Abstract
Stem cells offer the basis for the promotion of robust new therapeutic approaches for a variety of human disorders. There are still many limitations to be overcome before clinical therapeutic application, including a better understanding of the mechanism by which stem cell therapies may lead to enhanced recovery. In vitro investigations are necessary to dissect the mechanisms involved and to support the potential development in stem cell‐based therapies. In spite of growing interest in human amniotic fluid stem cells, not much is known about the characteristics of their secretome and regarding the potential neuroprotective mechanism in different pathologies, including stroke. To get more insight on amniotic fluid cells therapeutic potential, signal transduction pathways activated by human amniotic fluid stem cells (hAFSCs)‐derived secretome in a stroke in vitro model (ischemia/reperfusion [I/R] model) were investigated by Western blot. Moreover, miRNA expression in the exosomal fraction of the conditioned medium was analyzed. hAFSCs‐derived secretome was able to activate pro‐survival and anti‐apoptotic pathways. MicroRNA analysis in the exosomal component revealed a panel of 16 overexpressed miRNAs involved in the regulation of coherent signaling pathways. In particular, the pathways of relevance in ischemia/reperfusion, such as neurotrophin signaling, and those related to neuroprotection and neuronal cell death, were analyzed. The results obtained strongly point toward the neuroprotective effects of the hAFSCs‐conditioned medium in the in vitro stroke model here analyzed. This can be achieved by the modulation and activation of pro‐survival processes, at least in part, due to the activity of secreted miRNAs.
Keywords: amniotic fluid, brain‐derived neurotrophic factor, cerebral ischemia, conditioned medium, exosome, ischemia‐reperfusion, miRNA, oxygen‐glucose deprivation, stem cells, stroke
Neuroprotective effects of human amniotic fluid stem cell‐secretome in an ischemia/reperfusion model.
Significance statement.
This article focuses on the therapeutic potential of human amniotic fluid stem cells (hAFSCs)‐derived secretome in an ischemia/reperfusion in vitro model. Interestingly, in the presented experimental conditions, hAFSCs‐derived secretome was able to activate pro‐survival and anti‐apoptotic pathways. Furthermore, microRNA analysis in the exosomal component revealed overexpressed miRNAs involved in neurotrophin signaling and those related to neuroprotection and neuronal cell death. In light of the data obtained, the use of conditioned medium and, in particular, exosomes, may constitute a potential approach to stimulate neuronal plasticity, ameliorate cognitive loss and neural replacement, and may represent a suitable treatment for ischemia/reperfusion injury.
1. INTRODUCTION
Stroke is one of the primary causes of death and long‐term disability in the world and represents a major public health problem. Stroke can be categorized into two major types, specifically ischemic and hemorrhagic stroke. Ischemic stroke is due to a block within a blood vessel distributing blood to the brain, while hemorrhagic stroke is due by the rupture of a blood vessel or an irregular vascular construct. 1 , 2 According to statistics, most stroke patients are affected by ischemic stroke. 3 Cerebral ischemia leads to different detrimental effects, including oxidative stress, ionic imbalance, apoptosis, and inflammation, which cause neuronal impairment and death. 4 This pathology lacks successful cures, due to both the limited time window for intervention before the development of deleterious consequences, and the absence of specificity of the treatments established so far. Furthermore, the inhibition of some molecular targets demonstrates to be ineffective due to secondary effects. To date, tissue plasminogen activator is the only therapeutic approach used in stroke patients. 4 , 5 , 6 Reperfusion injury occurring in consequence of ischemic stroke is an intricate process involving several processes. Also, thrombolytic therapy itself can lead to brain injuries, named cerebral ischemia/reperfusion (I/R) injury. 7 , 8 I/R injury is one of the major reasons for disability, high morbidity, and mortality worldwide. Due to the lack of effective neuroprotective treatments, the care for I/R injury continues to be a main medical concern and needs to be thoroughly investigated.
Due to its characteristics, the oxygen and glucose deprivation (OGD) challenge of neurons is a useful model of cerebral ischemia and provides the analysis of molecular pathways underlying stroke. 9 , 10 , 11 This challenge entails placing the cells in a glucose‐free medium in an anaerobic condition; thus, merging the lack of these two components mimics the clinical situation in the brain during cerebral ischemia but in a simplified system. 11 , 12 Successively, to mimic in vitro the I/R injury, the OGD model is subjected to reperfusion (OGD/R or I/R) in normoxia condition. 13
Differentiated SH‐SY5Y are widely accepted for in vitro experiments requiring neuronal‐like cells. Differentiated SH‐SY5Y are low cost to culture, and the ethical concerns related with primary human neuronal culture are avoided. Furthermore, since SH‐SY5Y cells are human‐derived, they express several human‐specific proteins and isoforms that would not be inherently present in rodent primary cultures. 14 , 15 Particularly, this cell line is used to develop the in vitro OGD/IR model. 15 , 16 , 17 , 18 , 19 , 20 , 21
Current findings in regenerative medicine have strengthened the search for new stem cell sources with beneficial properties; in particular, amniotic fluid has been identified as a valid resource of stem cells in this field. Adult stem cells, even after reprogramming, could preserve epigenetic alterations, thus representing a limitation in their application. 22 On the other hand, fetal stem cells may overcome this limitation, and furthermore, ethical issues related to its isolation are minimal, in particular regarding amniotic fluid stem cells, as they are collected during routine amniocentesis, third‐trimester amnio‐reduction or cesarean section. 23
The amniotic fluid is a protective liquid for fetus growth and offers mechanical support as well as essential nutrients during embryogenesis. 24 It is constituted primarily of water, cells, and chemical elements. 25 These cells are heterogeneous in morphology, in vitro, and in vivo characteristics. 26 They are mainly of fetal derivation (respiratory, epithelial, urinary, and intestinal tract), but also amniotic membranes and connective tissues. Furthermore, the amniotic fluid presents various cellular subcategories (amniotic, fibroblastic, and epithelioid), which differ in percentage depending on gestational period. 27 Amniotic fluid mesenchymal stem cells (AFMSCs) are really attractive due to their potential therapeutic purposes, and numerous procedures of expansion and isolation have been reported. AFMSCs show a wide differentiation potential toward mesenchymal lineages and have the capability to differentiate toward chondrogenic, osteogenic, and adipogenic, thus representing a suitable cell source for regenerative and therapeutic approaches. 28
However, two processes exerting a crucial part in regenerative processes are reported. One process concerns the differentiation of the engrafted stem cells toward the specific cytotype of the injured area. This mechanism has been depicted by different authors, 29 , 30 , 31 , 32 but the small amount of exogenous cells constantly and directly engrafted into the regenerated tissue do not entirely clarify the achieved regenerative effect. The incomplete integration may be due to the heterogenic level of pre‐differentiation produced in vitro before the transplant. In fact, the entire cell population stimulated might not undertake the differentiation process. This subpopulation could preserve low immunogenic characteristics but, notably, can influence the immune system and promote the recruitment of resident progenitor cells to regenerate injured tissue. Numerous investigations corroborated this hypothesis, indicating the protective activity of the stem cell‐released molecules alone (conditioned medium) in the regeneration of the damaged area where, typically, a chronic inflammation condition is present. 33 , 34 , 35 , 36
Founding on these theories, in the last decade, more interest has been focused on stem cells secretome and, consequently, on the paracrine effect. 37 , 38 , 39 Conditioned medium (CM) consists of extracellular vesicles (EV) and soluble factors. Recently, besides exerting role as biomarkers, secreted EV has been included among the players promoting mesenchymal stem cells (MSCs) regenerative potential 40 , 41 , 42 , 43 although, so far, a complete molecular description of their “cargo” is lacking.
Exosomes represent a subpopulation of microvesicles, ranging from 40‐100 nm, which, initially, were identified as artifacts under the electron microscope. Recently, different investigations were focused on exosomes for their cell‐to‐cell communication, storage of biological information, the use as biomarkers, and, in particular, for the potential application in regeneration and neural protection. 44 , 45 , 46 Exosomes have cup‐shaped morphology and derive from the endocytic pathway after the fusion of multivesicular bodies with the plasma membrane and then release into the extracellular environment. 44
Exosomes contain several proteins, miRNA, DNA, saccharides, and lipids, indicated with the term “cargo.” 47 , 48 Because of their nano‐size and non‐complexed structure, exosomes efficiently cross the blood‐brain barrier, consequently, represent innovative approaches to design therapeutic strategies for different cerebral diseases. Thus, exosomes offer an alternative therapeutic approach as a substitute for cell transplantation. 49
On these bases, in the present work, the signal transduction pathways activated by hAFSCs‐secretome on a stroke in vitro model (I/R) and the main miRNA involved in the modulation of the observed effects were investigated, shedding light on some of the possible mechanisms of hAFSCs‐induced neuroprotection.
2. MATERIALS AND METHODS
2.1. Cell culture and treatments
The human neuroblastoma cell line SH‐SY5Y has comparable electrophysiological, morphological, and neurochemical characteristics of neurons and was bought from the European Collection of Authenticated Cell Cultures.Cells were maintained in DMEM high‐glucose medium complemented with 10% FBS and 1% antibiotics (Corning) and were maintained in a humidified condition with 5% carbon dioxide at 37°C. Cells were seeded at 104 cells/cm2; after 24 hours, to induce differentiation, medium was replaced with DMEM 1% FBS, and N2 supplement (Gibco) for 7 days, as previously reported, 50 and then subjected to OGD. Differentiated SH‐SY5Y are widely accepted for in vitro experiments requiring neuronal‐like cells. 17
2.1.1. OGD model
In brief, cells were gently rinsed with phosphate‐buffered saline, and the medium was substituted with EBSS containing L‐glutamine (Sigma‐Aldrich) that had been deoxygenated with an anaerobic gas mixture (0.1% O2) for 30 minutes before use. Cells were then incubated in a hypoxic condition incubator, 0.1% O2 at 37°C at different time points. To set the experimental conditions, we measured cell viability after different OGD timepoints. In particular, 3 hours OGD insult showed a 50% reduction in cell viability and was chosen for the following experiments.
2.1.2. I/R model
We set 3 hours OGD as timepoint, then to perform reperfusion conditions, medium was substituted with DMEM (for I/R control) or conditioned medium (I/R + CM), and cells were incubated for 24 hours in 95% air/5% CO2 in a humidified incubator (reperfusion). Control cells were exposed to the identical experimental processes with vehicle only and with no exposure to anoxia and glucose‐free medium (Normoxia). About 24 hours after OGD/Reperfusion we assayed again the cell viability to understand if conditioned media could exert positive effects.
2.1.3. CM preparation
Cells used to collect the conditioned medium were approved by the ethic committee for biomedical research of the G. D'Annunzio University of Chieti‐Pescara, Italy. Two milliliters of amniotic fluid were collected after informed written consent and centrifuged at 1200 rpm for 5 minutes and the pellet was utilized to establish the cell line. 51
hAFS cells were cultivated in Iscove's Modified Dulbecco's medium (Corning), complemented with 10% FBS (Corning), 100 μg/mL streptomycin, 100 U/mL penicillin, 2 mM l‐glutamine (Corning), and 5 ng/mL basic FGF 2 (Peprotech, United Kingdom), and cultured at 37°C in a humidified atmosphere with 5% CO2. The medium was replaced every 2 days.
To collect conditioned medium, when the culture reached the confluence of 70% the medium was replaced with IDMEM supplemented with exosome‐free FBS (depleted using a kit by Norgen Biotek, Canada) and collected after 72 hours.
2.1.4. Exosome preparation
To isolate and purify the conditioned media exosomes different ultracentrifuge procedures were performed. Briefly, the conditioned media were subjected to centrifuge for 5 minutes at 200g, then 10 minutes at 200g in order to remove cell debris. Then, the supernatant was collected and centrifuged for 30 minutes at 16 500g (+4°C); the supernatant was again collected and centrifuged at 120 000 g for 90 minutes (+4°C). The pellet obtained contains the exosomes and was washed with Ultrapure water (90 minutes, 120 000g at 4°C).
2.2. MTS assay
Cell viability at different time points and conditions were determined, using Cell Proliferation Assay (Promega) a colorimetric assay established on the amount of formazan produced, as a function of viability. The assay was read at 490 nm using a plate reader, Infinite F200 (Tecan, Swiss), and analyzed in triplicate.
2.3. Immunofluorescence
Treated and untreated cells were fixed in 4% paraformaldehyde in PBS for 15 minutes at RT and permeabilized in cold methanol for 15 minutes. Unspecific sites were blocked using 4% BSA for 20 minutes. As primary antibody, rabbit anti‐NHF (1:200, Invitrogen by Thermo Fisher Scientific) diluted in bovine serum albumin was used. Cells were then washed with PBS thoroughly and then incubated with the secondary antibody, goat anti‐rabbit conjugated with Alexa Fluor 633 (1:2000; Life Technologies) for 40 minutes. Coverslips were thoroughly washed, Vectashield mounting medium with DAPI (Vector Laboratories) was used, and then observed at fluorescence microscopy AXIOPHOT (Zeiss microscope, Germany).
2.4. Neurite analyses
Neuronal cells were photographed from 8 to 10 random fields per coverslip from three independent experiments using an immunofluorescence microscope (AXIOPHOT, Zeiss microscope). Cell clusters were excluded from morphometric analyses. Neurite length was defined as the distance from the soma to the tip of the longest primary neurite. Cells were traced using NeuronJ (plugin of Fiji software). The neurite length was then divided on soma diameter (cell body). All the detailed points can be found in the article by Pemberton et al, 2018. 52
2.5. Protein assay
Protein amount was assayed using Pierce BCA Protein Assay (Pierce) and the absorbance was read at 550 nm.
2.6. Western blotting
Treated and untreated cell cultures were collected and lysated in ice‐cold RIPA buffer, as previously reported. 53 , 54 Protein lysates (30‐40 μg) were run on 9‐13% SDS‐polyacrylamide gel and blotted on polyvinyldifluoride membrane (PVDF; Sigma‐Aldrich). Regarding the extraction of proteins from CM and EXO components, RIPA buffer was used as the above described, and then 50 μg of protein content was run on gradient SDS gel (Thermo). Unspecific sites were blocked in 5% lyophilized blocking buffer Blotto (Santa Cruz) diluted in Tris‐buffered saline with Tween for 1 hour at RT. Membranes were then incubated overnight at 4°C with the below primary antibodies, prepared in blocking buffer: rabbit anti‐HIF‐1α 1:200 (Santa Cruz); rabbit anti‐BDNF 1:1000 (Abcam, United Kingdom); rabbit anti‐p‐TrkB and TrKB 1:500 (Abcam, United Kingdom); rabbit anti‐p‐ERK5 and ERK5 1:1000 (Cell Signaling), rabbit anti PSD95 1:1000 (Cell Signaling); rabbit anti‐p‐CREB and CREB 1:1000 (Cell Signaling), rabbit anti P75 1:1000 (Abcam, United Kingdom); mouse anti‐RhoA 1:500 (Santa Cruz); rabbit anti‐p‐JNK and JNK 1:500 (Santa Cruz); mouse anti‐ProBDNF 1:500 (Invitrogen). After extensive washings, membranes were incubated with peroxidase‐conjugated anti‐rabbit or mouse IgG (1:10 000; Vector Laboratories). To visualize, the immunoreactive bands were incubated with luminol (Bio‐Rad Laboratories), according to the producer's directions. The bands were revealed using Uvitec (United Kingdom) machine and digital images were collected. To reprobe the same membrane, ReBlot Plus Strong Antibody Stripping Solution was used following manufacturer's protocol (Sigma). Relative densities were analyzed using Fiji software and to normalize and check the loading, HRP‐conjugated actin was used (Cell signaling). Phosphorylated proteins are normalized upon their respective total protein. Values were reported as relative units.
2.7. BDNF ELISA kit
mBDNF ELISA kit was purchased from Aviscera Bioscience, United States. Both the exosome component and liquid component were separated by ultracentrifugation. Then we assayed the kit following the manufacturer's protocols and finally, we read the optical density, using a microplate reader set to 450 nm. Data were expressed as mBDNF (pg/mL).
2.8. RNA extraction and miRNAs analysis
Total RNA was extracted from CM exosomes, obtained as above described, and exosome‐free control medium using the Plasma/Serum RNA Purification Mini Kit (Norgen Biotek, Canada) according to the manufacturer's instructions.
MiRNAs analysis in exosomal component was investigated by quantitative Real‐Time PCR (qRT‐PCR) using TaqMan Advanced miRNA Human Serum/Plasma Card (Applied Biosystem). This method allows evaluating the expression of 188 miRNAs, each of them in duplicate, with high sensitivity and specificity starting from a very low amount of total RNA input.
The analysis was performed by examining 2 replicates, by using RNAs extracted by two distinct CM‐exosome preparations compared with control medium. Comparative expression analysis was performed by QuantStudio Software v 1.3 and Expression Suite software v 1.3 (Applied Biosystems). Ath‐miR159a was used as exogenous control for normalization of data, and global normalization analysis was conducted as well. ΔΔCt method was applied to determine the relative miRNAs expression levels. Furthermore, manual analysis focused on PCR amplification plots profiles was performed, and miRNAs showing mean Ct less than 30 and relative quantification (RQ) values more than twofold (linear scale) were considered.
2.9. In silico target genes and pathways analysis
Pathway‐based analysis and target gene prediction of miRNAs identified were performed using DIANA miRPath v.3 software (http://snf-515788.vm.okeanos.grnet.gr/). 55 We used Tarbase analysis, 56 which provide data about experimentally supported target genes and pathways and KEGG database annotation with standard statistics and genes union settings for exploratory functional analysis. Graphs of miRNAs/target genes interactions were obtained by R software (www.r-project.org).
2.10. Statistical analyses
Data were reported as means ± SE (n = 3). Statistical analysis was performed through Graphpad software, and data were evaluated using one‐way analysis of variance. The level of significance was set at P < .05.
3. RESULTS
3.1. Phenotypic characterization of the hAFSCs
The hAFSC line used for this study was assessed for various intracellular and surface markers to determine that the cells are in an intermediary state between pluripotent stem cells and lineage‐limited adult progenitor cells. As previously described, 51 the hAFSCs do not express hematopoietic surface markers (ie, CD34, CD14, and CD45), while showing different mesenchymal markers (ie, CD90, CD73, and CD105), numerous related surface adhesion molecules (ie, CD146, CD44), and stemness markers, such as Sox‐2, SSEA‐4, and Oct3/4 (Data already published 51 ).
3.2. I/R model
To establish the I/R model, differentiated SH‐SY5Y cells (using N2 supplement, as explained in the method section) were subjected to OGD for 1 hour, 3 hours, 6 hours, or 12 hours. MTS assay revealed that cell viability gradually diminished with increasing OGD time (Figure 1A). Data confirmed also by contrast phase microscopy analyses (Figure 1B). About 3 hours OGD insult showed a 50% reduction in cell viability and was chosen for the following experiments. To further validate the model, the hypoxia inducible factor (HIF), a transcriptional activator that directs evolutionarily conserved adaptive reactions to hypoxia, 12 , 57 was assayed by Western blotting. HIF is induced in cerebral ischemia and the heterodimer HIF1α is regulated by oxygen‐dependent degradation. Indeed, in our experimental condition, it is possible to appreciate that the protein level is significantly increased in the OGD condition compared to normoxia (Figure 1C).
FIGURE 1.
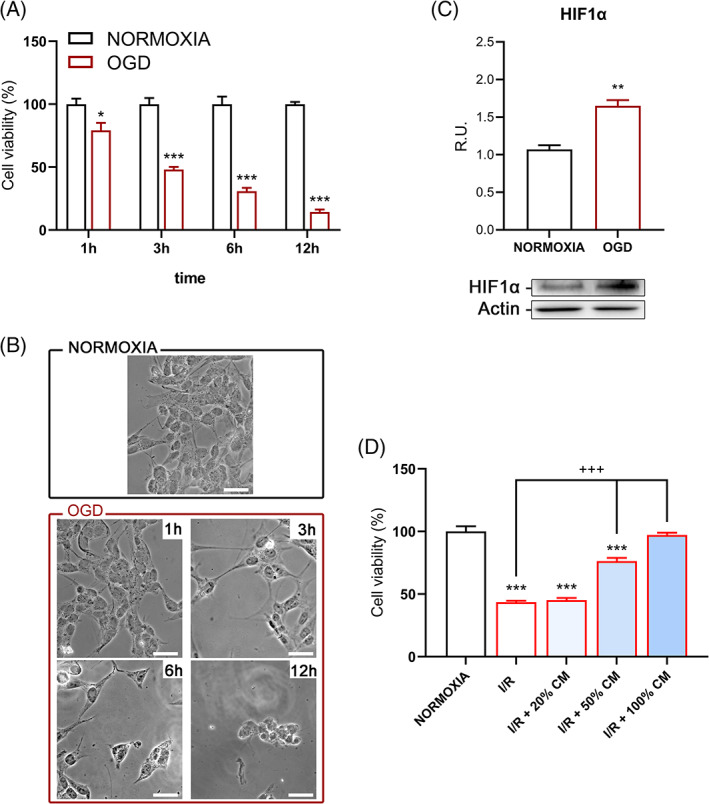
A, Viability test upon different OGD timepoints. B, Contrast phase representative pictures of differentiated SH‐SY5Y upon normoxia and OGD conditions. Bar = 20 μm. C, Western blotting and relative densitometric analysis for HIF‐1α. D, Viability test upon different concentration of CM. Results are mean ± SE of 3 experiments (n = 3). *P < .05, **P < .005, ***P < .0005 vs Normoxia; +++P < .0005 vs I/R. Representative WB images are shown
Regarding the reperfusion, 24 hours time point was chosen, and different percentages of hAFSCs‐conditioned medium were tested. In Figure 1D, it is possible to appreciate that the conditioned medium significantly increased cell viability respect to the control reperfusion. To confirm these data and show a similarity in hAFSCs‐derived CM, its therapeutic effect in primary culture was conducted, in particular preliminary experiments on rat cortical neurons were performed as reported in File S1. Once we confirmed the protective effect and the similarity with our I/R model, to better understand the “protective” mechanism, the pathways involved in ischemia‐reperfusion injury in differentiated SH‐SY5Y I/R model were dissected.
3.3. Protective mechanism analyses
To investigate the potential mechanisms underlying the conditioned media protective effects, the BDNF pathway upon both normoxic and I/R conditions, by Western blot analysis was examined.
Evidence demonstrated that BDNF resulted in increased neuronal survival via anti‐apoptotic effect. 53 , 58 , 59 , 60 Accordingly, in Figure 2A, it is possible to appreciate that the conditional medium can increase mature BDNF protein level respect to the reperfusion with normal media.
FIGURE 2.
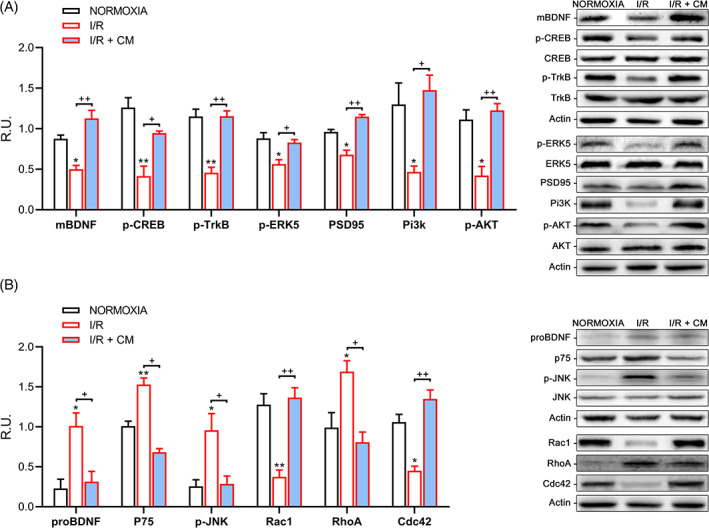
A, Western blotting and relative densitometric analysis for the neuroprotective pathway. Results are mean ± SE of 3 experiments (n = 3). *P < .05, **P < .005 vs Normoxia; +P < .05, ++P < .005 vs I/R. Representative WB images are reported. B, Western blotting and relative densitometric analysis for the neuronal death pathway and for Rho‐family GTPases. Results are mean ± SE of 3 experiments (n = 3). *P < .05, **P < .005 vs Normoxia; +P < .05, ++P < .005 vs I/R. Representative WB images are reported. Phosphorylated proteins are normalized upon their respective total protein
BDNF exerts multiple biological actions through TrkB (tropomyosin receptor kinase) receptors, it binds TrkB triggering autophosphorylation of the tyrosine residue in its intracellular domain, inducing ligand‐induced dimerization in each receptor, which triggers numerous intracellular signaling cascades with many roles; three enzymes represent the key regulators: mitogen‐activated protein kinase (MAPK), phosphatidylinositol‐3 kinase (PI3K), and phospholipase C γ (PLCγ). 53 , 58
In our experimental condition, also TrkB protein levels were significantly increased upon conditioned medium treatment, as well as the active forms of CREB (cAMP response element‐binding protein) and ERK5 (Extracellular signal Regulated kinase 5), all of which are involved in neuronal survival pathways (Figure 2A).
Therefore, it has been examined whether the conditioned media‐driven increase of BDNF and TrkB translated into variations in PSD95 (postsynaptic density protein 95) levels, a marker of post‐synaptic integrity. It is possible to observe a significative increase in the PSD95 protein level upon CM reperfusion (Figure 2A). PI3K/Akt signaling pathway was also analyzed and, as it is shown in the same figure (Figure 2A), the conditioned medium was able to restore stroke‐induced decrease.
Further, it has been examined whether the observed neuroprotective effect of the BDNF‐TrkB signaling was related to alterations in the proBDNF‐p75NTR signaling cascade. WB analysis of these pathways confirmed that compared to control reperfusion, CM treatment promoted a strong decrease in p75NTR levels as well as in proBDNF levels, paralleled with low levels of phosphorylated (active form) of JNK (p‐JNK) (Figure 2B).
Rho family GTPases received substantial appreciation as regulators of actin cytoskeletal organization. In addition, Rac and/or Rho GTPase dysregulation has been described in different neuronal injuries and neurodegenerative disorders, including I/R. Normally, Rac and its downstream effectors stimulate neuronal survival, while Rho and its downstream effectors can induce neuronal apoptosis. 61 , 62 , 63 Indeed, in our experimental conditions, a strong decrease in RhoA protein levels upon conditioned media reperfusion was observed, while both Rac and Cdc42 are significantly increased, as it is shown in Figure 2B, thus, suggesting a protective role exerted by the CM.
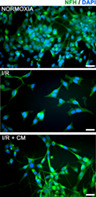
In Figure 3, immunofluorescence analyses for NFH (Neurofilament Heavy) is reported. Interestingly, upon CM reperfusion, a restore of neurite branches was observed, and this is even more evident in the graph, in which the length of the neurites was compared with the diameter of the soma. In fact, in control I/R, a significant neurites length reduction was observed, while, upon CM reperfusion, the length of the neurites was sharply increased, thus, suggesting that the CM exerted neuroprotective activities.
FIGURE 3.

Immunofluorescence for NFH and neurite analyses graphs. Bar = 10 μm. Results are mean ± SE of 3 experiments (n = 3). ***P < .0005 vs Normoxia; +++P < .0005 vs I/R. Representative IF images are shown
Afterward, to compare to the whole conditioned media with the exosomal fraction, the viability assay on the same model, treating the cells with the soluble component (CM w/o EXO) and the exosome extract (EXO) was performed. It is possible to notice in Figure 4A that, compared to I/R control, both the components increased cell viability, but the exosomal fraction (EXO) more significantly than the soluble component (CM w/o EXO).
FIGURE 4.
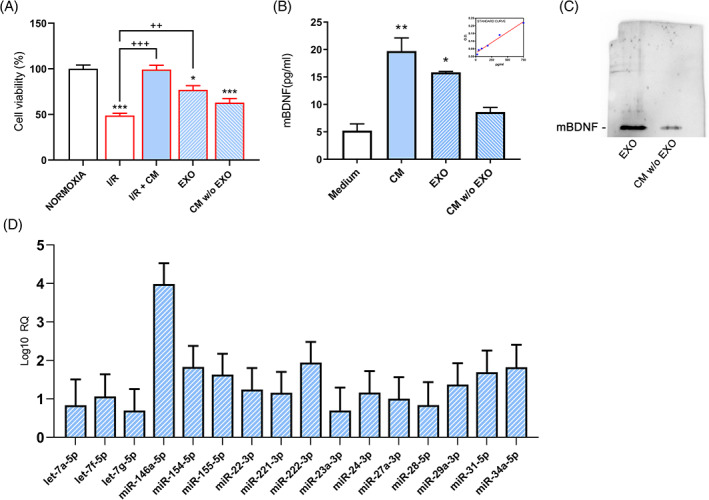
A, Viability test upon I/R, I/R+ CM, exosomal component (EXO) and soluble component (CM w/o EXO). B, mBDNF levels through ELISA kit and its relative standard curve. C, Western blotting analysis for mBDNF in the exosome protein lysates and CM w/o EXO. Results are mean ± SE of 3 experiments (n = 3). *P < .05, **P < .005, **P < .0005 vs Normoxia; ++P < .005, ++P < .0005 vs I/R. Representative WB image is reported. D, overexpressed miRNAs detected in hAFSC‐CM derived exosomes. RQ ± SE values obtained are reported and shown in the logarithmic (log10) scale
Further, mature BDNF levels through ELISA kit in whole CM, exosomal component, and CM without exosomes were analyzed. Interestingly, elevated levels of mBDNF in CM and exosomal components (EXO) compared to the control medium were observed (Figure 4B); these data were also confirmed by Western blot analysis in proteins extracted from exosomes compared to CM w/o EXO (Figure 4C).
miRNAs expression profile in conditioned media‐derived exosomes was examined to investigate a possible role of miRNAs as an exosomal component involved in the neuroprotective effects.
Among the miRNAs analyzed, we identified 16 hyper‐expressed miRNAs in CM hAFSC‐derived exosomes; these included let‐7a‐5p, let‐7f‐5p, let‐7g‐5p, miR‐146a‐5p, miR‐154‐5p, miR‐155‐5p, miR‐221‐3p, miR‐222‐3p, miR‐22‐3p, miR‐23a‐3p, miR‐24‐3p, miR‐27a‐3p, miR‐28‐5p, miR‐29a‐3p, miR‐31‐5p, miR‐34a‐5p (Figure 4D).
In some of the miRNA assays, wide error bars can be detected, arguably due to the nature of samples subjected to analysis, obtained from heterogeneous, and most probably variable, secreted exosomal components.
Pathway‐based analysis, predicting the putative biological functions of the 16 miRNAs, suggested their involvement in a high number of KEGG signaling pathways (File S2). Among them, we focused on pathways relevant in I/R model, such as neurotrophin signaling and other pathways related to neuroprotection and neuronal cell death (Table 1).
TABLE 1.
Pathway‐based analysis of the 16 upregulated miRNAs in conditioned media exosomes
| KEGG pathway | P‐value | #genes | #miRNAs |
|---|---|---|---|
| Hippo signaling pathway (hsa04390) | 7.36E‐08 | 103 | 16 |
| TNF signaling pathway (hsa04668) | 3.76E‐05 | 78 | 16 |
| mTOR signaling pathway (hsa04150) | 6.70E‐05 | 48 | 16 |
| Neurotrophin signaling pathway (hsa04722) | .000235281 | 85 | 15 |
| HIF‐1 signaling pathway (hsa04066) | .000639284 | 75 | 16 |
| Apoptosis (hsa04210) | .003465202 | 62 | 15 |
| PI3K‐Akt signaling pathway (hsa04151) | .019922794 | 209 | 16 |
| MAPK signaling pathway (hsa04010) | .043639368 | 152 | 15 |
| Axon guidance (hsa04360) | .04536589 | 78 | 16 |
Note: Only terms considered as the most relevant among the significant ones are reported.
Further, we generated the list of experimentally supported target genes (unique n = 582) involved in the pathways mentioned above (File S3). Among them, several genes are known to play a role in inducing cell proliferation and survival (eg, PI3K, AKT, RAS, ABL, SHC, BCL2, NF‐kB), on the contrary, others are involved in negative regulation of the signals mentioned above (eg, BAX, MAPK8, CDC42, RAC1, GSK3B, JUN, TP53). Consequently, depending on the specific role in such networks of genes overall targeted by miRNAs here considered, both pro‐survival/proliferation and apoptotic or inflammatory signals inhibition would be induced but contextually, fine‐tuned too.
With this regard, we focused on the analysis of four of the most interesting pathways listed in Table 1: HIF‐1 signaling, apoptosis, and neurotrophin signaling pathway and PI3K signaling. In Figures 5 and 6, pathways representations and miRNA/target genes interactions are reported, while relative target gene lists in File S4 are reported. Of note, most of the genes involved in the HIF‐1 signaling pathway were targeted by the 16 miRNAs here identified, with consequent extensive putative down‐regulation possibly driving to a global pathway switching off (Figure 5A).
FIGURE 5.
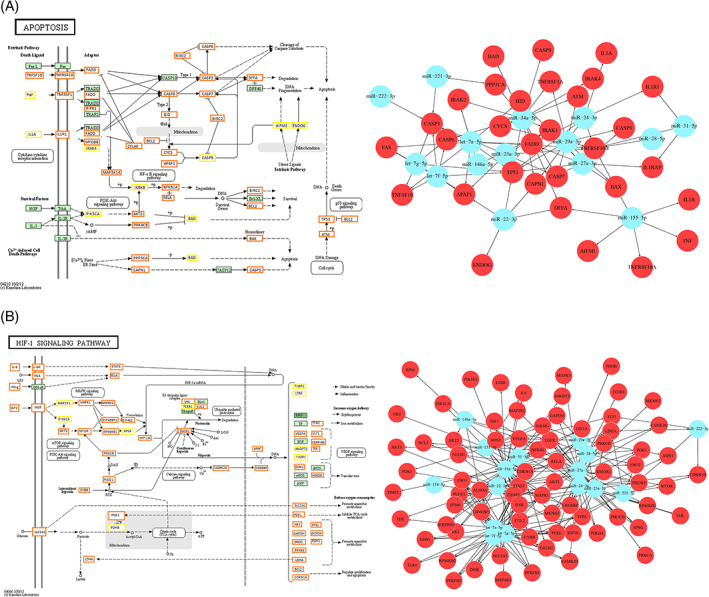
A, On the left, HIF‐1 signaling pathway (modified from KEGG hsa04066), showing genes targeted by miRNAs (n = 16) involved in its modulation. Orange boxes, genes targeted by more than 1 miRNA; yellow boxes, genes targeted by 1 miRNA and in green, not targeted genes. The complete list of target genes is reported in File S3. On the right, the graph showing interactions between miRNAs and related target genes. B, On the left, the Apoptosis pathway (modified from KEGG hsa04201), showing genes targeted by miRNAs (n = 15) involved in its modulation. Orange boxes, genes targeted by more than 1 miRNA; yellow boxes, genes targeted by 1 miRNA and in green, not targeted genes. The complete list of target genes is reported in File S3. On the right, the graph showing interactions between miRNAs and related target genes with pro‐apoptosis and pro‐inflammatory functions
FIGURE 6.
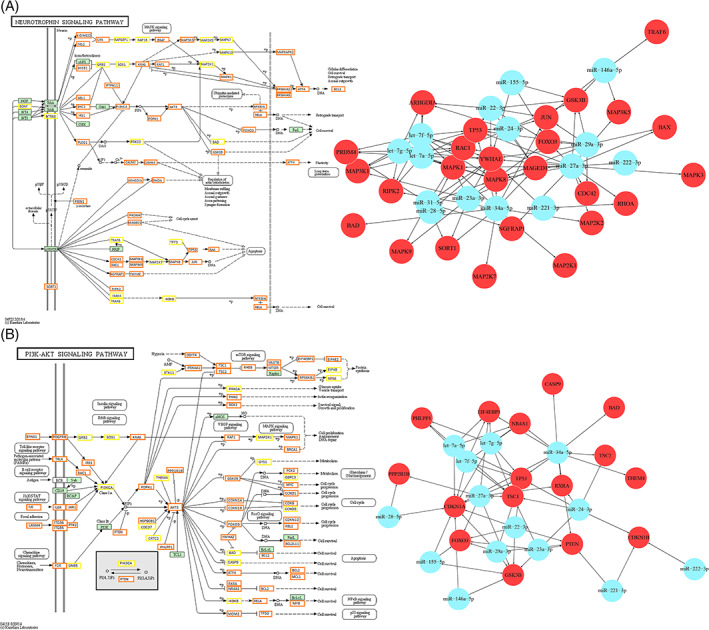
A, On the left, the Neurotrophin signaling pathway (modified from KEGG hsa04722), showing genes targeted by miRNAs (n = 15) involved in its modulation. Orange boxes, genes targeted by more than 1 miRNA; yellow boxes, genes targeted by 1 miRNA and in green, not targeted genes. The complete list of target genes is reported in File S3. On the right, the graph showing interactions between miRNAs and related target genes involved in neuronal death. B, On the left, the PI3K‐Akt signaling pathway (modified from KEGG hsa04151), showing genes targeted by miRNAs (n = 16) involved in its modulation. Orange boxes, genes targeted by more than 1 miRNA; yellow boxes, genes targeted by 1 miRNA and in green, not targeted genes. The complete list of target genes is reported in File S3. On the right, the graph showing interactions between miRNAs and related target genes with anti‐survival and anti‐proliferative functions
Effects leading to apoptosis and inflammation may be deduced by observing the apoptosis pathway map, where a relevant number of target genes (Figure 5, complete list in File S4) with physiological pro‐apoptotic and pro‐inflammatory functions, here supposed to be downregulated, are shown. Interactions among the target mentioned above genes (red circles) and related miRNAs (light‐blue circles) are displayed as well in a graph (Figure 5B, right).
Similarly, many genes known to be implicated in neuronal death, here presumed with decreased levels, are included and highlighted in the neurotrophin signaling pathway (complete list in File S4), in particular downstream p75NTR (Figure 6, left).
Of note, among the target genes, there is RhoA, down‐regulation of which was also described by Western blotting in I/R + CM conditions (Figure 2B). Also, in this case, interactions among target genes playing a role in neuronal death (red circles) and related miRNAs (light‐blue circles) are shown in the graph (Figure 6, right).
Conversely, especially in the latter two pathways, several genes, physiologically involved in promoting cell survival and proliferation, were included in the list of miRNAs target genes as well. For this reason, we hypothesize that even if supposed downregulated in our model, with consequent inhibition of such pathways, the complex microenvironment, where they play their function, may influence the effective downregulation of these players. This phenomenon, however, can be expected, being miRNAs fine regulators at the epigenetic level, and thus contributing to the balance of different functions within a broad multitude of cell process networks. Furthermore, due to the complexity of such networks, even though we refer to an experimentally supported database for miRNA‐target genes interactions in in silico analysis, it has to be considered that also additional regulatory mechanisms able to define the cell fate can play a role. The well‐known PI3K‐AKT survival pathway, already studied by Western blotting, was analyzed as well (Figure 6B). A relevant part of miRNA target genes promoting cell proliferation was present, but also others with pro‐apoptotic and anti‐proliferative functions (complete list in File S4), represented in the target genes/miRNA interaction graph (Figure 6, right). Interestingly, most of those factors here hypothesized to be downregulated as miRNAs targets, act downstream the major player of this pathway, possibly further enhancing pro‐survival and proliferation processes.
4. DISCUSSION
Ischemic stroke is one of the leading causes of morbidity, mortality, and disability in aging patients. 64 Appropriate reperfusion of the ischemic region is the standard approach to preserve brain structure and function. 8 However, reperfusion approaches after ischemic stroke trigger further brain injuries, described as cerebral I/R injury. I/R injury decreases the clinical benefits of reperfusion approaches for patients with ischemic stroke. 65 , 66 Consequently, defining the molecular mechanisms underlying the I/R injury can improve the success of reperfusion treatment and enhance the clinical advantages for patients with stroke. 67 Numerous biological processes involving calcium excess, oxidative stress, inflammatory reactions, and endoplasmic reticulum stress are closely related to the brain I/R injury. 65 , 68
Interestingly, each of these activities can be influenced by mitochondria. In reply to the brain I/R injury, mitochondria cannot generate sufficient energy and thus affect calcium reuptake by the endoplasmic reticulum, which induces calcium excess. Moreover, alterations in mitochondria can be due to excessive ROS, which causes oxidative stress. 69 Consequently, altered mitochondria can stimulate pro‐apoptotic factors in the cytoplasm or the nucleus to begin mitochondria‐supported cellular apoptosis. 70
In the present study, for the first time, the efficacy of hAFSCs‐secretome in protecting cells by death signals in I/R in vitro model was studied.
As mentioned above, the OGD/R model is a widely studied model to mimic ischemia. Differentiated SH‐SY5Y cells have been largely utilized to investigate neuronal activity since they have comparable morphology and biochemical characteristics to mature neurons. 71 , 72 , 73 Differentiation of SH‐SY5Y cells with N2 supplement demonstrated upregulated neuron‐specific markers, such as β‐tubulin III, Neurofilament 200, and GAP43. 74
Previous reports demonstrated that in ischemic conditions, different pathways were altered, including PI3K/AKT, AKT/GSK‐3β/β‐catenin, ERK1/2, apoptotic pathways, mTOR pathway as well as BDNF pathway. 60 , 75 , 76
Mostly, MSCs or MSCs‐CM administration showed structural and functional advantages: it can diminish apoptosis at the injured area, to modulate pro‐inflammatory reaction, to offer a permissive environment for axonal expansion, to stimulate neurogenesis and enhance neurological impairments. 36 , 77 , 78 The composition of the CM secretome “cargo” determines the therapeutic potential. 79 CM contains a variety of growth factors and tissue‐regenerative agents, which were released by the stem cells. Indeed, proteomic studies showed the presence of various growth factors and other cytokines in the CM. 80 , 81 Besides, MSCs are the most efficient exosome‐producing cells. 82
Based on this evidence, and on the paracrine theory which establishes that the positive effects of stem cell therapeutic approach are due to stimuli of resident cells by the release of bioactive molecules and EV, the use of secretome could offer several advantages over MSCs such as a superior safety profile. Different reports demonstrated that secretome released from MSCs could decrease cognitive impairments related to numerous neurological disease models, comprising Traumatic Brain Injury, Parkinson's disease, Alzheimer's disease, and stroke. 28 , 83 , 84 , 85 , 86 Secretome and conditioned media showed the ability to interact and promote the release of neurotrophins, comprising vascular endothelial growth factor, BDNF. Nerve growth factor, improving neurite development, stimulating neurorestorative and neurological improvement. 78 , 87
Exosome release is deemed an adaptation mechanism and its composition, biogenesis, and secretion are influenced by microenvironment with which cells interrelate. 78 , 87
The high proliferative capacity, the low immunogenicity, and tumorgenicity and the anti‐inflammatory activity support the hAFSCs use as safe and effective donor cells for stroke therapy. 88 Furthermore, human stem cell utilization is useful and helpful for clinical trial translation. In vitro systems have a partial capacity to reflect the complicated situation that occurs during stroke in vivo. Still, they represent a useful research tool to understand cellular and molecular mechanisms that are difficult to dissect in vivo.
Thus, the growing availability of human cells renders in vitro systems a solid low‐cost base for the drug discovery pyramid and for strengthened the in vivo research, improving clinical translation.
In this study, we established an I/R model and analyzed the downstream responses to hAFSC‐secretome exposure. Our findings indicated that the CM and, in particular, the exosomal fraction played a crucial role in promoting cell survival. This pro‐survival effect was paralleled by the activation of the neurotrophic pathways BDNF/TrkB and by the suppression of the death pathways (p75/JNK). These effects were paralleled by the increase of the survival pathway PI3K/Akt and ERK5.
Exosomes encapsulate and transfer numerous functional molecules, including proteins, lipids, and regulatory RNAs, which can alter cell metabolism. 79 Notably, in the MSC‐derived exosomes protein component, we identified a high level of mBDNF. Numerous studies showed that exosomes carry different neuronal proteins and that they can cross the blood‐brain barrier. 89 However, till now, it was not clear whether BDNF was present in the exosomes and the soluble component of CM, and, interestingly, in our experimental condition, we found that both exosomal fractions and soluble CM contains high levels of mBDNF, with exosomes particularly endowed of this neurotrophin.
Further, within exosomal cargo, a broad variety of miRNAs has been detected, which can influence functions associated with neural remodeling and neurogenic processes. Several miRNAs, already identified as players in neuroprotective mechanisms (miR‐146a‐5p, miR‐154‐5p, miR‐22‐3p, miR‐23a‐3p, miR‐27a‐3p, miR‐29a‐3p, and miR‐31‐5p), were also identified in our hAFSC‐derived exosomes. Interestingly, miR‐146a was already described in the negative regulation of inflammation 90 , 91 and in promoting oligodendrogenesis 92 in the post‐injury perinatal brain. MiR‐154 was detected in astrocyte‐derived extracellular vesicles, involved in regulating neurotrophic signaling in neurons. 93 Moreover, miR‐154 is known to target DKK2 (Dickkopf‐related protein 2) with consequent β‐catenin up‐regulation and classical Wnt signaling pathway activation, 94 crucial both for the maintenance of synaptic structures and neuronal survival. 95 MiR‐22‐3p, 23a, and 27a are involved in neuroprotection mechanisms through apoptosis inhibition 96 , 97 , 98 , 99 , 100 Furthermore, miR‐22‐3p can regulate neuronal morphology during migration by targeting the CoREST/REST complex, 101 and miR‐27a is involved in controlling neuroinflammation. 99 Also, among the other miRNA identified, miR‐29a was already described to protect against cell damage after ischemia‐like stress and astrocyte ischemic injury 102 , 103 ; miR‐31‐5p, also identified in our exosomal component, is known to reduce the inflammatory response and oxidative stress‐induced neuronal damage 104 and, further, shows pro‐angiogenic activity. 105
On the other hand, regarding other miRNAs here identified, such as miR‐221‐3p, miR‐222‐3p, miR‐24‐3p, and miR‐28‐5p, limited or conflicting data about their function and implication in neuronal processes have been reported. 106 , 107 , 108 , 109
Besides neurotrophin signaling, interestingly, the pathway‐based analysis revealed that these miRNAs act on pathways known to be altered in ischemic conditions and involved in neuroprotection, such as TNF, Hippo, and PI3K. 110 , 111 , 112 Among the miRNAs targets here identified, supposed to be downregulated in our model, we highlighted several genes involved in apoptosis and inflammation processes.
Interestingly, based on the experimentally supported miRNAs/target genes, in HIF‐1 pathway, a sort of global switch‐off mechanism can be observed. In contrast, other pathways of interest, including apoptosis, neurotrophins, and PI3K‐AKT, showed several groups of genes, all supposed to be downregulated in response to miRNA activity, physiologically playing a role in inducing or, conversely repressing different processes, such as proliferation, apoptosis, inflammation. This is, however, consistent with the pleiotropic and versatile activity and function of miRNAs, molecules specifically designated to regulate and fine tune, at the epigenetic level, expression of multiple target genes within complex molecular circuitries. 113 Further studies are needed to confirm the involvement and biological impact of miRNAs identified in this in silico study regulating neurogenic processes after stroke, as well as to shed light on molecular mechanisms underlying their possible neuroprotective role, even if the biochemical data obtained strongly support the involvement of these miRNAs in regulating several actors of the analyzed pathways, that is, pro‐BDNF, PI3K‐Akt, Rho/Rac.
The central nervous system includes various cells other than neurons, thus, undoubtedly it is important also to study the effect of the conditioned media derived from hAFSCs in vivo; future studies will be devoted to clarify the therapeutic effect of hAFSCs‐CM in stroke in vivo models.
5. CONCLUSIONS
These data indicate that miRNAs represent a relevant component of the exosomal cargo and that the effect of CM‐derived exosomes could be in part mediated by secreted miRNAs. Specifically, our findings suggest that miRNAs may mediate neuroprotection mainly through anti‐apoptotic and pro‐survival pathways. However, it is conceivable that some effects may also be due to the soluble component of the CM, being this latter endowed by neurotrophic activity.
Taking this into consideration, the use of CM, and in particular, exosomes, may constitute a potential approach to stimulate neuronal plasticity, ameliorate cognitive loss, and neural replacement in stroke. Consequently, it is possible to indicate that the exosomal fraction of the hAFSC‐CM may represent a suitable treatment for I/R injury.
CONFLICT OF INTEREST
C.B. disclosed employment/leadership position with University of South Florida, patent holder and stock ownership with Sanbio Inc, Athersys Inc, advisory role with KMPHC, Chiesi, research funding from NIH, Asterias, Astellas. The other authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
V.C.: conception and design, experiments execution, collection and assembly of data, data analysis and interpretation, manuscript writing; I.A.: provision of study material, supervision of experiments, critical review of the manuscript; M.d.A.: experiments execution, data analysis and interpretation, figure preparation; A.T., V.Z.: miRNA experiments and analysis, critical review of the final manuscript; E.B., C.F.: critical review of the manuscript; G.D.: critical review of the manuscript, final approval of the manuscript. C.B.: critical review of the manuscript and experimental execution, final approval of the manuscript; L.S.: supervision, critical review of manuscript and experimental execution, final approval of the manuscript; A.C.: conception and design, supervision, data analysis and interpretation, manuscript writing, financial support.
ETHICS STATEMENT
Amniotic fluid was used upon informed written consent.
Supporting information
File S1. List of significant pathways induced by the group of 16 exosomal miRNAs. For each pathway, P‐value, number of targeted genes involved in and number of modulating miRNAs are reported.
File S2. Experimentally supported target genes (unique n = 582) involved in pathways of interest.
File S3. Lists of experimentally supported (Tarbase database) miRNAs' target genes in apoptosis, HIF‐1, neurotrophin, PI3K‐AKT signalling pathways.
Figure S1. Supporting information.
ACKNOWLEDGEMENTS
The authors thank University of L'Aquila, RIA funding for their support.
Castelli V, Antonucci I, d'Angelo M, et al. Neuroprotective effects of human amniotic fluid stem cells‐derived secretome in an ischemia/reperfusion model. STEM CELLS Transl Med. 2021;10:251–266. 10.1002/sctm.20-0268
Vanessa Castelli and Ivana Antonucci equally contributed.
Contributor Information
Liborio Stuppia, Email: stuppia@unich.it.
Annamaria Cimini, Email: annamaria.cimini@univaq.it.
DATA AVAILABILITY STATEMENT
The datasets generated during the current study are available from the corresponding authors upon reasonable requests.
REFERENCES
- 1. Donkor ES. Stroke in the 21st century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. 2018;2018:3238165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hui C, Tadi P, Patti L. Ischemic Stroke. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. http://www.ncbi.nlm.nih.gov/books/NBK499997/. Accessed May 7, 2020. [Google Scholar]
- 3. Maestrini I, Ducroquet A, Moulin S, Leys D, Cordonnier C, Bordet R. Blood biomarkers in the early stage of cerebral ischemia. Rev Neurol (Paris). 2016;172:198‐219. [DOI] [PubMed] [Google Scholar]
- 4. Juan W‐S, Lin H‐W, Chen Y‐H, et al. Optimal Percoll concentration facilitates flow cytometric analysis for annexin V/propidium iodine‐stained ischemic brain tissues. Cytometry. 2012;81A:400‐408. 10.1002/cyto.a.22021. [DOI] [PubMed] [Google Scholar]
- 5. Lee J‐Y, Castelli V, Bonsack B, et al. Eyeballing stroke: blood flow alterations in the eye and visual impairments following transient middle cerebral artery occlusion in adult rats. Cell Transplant. 2020;29:963689720905805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee J‐Y, Castelli V, Bonsack B, et al. A novel partial MHC class II construct, DRmQ, inhibits central and peripheral inflammatory responses to promote neuroprotection in experimental stroke. Transl Stroke Res. 2020;11(4):831‐836. 10.1007/s12975-019-00756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin L, Wang X, Yu Z. Ischemia‐reperfusion injury in the brain: mechanisms and potential therapeutic strategies. Biochem Pharmacol. 2016;5(4):213‐215. 10.4172/2167-0501.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu W, Liu L. Therapeutic window beyond cerebral ischemic reperfusion injury In: Jiang W, Yu W, Qu Y, Shi Z, Luo B, Zhang JH, eds. Cerebral Ischemic Reperfusion Injuries (CIRI). Cham: Springer International Publishing; 2018:245‐259. 10.1007/978-3-319-90194-7_16. [DOI] [Google Scholar]
- 9. Tasca CI, Dal‐Cim T, Cimarosti H. Vitro oxygen‐glucose deprivation to study ischemic cell death In: Lossi L, Merighi A, eds. Neuronal Cell Death. New York, NY: Springer New York; 2015:197‐210. 10.1007/978-1-4939-2152-2_15. [DOI] [PubMed] [Google Scholar]
- 10. Sommer CJ. Ischemic stroke: experimental models and reality. Acta Neuropathol. 2017;133:245‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y‐J, Peng Q‐Y, Deng S‐Y, et al. Hemin protects against oxygen‐glucose deprivation‐induced apoptosis activation via neuroglobin in SH‐SY5Y cells. Neurochem Res. 2017;42:2208‐2217. [DOI] [PubMed] [Google Scholar]
- 12. Niu G, Zhu D, Zhang X, Wang J, Zhao Y, Wang X. Role of hypoxia‐inducible factors 1α (HIF1α) in SH‐SY5Y cell autophagy induced by oxygen‐glucose deprivation. Med Sci Monit. 2018;24:2758‐2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryou M, Mallet RT. An in vitro oxygen–glucose deprivation model for studying ischemia–reperfusion injury of neuronal cells In: Tharakan B, ed. Traumatic and Ischemic Injury. New York, NY: Springer New York; 2018:229‐235. 10.1007/978-1-4939-7526-6_18. [DOI] [PubMed] [Google Scholar]
- 14. Kovalevich J, Langford D. Considerations for the use of SH‐SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol. 2013;1078:9‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhi S‐M, Fang G‐X, Xie X‐M, et al. Melatonin reduces OGD/R‐induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur Rev Med Pharmacol Sci. 2020;24:1524‐1536. [DOI] [PubMed] [Google Scholar]
- 16. Xin L, Junhua W, Long L, Jun Y, Yang X. Exogenous hydrogen sulfide protects SH‐SY5Y cells from OGD/R induced injury. Curr Mol Med. 2017;17:563‐567. [DOI] [PubMed] [Google Scholar]
- 17. Liu Y, Eaton ED, Wills TE, McCann SK, Antonic A, Howells DW. Human ischaemic cascade studies using SH‐SY5Y cells: a systematic review and meta‐analysis. Transl Stroke Res. 2018;9:564‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piccirillo S, Castaldo P, Macrì ML, Amoroso S, Magi S. Glutamate as a potential “survival factor” in an in vitro model of neuronal hypoxia/reoxygenation injury: leading role of the Na+/Ca2+ exchanger. Cell Death Dis. 2018;9:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Z‐H, Wang Y‐R, Li F, et al. Circ‐camk4 involved in cerebral ischemia/reperfusion‐induced neuronal injury. Sci Rep. 2020;10:7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu T, Zou Y, Zhou X, Peng W, Hu Z. The mechanism on phosphorylation of Hsp20Ser16 inhibit GA stress and ER stress during OGD/R. PLoS One. 2019;14:e0213410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han J, Luk B, Lee FJS. Neuroprotective effects of extracellular DJ‐1 on reperfusion injury in SH‐SY5Y cells. Synapse. 2017;71:1‐5. [DOI] [PubMed] [Google Scholar]
- 22. Tuazon JP, Castelli V, Lee J‐Y, et al. Neural Stem Cells. Adv Exp Med Biol. 2019;1201:79‐91. [DOI] [PubMed] [Google Scholar]
- 23. Loukogeorgakis SP, De Coppi P. Concise review: amniotic fluid stem cells: the known, the unknown, and potential regenerative medicine applications: amniotic fluid stem cells in regenerative medicine. stem cells. 2017;35:1663‐1673. 10.1002/stem.2553. [DOI] [PubMed] [Google Scholar]
- 24. Roubelakis MG, Trohatou O, Anagnou NP. Amniotic fluid and amniotic membrane stem cells: marker discovery. Stem Cells Int. 2012;2012:107836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prasongchean W, Ferretti P. Autologous stem cells for personalized medicine. N Biotechnol. 2012;29:641‐650. [DOI] [PubMed] [Google Scholar]
- 26. Maraldi T, Riccio M, Resca E, et al. Human amniotic fluid stem cells seeded in fibroin scaffold produce in vivo mineralized matrix. Tissue Eng Part A. 2011;17:2833‐2843. [DOI] [PubMed] [Google Scholar]
- 27. Loukogeorgakis SP, Maghsoudlou P, De Coppi P. Recent developments in therapies with stem cells from amniotic fluid and placenta. Fetal Matern Med Rev. 2013;24:148‐168. [Google Scholar]
- 28. Tuazon JP, Castelli V, Borlongan CV. Drug‐like delivery methods of stem cells as biologics for stroke. Expert Opin Drug Deliv. 2019;16:823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdelwahid E, Kalvelyte A, Stulpinas A, de Carvalho KAT, Guarita‐Souza LC, Foldes G. Stem cell death and survival in heart regeneration and repair. Apoptosis. 2016;21:252‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iismaa SE, Kaidonis X, Nicks AM, et al. Comparative regenerative mechanisms across different mammalian tissues. NPJ Regen Med. 2018;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cell. 2019;8(8):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palladino A, Mavaro I, Pizzoleo C, et al. Induced pluripotent stem cells as vasculature forming entities. J Clin Med. 2019;8(11):1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen Z‐Y, Hu Y‐Y, Hu X‐F, Cheng L‐X. The conditioned medium of human mesenchymal stromal cells reduces irradiation‐induced damage in cardiac fibroblast cells. J Radiat Res. 2018;59:555‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El Moshy S, Radwan IA, Rady D, et al. Dental stem cell‐derived secretome/conditioned medium: the future for regenerative therapeutic applications. Stem Cells Int. 2020;2020:7593402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jha KA, Pentecost M, Lenin R, et al. Concentrated conditioned media from adipose tissue derived mesenchymal stem cells mitigates visual deficits and retinal inflammation following mild traumatic brain injury. Int J Mol Sci. 2018;19(7):2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sriramulu S, Banerjee A, Di Liddo R, et al. Concise review on clinical applications of conditioned medium derived from human umbilical cord‐mesenchymal stem cells (UC‐MSCs). Int J Hematol Oncol Stem Cell Res. 2018;12:230‐234. [PMC free article] [PubMed] [Google Scholar]
- 37. Drago D, Cossetti C, Iraci N, et al. The stem cell secretome and its role in brain repair. Biochimie. 2013;95:2271‐2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. d’Angelo M, Cimini A, Castelli V. Insights into the effects of mesenchymal stem cell‐derived secretome in parkinson’s disease. Int J Mol Sci. 2020;21(15):5241 10.3390/ijms21155241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Balbi C, Bollini S. Fetal and perinatal stem cells in cardiac regeneration: moving forward to the paracrine era. Placenta. 2017;59:96‐106. [DOI] [PubMed] [Google Scholar]
- 40. Campanella C, Caruso Bavisotto C, Logozzi M, et al. On the choice of the extracellular vesicles for therapeutic purposes. Int J Mol Sci. 2019;20:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park K‐S, Bandeira E, Shelke GV, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell‐derived extracellular vesicles. Stem Cell Res Ther. 2019;10:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taverna S, Pucci M, Alessandro R. Extracellular vesicles: small bricks for tissue repair/regeneration. Ann Transl Med. 2017;5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baek G, Choi H, Kim Y, Lee H, Choi C. Mesenchymal stem cell‐derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl Med. 2019. 10.1002/sctm.18-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalani A, Tyagi N. Exosomes in neurological disease, neuroprotection, repair and therapeutics: problems and perspectives. Neural Regen Res. 2015;10:1565‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cell. 2019;8:727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gurunathan S, Kang M‐H, Jeyaraj M, Qasim M, Kim J‐H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cell. 2019;8:307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vyas N, Dhawan J. Exosomes: mobile platforms for targeted and synergistic signaling across cell boundaries. Cell Mol Life Sci. 2017;74:1567‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schey KL, Luther JM, Rose KL. Proteomics characterization of exosome cargo. Methods. 2015;87:75‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aryani A, Denecke B. Exosomes as a nanodelivery system: a key to the future of neuromedicine? Mol Neurobiol. 2016;53:818‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Castelli V, Melani F, Ferri C, et al. Neuroprotective activities of bacopa, lycopene, astaxanthin, and vitamin B12 combination on oxidative stress‐dependent neuronal death. J Cell Biochem. 2020. 10.1002/jcb.29722. [DOI] [PubMed] [Google Scholar]
- 51. Pipino C, Pierdomenico L, Di Tomo P, et al. Molecular and phenotypic characterization of human amniotic fluid‐derived cells: a morphological and proteomic approach. Stem Cells Dev. 2015;24:1415‐1428. 10.1089/scd.2014.0453. [DOI] [PubMed] [Google Scholar]
- 52. Pemberton K, Mersman B, Xu F. Using ImageJ to assess neurite outgrowth in mammalian cell cultures: research data quantification exercises in undergraduate neuroscience lab. J Undergrad Neurosci Educ. 2018;16:A186‐A194. [PMC free article] [PubMed] [Google Scholar]
- 53. Castelli V, d'Angelo M, Lombardi F, et al. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson's disease models. Aging. 2020;12:4641‐4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fidoamore A, Cristiano L, Laezza C, et al. Energy metabolism in glioblastoma stem cells: PPARα a metabolic adaptor to intratumoral microenvironment. Oncotarget. 2017;8:108430‐108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vlachos IS, Zagganas K, Paraskevopoulou MD, et al. DIANA‐miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43:W460‐W466. 10.1093/nar/gkv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karagkouni D, Paraskevopoulou MD, Chatzopoulos S, et al. DIANA‐TarBase v8: a decade‐long collection of experimentally supported miRNA‐gene interactions. Nucleic Acids Res. 2018;46:D239‐D245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shi H. Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr Med Chem. 2009;16:4593‐4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 2018;38:579‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Castelli V, Benedetti E, Antonosante A, et al. Neuronal cells rearrangement during aging and neurodegenerative disease: metabolism, oxidative stress, and organelles dynamic. Front Mol Neurosci. 2019;12:132 10.3389/fnmol.2019.00132/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rahman M, Luo H, Sims NR, Bobrovskaya L, Zhou X‐F. Investigation of mature BDNF and proBDNF signaling in a rat photothrombotic ischemic model. Neurochem Res. 2018;43:637‐649. [DOI] [PubMed] [Google Scholar]
- 61. Stankiewicz TR, Linseman DA. Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Front Cell Neurosci. 2014;8:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen F, Liu Z, Peng W, et al. Activation of EphA4 induced by EphrinA1 exacerbates disruption of the blood‐brain barrier following cerebral ischemia‐reperfusion via the rho/ROCK signaling pathway. Exp Ther Med. 2018;16:2651‐2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Karabiyik C, Fernandes R, Figueiredo FR, et al. Neuronal rho GTPase Rac1 elimination confers neuroprotection in a mouse model of permanent ischemic stroke. Brain Pathol. 2018;28:569‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lui SK, Nguyen MH. Elderly stroke rehabilitation: overcoming the complications and its associated challenges. Curr Gerontol Geriatr Res. 2018;2018:9853837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2016;7:113‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xia C‐F, Yin H, Yao Y‐Y, Borlongan CV, Chao L, Chao J. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Hum Gene Ther. 2006;17:206‐219. [DOI] [PubMed] [Google Scholar]
- 67. Hentia C, Rizzato A, Camporesi E, et al. An overview of protective strategies against ischemia/reperfusion injury: the role of hyperbaric oxygen preconditioning. Brain Behav. 2018;8:e00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Enzmann G, Kargaran S, Engelhardt B. Ischemia‐reperfusion injury in stroke: impact of the brain barriers and brain immune privilege on neutrophil function. Ther Adv Neurol Disord. 2018;11:1756286418794184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu F, Lu J, Manaenko A, Tang J, Hu Q. Mitochondria in ischemic stroke: new insight and implications. Aging Dis. 2018;9:924‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yang J‐L, Mukda S, Chen S‐D. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018;16:263‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xiao B, Chai Y, Lv S, et al. Endothelial cell‐derived exosomes protect SH‐SY5Y nerve cells against ischemia/reperfusion injury. International Journal of Molecular Medicine. 2017;40 (4):1201‐1209. 10.3892/ijmm.2017.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shipley MM, Mangold CA, Szpara ML. Differentiation of the SH‐SY5Y human neuroblastoma cell line. J Vis Exp. 2016(108);53193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xiong S, Xu Y, Ma M, et al. Neuroprotective effects of a novel peptide, FK18, under oxygen‐glucose deprivation in SH‐SY5Y cells and retinal ischemia in rats via the Akt pathway. Neurochem Int. 2017;108:78‐90. [DOI] [PubMed] [Google Scholar]
- 74. Di Giacomo E, Benedetti E, Cristiano L, et al. Roles of PPAR transcription factors in the energetic metabolic switch occurring during adult neurogenesis. Cell Cycle. 2017;16:59‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang Z, Yao L, Yang J, Wang Z, Du G. PI3K/Akt and HIF‐1 signaling pathway in hypoxia‐ischemia (review). Mol Med Rep. 2018;18:3547‐3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lasek‐Bal A, Jędrzejowska‐Szypułka H, Różycka J, et al. Low concentration of BDNF in the acute phase of ischemic stroke as a factor in poor prognosis in terms of functional status of patients. Med Sci Monit. 2015;21:3900‐3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qu J, Zhang H. Roles of mesenchymal stem cells in spinal cord injury. Stem Cells Int. 2017;2017:5251313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Reza‐Zaldivar EE, Hernández‐Sapiéns MA, Minjarez B, Gutiérrez‐Mercado YK, Márquez‐Aguirre AL, Canales‐Aguirre AA. Potential effects of MSC‐derived exosomes in neuroplasticity in Alzheimer's disease. Front Cell Neurosci. 2018;12:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J. Exosomes: new molecular targets of diseases. Acta Pharmacol Sin. 2018;39:501‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zagoura DS, Roubelakis MG, Bitsika V, et al. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut. 2012;61:894‐906. [DOI] [PubMed] [Google Scholar]
- 82. Cosenza S, Toupet K, Maumus M, et al. Mesenchymal stem cells‐derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8:1399‐1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida‐Porada G, Gonçalves RM. Mesenchymal stromal cell Secretome: influencing therapeutic potential by cellular pre‐conditioning. Front Immunol. 2018;9:2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maacha S, Sidahmed H, Jacob S, et al. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020;2020:4356359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hasan A, Deeb G, Rahal R, et al. Mesenchymal stem cells in the treatment of traumatic brain injury. Front Neurol. 2017;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Marques CR, Marote A, Mendes‐Pinheiro B, Teixeira FG, Salgado AJ. Cell secretome‐based approaches in Parkinson's disease regenerative medicine. Expert Opin Biol Ther. 2018;18:1235‐1245. 10.1080/14712598.2018.1546840. [DOI] [PubMed] [Google Scholar]
- 87. Harting MT, Srivastava AK, Zhaorigetu S, et al. Inflammation‐stimulated mesenchymal stromal cell‐derived extracellular vesicles attenuate inflammation: stimulated EVs attenuate inflammation. stem cells. 2018;36:79‐90. 10.1002/stem.2730. [DOI] [PubMed] [Google Scholar]
- 88. Elias M, Hoover J, Nguyen H, Reyes S, Lawton C, Borlongan CV. Stroke therapy: the potential of amniotic fluid‐derived stem cells. Future Neurol. 2015;10:321‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kang X, Zuo Z, Hong W, Tang H, Geng W. Progress of research on exosomes in the protection against ischemic brain injury. Front Neurosci. 2019;13:1149 10.3389/fnins.2019.01149/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gaudet AD, Fonken LK, Watkins LR, Nelson RJ, Popovich PG. MicroRNAs: roles in regulating neuroinflammation. Neuroscientist. 2018;24:221‐245. 10.1177/1073858417721150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Omran A, Peng J, Zhang C, et al. Interleukin‐1β and microRNA‐146a in an immature rat model and children with mesial temporal lobe epilepsy: neuroinflammation and MTLE development. Epilepsia. 2012;53:1215‐1224. 10.1111/j.1528-1167.2012.03540.x. [DOI] [PubMed] [Google Scholar]
- 92. Liu XS, Chopp M, Pan WL, et al. MicroRNA‐146a promotes oligodendrogenesis in stroke. Mol Neurobiol. 2017;54:227‐237. 10.1007/s12035-015-9655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chaudhuri AD, Dastgheyb RM, Yoo S‐W, et al. TNFα and IL‐1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 2018;9:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sun L‐Y, Bie Z‐D, Zhang C‐H, Li H, Li L‐D, Yang J. MiR‐154 directly suppresses DKK2 to activate Wnt signaling pathway and enhance activation of cardiac fibroblasts: MiR‐154 regulates DKK2 to promote Wnt and CF activation. Cell Biol Int. 2016;40:1271‐1279. 10.1002/cbin.10655. [DOI] [PubMed] [Google Scholar]
- 95. Chen C‐M, Orefice LL, Chiu S‐L, et al. Wnt5a is essential for hippocampal dendritic maintenance and spatial learning and memory in adult mice. Proc Natl Acad Sci U S A. 2017;114:E619‐E628. 10.1073/pnas.1615792114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jovicic A, Zaldivar Jolissaint JF, Moser R, Silva Santos M d F, Luthi‐Carter R. MicroRNA‐22 (miR‐22) overexpression is neuroprotective via general anti‐apoptotic effects and may also target specific Huntington's disease‐related mechanisms. PLoS One. 2013;8:e54222 10.1371/journal.pone.0054222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sabirzhanov B, Zhao Z, Stoica BA, et al. Downregulation of miR‐23a and miR‐27a following experimental traumatic brain injury induces neuronal cell death through activation of proapoptotic Bcl‐2 proteins. J Neurosci. 2014;34:10055‐10071. 10.1523/JNEUROSCI.1260-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen Q, Xu J, Li L, et al. MicroRNA‐23a/b and microRNA‐27a/b suppress Apaf‐1 protein and alleviate hypoxia‐induced neuronal apoptosis. Cell Death Dis. 2014;5:e1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cho KHT, Xu B, Blenkiron C, Fraser M. Emerging roles of miRNAs in brain development and perinatal brain injury. Front Physiol. 2019;10:227 10.3389/fphys.2019.00227/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ma J, Shui S, Han X, Guo D, Li T, Yan L. microRNA‐22 attenuates neuronal cell apoptosis in a cell model of traumatic brain injury. Am J Transl Res. 2016;8:1895‐1902. [PMC free article] [PubMed] [Google Scholar]
- 101. Volvert M‐L, Prévot P‐P, Close P, et al. MicroRNA targeting of CoREST controls polarization of migrating cortical neurons. Cell Rep. 2014;7:1168‐1183. [DOI] [PubMed] [Google Scholar]
- 102. Ouyang Y‐B, Xu L, Lu Y, et al. Astrocyte‐enriched miR‐29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia: glia‐enriched miR‐29a protects from stroke. Glia. 2013;61:1784‐1794. 10.1002/glia.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zheng Y, Pan C, Chen M, Pei A, Xie L, Zhu S. miR‑29a ameliorates ischemic injury of astrocytes in vitro by targeting the water channel protein aquaporin 4. Oncol Rep. 2019;41(3):1707‐1717. 10.3892/or.2019.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li J, Lv H, Che Y. Upregulated microRNA‐31 inhibits oxidative stress‐induced neuronal injury through the JAK/STAT3 pathway by binding to PKD1 in mice with ischemic stroke. J Cell Physiol. 2020;235:2414‐2428. 10.1002/jcp.29146. [DOI] [PubMed] [Google Scholar]
- 105. Yin K‐J, Hamblin M, Chen YE. Angiogenesis‐regulating microRNAs and ischemic stroke. Curr Vasc Pharmacol. 2015;13:352‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang J, Gao F, Zhang Y, Liu Y, Zhang D. Buyang Huanwu decoction (BYHWD) enhances angiogenic effect of mesenchymal stem cell by upregulating VEGF expression after focal cerebral ischemia. J Mol Neurosci. 2015;56:898‐906. 10.1007/s12031-015-0539-0. [DOI] [PubMed] [Google Scholar]
- 107. Kan AA, van Erp S, Derijck AAHA, et al. Genome‐wide microRNA profiling of human temporal lobe epilepsy identifies modulators of the immune response. Cell Mol Life Sci. 2012;69:3127‐3145. 10.1007/s00018-012-0992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Liu W, Chen X, Zhang Y. Effects of microRNA‐21 and microRNA‐24 inhibitors on neuronal apoptosis in ischemic stroke. Am J Transl Res. 2016;8:3179‐3187. [PMC free article] [PubMed] [Google Scholar]
- 109. Annis RP, Swahari V, Nakamura A, Xie AX, Hammond SM, Deshmukh M. Mature neurons dynamically restrict apoptosis via redundant premitochondrial brakes. FEBS J. 2016;283:4569‐4582. 10.1111/febs.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience. 2015;302:2‐22. [DOI] [PubMed] [Google Scholar]
- 111. Gong P, Zhang Z, Zou C, et al. Hippo/YAP signaling pathway mitigates blood‐brain barrier disruption after cerebral ischemia/reperfusion injury. Behav Brain Res. 2019;356:8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sánchez‐Alegría K, Flores‐León M, Avila‐Muñoz E, Rodríguez‐Corona N, Arias C. PI3K signaling in neurons: a central node for the control of multiple functions. IJMS. 2018;19:3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chiu H, Alqadah A, Chang C. The role of microRNAs in regulating neuronal connectivity. Front Cell Neurosci. 2014;7:283. 10.3389/fncel.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. List of significant pathways induced by the group of 16 exosomal miRNAs. For each pathway, P‐value, number of targeted genes involved in and number of modulating miRNAs are reported.
File S2. Experimentally supported target genes (unique n = 582) involved in pathways of interest.
File S3. Lists of experimentally supported (Tarbase database) miRNAs' target genes in apoptosis, HIF‐1, neurotrophin, PI3K‐AKT signalling pathways.
Figure S1. Supporting information.
Data Availability Statement
The datasets generated during the current study are available from the corresponding authors upon reasonable requests.


