Abstract
Background
The United States Food and Drug Administration is investigating possible diet‐associated dilated cardiomyopathy (DCM) in dogs and cats.
Objectives
To retrospectively review DCM cases for signalment, diet information, echocardiographic changes, and survival.
Animals
Client‐owned dogs (n = 71).
Methods
Medical records of dogs diagnosed with DCM between January 1, 2014 and September 30, 2018 were reviewed. Dogs were grouped into “traditional” or “nontraditional” diet categories and whether or not diet was changed after diagnosis.
Results
For dogs eating nontraditional diets, those that had their diets changed had a larger percentage decrease in normalized systolic left ventricular internal dimension (P = .03) and left atrial:aorta ratio (P < .001) compared to those that did not have their diets changed. Survival time was significantly longer for dogs with DCM eating nontraditional diets that had their diets changed (median survival, 337 days; range, 9‐1307 days) compared to dogs eating nontraditional diets that did not have their diets changed (median survival, 215 days; range, 1‐852 days; P = .002).
Conclusions and Clinical Importance
Dogs with DCM eating nontraditional diets can experience improvement in cardiac function after diet change but additional research is needed to examine possible associations between diet and DCM.
Keywords: cardiology, congestive heart failure, grain‐free, nutritional
Abbreviations
- 2‐D
2‐dimensional
- CHF
congestive heart failure
- DCM
dilated cardiomyopathy
- FDA
United States Food and Drug Administration
- LVIDd/s
left ventricular internal diameter in diastole/systole
- nLVIDd
normalized LVIDd
- nLVIDs
normalized LVIDs
- WSAVA
World Small Animal Veterinary Association
1. INTRODUCTION
Historically, dilated cardiomyopathy (DCM) affects large and giant breed dogs and is associated with a known genetic mutation or established inheritance pattern in some breeds. 1 , 2 , 3 , 4 Beginning in 1995, a nutritional form of DCM in dogs was reported to be associated with dietary taurine deficiency—most commonly in American Cocker Spaniels, Golden Retrievers, and Newfoundlands, but also in other breeds. 5 , 6 , 7 , 8 , 9 Uniquely, the myocardial changes associated with taurine deficiency improved to some extent with taurine supplementation in some dogs. 6 , 7 , 8 , 9 In some cases, however, diet also was changed when medications were administered, and the specific role of taurine supplementation in improvement, where it occurred, remains unknown.
In July 2018, the United States Food and Drug Administration (FDA) released a report on its investigation of a potential connection between diet and DCM. 10 The FDA reported on cases of DCM in dogs eating pet foods containing a high proportion of peas, lentils, other pulses, or potatoes, many of which were labeled grain‐free. The FDA first began receiving reports in 2014 (although only 7 cases were reported between 2014 and 2017) and many cases have occurred in breeds not known to have a genetic predisposition to DCM. The FDA has since released 2 updates elaborating upon the relationship between diet and DCM. 11 , 12 In addition, 2 studies have provided valuable information on this possible association. 13 , 14 One study 13 focused on Golden Retrievers with DCM, whereas the second study 14 presented findings from >15 dog breeds with DCM that appeared to be diet‐associated. In addition to differences in breeds examined or included, all dogs in the study of Golden Retrievers had low circulating taurine concentrations, 13 whereas low taurine concentrations were uncommon in the other study. 14 What was consistent between the studies, however, is that most dogs had been fed grain‐free or pulse‐rich diets, and many of the dogs that had follow‐up examinations showed clinical and echocardiographic improvement after diet change (and taurine supplementation in all dogs in the Golden Retriever study 13 and some dogs in the other study 14 ). Details on survival were not provided for either study. Despite these important studies, additional data are needed on diets and outcomes of dogs with DCM to identify possible causes. To begin to address current gaps in information, the objective of our study was to retrospectively describe all cases of DCM at a tertiary referral hospital between 2014 and 2018 to determine demographics, dietary associations, and outcomes.
2. MATERIALS AND METHODS
Electronic medical records were searched to identify all dogs with DCM evaluated by the Cardiology or Nutrition Services at the Cummings School of Veterinary Medicine at Tufts University's Foster Hospital for Small Animals between January 1, 2014 and September 30, 2018. The start date was determined by the earliest date included in the FDA's investigation of diet‐associated DCM. The end date for retrospective data collection was the date that we began enrolling dogs in a separate prospective study. To be eligible for this retrospective study, dogs had to be newly diagnosed with DCM during the study period (ie, not reevaluations of dogs diagnosed with DCM before 2014). Criteria for diagnosis of DCM included a fractional shortening <25%, normalized left ventricular internal diameter in diastole (nLVIDd) >1.8, and normalized left ventricular internal diameter in systole (nLVIDs) >1.2.14‐16 Dogs with sepsis or serious systemic illness, primary valvular disease, cardiac shunts, and myocardial dysfunction suspected to be caused by tachycardia were excluded. Echocardiography was performed using standard techniques by a board‐certified veterinary cardiologist or a supervised cardiology resident. 17
Medical records were retrospectively reviewed using a standardized form to collect the following data from the visit at which DCM was diagnosed: signalment, body weight, heart rate, presence of arrhythmias (based on simultaneous ECG during echocardiography, diagnostic ECG, or in‐house ECG monitoring), murmur grade, echocardiographic measurements, CBC and serum biochemistry results, body condition score (on a 1‐9 scale; dogs were categorized as underweight if body condition score = 1‐3, normal if body condition score = 4‐5, and overweight if body condition score = 6‐9), and muscle condition score (normal, mild, moderate, or severe muscle loss), and medications. 18 The dog's main diet (ie, the diet providing the majority of the dog's calories) at the time of diagnosis was recorded, as was whether or not the diet was changed and each dog's final main diet. For the purposes of the study, diets were classified as traditional when they were grain‐inclusive extruded diets that did not contain peas, lentils, or potatoes as main ingredients (ie, top 10 ingredients on the ingredient list), and the manufacturer of which met the World Small Animal Veterinary Association (WSAVA) Global Nutrition Committee recommendations. 19 Nontraditional extruded diets were defined as those that were grain‐free, contained nontraditional ingredients (eg, peas, lentils) as main ingredients, or whose manufacturer did not meet the WSAVA Global Nutrition Committee recommendations. Taurine concentrations (plasma or whole blood), when measured, were recorded. The majority of taurine analyses were performed at a single commercial laboratory (Amino Acid Laboratory, University of California Davis, Davis, California). Low taurine concentration was defined as <60 nmol/mL (plasma) and <200 nmol/mL (whole blood). 20 The last available echocardiographic measurements for each dog also was recorded. The date and cause of death, if not still alive, were recorded. If survival information was not available in the medical record, the primary care veterinarian was contacted or, if any information was still unavailable, the owner was contacted. If the owner could not be contacted, the dog was considered lost to follow‐up.
2.1. Statistical analysis
Data distributions were examined graphically and using Shapiro‐Wilks tests. Percentages were compared between groups using Chi squared analysis. Independent t tests (for normally distributed variables) or Mann‐Whitney U tests (for skewed variables) were used to compare continuous variables between 2 groups, whereas analysis of variance (ANOVA) with Fisher's least significant difference post hoc tests and Kruskall‐Wallis tests was used to compare continuous variables among 3 groups. Variables that were significantly associated with changes in echocardiographic variables (eg, diet group, taurine supplementation, and presence of congestive heart failure [CHF] with percentage change in nLVIDs) on univariable analysis (P < .05) were included in a linear regression model. Survival times were calculated from the time of diagnosis of DCM until the time of death or euthanasia from all causes. Dogs were right‐censored if they were alive at the time of analysis or if they were lost to follow‐up. Survival times were compared between groups (eg, diet groups, presence of CHF, use of individual medications and supplements, and presence of arrhythmias) using Kaplan‐Meier curves and log‐rank tests. Variables that were significantly associated with survival in the univariable analysis (P < .05) were analyzed further using Cox proportional hazards analysis with a forward stepwise elimination strategy with modeling stopped once all of the variables remaining had a P‐value <.05. P‐values, hazard ratios, and 95% confidence intervals (CI) for the final explanatory multivariable model were calculated. All statistical tests were carried out using commercial statistical software (Systat version 13.0, Systat, San Jose, California; SPSS version 26.0, IBM Corp, Armonk, New York) and P‐values ≤.05 were considered significant.
3. RESULTS
Seventy‐five dogs with DCM were evaluated between January 2014 and September 2018. The number of dogs varied by year of diagnosis: 2014 (n = 9), 2015 (n = 8), 2016 (n = 21), 2017 (n = 16), 2018 (January‐September only; n = 21; P = .03). Diet information from the time of diagnosis was available for 73 of 75 dogs (97.3%). The 2 dogs without diet histories were both Doberman Pinschers diagnosed in 2016. Neither dog had a follow‐up echocardiogram and both died or were euthanized within 15 days of diagnosis. These 2 dogs were excluded from all further analyses. One dog was eating a home‐cooked diet at the time of diagnosis of DCM (2017) and was not included in subsequent analyses. Finally, at the time of diagnosis of DCM (2014), 1 dog had concurrent hypoadrenocorticism, newly diagnosed hypothyroidism, pulmonary infiltrates, and respiratory distress requiring support on a ventilator and also was excluded from further analyses. Therefore, all subsequent analyses were for 71 dogs. Fifty‐six of 71 dogs (78.9%) were eating nontraditional commercial diets and 15 dogs (21.1%) were eating traditional diets. The mean (SD) age was 7.2 ± 3.0 years, with a 2.4:1 ratio of males to females (Table 1). The most common breeds were Doberman Pinscher (n = 18), Great Dane (n = 16), Boxer (n = 6), Golden Retriever (n = 5), Labrador Retriever (n = 5), and French Bulldog (n = 3), but 18 different breeds were represented (Table 1). Thirty‐nine of the 71 dogs (55%) had supraventricular (n = 19, including 15 with atrial fibrillation) or ventricular (n = 25) arrhythmias; 5 dogs had both supraventricular and ventricular arrhythmias (Table 1). Echocardiographic measurements at baseline are summarized in Table 2.
TABLE 1.
Baseline comparison of signalment, clinical, and laboratory variables for 71 dogs with dilated cardiomyopathy (DCM) diagnosed between 2014 and September of 2018. In addition to total numbers, the 71 dogs are categorized based on diet into traditional or nontraditional diet groups. Continuous data are presented as mean ± SD or median (range), and categorical data are presented as number (%). P values are for comparison of the traditional vs nontraditional diet groups
| Variable | All dogs | Traditional diet | Nontraditional diet | P value |
|---|---|---|---|---|
| n | 71 | 15 | 56 | … |
| Age (years) | 7.2 ± 3.0 | 7.2 ± 2.0 | 7.2 ± 3.2 | .95 |
| Sex | .20 | |||
| Male | 50 (43 castrated) | 13 (11 castrated) | 37 (32 castrated) | |
| Female | 21 (19 spayed) | 2 (1 spayed) | 19 (18 spayed) | |
| Breed | .73 | |||
| Doberman Pinscher | 18 (25.4%) | 5 (33.3%) | 13 (23.2%) | |
| Great Dane | 16 (22.5%) | 2 (13.3%) | 14 (25.0%) | |
| Boxer | 6 (8.5%) | 3 (20.0%) | 3 (5.4%) | |
| Golden Retriever | 5 (7.0%) | 0 (0.0%) | 5 (8.6%) | |
| Labrador Retriever | 5 (7.0%) | 2 (13.3%) | 3 (5.4%) | |
| Mixed Breed | 4 (5.6%) | 1 (6.7%) | 3 (5.4%) | |
| French Bulldog | 3 (4.2%) | 0 (0.0%) | 3 (5.4%) | |
| Other breeds a | 14 (19.7%) | 2 (13.3%) | 12 (21.4%) | |
| Weight (kg) | 40.4 ± 15.6 | 40.9 ± 11.1 | 40.3 ± 16.7 | .87 |
| Body condition score | .25 | |||
| Underweight | 3 (4.2%) | 0 (0.0%) | 3 (5.4%) | |
| Normal | 42 (59.2%) | 7 (46.7%) | 35 (62.5%) | |
| Overweight | 26 (36.6%) | 8 (53.3%) | 18 (32.1%) | |
| Muscle condition score | .25 | |||
| Normal | 33 (46.5%) | 10 (66.7%) | 23 (41.1%) | |
| Mild | 31 (43.7%) | 5 (33.3%) | 26 (46.4%) | |
| Moderate | 3 (4.2%) | 0 (0.0%) | 3 (5.4%) | |
| Severe | 4 (5.6%) | 0 (0.0%) | 4 (7.1%) | |
| Congestive heart failure | 50 (70.4%) | 7 (46.7%) | 43 (76.8%) | .02 |
| Heart rate (/min) | 145 ± 42 | 138 ± 37 | 147 ± 44 | .47 |
| Murmur | 2 (0‐4) | 2 (0‐3) | 2 (0‐4) | .61 |
| Arrhythmia | ||||
| Any arrhythmia | 39 (54.9%) | 10 (66.7%) | 29 (51.8%) | .30 |
| Supraventricular | 19 (26.8%) | 2 (13.3%) | 17 (30.4%) | .19 |
| Ventricular | 25 (35.2%) | 9 (60.0%) | 16 (28.6%) | .02 |
Other breeds included German Shepherd (n = 2), Pit Bull (n = 2), and 1 each of Australian Cattle Dog, Bull Mastiff, Caucasian Shepherd Dog, German Shorthaired Pointer, Irish Wolfhound, Mastiff, Miniature Schnauzer, Portuguese Water Dog, Saint Bernard, and Samoyed.
TABLE 2.
Baseline comparison of echocardiographic variables for 71 dogs with dilated cardiomyopathy (DCM) diagnosed between 2014 and September of 2018. In addition to total numbers, the 71 dogs are categorized based on diet into traditional or nontraditional diet groups. Data are presented as mean ± SD. P values are for comparison of the traditional vs. nontraditional diet groups
| Variable | All dogs | Traditional diet | Nontraditional diet | P value |
|---|---|---|---|---|
| n | 71 | 15 | 56 | … |
| Echocardiography | ||||
| M‐mode | ||||
| LVIDd (cm) | 6.15 ± 1.10 | 5.98 ± 0.88 | 6.19 ± 1.16 | .51 |
| IVSd (cm) | 1.03 ± 0.23 | 1.08 ± 0.30 | 1.02 ± 0.21 | .48 |
| LVWd (cm) | 0.93 ± 0.21 | 0.92 ± 0.21 | 0.93 ± 0.22 | .95 |
| LVIDs (cm) | 5.16 ± 0.98 | 4.94 ± 0.80 | 5.22 ± 1.02 | .33 |
| IVSs (cm) | 1.24 ± 0.32 | 1.35 ± 0.38 | 1.21 ± 0.30 | .13 |
| LVWs (cm) | 1.11 ± 0.29 | 1.18 ± 0.31 | 1.09 ± 0.29 | .33 |
| Fractional shortening (%) | 15.99 ± 5.00 | 17.38 ± 5.54 | 15.62 ± 4.84 | .23 |
| nLVIDd | 2.11 ± 0.32 | 2.03 ± 0.27 | 2.13 ± 0.33 | .28 |
| nLVIDs | 1.65 ± 0.29 | 1.55 ± 0.26 | 1.68 ± 0.30 | .13 |
| 2‐D | ||||
| Left atrium:aorta | 2.13 ± 0.55 | 2.04 ± 0.57 | 2.16 ± 0.54 | .47 |
Abbreviations: 2‐D, 2‐dimensional; IVSd/s, interventricular septal thickness in diastole/systole; LVIDd/s, left ventricular internal diameter in diastole/systole; LVWd/s, left ventricular free wall in diastole/systole; nLVIDd, normalized LVIDd; nLVIDs, normalized LVIDs.
At the time of diagnosis, no significant differences were found between dogs eating traditional and nontraditional diets for age, sex, breed, weight, body condition score, muscle condition score, CBC (performed in 45 dogs; data not shown) or serum biochemistry profile variables (performed in 63 dogs; data not shown), murmur grade, presence of any arrhythmia or presence of supraventricular arrhythmias, presence of CHF, or echocardiographic measurements (Tables 1 and 2). Dogs eating nontraditional diets were more likely to have CHF (P = .02) and less likely to have ventricular arrhythmias (P = .02) compared to dogs eating traditional diets (Table 1).
Taurine was measured in 20 of the 71 dogs (28%; 19 in the nontraditional diet group and 1 in the traditional diet group). Both plasma and whole blood taurine concentrations were measured in 12 dogs (all 12 in the nontraditional diet group), whereas whole blood taurine concentration only was measured in 6 dogs (5 in the nontraditional diet group and 1 in the traditional diet group) and plasma taurine concentration only was measured in 2 dogs (both in the nontraditional diet group). Four dogs (all in the nontraditional diet group) had low plasma taurine concentrations (29, 40, 47, and 57 nmol/mL; reference range, 60‐120 nmol/mL). For 2 of these dogs, only plasma taurine concentration was measured with no corresponding whole blood measurement, whereas for the other 2 dogs with low plasma taurine concentration, the corresponding whole blood taurine concentration was not low. Therefore, no dogs had both low plasma and low whole blood taurine concentration. For 3 of the 4 low plasma taurine concentration results, analyses were not performed at the usual laboratory. Of the 18 dogs that had whole blood taurine concentrations measured, only 1 dog had a low whole blood taurine concentration (52 nmol/mL; reference range, 200‐350 nmol/mL). This dog was eating a nontraditional (commercial vegan) diet. Five of 19 dogs tested for taurine concentrations in the nontraditional diet group had low concentrations compared to 0/1 tested in the traditional diet group (P = .55).
Over the course of the dogs' disease, many cardiac medications were used, including pimobendan (n = 69), an angiotensin converting‐enzyme inhibitor (n = 57), furosemide (n = 56), diltiazem (n = 22), amiodarone (n = 19), digoxin (n = 16), spironolactone (n = 11), torsemide (n = 9), mexiletine (n = 7), sotalol (n = 5), carvedilol (n = 3), sildenafil (n = 2), atenolol (n = 1), and clopidogrel (n = 1). In addition, 30 dogs received a taurine supplement (28 in the nontraditional diet group and 2 in the traditional diet group) and 3 dogs received an L‐carnitine supplement (all in the nontraditional diet group, with all 3 also receiving taurine). For the 56 dogs in the nontraditional diet group, 31/56 dogs (55%) had their diets changed after diagnosis, whereas 25/56 (45%) did not have their diets changed (Supplemental Information). For the 15 dogs in the traditional diet group, 6/15 (40%) had their diets changed after diagnosis, whereas 9/15 (60%) did not have their diets changed (Supplemental Information).
Forty‐five of the 71 dogs (63.4%) had a follow‐up echocardiogram at least 90 days after the original diagnosis. The median time between echocardiograms for all dogs was 287 days (range, 93‐1294 days); dogs eating nontraditional diets that had their diets changed (median, 326 days; range, 103‐1087 days); dogs eating nontraditional diets that did not have their diets changed (median, 265 days; range, 93‐478 days); and dogs eating traditional diets (median, 274 days; range, 108‐1294 days; P = .39). Dogs in the nontraditional diet group that had their diets changed and had follow‐up echocardiography (n = 23) had a significantly larger percentage decrease in nLVIDs (P = .03) and 2‐dimensional (2‐D) left atrial:aortic ratio (LA:Ao; P < .001) compared to dogs in the nontraditional diet group that did not have their diets changed (n = 13; Figure 1). No significant differences were found between the nontraditional diet group that had their diets changed and the nontraditional diet group that did not have their diets changed for percentage change in nLVIDd (P = .08) or fractional shortening (M‐mode; P = .21; Figure 1). No significant differences were found for nLVIDd, nLVIDs, LA:Ao, or fractional shortening between the traditional diet group that had a follow‐up echocardiogram (n = 9) and either of the nontraditional diet groups (those that had their diets changed and those that did not have their diets changed; Figure 1). There were too few dogs in the traditional diet group that had follow‐up echocardiograms to perform statistical analyses on those that had their diets changed vs those that did not have their diets changed.
FIGURE 1.

Comparison of echocardiographic changes in 45 of 71 dogs with dilated cardiomyopathy (DCM) that had a follow‐up echocardiogram at least 90 days after diagnosis. Dogs are categorized as eating nontraditional diets that had their diets changed after diagnosis (NT‐change), dogs with DCM eating nontraditional diets that did not have their diets changed (NT‐no change), and dogs with DCM eating traditional diets (Traditional). Bars represent the mean percent change (±SD) in the echocardiographic measurement between the time of diagnosis and last measurement. FS, fractional shortening; LA:Ao, left atrial diameter:aortic diameter (2‐dimensional); nLVIDd, normalized left ventricular internal diameter in diastole; nLVIDs, normalized left ventricular internal diameter in systole
For dogs that had a follow‐up echocardiogram, no significant differences were found in any of the cardiac medications among diet groups. For dogs that had a follow‐up echocardiogram, dogs in the nontraditional diet group that had their diets changed were significantly more likely to have received taurine (15/23) compared to dogs in the nontraditional diet group that did not have their diets changed (4/13; P = .05) and compared to the traditional diet group (2/9; P = .03). In addition to the previously mentioned significant changes in nLVIDs and LA:Ao in dogs that were in the nontraditional diet group that had their diets changed, dogs that received taurine supplementation also had a higher percentage decrease in nLVIDs (P = .007) but not a higher percentage decrease in LA:Ao (P = .18) compared to dogs that did not receive taurine. The absence of CHF also was significantly associated with a higher percentage decrease in nLVIDs as compared to dogs with CHF (P = .02), but CHF was not significantly associated with LA:Ao changes (P = .25). On multivariable analysis, only diet group (P = .03) and CHF (P = .008) remained significantly associated with the change in nLVIDs.
At the time of analysis, 11 dogs were still alive: 10/31 dogs (32.3%) in the nontraditional diet group that had their diets changed, 0/25 dogs (0.0%) in the nontraditional diet group that did not have their diets changed, and 1/15 dogs (6.7%) in the traditional diet group (P = .002). Of the 60 dogs that were no longer alive, 21 dogs died suddenly and 38 dogs were euthanized because of worsening CHF or other causes; 1 dog was no longer alive but the cause of death was unknown. The most common cause of death was CHF (n = 35) or sudden death (n = 21), with only 3 dogs having a non‐cardiac cause of death (1 each of cancer, gastric dilatation‐volvulus, and osteoarthritis). No significant difference was found in the cause of death between the nontraditional diet group that had their diets changed (10 CHF, 9 sudden death, 2 other), the nontraditional group that did not have their diets changed (14 CHF, 10 sudden death, 1 unknown), and the traditional diet groups (11 CHF, 2 sudden death, 1 other; P = .21). Using log‐rank analysis, survival time was significantly longer for dogs in the nontraditional diet group that had their diets changed (median survival time, 337 days; range, 9‐1307 days) compared to dogs in the nontraditional diet group that did not have their diets changed (median survival, 215 days; range, 1‐852 days; P = .002; Figure 2). Survival time for dogs in the traditional diet group (median survival, 435 days; range, 59‐1506 days) was not significantly different compared to either of the nontraditional diet groups (compared to nontraditional diet group that had their diets changed [P = .33]; compared to nontraditional diet group that did not have their diets changed [P = .07]). The nontraditional diet groups had more dogs with CHF at the time of diagnosis than did the traditional diet group, which could have impacted survival. Therefore, survival time also was compared among diet groups for only dogs with CHF at the time of diagnosis (Figure 3). Dogs in the nontraditional diet group with CHF that had their diets changed (n = 25) had significantly longer survival time compared to dogs in the nontraditional diet group with CHF that did not have their diets changed (n = 18; P = .01). No significant differences in survival time were found between dogs in the traditional diet group with CHF (n = 7) and either the nontraditional diet group with CHF that had their diets changed (P = .88) or the nontraditional diet group with CHF that did not have their diets changed (P = .16).
FIGURE 2.
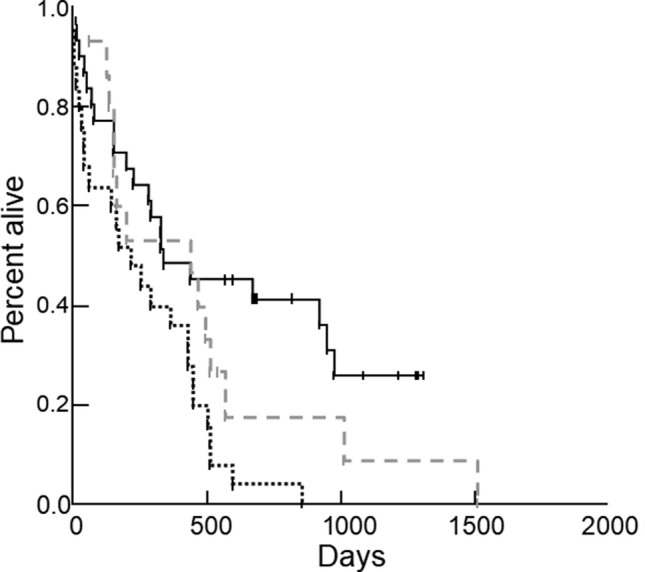
Kaplan‐Meier survival curves comparing survival time in 71 dogs with DCM: 31/56 dogs eating nontraditional diets that had their diets changed after diagnosis (black solid line) and 25/56 dogs eating nontraditional diets that did not have their diets changed after diagnosis (black dotted line). Survival time was significantly longer in dogs eating nontraditional diets that had their diets changed compared to dogs eating nontraditional diets that did not have their diets changed (P = .002). Survival time for dogs in the traditional diet group (n = 15; gray dashed line) was not significantly different compared to the nontraditional diet group that had their diets changed (P = .33) or to the nontraditional diet group that did not have their diets changed (P = .07)
FIGURE 3.

Kaplan‐Meier survival curves comparing survival time in 50 dogs with dilated cardiomyopathy (DCM) that had congestive heart failure (CHF) at the time of diagnosis. Dogs with DCM and CHF in the nontraditional diet group that had their diets changed (n = 25; black solid line) had a significantly longer survival time compared to dogs in the nontraditional diet group that did not have their diets changed (n = 18; black dotted line; P = .01). No significant differences were found between dogs in the traditional diet group with CHF (n = 7; gray dashed line) and either the nontraditional diet group that had their diets changed (P = .88) or the nontraditional diet group that did not have their diets changed (P = .16)
Survival times also were compared for all 71 dogs with other variables using log‐rank analyses. Dogs that received taurine supplementation during the course of their disease had a significantly longer survival time than did dogs not receiving taurine supplementation (P = .003), but the presence of low plasma or whole blood taurine concentration was not associated with survival (P = .65). Dogs with ventricular arrhythmias at the time of diagnosis (compared to dogs without ventricular arrhythmias; P = .004), dogs with CHF during the course of their disease (n = 56; compared to dogs without CHF; P = .01), and dogs that received furosemide during the course of their disease (compared to dogs not receiving furosemide; P = .01) had significantly shorter survival times. No other medications or other variables were significantly associated with survival time on univariable analyses. The significant variables (ie, diet group, taurine supplementation, ventricular arrhythmias, CHF, and furosemide) were analyzed using Cox proportional hazards analysis. In the final multivariable model, the presence of CHF during the course of their disease (P = .02) or ventricular arrhythmias (P = 0.01) was significantly associated with shorter survival time (Table 3), whereas diet group (ie, dogs that were in the nontraditional diet group that had their diets changed) was significantly associated with longer survival time (P = .001; Table 3).
TABLE 3.
Final Cox proportional hazards model for survival time in 71 dogs with dilated cardiomyopathy diagnosed between 2014 and September of 2018
| Variable | P‐value | Hazard ratio | 95% CI for HR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Diet group | ||||
| Dogs eating nontraditional diets that did not change diets (reference) | … | 1.000 | … | … |
| Dogs eating nontraditional diets that changed diets | .001 | 0.354 | 0.190 | 0.660 |
| Dogs eating traditional diets | .08 | 0.535 | 0.263 | 1.091 |
| Congestive heart failure present (reference, no CHF) | .02 | 2.371 | 1.177 | 4.774 |
| Ventricular arrhythmias present (reference, no ventricular arrhythmias) | .01 | 2.152 | 1.204 | 3.847 |
Abbreviations: CHF, congestive heart failure; CI, confidence intervals; HR, hazard ratio.
4. DISCUSSION
Our results showed that for dogs eating nontraditional diets, those that had their diets changed had a significantly longer survival time compared to those that did not have their diets changed. Although this observation supports a benefit of diet change and a possible role of diet in the etiology of some cases of DCM, additional research is needed to show a causal association. In our retrospective study, median survival time for dogs eating nontraditional diets that had their diets changed was 337 days, but there was a wide range (9‐1307 days). Survival time of dogs with presumed diet‐associated DCM was not reported in previous studies. 13 , 14 Median survival of dogs with primary DCM varies based on several factors, such as breed, whether or not they have developed CHF or serious arrhythmias, or if they have occult disease. In general, dogs with primary DCM typically live for <1 year after the onset of CHF. 21 , 22 Survival time for dogs eating traditional diets (435 days) was not significantly longer than that of dogs in either of the nontraditional diet groups, but fewer dogs in the traditional diet group had CHF at the time of diagnosis and thus, as a group, were less severely affected than were dogs eating nontraditional diets. There also was a wide range of survival times for dogs eating traditional diets.
Our results also show that dogs with DCM that had been eating nontraditional diets experienced improvement in some echocardiographic measurements after diet change, with a significantly higher percentage decrease in left atrial:aortic ratio and nLVIDs compared to dogs eating nontraditional diets that did not have their diets changed. These results support the findings of 2 previous studies that showed significant improvement in 23/24 (96%) of Golden Retrievers with DCM and taurine deficiency fed grain‐free or pulse‐rich diets that had their diets changed and received taurine supplementation 13 and in 7 dogs of multiple breeds with DCM eating grain‐free diets that had their diets changed to traditional diets and then were reevaluated. 14 The cause for this improvement in cardiac size and function with diet change is unclear, but could be related to correction of a nutritional deficiency that developed as a result of the nontraditional diet, or to removing a toxic factor that was introduced in the nontraditional diets. It is also possible that changes in echocardiographic measurements in our study and in the 2 previous studies are unrelated to diet and could be the result of other factors, such as medications or genetic factors. When comparing echocardiographic measurements for the nontraditional diet group that had their diets changed to measurements for the traditional diet group, none of the differences were significant, but this result could have been related to the small size of the traditional diet group (only 15 dogs in the traditional diet group and only 9 had follow‐up echocardiography). The traditional diet group also was less severely affected because there were significantly fewer dogs that had CHF compared to either of the nontraditional diet groups.
Although dogs in the nontraditional diet group that had their diets changed showed improvements in some echocardiographic measurements as a group, the response was variable among individual dogs. One reason for this finding could be that some of the dogs in the nontraditional diet group may have had primary DCM or a combination of primary DCM and diet‐associated DCM, or the variation could have been a consequence of other factors. Breeds typically affected by primary DCM, such as Doberman Pinschers, Great Danes, and Boxers, made up more than 50% of the cases in the nontraditional diet group. When DCM is diagnosed in an atypical breed, such as a Miniature Schnauzer, it is reasonable to suspect a nutritional etiology, especially when clear cardiac improvement is noted after a dietary intervention. However, it is more difficult to predict if a Doberman Pinscher with DCM eating a nontraditional diet has primary DCM, diet‐associated DCM, or combination of both. However, echocardiographic improvements were seen in all breeds, even in breeds predisposed to primary DCM. Conversely, it is unknown how many dogs of breeds typically associated with primary DCM eating nontraditional diets that did not have their diets changed might have shown improvement.
The variable response in echocardiographic changes also could be a consequence of the retrospective design of the study because dogs were reevaluated echocardiographically between 93 and 1294 days after diagnosis. Although the time course of improvement for dogs with DCM eating nontraditional diets is not yet completely understood, some dogs continue to improve for up to 9 months or more after diet change. Additional research is needed to better understand the time course of possible improvement for dogs with DCM eating nontraditional diets, as well as how to determine which dogs of breeds typically associated with primary DCM are most likely to have diet‐associated as compared with primary DCM.
The reason for the higher prevalence of CHF at the time of diagnosis in dogs eating nontraditional diets is unclear but could be related to diagnosis later in the course of the disease, to faster progression of the disease, or to other factors. Dogs eating nontraditional diets also were less likely to have ventricular arrhythmias compared to dogs eating traditional diets. The presence of arrhythmias was not reported in the 2 previous studies on diet‐associated DCM, 13 , 14 and it is unclear whether or not this finding is consistent, a question that will require further research. However, even in the nontraditional diet group, 28.6% of dogs had ventricular arrhythmias at the time of diagnosis and 19/56 dogs (33.9%) experienced sudden death (9 in the nontraditional diet group that had their diets changed and 10 in the nontraditional group that did not have their diets changed). No other significant differences were found between dogs in the nontraditional compared to traditional diet groups in signalment, laboratory results, or echocardiographic measurements at the time of diagnosis. Sphericity index, which was reported to be significantly lower in dogs eating 1 specific grain‐free diet compared to other grain‐free diets in a previous study, 14 was not routinely measured in our hospital during this time period and could not be evaluated in our study. However, this measurement should be included in future prospective studies to determine if it could help distinguish dogs with diet‐associated DCM from dogs with primary DCM. Global longitudinal strain also might provide useful information, especially for following changes in cardiac performance over time after diet change.
The underlying cause of diet‐associated DCM remains unknown but is being actively investigated by the FDA and several researchers. Diet‐associated deficiencies, toxins, or a multifactorial etiology (eg, nutritional, other environmental factors, genetics) remains possible explanations. A nutritional deficiency could be caused either by low concentrations of a nutrient in the diet or a relative deficiency caused by decreased bioavailability or nutrient‐nutrient interactions (eg, decreased bioavailability of a nutrient because of high dietary fiber content). Although ingredients used to replace grains in grain‐free diets, such as peas and lentils or potatoes, are suspected to play a role, it is not yet clear whether or not these are the source of the problem. Deficiency of taurine or its precursors, methionine and cysteine, also has been suggested to be a possible cause of diet‐associated DCM. Most dogs that were tested in our study did not have low taurine concentrations, but taurine concentrations were only measured in 20 dogs, and not all analyses were performed at the same laboratory. In a previous retrospective study 14 of dogs with diet‐associated DCM, taurine concentrations below the reference range were identified in only 2 dogs eating grain‐based diets, but in none of the dogs eating grain‐free diets. In our retrospective study, 30/71 dogs were supplemented with taurine, and thus some dogs could have improved as a result of correcting undetected taurine deficiency rather than as a result of diet change. Taurine supplementation also potentially could have benefits even if taurine deficiency was not present, because it has some antioxidant and positive inotropic effects. 23 Although supplementation of taurine was not associated with survival in our multivariable analysis, sample size was relatively small and an effect may not have been detected. Ultimately, taurine deficiency as a cause for diet‐associated DCM seems unlikely from our results because taurine supplementation was not associated with survival or echocardiographic changes on multivariable analysis, but taurine still could play a role in some breeds or could be part of a multifactorial etiology. Regardless, more research is needed on the role of taurine in DCM, including any potential benefits of supplementation, whether or not taurine deficiency is present.
The number of dogs diagnosed with DCM in our hospital has increased significantly over time, from 9 cases in 2014 to 21 cases in the first 9 months of 2018 (P = .03). This increase could be a consequence of increased awareness of the potential association with diet and subsequently more referrals of DCM cases. However, the increase in case numbers first occurred in 2016, before information on this issue was widely disseminated, and thus these numbers also could correspond to a true increase in prevalence of DCM. It is impossible to determine whether these numbers were due solely to an increase in prevalence or to other factors, such as hospital caseload, economic factors, and other possible confounders. In 2018, grain‐free diets were reported to represent 46% of the US pet food market. 24 In a retrospective study of dogs with DCM diagnosed between January 1, 2015 and May 1, 2018, 75% were eating grain‐free diets. 14 Overall, 79% of dogs in our study (diagnosed between January 2014 and September 2018) were eating nontraditional diets. A recent abstract reported that 95% of dogs with DCM seen between May and September 2018 were eating nontraditional diets compared to 47% of dogs with myxomatous mitral valve disease (P < .001; Freid et al. Retrospective investigation of diet and dilated cardiomyopathy [DCM] in dogs. J Vet Intern Med 2020, abstract). The abstract represented only 5 months of data from a single hospital, and thus further research is warranted to investigate a possible role of diet in some dogs with DCM.
Our study had a number of limitations. Most importantly, although these retrospective data allow for exploration of hypotheses of associations between diet and DCM, a causal relationship cannot be determined. In addition, evaluating a large number of variables with a relatively small sample size increases the risk of type 1 statistical error. Additional research (eg, larger retrospective studies as well as laboratory and prospective studies) is needed to definitely identify a link and better understand this issue. Dogs were categorized into the traditional and nontraditional diet categories based on their primary diet at the time of diagnosis, but information on the length of time dogs had been eating a particular diet and on additional foods or treats they may have received was not usually available from the medical record. It is not yet known how many dogs eating nontraditional diets might develop DCM or other cardiac problems, if certain ingredients are primary causes, or if there is a threshold for how long dogs need to eat suspected diets before developing DCM. However, in 1 study of Golden Retrievers with DCM and taurine deficiency, 13 the length of time dogs had been fed the diet at the time of diagnosis ranged from 182 to 3558 days (median, 815 days). The extent to which disease severity and likelihood of improvement correspond to the duration of time a dog is eating a nontraditional diet is also unknown. Eating other foods or treats in addition to the main diet could decrease the proportion of a dog's intake of the main diet, which theoretically could decrease intake of a toxic factor (and decrease likelihood of DCM) or decrease intake of an important nutrient (and increase likelihood of DCM), thus future studies should include all relevant dietary information. Similarly, in the study of Golden Retrievers with DCM and taurine deficiency, 13 nearly all dogs in which calorie intake could be calculated were consuming less than their predicted energy requirements, which could increase the risk for nutritional deficiencies. Information on calorie intake was not available for our study, and it is therefore unknown if calorie intake in our population of dogs would be similar to those in the previous study. 13 As a result of the retrospective nature of our study, the exact timing of the diet change was not always available in the medical record. Not all dogs were changed from a nontraditional to traditional diet; several were changed to either another nontraditional diet or a home‐cooked diet. Additionally, only 63% of dogs had follow‐up echocardiography and these studies were not conducted at consistent intervals after diagnosis, and thus possible changes in echocardiographic parameters could have been missed. Also, bias could have existed in the follow‐up of dogs with CHF compared to those with occult disease because follow‐up might be less frequently carried out in dogs with occult disease.
Our results are consistent with the results of 2 previous studies suggesting that in dogs with DCM eating nontraditional diets, diet change can be associated with significant improvement in some echocardiographic measurements. In addition, dogs eating nontraditional diets that had their diets changed had a significantly longer survival time compared with those that did not have their diets changed. The underlying cause and mechanisms of diet‐associated DCM are still not understood, but our findings emphasize the importance of prospective studies to better understand this issue as soon as possible.
CONFLICT OF INTEREST DECLARATION
In the last 3 years, Dr. Freeman has received research funding from, given sponsored lectures for, and/or provided professional services to Aratana Therapeutics, Elanco, Hill's Pet Nutrition, Nestlé Purina PetCare, P&G Pet Care (now Mars), and Royal Canin. In the last 3 years, Dr. Rush has received research funding from, given sponsored lectures for, and/or provided professional services to Aratana Therapeutics, Boehringer Ingelheim, Elanco, IDEXX, Nestlé Purina PetCare, and Royal Canin.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplemental Table 1 Diet changes made after diagnosis in 71 dogs with dilated cardiomyopathy.
ACKNOWLEDGMENTS
Funding provided by the Barkley Fund. The authors acknowledge the excellent clinical care and assistance from Ms Kristen Antoon, Ms Kelsey Weeks, Ms Michelle Maillet, Dr Amelie Beaumier, and Dr Luis Dos Santos.
Freid KJ, Freeman LM, Rush JE, et al. Retrospective study of dilated cardiomyopathy in dogs. J Vet Intern Med. 2021;35:58–67. 10.1111/jvim.15972
Funding information Barkley Fund
REFERENCES
- 1. Martin MWS, Stafford Johnson MJ, Celona B. Canine dilated cardiomyopathy: a retrospective study of signalment, presentation and clinical findings in 369 cases. J Small Anim Pract. 2009;50(1):23‐29. [DOI] [PubMed] [Google Scholar]
- 2. Meurs KM, Lahmers S, Keene BW, et al. A splice site mutation in a gene encoding for PDK4, a mitochondrial protein, is associated with the development of dilated cardiomyopathy in the Doberman pinscher. Hum Genet. 2012;131(8):1319‐1325. [DOI] [PubMed] [Google Scholar]
- 3. Oyama MA, Chittur SV, Reynolds CA. Decreased triadin and increased calstabin2 expression in Great Danes with dilated cardiomyopathy. J Vet Intern Med. 2009;23(5):1014‐1019. [DOI] [PubMed] [Google Scholar]
- 4. Simpson S, Dunning MD, Brownlie S, et al. Multiple genetic associations with Irish Wolfhound dilated cardiomyopathy. BioMed Research Intl. 2016;2016:1‐14. 10.1155/2016/6374082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kramer GA, Kittleson MD, Fox PR, Lewis J, Pion PD. Plasma taurine concentrations in normal dogs and in dogs with heart disease. J Vet Intern Med. 1995;9(4):253‐258. [DOI] [PubMed] [Google Scholar]
- 6. Kittleson MD, Keene B, Pion PD, Loyer CG. Results of the multicenter spaniel trial (MUST): taurine‐and carnitine‐responsive dilated cardiomyopathy in American Cocker Spaniels with decreased plasma taurine concentration. J Vet Intern Med. 1997;11(4):204‐211. [DOI] [PubMed] [Google Scholar]
- 7. Fascetti AJ, Reed JR, Rogers QR, Backus RC. Taurine deficiency in dogs with dilated cardiomyopathy: 12 cases (1997‐2001). J Am Vet Med Assoc. 2003;223(8):1137‐1141. [DOI] [PubMed] [Google Scholar]
- 8. Backus RC, Cohen G, Pion PD, Good KL, Rogers QR, Fascetti AJ. Taurine deficiency in Newfoundlands fed commercially available complete and balanced diets. J Am Vet Med Assoc. 2003;223(8):1130‐1136. [DOI] [PubMed] [Google Scholar]
- 9. Bélanger MC, Ouellet M, Queney G, Moreau M. Taurine‐deficient dilated cardiomyopathy in a family of Golden Retrievers. J Am Anim Hosp Assoc. 2005;41(5):284‐291. [DOI] [PubMed] [Google Scholar]
- 10. United States Food and Drug Administration . FDA investigating potential connections between diet and cases of canine heart disease; July 12, 2018. https://www.fda.gov/animal-veterinary/cvm-updates/fda-investigating-potential-connection-between-diet-and-cases-canine-heart-disease. Accessed November 29, 2020.
- 11. United States Food and Drug Administration . FDA provides update on investigation into potential connection between certain diets and cases of canine heart disease; February 19, 2019. https://www.fda.gov/animal-veterinary/news-events/fda-investigation-potential-link-between-certain-diets-and-canine-dilated-cardiomyopathy-february. Accessed November 29, 2020.
- 12. United States Food and Drug Administration . FDA provides third status report on investigation into potential connection between certain diets and cases of canine heart disease; June 27, 2019. https://www.fda.gov/animal-veterinary/cvm-updates/fda-provides-third-status-report-investigation-potential-connection-between-certain-diets-and-cases. Accessed November 29, 2020.
- 13. Kaplan JL, Stern JA, Fascetti AJ, et al. Taurine deficiency and dilated cardiomyopathy in Golden Retrievers fed commercial diets. PLoS One. 2018;13(12):e0209112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adin D, DeFrancesco TC, Keene B, et al. Echocardiographic phenotype of canine dilated cardiomyopathy differs based on diet type. J Vet Cardiol. 2019;21:1‐9. [DOI] [PubMed] [Google Scholar]
- 15. Dukes‐McEwan J, Borgarelli M, Tidholm A, Vollmar AC, Häggström J, ESVC Taskforce for Canine Dilated Cardiomyopathy . Proposed guidelines for the diagnosis of canine idiopathic dilated cardiomyopathy. J Vet Cardiol. 2003;5(2):7‐19. [DOI] [PubMed] [Google Scholar]
- 16. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med. 2004;18(3):311‐321. [DOI] [PubMed] [Google Scholar]
- 17. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med. 1993;7(4):247‐252. [DOI] [PubMed] [Google Scholar]
- 18. World Small Animal Veterinary Association Global Nutrition Committee . Muscle condition score chart. https://wsava.org/wp-content/uploads/2020/01/Muscle-Condition-Score-Chart-for-Dogs.pdf. Accessed November 29, 2020.
- 19. World Small Animal Veterinary Association Global Nutrition Committee . Recommendations on selecting pet foods. https://wsava.org/wp-content/uploads/2020/01/Selecting-the-Best-Food-for-your-Pet.pdf. Accessed November 29, 2020.
- 20. Delaney SJ, Kass PH, Rogers QR, Fascetti AJ. Plasma and whole blood taurine in normal dogs of varying size fed commercially prepared food. J Anim Physiol Anim Nutr. 2003;87(5–6):236‐244. [DOI] [PubMed] [Google Scholar]
- 21. Martin MWS, Stafford Johnson MJ, Strehlau G, King JN. Canine dilated cardiomyopathy: a retrospective study of prognostic findings in 367 clinical cases. J Small Anim Pract. 2010;51(8):428‐436. [DOI] [PubMed] [Google Scholar]
- 22. Vollmar C, Vollmar A, Keene BW, Fox PR, Reese S, Kohn B. Dilated cardiomyopathy in 151 Irish Wolfhounds: characteristic clinical findings, life expectancy and causes of death. Vet J. 2019;245:15‐21. [DOI] [PubMed] [Google Scholar]
- 23. Bkaily G, Jaalouk D, Haddad G, et al. Modulation of cytosolic and nuclear Ca2+ and Na+ transport by taurine in heart cells. Molec Cell Biochem. 1997;170(1‐2):1‐8. [DOI] [PubMed] [Google Scholar]
- 24. Petfood Industry . How pet food retail shifts are playing out in each channel; Mar 11, 2019. https://www.petfoodindustry.com/blogs/7-adventures-in-pet-food/post/7948-how-pet-food-retail-shifts-are-playing-out-in-each-channel. Accessed May 11, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Diet changes made after diagnosis in 71 dogs with dilated cardiomyopathy.


