgical excision with complete margins may have been problematic (eg, on lower limbs), and for patient groups at higher anesthetic and surgical risk. The drug was well tolerated with dogs exhibiting a relatively good QoL when assessed by their owners and with the majority of AEs requiring no veterinary intervention. We also identified 3 important determinants of efficacy of the drug: (1) the formation of a wound after slough of the treated tumor, (2) the size of that wound relative to original tumor volume, and (3) tumor cytological grade.
The efficacy results from this study are consistent with:
Five pilot clinical MCT studies in Australia, using the same dosing protocols, where CR at 28 days ranged from 70% to 90%, with an overall CR for 72 TT treated dogs of 82% (Table S3); and,
A thermo‐imaging study that followed response in 21 individual MCTs injected with TT and recorded 76% CR after 28 days (Matera et al, University of Sao Paulo, Brazil, manuscript in preparation).
The efficacy data also suggest the suitability of TT treatment for situations where surgical options for treating MCT can be difficult or risky. For example, almost 50% of the tumors treated in the study were located in peripheral areas such as on lower limbs or the perineal region, sites where routine surgical excision with clean margins often would be difficult in a primary care setting and could risk complications such as damage to surrounding vital tissues, dehiscence of the closed excisional wound or both. The CR achieved in our study at these sites after a single treatment with TT was similar to that at other locations more amenable to routine surgery (eg, trunk and upper limbs). For example, CR at 84 days on lower limbs was 73% compared to 74% for upper limbs (Table 5) and, when the 6 perineal cases are separated from the body category in this table, their CR of 67% (4 of 6 dogs) is similar to that for the rest of the body category. Additionally, many of the dogs in the study were from demographics associated with higher anesthetic death risk, including breed, age, and having clinical relevant comorbidities. 13 , 14 Thirty‐two percent of TT treated dogs were considered high risk breeds including brachycephalics, spaniels or toy breeds, and almost half of these dogs also had clinically relevant comorbidities. The CR for these higher risk dogs at 84 days was 73% (24/33) compared to 78% (53/68) CR for lower risk breeds. Similarly, in relation to age, some studies suggest that age may increase the risk of anesthetic deaths. 13 Dogs >8 years old comprised slightly more than half of the patient population in our study, with the CR at 84 days for this age group being 88% (42/48). Although sedation was used by investigators to facilitate treatment of some dogs >8 years of age (31%; 21/68) and in some high anesthetic death risk breeds (36%; 12/33), the ability to use TT for local treatment of MCTs in such dogs without the necessity for general anesthesia suggests the drug could be a particularly attractive approach for such higher risk patient groups.
Wounds resulting from the destruction of the MCT by TT are an expected feature of successful treatment. Consequently, it is not surprising that wound formation after slough of the treated tumor and the size of the resulting wound relative to the volume of that tumor were critical determinants of TT efficacy. Because dosing of TT is calculated on tumor volume, wound size relative to tumor volume also is directly related to the wound size per mL TT dose administered to the target tumor. Essentially, wound size‐to tumor volume can be seen as a measure of whether an effective dose has been delivered to achieve complete tumor resolution. It is notable that all 6 dogs that did not develop wounds in our study had small tumors (0.1‐0.6 cm3) and none achieved CR. Furthermore, within this <0.6 cm3 tumor volume category, when wound size relative to original tumor volume (W SA/T vol) also was low (<2 cm2/cm3), CR at 84 days was only 36% (4 of 11 dogs). This observation reinforces the importance of wound size relative to tumor volume as a measure of efficacy and suggests that some difficulty may have occurred with effective dose administration into some smaller tumors. Difficulties in accurately measuring small tumors and uniformly injecting small volumes of drug also have been recognized recently for intratumoral dosing in immunotherapy studies in humans. 30
Tumor cytological grade was identified as a third determinant of efficacy of TT. Based on cytological grading alone, high‐grade MCTs, which occurred in 11% of the patient population in our study, were apparently less responsive to a single TT treatment than were low‐grade MCTs. Although tentative because based on a small number of dogs, this outcome is not unsurprising because high‐grade MCTs (based on histological grading) are well known to have poor outcomes in response rates to single or combination treatments 8 and in median survival times. 29 , 31 Nonetheless, TT did deliver clinical benefit to 4 dogs in the study with high‐grade MCT with enlarged locoregional LNs where metastasis was not confirmed on FNAs.
In terms of safety and tolerability, most AEs were low‐grade, transient, and manageable. Wound formation at the treatment site, the most frequently reported AE, was considered low grade in all but 3 cases and, as demonstrated in our study, is essential for efficacy. Four other frequent AEs: injection site pain; injection site bruising, erythema and edema; lameness in treated limbs; and locoregional LN enlargement, also are directly associated with the mode of action of TT in inducing a localized, acute, and transient inflammatory response. 15 This inflammatory response contributes to tumor destruction and site healing by rapidly isolating the tumor mass, recruiting innate immune cells to the treatment site, and initiating subsequent cytokine and chemokine and immune‐mediated wound healing responses to resolve the tissue deficit after tumor slough. 15 , 16 , 17 These 5 event types comprised approximately 38% (222/587) of all AEs. As well as being AEs, they also could be described as positive indicators of efficacy. Of the 9 other AE types recorded by investigators at a frequency of >5% and considered possibly TT related, all occurred in ≤20% of treatments. Most of the second group of TT related events likely represent responses of the patients to the localized pain associated with the inflammatory and tumor necrosis responses at the treatment site. Although a low level of very short‐term systemic exposure to TT has been reported after intratumoral treatment with the drug, 16 , 18 this explanation is much less likely because these AEs are not consistent with results seen in IV toxicity studies using the drug. This overall profile of low‐grade and transient AEs within the first days after treatment is consistent with owner assessments from the QoL survey and shows that the transient AEs of the drug are manageable and well tolerated.
Our study had some limitations. It relied on cytological grading, complete clinical staging of MCT disease was not undertaken and the relatively short 84‐day post‐treatment follow‐up time was relatively short. Although cytological grading is widely used and is gaining traction as an alternative that is less invasive, 32 histological grading still is considered the gold standard. The nature and logistics of our study in delivering an intratumoral agent however necessitated the use of cytological grading to avoid leakage of the drug from the biopsy site that would have been required for histology. To minimize potential differences in cytological grading results in our study, a single centralized laboratory was used for all assessments although different clinical pathologists did score some FNAs. Staging in our study also was limited to ruling out metastasis by the absence of clinical signs and assessing possible local metastasis of any palpably enlarged locoregional LNs by FNAs. It is acknowledged that this approach is not complete staging because even palpably normal LNs can harbor metastases that can be missed on FNA. 32 , 33 Although staging is gold standard, especially in the referral practice, in the general care setting staging is rarely pursued further unless a high‐grade diagnosis is obtained either on FNA cytology or histological examination, or if clinical signs of metastasis are evident. Because of the rapid mode of action of the drug in tumor destruction, we believe the 84 day assessment time provided a strong indication of the likely prolonged treatment response but we also understand that longer term follow‐up will be needed to provide longitudinal evidence of the durability of response and better facilitate future comparison to other treatment options.
Overall, TT was an easy‐to‐administer local intratumoral treatment that was efficacious and provides a new addition to methods veterinarians currently use to treat MCTs in dogs, particularly in the primary care setting.
CONFLICT OF INTEREST DECLARATION
Drs T. R. De Ridder, J. E. Campbell, P. D. Jones, P. F. Schmidt, V. Gordon, and P. Reddell are employed by QBiotics Group Limited. QBiotics Group Limited owns the intellectual property and patents associated with tigilanol tiglate. Drs C. M. J. and J. M. receive payments as independent consultants and service providers to QBiotics Group Limited. Drs C. Burke‐Schwarz, D. Clegg, E. L. Elliot, S. Geller, W. Kozak, S. T. Pittenger, J. B. Pruitt, J. Riehl, J. White, and M. L. Wiest are employed at the veterinary study trial sites that received compensation for participating in the study on a set fee per case enrolled and treated.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Institutional animal ethics was not required for this study as it was under a United States Center for Veterinary Medicine—Food and Drug Administration Protocol—Investigational New Animal Drug (INAD) No. I‐012436 (July 25, 2016).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We gratefully acknowledge the clinicians who contributed to the case data at each trial site. We also thank Dr Stewart Lowden and other members of the QBiotics Group technical team for their support during implementation of the study. Triveritas Limited provided independent VICH GCP study management for the trial. Sheryl Pacchiardi formatted and proof‐read the manuscript. This study was funded by QBiotics Group Limited. The data sets generated and analyzed for this study are not publicly available due to being part of a data package to support the evaluation of TT with regulatory authorities for the purpose of registration and commercialization. They may be available from QBiotics Group upon reasonable requests after an assessment process.
De Ridder TR, Campbell JE, Burke‐Schwarz C, et al. Randomized controlled clinical study evaluating the efficacy and safety of intratumoral treatment of canine mast cell tumors with tigilanol tiglate (EBC‐46). J Vet Intern Med. 2021;35:415–429. 10.1111/jvim.15806
Funding information QBiotics Group Limited
REFERENCES
- 1. Welle MM, Bley CR, Howard J, Rüfenacht S. Canine mast cell tumours: a review of the pathogenesis, clinical features, pathology and treatment. Vet Dermatol. 2008;19(6):321‐339. [DOI] [PubMed] [Google Scholar]
- 2. Blackwood L, Murphy S, Buracco P, et al. European consensus document on mast cell tumours in dogs and cats. Vet Comp Oncol. 2012;10(3):1‐29. [DOI] [PubMed] [Google Scholar]
- 3. London CA, Seguin B. Mast cell tumors in the dog. Vet Clin North Am Small Anim Pract. 2003;33(3):473‐489. [DOI] [PubMed] [Google Scholar]
- 4. Simpson AM, Ludwig LL, Newman SJ, Bergman PJ, Hottinger HA, Patnaik AK. Evaluation of surgical margins required for complete excision of cutaneous mast cell tumors in dogs. J Am Vet Med Assoc. 2004;224(2):236‐240. [DOI] [PubMed] [Google Scholar]
- 5. Milovancev M, Townsend KL, Tuohy JL, et al. Long‐term outcomes of dogs undergoing surgical resection of mast cell tumors and soft tissue sarcomas: a prospective 2‐year‐long study. Vet Surg. 2019;49(1):96‐105. [DOI] [PubMed] [Google Scholar]
- 6. Schultheiss PC, Gardiner DW, Rao S, Olea‐Popelka F, Tuohy JL. Association of histologic tumor characteristics and size of surgical margins with clinical outcome after surgical removal of cutaneous mast cell tumors in dogs. J Am Vet Med Assoc. 2011;238(11):1464‐1469. [DOI] [PubMed] [Google Scholar]
- 7. Pratschke KM, Atherton MJ, Sillito JA, Lamm CG. Evaluation of a modified proportional margins approach for surgical resection of mast cell tumors in dogs: 40 cases (2008–2012). J Am Vet Med Assoc. 2013;243(10):1436‐1441. [DOI] [PubMed] [Google Scholar]
- 8. Donnelly L, Mullin C, Balko J, et al. Evaluation of histological grade and histologically tumour‐free margins as predictors of local recurrence in completely excised canine mast cell tumours. Vet Comp Oncol. 2015;13(1):70‐76. [DOI] [PubMed] [Google Scholar]
- 9. Sfiligoi G, Rassnick KM, Scarlett JM, Northrup NC, Gieger TL. Outcome of dogs with mast cell tumors in the inguinal or perineal region versus other cutaneous locations: 124 cases (1990‐2001). J Am Vet Med Assoc. 2005;226(8):1368‐1374. [DOI] [PubMed] [Google Scholar]
- 10. Garrett L. Canine mast cell tumors: diagnosis, treatment, and prognosis. Vet Med Res Rep. 2014;49:49‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thamm DH, Turek MM, Vail DM. Outcome and prognostic factors following adjuvant prednisone/vinblastine chemotherapy for high‐risk canine mast cell tumour: 61 cases. J Vet Med Sci. 2006;68:581‐587. [DOI] [PubMed] [Google Scholar]
- 12. Thamm DH. Mast cell tumors In: Withrow SJ, Vail DM, Page RL, eds. Withrow & MacEwen's Small Animal Clinical Oncology. St. Louis, Missouri: W.B. Saunders; 2007:402‐424. https://www.sciencedirect.com/science/article/pii/B9780721605586500228. Accessed July 18, 2019. [Google Scholar]
- 13. Brodbelt DC, Pfeiffer DU, Young LE, Wood JLN. Results of the confidential enquiry into perioperative small animal fatalities regarding risk factors for anesthetic‐related death in dogs. J Am Vet Med Assoc. 2008;233(7):1096‐1104. [DOI] [PubMed] [Google Scholar]
- 14. Brodbelt D. Perioperative mortality in small animal anaesthesia. Vet J. 2009;182(2):152‐161. 10.1016/j.tvjl.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 15. Boyle GM, D'Souza MMA, Pierce CJ, et al. Intra‐lesional injection of the novel PKC activator EBC‐46 rapidly ablates tumors in mouse models. PLoS One. 2014;9(10):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Panizza BJ, de Souza P, Cooper A, Roohullah A, Karapetis CS, Lickliter JD. Phase I dose‐escalation study to determine the safety, tolerability, preliminary efficacy and pharmacokinetics of an intratumoral injection of tigilanol tiglate (EBC‐46). EBioMedicine. 2019;50:433‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnett CME, Broit N, Yap P‐Y, et al. Optimising intratumoral treatment of head and neck squamous cell carcinoma models with the diterpene ester Tigilanol tiglate. Invest New Drugs. 2019;37(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 18. Miller J, Campbell J, Blum A, et al. Dose characterization of the investigational anticancer drug tigilanol tiglate (EBC‐46) in the local treatment of canine mast cell tumors. Front Vet Sci. 2019;6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell J, Poulos C, Lowden S. Using triamcinolone in combination with the investigational anticancer agent EBC‐46 ( tigilanol tiglate ) in the local treatment of a canine subcutaneous mast cell tumour. CVE Control Ther Ser. 2017;46(286):11‐17. [Google Scholar]
- 20. Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2015;13(3):176‐183. [DOI] [PubMed] [Google Scholar]
- 21. Sabattini S, Scarpa F, Berlato D, Bettini G. Histologic grading of canine mast cell tumor. Vet Pathol. 2015;52(1):70‐73. [DOI] [PubMed] [Google Scholar]
- 22. Valladão ML, Scarpelli KC, Metze K. Clinical utility of a life quality score in dogs with canine transmissible venereal tumor treated by vincristine chemotherapy. Arq Bras Med Vet Zootec. 2010;62(5):1086‐1093. [Google Scholar]
- 23. Celikoglu F, Celikoglu SI, Goldberg EP. Techniques for intratumoral chemotherapy of lung cancer by bronchoscopic drug delivery. Cancer Ther. 2008;6(6):545‐552. [Google Scholar]
- 24. Monga SP, Wadleigh R, Sharma A, et al. Intratumoral therapy of cisplatin/epinephrine injectable gel for palliation in patients with obstructive esophageal cancer. Am J Clin Oncol. 2000;23(4):386‐392. [DOI] [PubMed] [Google Scholar]
- 25. VCOG‐CTCAE . Veterinary cooperative oncology group ‐ common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2016;14(4):417‐446. 10.1111/vco.283. [DOI] [PubMed] [Google Scholar]
- 26. US Government . CFR ‐ Code of Federal Regulations Title 21. Part 312.32 (a) ‐ Investigational New Drugs Application (IND). US Food & Drug Administration (FDA); 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=312.32. Accessed October 29, 2019.
- 27. Lynch S, Savary‐Bataille K, Leeuw B, Argyle DJ. Development of a questionnaire assessing health‐related quality‐of‐life in dogs and cats with cancer. Vet Comp Oncol. 2011;9(3):172‐182. [DOI] [PubMed] [Google Scholar]
- 28. Shoop SJ, Marlow S, Church DB, et al. Prevalence and risk factors for mast cell tumours in dogs in England. Canine Genet Epidemiol. 2015;2(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Şmiech A, Şlaska B, Łopuszyński W, Jasik A, Bochyńska D, Dabrowski R. Epidemiological assessment of the risk of canine mast cell tumours based on the Kiupel two‐grade malignancy classification. Acta Vet Scand. 2018;60(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marabelle A, Andtbacka R, Harrington K, et al. Starting the fight in the tumor: expert recommendations for the development of human intratumoral immunotherapy (HIT‐IT). Ann Oncol. 2018;29(11):2163‐2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Camus MS, Priest HL, Koehler JW, et al. Cytologic criteria for mast cell tumor grading in dogs with evaluation of clinical outcome. Vet Pathol. 2016;53(6):1‐7. [DOI] [PubMed] [Google Scholar]
- 32. Kiupel M, Camus M. Diagnosis and prognosis of canine cutaneous mast cell tumors. Vet Clin North Am Small Anim Pract. 2019;49(5):819‐836. [DOI] [PubMed] [Google Scholar]
- 33. Mutz ML, Boudreaux BB, Royal A, et al. Cytologic comparison of the percentage of mast cells in lymph node aspirate samples from clinically normal dogs versus dogs with allergic dermatologic disease and dogs with cutaneous mast cell tumors. J Am Vet Med Assoc. 2017;251(4):421‐428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


