Abstract
Melanoma is an aggressive malignancy with a poor prognosis. Current studies show that imatinib treatment is a promising approach in treating advanced melanoma patients harboring c-Kit mutations or amplifications. We retrospectively analyzed the clinical medical records of 78 patients with metastatic melanoma harboring c-Kit mutations or amplifications. These patients were treated with imatinib at a dose of 400 mg/day continuously unless intolerable toxicities or disease progression occurred. Endpoints for exploration included overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease of control rate (DCR). The median OS and PFS of all patients were 13.1 and 4.2 months, respectively. ORR and DCR were 21.8% and 60.3%, respectively. The survival time of patients who achieved partial response or stable disease was significantly superior to those with disease progression. Cox regression analysis showed that patients with M1c stage, subtype of cutaneous melanoma, or elevated LDH level (>upper limit of normal) had higher hazard ratios for overall survival. Our study, combined with those studies targeting patients with a c-Kit alteration, validates the role of imatinib as an important and promising therapeutic agent in the treatment of patients with advanced melanoma.
Key words: Metastatic melanoma, c-Kit aberrations, Imatinib, Efficacy, Progression-free survival (PFS), Overall survival (OS), Response rate
INTRODUCTION
Melanoma is an aggressive malignancy with an overall poor prognosis. The incidence and mortality of melanoma have been increasing dramatically1,2. Over the past 18 years, over 2,000 registered clinical trials (https://clinicaltrials.gov/ ) have been conducted to investigate novel agents and combination regimens for metastatic melanoma. Medical treatments were at a standstill prior to the advent of more novel therapies, such as targeted agents and immune checkpoint immunotherapy agents. Several of these agents have now been approved for clinical practice by the various regulatory agencies, and these newer treatment strategies have clearly shown greater clinical benefit when compared to standard chemotherapy3–6.
The most prevalent subtypes among Chinese melanoma patients are acral and mucosal melanoma (>70%), which constitute only a very small proportion of melanomas among Caucasians (<5%)7–9. The c-Kit gene mutation rate within these two subtypes is significantly higher than that of other subtypes, and the rates in acral and mucosal melanoma were 17.6% and 19.2%, respectively10,11. Therefore, individualized targeted therapy for c-Kit may be especially relevant for Chinese melanoma patients.
Previous studies have shown that treatment with imatinib is a promising approach among advanced melanoma patients harboring c-Kit mutations or amplifications10,12,13. A phase II clinical trial of imatinib (Novartis Pharma Stein AG Stein, Switzerland) in melanoma patients with c-Kit mutation/amplifications (n = 43) was conducted by our group between 2008 and 201010. In this study, we observed that imatinib demonstrated significant activity with benefits in overall survival (OS), progression-free survival (PFS), and objective response rate (ORR). However, the long-term efficacy of imatinib is still uncertain and unclear. In this retrospective study, we further investigated the clinical efficacy of imatinib in 78 patients with advanced or metastatic melanoma with c-Kit alterations.
PATIENTS AND METHODS
Patients
We retrospectively analyzed the clinical information of patients whose admission dates ranged from January 1, 2008 to September 30, 2015. To reduce potential bias and heterogeneity, eligibility criteria were set according to the following: (1) histologically and clinically/radiologically confirmed melanoma diagnosis by the Department of Pathology of Peking University Cancer Hospital and Institute; (2) evidence for the presence of mutations and/or gene amplification of c-Kit; (3) Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 1; (4) lesion assessment as defined by the Response Evaluation Criteria in Solid Tumors 1.0 (RECIST 1.0)14; (5) adequate hepatic, renal, and hematologic functions, including WBC greater than 3,000/μl, absolute neutrophil count greater than 1,500/μl, platelets greater than 100,000/μl, serum creatinine less than two times the upper limit of normal (ULN), bilirubin less than 1.5 × ULN, AST less than 2.5 × ULN, ALT less than 5.0 × ULN, international normalized ratio less than 1.5 ULN, and partial thromboplastin time less than ULN. A total of 78 patients were eligible for clinical evaluation. This study was carried out with the approval of the Institutional Review Board of Peking University Cancer Hospital and Institute.
Determination of c-Kit Mutation and Gene Amplification
Genomic DNA was extracted from formalin-fixed, paraffin-embedded (FFPE) sections by QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). Aiming at detecting hotspot mutations, we amplified exons 9, 11, 13, 17, and 18 of the c-Kit gene by polymerase chain reaction (PCR) in at least two separate preparations of genomic DNA and purified PCR products with QIAquick (Qiagen). The PCR products were then directly sequenced using Big Dye Terminator sequencing chemistry on an ABI3130 automated sequencer (Applied Biosystems, Foster City, CA, USA). All mutations were confirmed by bidirectional sequencing repeat on the ABI sequencer. To determine the gene copy number of c-Kit, quantitative real-time PCR was performed using ribonuclease P (RNaseP) as a control gene. Relative copy numbers were calculated using the ΔΔCt method11.
Treatment and Assessment of Response
Patients received continuous therapy with imatinib (400 mg/day) until intolerable toxicities or disease progression occurred. Response of disease was assessed by RECIST 1.0. Computed tomography (CT) scans of the chest, abdomen, and pelvis were performed at baseline, after 4 weeks of the initial treatment of imatinib, and at 2-month intervals. Magnetic resonance imaging (MRI) scans of the brain were obtained if clinically indicated. Adverse events were assessed and recorded per the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0). All clinical data of patients were obtained from the medical records.
The following four outcome measures evaluated treatment efficacy and the patient status: (1) OS: the time from initiation of imatinib to the date of death due to any cause or last follow-up; (2) PFS: the time from initiation of imatinib to the date of disease progression or death; (3) ORR: consisting of complete response (CR) and partial response (PR); (4) disease control rate (DCR): an aggregate score of CR, PR, and stable disease (SD). ORR and DCR were assessed according to RECIST 1.0 criteria.
Statistical Analyses
OS and PFS distributions were calculated using the Kaplan–Meier method. The difference comparisons of PFS and OS between mutation types (exon 11 or 13 vs. others, multiple mutation vs. others), responses (PR and SD vs. PD), and M stages (according to AJCC 7th edition, M1a vs. M1b vs. M1c) were calculated by log-rank tests. We also used chi-square tests to compare the response rates in different mutation types. For further analysis of the correlation between OS, PFS, and clinical characteristics, we also ran the Cox regression models of OS and PFS on M stages, primary sites, age, sex, and lactate dehydrogenase (LDH) level. Hazard ratios and 95% confidence interval (95% CI) were estimated.
All analyses were performed using IBM SPSS Statistics 19.0 software. The significance two-sided α level was set as 0.05.
RESULTS
Patients
Table 1 shows the demographics and baseline disease characteristics of the 78 patients included in this analysis. Between January 1, 2008 and September 30, 2015, 78 patients harboring c-Kit alterations were admitted to the Renal Cancer and Melanoma Department, with a median age of 54 years old. As for the primary site classification, there were 33 (42.3%) cases of acral melanoma, 18 (23.1%) cases of mucosal melanoma, 15 (19.2%) cases of cutaneous melanoma, and 12 (15.4%) cases of other subtypes. Nineteen, 24, and 35 patients had American Joint Commission on Cancer (AJCC) staging M1a, M1b, and M1c disease, respectively. Thirty-seven of the 78 (47.4%) patients were treatment naive. Thirty cases (38.5%) and 11 cases (47.4%) had received first- and second-line or beyond systemic treatments, respectively. In addition, 31 (39.7%) and 16 (20.5%) patients harbored single mutations in exon 11 and exon 13, respectively. Twenty-three patients (29.5%) harbored mutations in other exons (exon 9, 17, or 18). Multiple c-Kit mutations were detected in 12 patients (16.7%) of the analyzed population. In addition to the presence of c-Kit mutations, BRAF status was determined, and 4 of 78 patients had the BRAF V600E mutation (Table 2).
Table 1.
Baseline Characteristics of Melanoma Patients
| Indicators | No. of Patients |
|---|---|
| Age [median (range)] | 54 (11–80) |
| Gender | |
| Male | 36 (46.2%) |
| Female | 42 (53.8%) |
| Clinical subtype | |
| Acral | 42 (53.8%) |
| Mucosal | 16 (20.5%) |
| Chronically sun damaged | 14 (18.0%) |
| Others | 6 (7.7%) |
| LDH levels > ULN | 25 (32.1%) |
| Lines of imatinib treatment | |
| First line | 37 (47.4%) |
| Second line | 30 (38.5%) |
| Third line | 11 (14.1%) |
| M stage | |
| M1a | 19 (24.4%) |
| M1b | 24 (30.8%) |
| M1c | 35 (44.9%) |
LDH, lactate dehydrogenase; ULN, upper limits of normal. Total percent may be not equal to 100 because of rounding.
Table 2.
c-Kit and BRAF Mutation Types and Response Rates
| Mutation Types | PR [n (%)] | SD [n (%)] | PD [n (%)] |
|---|---|---|---|
| c-Kit | |||
| Exon 9 | 1 (14.2%) | 3 (42.9%) | 3 (42.9%) |
| Exon 11 | 6 (20.7%) | 13 (44.8%) | 10 (34.5%) |
| Exon 13 | 5 (31.3%) | 6 (37.4%) | 5 (31.3%) |
| Exon 17 | 1 (20.0%) | 2 (40.0%) | 2 (40.0%) |
| Exon 18 | 1 (33.3%) | 2 (66.7%) | 0 |
| Multiple mutations | 2 (18.2%) | 4 (36.4%) | 5 (45.4%) |
| Amplification | 1 (50.0%) | 0 | 1 (50.0%) |
| BRAF | |||
| V600E | 0 | 3(75.0%) | 1 (25.0%) |
PR, partial response; SD, stable disease; PD, progressive disease.
PFS, OS, and ORR
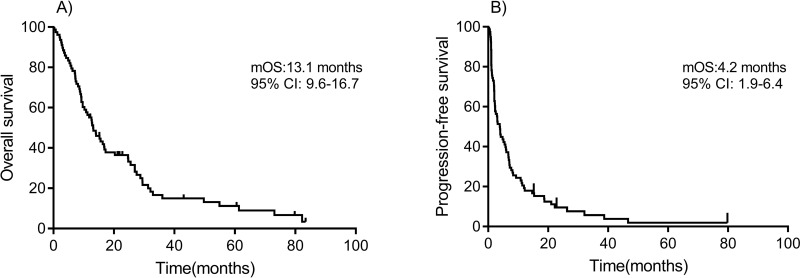
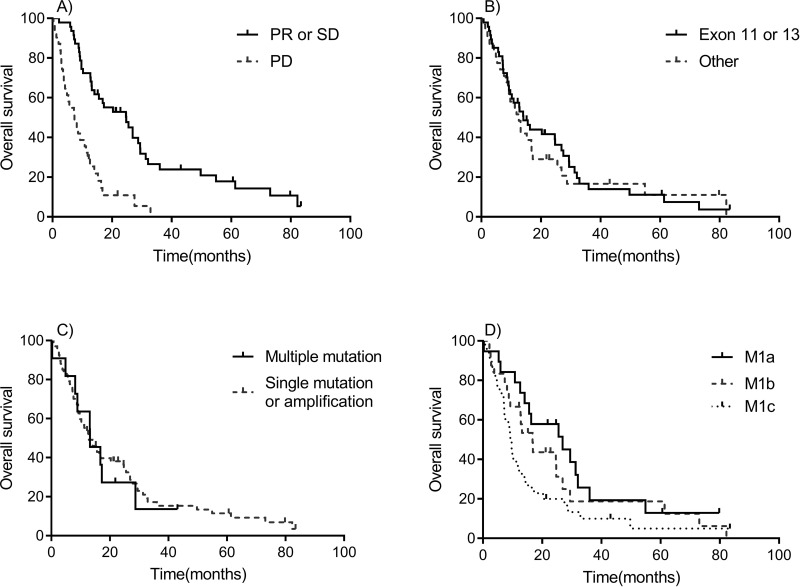
Median follow-up time for survival was 12.5 months (ranging from 0.3 to 82.2 months). At the time of this analysis, 2 patients remained alive without disease progression, 9 were alive with disease progression, and 67 patients had died. The 1- and 2-year OS rate was 55.1% and 29.5%, respectively. The median OS (mOS) was 13.1 months (95% CI: 9.6–16.7 months) (Fig. 1A). The OS of patients who achieved PR or SD was significantly superior compared to those with disease progression (mOS: 24.7 vs. 7.2 months, p < 0.001) (Fig. 2A). The mOS of patients who harbored exon 11 or 13 mutations was similar to that of patients with other mutations (14.1 vs. 12.6 months, p = 0.640) (Fig. 2B). The survival distributions of patients with multiple mutations or others (single mutation or amplification) were also similar (mOS: 13.1 vs. 12.8 months, p = 0.727) (Fig. 2C). Patients with the M stage of M1c had a shorter survival time (mOS: 9.3 months) than those with M1a or M1b (mOS: 26.9 and 16.8 months) (Fig. 2D).
Figure 1.
Kaplan–Meier overall survival (OS) and progression-free survival (PFS) curves. (A) OS. (B) PFS.
Figure 2.
Kaplan–Meier OS curves for different subgroups. (A) Partial response (PR) and stable disease (SD) versus progressive disease (PD). (B) Exon 11 or 13 versus Other. (C) Multiple mutation versus single mutation or amplification. (D) M1a versus M1b versus M1c.
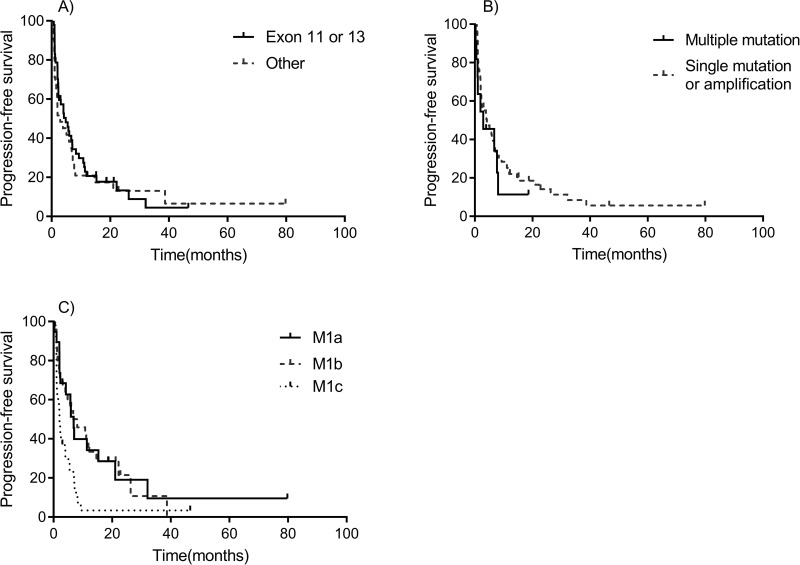
The median PFS (mPFS) of all patients was 4.2 months (95% CI: 1.9–6.4 months) (Fig. 1B). PFS comparisons between different factors are shown in Figure 3. mPFS were 7.2, 4.0, and 1.6 months among patients treated with imatinib as first, second, and third line or greater, respectively. The mPFS of patients who harbored exon 11 or 13 mutations was only marginally longer than that of other aberrations (4.2 vs. 3.9 months, p = 0.770). The mPFS of patients with multiple mutations was a little shorter than others (2.9 vs. 4.8 months, p = 0.186). Patients with the M stage of M1c, M1b, and M1c had mPFS of 5.9, 6.7, and 2.0 months (p = 0.002), respectively.
Figure 3.
Kaplan–Meier PFS curves for different subgroups. (A) Exon 11 or 13 versus Other. (B) Multiple mutations versus single mutation or amplification. (C) M1a versus M1b versus M1c.
The best overall responses were as follows: PR, SD, and PD were observed in 17 patients (21.8%), 30 patients (38.5%), and 29 patients (37.2%), respectively. The disease control rate (PR and SD) was 60.3%. Among 17 patients who experienced a partial response, 11 patients were confirmed to have exon 11 or 13 mutations, which accounted for 64.7% (Table 2). The ORR of patients carrying the exon 11 or 13 mutations was 24.4%, whereas ORR in patients with other types of mutations was 19.4%. Ten of 37, 5/30, and 2/11 patients treated with imatinib as first, second, and third or above lines achieved PR, but without significance (χ2 = 1.141, p = 0.565). DCR of the exon 11 or 13 group was 66.7%, whereas the DCR of the group with other aberrations was 54.8%. No significant difference was observed (χ2 = 1.088, p = 0.297) in DCR between the sensitive mutation types (exon 11 or 13) and other genetic alterations.
Cox Regression Analysis
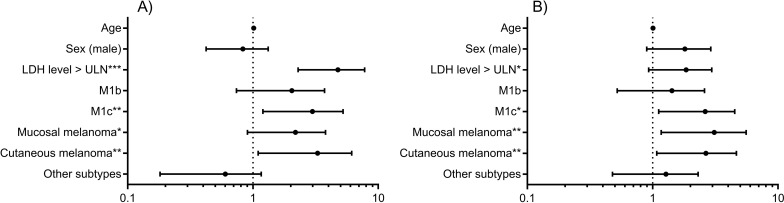
Figure 4 shows the results of the Cox regression analysis. After adjusting for the different treatment lines, patients with M1c, cutaneous melanoma, and elevated LDH level (>ULN) had higher hazard ratios for OS (with the HRs of 2.510, 2.601, and 4.228, respectively). In particular, patients with M1c and cutaneous/mucosal melanoma had a higher hazard for PFS with statistical significance. Age and sex did not correlate with OS and PFS. The hazard ratio of LDH level for PFS is greater than 1 but insignificant at the level of 0.05 (HR = 1.660, p = 0.087).
Figure 4.
Cox regression results. (A) OS. (B) PFS. Variables for regression include M stages (M1a, M1b, M1c), primary sites (acral, mucosal, cutaneous, other subtypes), age, sex (male, female), and LDH level (>ULN, normal).
Imatinib was received as the regimen of different lines in this study. Among those 37 patients treated with imatinib as the first line, 10 patients continued to receive the combination of chemotherapy and antiangiogenic agents as the second-line treatment. One patient was treated with pembrolizumab. Further cancer-related therapy did not occur in the rest of the patients. All patients receiving imatinib as non-first-line treatment were chemotherapy refractory, and only a small proportion received different chemotherapy regiments again after disease progression. None of them received immunotherapy as the further treatment. As for those four patients with BRAF V600E mutation, two patients continued to receive vemurafenib treatment after disease progression, and the other two did not received any treatment. It can be seen that concomitant cancer therapies occurring before or after the imatinib treatment were limited to regular treatment regimens.
Adverse Events
The imatinib adverse event profile observed in our study was generally consistent with data observed in previous studies10,12,13. The most common adverse events were edema (50%), rash (18%), fatigue (9%), anorexia (7%), nausea (5%), and neutropenia (2%). Other adverse events included vomiting, psychiatric symptoms, and elevated ALT or AST, which occurred in a fraction of patients. However, adverse events were generally mild to moderate in severity and were easily managed by dose reduction or supportive medical treatment. Imatinib treatment was terminated in two patients (2.6%) due to the development of refractory serious pleural and pericardial cavity effusion, and intolerable diarrhea. Another two patients had a reduced imatinib dosage as a result of diarrhea and skin rash. No treatment-related deaths were recorded.
DISCUSSION
With respect to the use of imatinib for the treatment of metastatic melanoma, there have been 10 registered clinical trials (https://clinicaltrials.gov/ ) conducted to date, exploring the efficacy or safety of imatinib with or without other agents. One clinical trial remains open for accrual with an estimated completion year of 2022. Six trials failed or were terminated due to intolerable toxicity or lack of clinical efficacy. Three of these 10 studies successfully demonstrated that patients with c-Kit mutations can benefit from imatinib in the clinical practice.
In accordance with those reported results10,12,13, our findings show that patients with c-Kit mutations respond to imatinib therapy, further confirming the clinical efficacy of imatinib based on a larger population. Carvajal et al.’s study12 (trial 1) was a phase II, single-arm trial with a sample size of 28 patients who had advanced unresectable melanoma; Guo et al.’s trial10 (trial 2) was an open-label and single-arm trial with a total of 43 enrolled patients with metastatic melanoma; Hodi et al.’s trial13 (trial 3) was designed as a phase II study and conducted in five sites in the US, and this trial enrolled a total of 25 patients. A mPFS of 4.2 months was observed in our study, longer than that in trial 1 to trial 3 [2.8 (time to progression, TTP), 3.5, 3.7 months (TTP)], but no dominant mOS was found (13.1 vs. 10.8, 12.5, 14.0 months, respectively, in trial 1 to trial 3). A higher ORR was observed in our study compared with trial 1 (21.8% vs. 16%), but it was slightly lower than in trial 2 (23.3%) and trial 3 (29%). We observed a higher DCR of 60.3%, an increase by nearly 17% than that of trials 2 and 3. One-year OS rate was demonstrated to be 55.1% longer than in previous trials.
In terms of the mutation status, the previous clinical studies observed that the clinical efficacy of imatinib was related to the specific mutation hotspot. c-Kit mutations are mainly located in exons 11, 13, and 17. Mutations present in exon 11 or 13 may be more sensitive compared to other mutation types10,12,13. In our study, single exon mutation of exon 11 or 13 accounted for 60.2% of the genetic alterations, of which the most frequent mutation hotspots were L576P and K642E (24.3%). The PFS and OS were slightly longer in patients with exon 11 or 13 mutations, with a better curative effect of imatinib, although this effect did not reach statistical significance. Compared with the previous study of trial 2, the difference of both mPFS and mOS between exon 11/13 mutation patients compared to patients with other types, multiple mutation patients versus others decreased slightly. However, there was still no statistical significance across different subgroups. Considering sample size and timeframe, a greater number of enrolled patients and a longer follow-up are required to observe a more accurate difference. The best response of two patients with NRAS mutation in this study was PR, consistent with the results of trial 3, which noted that the presence of NRAS mutations might be a likely cause for primary resistance to KIT targeting in some melanoma. However, further studies with larger sample size are needed to further explore the potential causal pathway.
Patients with gastrointestinal stromal tumors (GIST) are characterized by gain-of-function mutations in the Kit proto-oncogene, most commonly involving exon 11 and less frequently involving exon 915. Several clinical studies have shown that GIST patients with exon 9 mutation are able to derive clinical benefit from imatinib treatment (ORR: 34%–48%)16–18. In addition, trial 1 reported that the clinical efficacy rate of exons 9 and 13 mutations in patients with imatinib treatment was approximately 33%. Some studies also have found mutation in the juxtamembrane region to be linked to better prognosis when compared with the kinase domain mutant cases19. In our study, the c-Kit mutation distribution shows a scattered pattern and that common mutations only comprise a small proportion. These scattered mutations with lower frequencies may be nonsense mutations, and they can be located in the kinase domain, which leads to the development of primary drug resistance.
To our knowledge, this study includes the largest number of metastatic melanoma patients with c-Kit aberrations. Other c-Kit inhibitors such as dasatinib and nilotinib have been also investigated in clinical trials. Kluger et al.20 and Kalinsky et al.21 found that dasatinib was poorly tolerated and that the overall response rate among Kit + melanoma patients was low (only 5%–18%). The proportion of partial response in those patients who received nilotinib treatment was also low22,23. Compared with other c-Kit inhibitors, imatinib therapy is associated with better clinical efficacy and safety profiles among melanoma patients.
In conclusion, our findings, combined with those studies targeting population with c-Kit alteration, provide further evidence for the role of imatinib as an important and promising therapeutic agent in metastatic melanoma patients with c-Kit genetic alterations.
ACKNOWLEDGMENTS
This work was supported by grants from the Major State Basic Research Development Program of China (2013CB911004), National Natural Science Foundation of China (81172196, 81102068, 81272991, 81301984), New Century Excellent Talents in University (NCET-13-0007), Beijing Municipal Natural Science Foundation (7152033), and Beijing Talents Fund (2014000021223ZK26). We would also like to thank all of the patients for their willingness to be part of this clinical study.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J, Wiggins CL, Wingo PA. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17–25. [DOI] [PubMed] [Google Scholar]
- 3. Curry JL, Torres-Cabala CA, Tetzlaff MT, Bowman C, Prieto VG. Molecular platforms utilized to detect BRAF V600E mutation in melanoma. Semin Cutan Med Surg. 2012;31(4):267–73. [DOI] [PubMed] [Google Scholar]
- 4. Queirolo P, Picasso V, Spagnolo F. Combined BRAF and MEK inhibition for the treatment of BRAF-mutated metastatic melanoma. Cancer Treat Rev. 2015;41(6):519–26. [DOI] [PubMed] [Google Scholar]
- 5. Hao C, Tian J, Liu H, Li F, Niu H, Zhu B. Efficacy and safety of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4 immunotherapy to advanced melanoma: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96(26):e7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hui SK, Tang WY, Wong TW, Lau KH, Lee S, Chong LY, Lo KK. Cutaneous melanoma: A population-based epidemiology report with 989 patients in Hong Kong. Clin Exp Dermatol. 2007;32(3):265–7. [DOI] [PubMed] [Google Scholar]
- 8. Lv J, Dai B, Kong Y, Shen X, Kong J. Acral melanoma in Chinese: A clinicopathological and prognostic study of 142 cases. Sci Rep. 2016;631432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton AJ, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–48. [DOI] [PubMed] [Google Scholar]
- 10. Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, Corless CL, Li L, Li H, Sheng X. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29(21):2904. [DOI] [PubMed] [Google Scholar]
- 11. Kong Y, Si L, Zhu Y, Xu X, Corless CL, Flaherty KT, Li L, Li H, Sheng X, Cui C, Chi Z, Li S, Han M, Mao L, Lu A, Guo J. Large-scale analysis of KIT aberrations in Chinese patients with melanoma. Clin Cancer Res. 2011;17(7):1684–91. [DOI] [PubMed] [Google Scholar]
- 12. Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, Panageas KS, Busam KJ, Chmielowski B, Lutzky J, Pavlick AC, Fusco A, Cane L, Takebe N, Vemula S, Bouvier N, Bastian BC, Schwartz GK. KIT as a therapeutic target in metastatic melanoma. JAMA 2011;305(22):2327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, Friedlander P, Gonzalez R, Weber JS, Gajewski TF, O’Day SJ, Kim KB, Lawrence D, Flaherty KT, Luke JJ, Collichio FA, Ernstoff MS, Heinrich MC, Beadling C, Zukotynski KA, Yap JT, Van den Abbeele AD, Demetri GD, Fisher DE. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31(26):3182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205. [DOI] [PubMed] [Google Scholar]
- 15. Stamatakos M, Douzinas E, Stefanaki C, Safioleas P, Polyzou E, Levidou G, Safioleas M. Gastrointestinal stromal tumor. World J Surg Oncol. 2009;7(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Casper ES, Conrad ER, DeLaney TF, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JR, Mayerson J, McGarry SV, Meyer C, O’Donnell RJ, Pappo AS, Paz IB, Pfeifer JD, Riedel RF, Schuetze S, Schupak KD, Schwartz HS, Van Tine BA, Wayne JD, Bergman MA, Sundar H. Gastrointestinal stromal tumors, version 2.2014. J Natl Compr Canc Netw. 2014;12:853–62. [DOI] [PubMed] [Google Scholar]
- 17. Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S, Stul M, Casali PG, Zalcberg J, Verweij J, Van Glabbeke M, Hagemeijer A, Judson I. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 2006;42(8):1093–103. [DOI] [PubMed] [Google Scholar]
- 18. Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet 2004;364(9440):1127–34. [DOI] [PubMed] [Google Scholar]
- 19. Frost MJ, Ferrao PT, Hughes TP, Ashman LK. Juxtamembrane mutant V560GKit is more sensitive to Imatinib (STI571) compared with wild-type c-kit whereas the kinase domain mutant D816VKit is resistant. Mol Cancer Ther. 2002;1(12):1115–24. [PubMed] [Google Scholar]
- 20. Kluger HM, Dudek AZ, McCann C, Ritacco J, Southard N, Jilaveanu LB, Molinaro A, Sznol M. A phase 2 trial of dasatinib in advanced melanoma. Cancer-Am Cancer Soc. 2011;117(10):2202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalinsky K, Lee S, Rubin KM, Lawrence DP, Iafrarte AJ, Borger DR, Margolin KA, Leitao MJ, Tarhini AA, Koon HB, Pecora AL, Jaslowski AJ, Cohen GI, Kuzel TM, Lao CD, Kirkwood JM. A phase 2 trial of dasatinib in patients with locally advanced or stage IV mucosal, acral, or vulvovaginal melanoma: A trial of the ECOG-ACRIN Cancer Research Group (E2607). Cancer-Am Cancer Soc. 2017;123(14):2688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo J, Carvajal RD, Dummer R, Hauschild A, Daud A, Bastian BC, Markovic SN, Queirolo P, Arance A, Berking C, Camargo V, Herchenhorn D, Petrella TM, Schadendorf D, Sharfman W, Testori A, Novick S, Hertle S, Nourry C, Chen Q, Hodi FS. Efficacy and safety of nilotinib in patients with KIT-mutated metastatic or inoperable melanoma: Final results from the global, single-arm, phase II TEAM trial. Ann Oncol. 2017;28(6):1380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carvajal RD, Lawrence DP, Weber JS, Gajewski TF, Gonzalez R, Lutzky J, O’Day SJ, Hamid O, Wolchok JD, Chapman PB, Sullivan RJ, Teitcher JB, Ramaiya N, Giobbie-Hurder A, Antonescu CR, Heinrich MC, Bastian BC, Corless CL, Fletcher JA, Hodi FS. Phase II study of nilotinib in melanoma harboring KIT alterations following progression to prior KIT inhibition. Clin Cancer Res. 2015;21(10):2289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]