Abstract
Background
Little is known about the effect of renin angiotensin aldosterone system‐inhibiting (RAASi) drugs on alternative angiotensin peptides (APs) such as angiotensin 1‐7 (Ang1‐7), which are mediated by angiotensin‐converting enzyme 2 (ACE2).
Hypothesis/Objectives
Angiotensin receptor blockers (ARBs) would alter balance of APs and differences would be magnified in vitro by incubation of plasma samples with recombinant human ACE2 (rhACE2).
Animals
Six cats with cardiomyopathy (CM), 8 healthy cats.
Methods
Prospective open label trial. Plasma equilibrium concentrations of APs were measured in healthy cats as well as in CM cats that first received no RAASi drugs (CMnoRAASi) and then after 14 days of PO telmisartan (CMARB). Plasma APs also were measured after in vitro incubation with rhACE2.
Results
No significant differences were found between healthy and CMnoRAASi groups. Concentrations of several APs, including angiotensin I (AT1) and angiotensin II (AT2) were significantly different between CMnoRAASi and CMARB groups. Incubation with rhACE2 decreased AT1 and AT2 in both groups. The geometric mean concentration of Ang1‐7 was significantly higher in CMARB (4.9 pg/mL; 95% confidence interval [CI], 3.7‐6.4 pg/mL) vs CMnoRAASi (3.2 pg/mL; 95% CI, 2.2‐4.7 pg/mL; P = .01) and in CMARB + ACE2 (5.0 pg/mL; 95% CI, 3.9‐6.4 pg/mL) vs CMnoRAASi + ACE2 (3.0 pg/mL; 95% CI, 1.7‐5.5 pg/mL; P = .01). The most favorable theoretical AP profile that maximized Ang1‐7 and other alternative APs was CMARB + ACE2.
Conclusions and Clinical Importance
Balance between traditional and alternative APs can be favorably shifted using ARBs and in vitro incubation with rhACE2. These data shed light on new AP‐targeting strategies in cats with CM.
Keywords: angiotensin 1‐7, angiotensin II, cardiomyopathy, renin‐angiotensin‐aldosterone‐system
Abbreviations
- ACE
angiotensin‐converting enzyme
- ACE2
angiotensin‐converting enzyme 2
- ACEIs
angiotensin‐converting enzyme inhibitors
- ACE‐S
surrogate measure of ACE activity
- ALT‐S
surrogate measure of alternative renin‐angiotensin‐aldosterone system pathway
- Ang1‐5
angiotensin 1‐5
- Ang1‐7
angiotensin 1‐7
- Ang1‐9
angiotensin 1‐9
- AoD
aortic root diameter
- APs
angiotensin peptides
- ARBs
angiotensin receptor blockers
- AT1
angiotensin I
- AT2
angiotensin II
- AT3
angiotensin III
- AT4 (3‐8)
angiotensin 4
- CHF
congestive heart failure
- CI
confidence interval
- CM
cardiomyopathy
- E : A
peak mitral E wave to A wave velocity
- HCM
hypertrophic cardiomyopathy
- HOCM
hypertrophic obstructive cardiomyopathy
- IVSd
thickness of the interventricular septum at end‐diastole
- LAD
left atrial diameter
- LAD : AoD
left atrium to aortic root diameter ratio
- LLOD
lower limit of detection
- PRA‐S
surrogate measure of plasma renin activity
- RAAS
renin‐angiotensin‐aldosterone system
- RAASi
renin‐angiotensin‐aldosterone system inhibiting
- RCM
restrictive cardiomyopathy
- rhACE2
recombinant human angiotensin converting enzyme 2
1. INTRODUCTION
Cardiomyopathy (CM) is an important cause of morbidity and mortality in cats, but relatively little is known about its neurohormonal features as they relate to various angiotensin peptides (APs), such as angiotensin I (AT1) and angiotensin II (AT2). The traditional view of the AP system involves conversion of angiotensinogen by renin to AT1, followed by subsequent cleavage of 2 amino acids from the C‐terminal end of AT1 by angiotensin‐converting enzyme (ACE) to form the octapeptide AT2. 1 The biological activity of chronic AT2 stimulation contributes to the pathophysiology of heart failure through water and sodium retention and vasoconstriction, as well as myocardial and vascular remodeling. 1 Studies of APs in cats with CM are limited. One study 2 reported high kidney renin production in cats with CM, but renin angiotensin aldosterone system‐inhibiting (RAASi) drugs such as angiotensin‐converting enzyme inhibitors (ACEIs) and spironolactone have failed to definitively demonstrate benefit. 3 , 4 , 5 , 6 As such, our understanding of the role of APs in cats with CM is incomplete.
In 2002, the monocarboxypeptidase angiotensin‐converting enzyme 2 (ACE2) was discovered. 7 , 8 Unlike ACE, which cleaves 2 amino acids, ACE2 removes a single amino acid from AT1 and AT2, forming angiotensin 1‐9 (Ang1‐9) and angiotensin 1‐7 (Ang1‐7), respectively (Figure 1). Despite a structural difference of just 1 amino acid, Ang1‐7, Ang1‐9, and other APs such as angiotensin 1‐5 (Ang1‐5) are vasodilatory, natriuretic, and cardioprotective. 9 This discovery has expanded our understanding of the AP system and use of traditional RAASi drugs, such as ACEI, beyond just AT1 and AT2. The existence of alternative APs is reason to reconsider RAASi drugs such as angiotensin receptor blockers (ARBs), which block AT2 receptors. Angiotensin receptor blockers are used for treatment of hypertension and congestive heart failure (CHF) in humans, occasionally used for systemic hypertension in dogs and cats, and rarely used for heart disease in either dogs or cats. 10 , 11 One highly selective ARB, telmisartan, is approved for treatment of systemic hypertension in cats. 12 , 13 The motivation behind development of ARBs stems from the existence of ACE‐independent pathways that can circumvent ACEI treatment and still produce AT2. 14 , 15 Although treatment with ARBs permits unabated AT2 production, harmful effects of AT2, regardless of its source, are prevented by AT2 receptor blockade. When viewed in light of ACE2, the fact that ARBs permit unchecked production of AT2 has potential therapeutic implications. Specifically, the large pool of AT2 might serve as substrate for ACE2 and be converted to Ang1‐7, promoting beneficial effects such as vasodilatation and natriuresis. 16 Previous studies in dogs 17 and humans 16 with heart disease indicated that in vitro incubation of plasma with recombinant human ACE2 (rhACE2) drove the balance of APs toward more beneficial APs and fewer maladaptive APs. The relative concentrations of APs, especially those related to ACE2, in cats with CM have not been extensively studied. We sought to explore the relative concentrations of traditional and alternative APs between healthy cats and cats with CM. We hypothesized that treatment of affected cats with telmisartan would alter the balance of APs, and that these differences would be further magnified after in vitro incubation of plasma samples with rhACE2 compared with untreated samples. These data would provide proof of concept that ACE2 supplementation alters the balance of APs. Our objective was to characterize traditional and alternative APs in healthy cats and cats with CM, first in the absence of RAASi drugs, then after treatment with telmisartan, and finally, after incubation with rhACE2 with particular attention to the balance between beneficial APs such as Ang1‐7, Ang1‐9, and Ang1‐5 and maladaptive APs such as AT1 and AT2.
FIGURE 1.
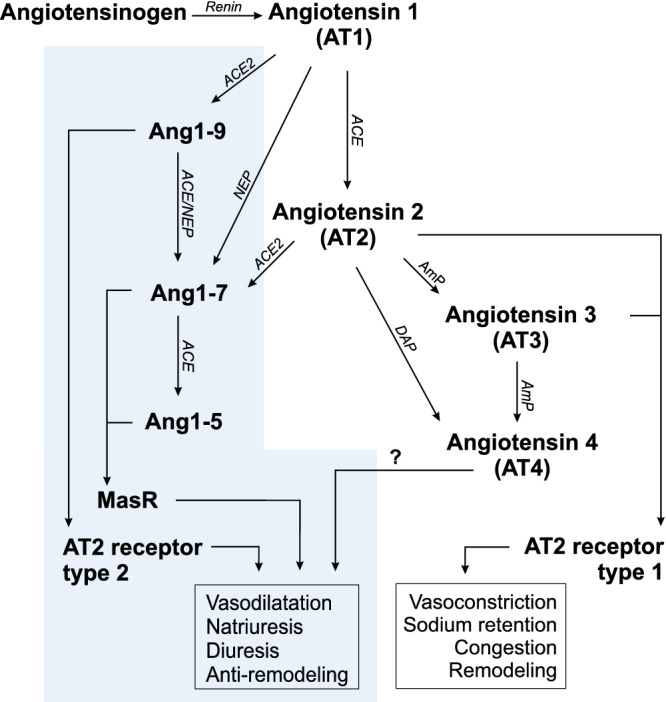
The traditional (unshaded background) and alternative (shaded background) renin‐angiotensin‐aldosterone system pathways. ACE, angiotensin‐converting enzyme; ACE2, angiotensin‐converting enzyme 2; AmP, aminopeptidase; Ang1‐5, angiotensin 1‐5; Ang1‐7, angiotensin 1‐7; Ang1‐9, angiotensin 1‐9; DAP, aspartyl aminopeptidase; NEP, neutral endopeptidase
2. MATERIALS AND METHODS
We designed a prospective study for healthy cats and cats with CM. The study protocol underwent institutional review and approval, and cat owners were required to provide informed consent. Inclusion criteria for cats with CM included an echocardiographic diagnosis of diastolic heart disease caused by either hypertrophic cardiomyopathy (HCM) or restrictive cardiomyopathy (RCM) phenotype using 2‐dimensional left atrium‐to‐aortic root dimension ratio (LAD : AoD) ≥1.6 within 90 days from date of inclusion. A diagnosis of HCM was based on idiopathic left ventricular (LV) hypertrophy defined as diffuse or regional interventricular septal diastolic wall thickness (IVSd) or LV diastolic caudal wall thickness (LVPWd) >6 mm, 18 whereas a diagnosis of RCM was based on IVSd and LVPWd <6 mm, and when measurable, a restrictive mitral inflow filling pattern defined as peak mitral E wave‐to‐A wave velocity (E : A) >2.0. 19 Inability to measure E : A because of fused waves did not exclude a diagnosis of RCM phenotype if LV and left atrial criteria were both present. Exclusion criteria included systolic blood pressure ≥ 180 mm Hg or ≤ 100 mm Hg, hyperthyroidism in cats >10 years of age, active CHF in the form of pulmonary edema or pleural effusion, congenital heart disease, or presence of concurrent systemic disease that was considered severe in the opinion of the investigators. Cats with CM receiving cardiac medications such as diuretics, beta‐blockers, and anti‐platelet agents were eligible, but those receiving ACEI were excluded. A group of healthy cats with IVSd and LVPWd <5 mm 20 and LAD : AoD <1.4 21 and not receiving any cardiac medications was recruited as a control. Echocardiographic studies (iE33, Philips Healthcare, Andover, Massachusetts) were performed without sedation. Measurements of IVSd, LVPWd, and LAD : AoD were performed from 2‐dimensional right parasternal short axis views. Cats with HCM were described as having hypertrophic obstructive cardiomyopathy (HOCM) if there was 2‐dimensional or M‐mode evidence of systolic cranial motion of the mitral valve and a left ventricular outflow tract velocity ≥2.5 m/s as measured from the left apical view. 18 At baseline, approximately 3 to 5 mL of venous blood was obtained and separated into plain tubes and tubes containing lithium heparin. Systolic blood pressure measurement was performed using a Doppler unit (Parks Medical Electronics, Inc, Aloha, Oregon) and an appropriately sized cuff. Cats with CM were prescribed open‐label liquid telmisartan (Semintra, Boehringer Ingelheim Vetmedica, St Joseph, Missouri) at a dosage of 2 mg/kg PO q24h for 14 to 21 days until the day of evaluation. Changes to other concurrent cardiac medications were not allowed during the treatment period. At evaluation, blood pressure measurement and blood collection were performed.
2.1. Blood assays
Serum was used to measure BUN, creatinine, sodium, potassium, and chloride. Lithium heparin plasma was stored at −80°C until batched measurement of equilibrium concentrations of APs, including AT1, AT2, Ang1‐9, Ang1‐7, Ang1‐5, angiotensin 3 (AT3), and angiotensin 4 (AT4) was performed as previously described. 1 , 16 , 17 , 22 , 23 Briefly, plasma was spiked with stable isotope‐labeled standards for each AP, allowed to reach equilibrium at 37°C, and assayed using liquid chromatography‐tandem mass spectrometry (Attoquant Diagnostics, Vienna, Austria). Use of equilibrium assays avoids the need for special collection and handling requirements at the time of blood collection, increases the signal‐to‐noise ratio of the assay, and results in AP concentrations higher than, but proportional to, plasma concentrations using enzyme inhibitors at time of blood collection. 16 , 24 To assess the effect of exogenous ACE2 on the relative concentrations of selected APs, plasma from cats with CM was assayed after in vitro incubation with 5 μg/mL of rhACE2 (R&D Systems, Minneapolis, Minnesota) at 37°C, followed by measurement of equilibrium concentrations of AT1, AT2, Ang1‐9, Ang1‐7, and Ang1‐5. 16 , 17
2.2. Statistical analysis
Previous to our study, data regarding equilibrium concentrations of APs in cats were not available, which hindered a priori sample size calculation. The number of cats in the study (8 healthy cats and 8 cats with CM) was chosen based on similar studies 17 conducted in dogs. Descriptive statistics regarding patient signalment, physical examination findings, and diagnostic test results were generated. Descriptive data are presented as mean (SD) or median (range). Comparisons of longitudinal blood pressure and routine blood test results in cardiomyopathic cats before and after ARB treatment were performed using paired t tests or Wilcoxon signed rank tests. Concentrations of APs were natural log transformed and assessed for normality using Kolmogorov‐Smirnov tests and visual inspection of histograms and QQ plots. Any AP result that was below the lower limit of detection (LLOD) was entered as 0.5 times the LLOD. 25 The central tendencies of APs are reported as geometric means (95% confidence interval [CI]). Comparisons of APs between healthy and CM cats were performed using unpaired t tests. The AP concentrations in CM cats were measured using 4 different plasma samples, including baseline in the absence of RAASi drugs (CMnoRAASi), after ARB (CMARB), and after rhACE2 incubation of each plasma type (CMnoRAASi + ACE2, CMARB + ACE2). These comparisons were performed using repeated measures of 1‐way analysis of variance followed by 4 predefined Holm‐Sidak multiple comparison tests, including the following pairs of results: CMnoRAASi vs CMARB, CMnoRAASi vs CMnoRAASi + ACE2, CMARB vs CMARB + ACE2, and CMnoRAASi + ACE2 vs CMARB + ACE2. Two surrogate and unitless measures of RAAS activity were calculated, including surrogate of plasma renin activity (PRA‐S) calculated as [AT2] + [AT1] and surrogate activity of ACE (ACE‐S) calculated as [AT2]/[AT1]. 17 , 26 We explored the relative concentrations of alternative APs to traditional APs as another unitless ratio, ALT : TRAD: ([Ang1‐9] + [Ang1‐7] + [Ang1‐5])/([AT2] + [AT1]). Correlations between AP concentrations and clinical variables were assessed by calculation of either Pearson or Spearman rank correlation coefficients. Figures displaying the different APs and their relationships were constructed using variably sized circles the sizes of which were proportional to the geometric mean equilibrium concentration of each AP. Statistical calculations were performed using statistical software (Prism 8, GraphPad Software, San Diego, California). Significance was defined as P < .05.
3. RESULTS
3.1. Study cohort
Eight healthy cats owned by staff or faculty and 8 cats with CM presented to the Veterinary Hospital of the University of Pennsylvania were recruited between March 2019 and March 2020. The owner of 1 cat with RCM was unable to consistently administer the ARB and dropped out of the study. One female spayed 6‐year‐old cat with HOCM and history of CHF that was receiving furosemide, spironolactone and clopidogrel and with systolic blood pressure of 110 mm Hg at the time of enrollment also was withdrawn from the study because of lethargy, vomiting, tachypnea, and hypotension (systolic blood pressure = 70 mm Hg) after receiving 2 doses of ARB. The cat was hospitalized for hypotension and CHF and was treated with parenteral furosemide and dopamine. The cat was discharged 20 hours after admission with resolution of CHF and improved systolic blood pressure of 105 mm Hg. The remaining 6 cats with CM were analyzed, including 3 cats with HOCM, 2 cats with HCM, and 1 cat with RCM. Cats received ARB for a median duration of 16 days (range, 15‐21 days). Baseline demographic information, medication history, and echocardiographic findings are presented in Table 1. Two of 6 (33%) cardiomyopathic cats had a history of CHF and were receiving furosemide. Small but significant increases in BUN and sodium were observed during ARB treatment as compared to baseline. No significant differences in blood pressure or serum creatinine, chloride, or potassium concentrations were observed (Table 2).
TABLE 1.
Demographic, medication, and echocardiographic characteristics of healthy cats and cats with cardiomyopathy
| Healthy (n = 8) | Cardiomyopathy (n = 6) | |
|---|---|---|
| Age (y) | 7 (3) | 11 (4‐12) |
| Body weight (kg) | 4.2 (0.6) | 5.2 (0.7) |
| Sex | 4M/4F | 6M/1F |
| Blood pressure (mm Hg) | 142 (26) | 130 (9) |
| Furosemide (Y/N) | 0/8 | 2/4 |
| ACEI (Y/N) | 0/8 | 0/6 |
| Clopidogrel (Y/N) | 0/8 | 3/3 |
| Atenolol (Y/N) | 0/8 | 4/2 |
| Spironolactone (Y/N) | 0/8 | 0/6 |
| LVIDd (cm) | 1.41 (0.08) | 1.62 (0.16) |
| LVIDs (cm) | 0.69 (0.08) | 0.77 (0.19) |
| IVSd (cm) | 0.42 (0.04) | 0.55 (0.08) |
| LVPWd (cm) | 0.42 (0.04) | 0.61 (0.08) |
| LAD (cm) | 1.20 (1.10‐1.30) | 1.89 (0.32) |
| AoD (cm) | 1.0 (0.88‐1.30) | 0.95 (0.11) |
| LA : Ao | 1.23 (0.14) | 2.00 (0.38) |
Note: Data are presented as mean (SD) or median (range).
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AoD, aortic root diameter; IVSd, thickness of the interventricular septum at end‐diastole; LAD, left atrial diameter; LVIDd, left ventricular internal diameter at end‐diastole; LVIDs, left ventricular internal diameter at end‐systole; LVPWd, left ventricular caudal wall thickness at end‐diastole.
TABLE 2.
Mean (SD) results of renal blood markers and serum electrolyte concentrations during treatment without renin‐angiotensin‐aldosterone system‐inhibiting drugs (no RAASi) and during treatment with an angiotensin receptor blocker (ARB) in 6 cats with cardimyopathy
| no RAASi | ARB | P value | |
|---|---|---|---|
| Blood pressure (mm Hg) | 130 (9) | 128 (17) | .58 |
| BUN (mg/dL) | 25 (4) | 28 (5) | .03 |
| Creatinine (mg/dL) | 1.58 (0.47) | 1.60 (0.58) | .74 |
| Sodium (mmol/L) | 151 (1) | 152 (2) | .03 |
| Chloride (mmol/L) | 119 (1) | 121 (2) | .11 |
| Potassium (mmol/L) | 4.1 (0.3) | 4.0 (0.1) | .5 |
Abbreviation: BUN, blood urea nitrogen.
3.2. Angiotensin peptides in healthy cats vs cats with cardiomyopathy
No significant differences in AP concentrations were observed between healthy and CMnoRAASi cats (Figure 2). Concentrations of Ang1‐9 were below the LLOD in all cats in both groups. No significant differences were observed in surrogate measures of AP system activity between the 2 groups, including PRA‐S (healthy, 152; 95% CI, 49‐473 vs CMnoRAASi, 260; 95% CI, 92‐737; P = .43), ACE‐S (healthy, 4.4; 95% CI, 3.2‐6.2 vs CMnoRAASi, 3.3; 95% CI, 2.5‐4.3; P = .12), or ALT : TRAD (healthy, 0.73; 95% CI, 0.57‐0.94 vs CM, .87; 95% CI, 0.73‐1.05; P = .23). No significant correlations were found among APs, LA : Ao, IVSd, LVPWd, BUN, creatinine, Na, or K in the CMnoRAASi samples (data not shown).
FIGURE 2.
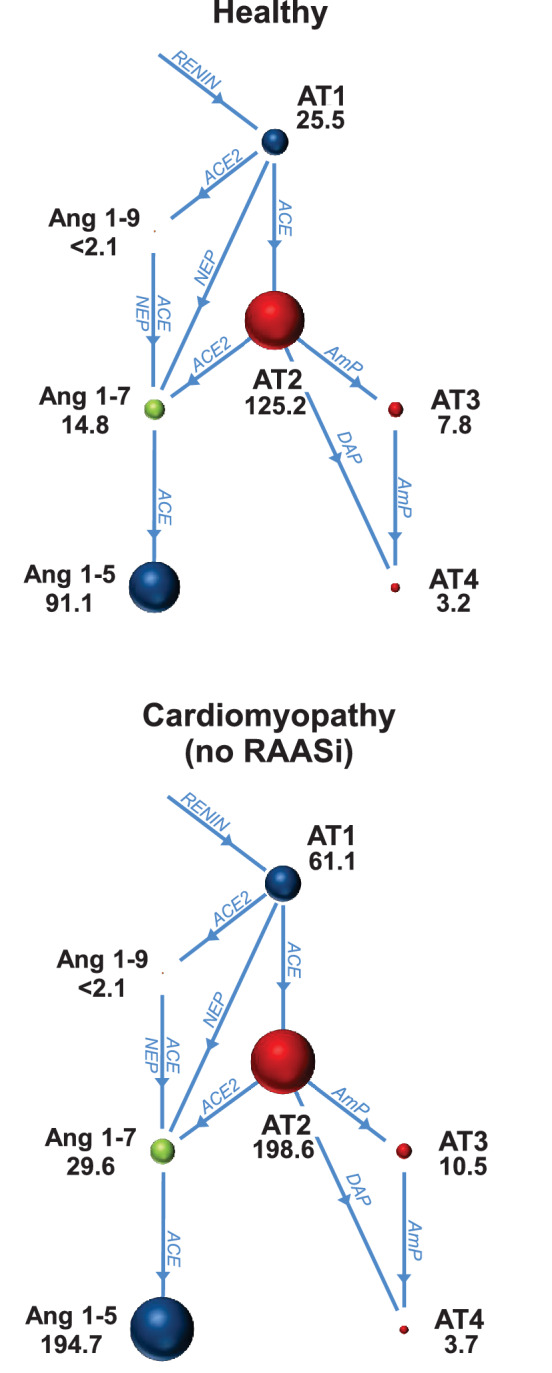
Plasma equilibrium concentrations and relationships of angiotensin peptides and angiotensin‐converting enzyme (ACE) and angiotensin‐converting enzyme 2 (ACE2) pathways in healthy cats and cats with cardiomyopathy receiving no renin‐angiotensin‐aldosterone system inhibiting drugs (no RAASi). There were no significant differences in angiotensin peptide concentrations between groups. The sizes of the circles are proportional to the geometric mean (pg/mL) listed beside each AP. AmP, aminopeptidase; Ang1‐5, angiotensin 1‐5; Ang1‐7, angiotensin 1‐7; Ang1‐9, angiotensin 1‐9; ARB, angiotensin receptor blocker; AT1, angiotensin 1; AT2, angiotensin 2; AT3, angiotensin 3; AT4, angiotensin 4; DAP, aspartyl aminopeptidase; NEP, neutral endopeptidase
3.3. Angiotensin peptides during ARB treatment and after incubation with rhACE2
Concentrations of APs and surrogate measures of AP system activity in the CMnoRAASi, CMARB, CMnoRAASi + ACE2, and CMARB + ACE2 samples are shown in Figure 3 and Table 3. The CMARB samples had significantly higher AT1, AT2, and Ang1‐7 concentrations and higher PRA‐S as compared with the CMnoRAASi samples. The ratio of ALT : TRAD was mildly but not significantly lower in CMARB samples as compared to CMnoRAASi. Incubation of both sample types with rhACE2 decreased AT1, AT2, PRA‐S and ACE‐S and increased ALT : TRAD as compared to baseline. Concentration of Ang1‐9 significantly increased in CMARB + ACE2 as compared to CMARB but did not significantly change between CMnoRAASi + ACE2 and CMnoRAASi. Concentrations of Ang1‐5 were mildly but significantly increased in the CMnoRAASi + ACE2 samples over baseline after rhACE2 incubation. Concentrations of Ang1‐7 after incubation with rhACE2 were not significantly different from baseline in either sample type, but concentrations of Ang1‐7 and Ang1‐9 remained significantly higher in CMARB + ACE2 vs CMnoRAASi + ACE2 samples. The AT2 concentration substantially decreased from baseline to below the LLOD after rhACE2 incubation in all cats in both groups. The relationships and relative amounts of specific APs in the 2 groups after incubation with rhACE2 are shown in Figure 4.
FIGURE 3.
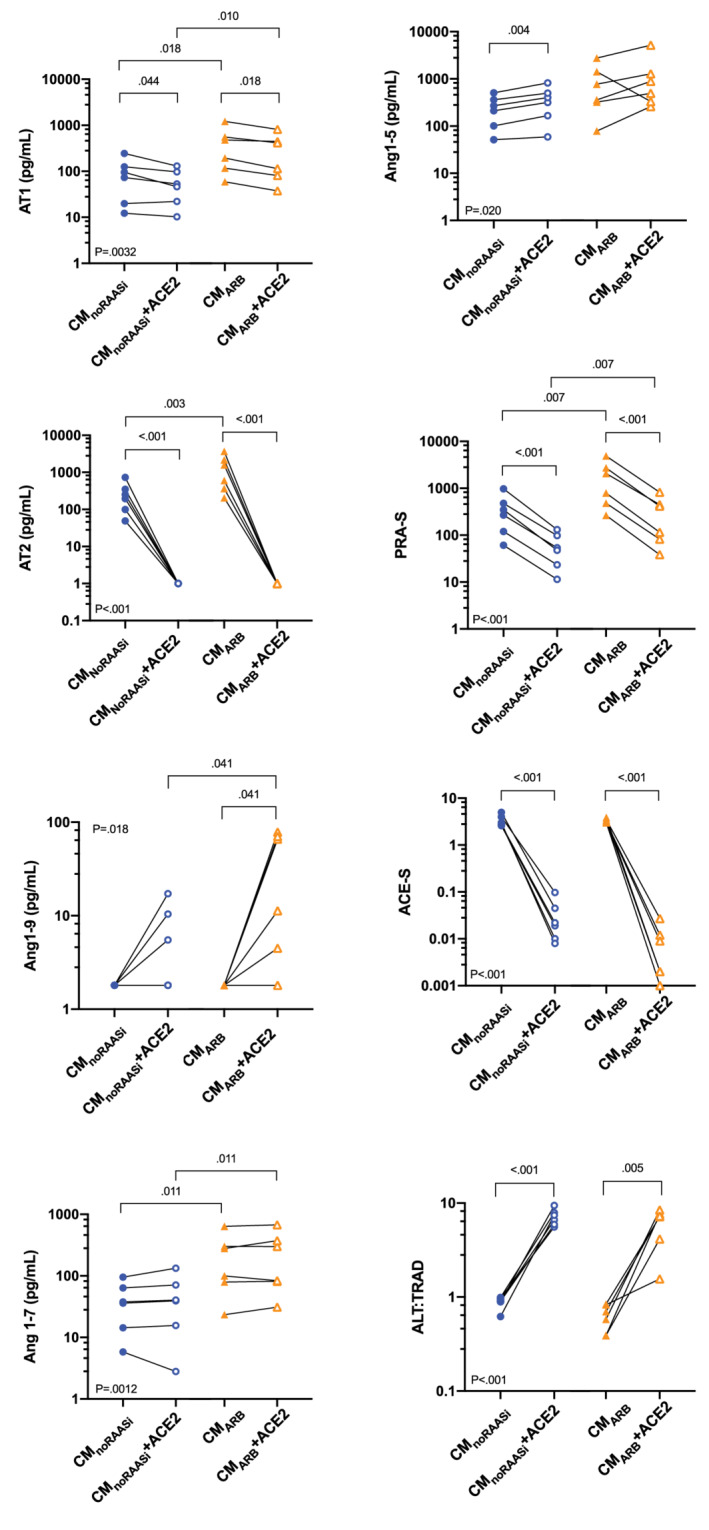
Plasma equilibrium concentrations of angiotensin peptides (APs) and surrogate measures AP system activity, including of plasma renin activity (PRA‐S) and the ratio of alternative APs to traditional APs (ALT : TRAD) from 6 cats with cardiomyopathy while receiving no RAAS‐inhibiting drugs (no RAASi) (blue solid circles), angiotensin receptor blocker (ARB) (purple solid triangles) and after in vitro incubation of no RAASi plasma with recombinant human angiotensin‐converting enzyme 2 (no RAASi + ACE2) (open blue circles) and ARB plasma with recombinant human angiotensin‐converting enzyme 2 (ARB + ACE2) (open purple triangles). Ang1‐5, angiotensin 1‐5; Ang1‐7, angiotensin 1‐7; Ang1‐9, angiotensin 1‐9; ARB, angiotensin receptor blocker; AT1, angiotensin 1; AT2, angiotensin 2; ln, natural log; LLOD, lower limit of detection
TABLE 3.
Geometric mean (95% confidence interval) of plasma equilibrium concentrations of angiotensin peptides (APs) and mean (SD) measures of surrogate markers of plasma renin activity (PRA‐S) and angiotensin converting enzyme (ACE‐S) and ratio of alternative to traditional APs (ALT : TRAD) in 6 cats with cardiomyopathy while receiving no renin‐angiotensin‐aldosterone system inhibiting drugs (no RAASi), while receiving angiotensin receptor blocker (ARB), and after in vitro incubation of plasma with recombinant human angiotensin converting enzyme‐2 (rhACE2)
| No RAASi (A) | ARB (B) | No RAASi + ACE2 (C) | ARB + ACE2 (D) | P value | |
|---|---|---|---|---|---|
| AT1 (pg/mL) | 61 | 276 | 44 | 194 | .003 |
| (19‐202) | (86‐891) | (16‐118) | (56‐678) | A vs B, P = .02 | |
| A vs C, P = .04 | |||||
| C vs D, P = .02 | |||||
| B vs D, P = .01 | |||||
| AT2 (pg/mL) | 199 | 913 | <LLOD a | <LLOD a | <.001 |
| (73‐539) | (282‐2955) | A vs B, P = .003 | |||
| A vs C, P < .001 | |||||
| B vs D, P < .001 | |||||
| Ang1‐9 (pg/mL) | <LLOD b | <LLOD b | 4.6 | 18 | .02 |
| (1.5‐12.1) | (3.3‐99) | C vs D, P = .04 | |||
| B vs D, P = .04 | |||||
| Ang1‐7 (pg/mL) | 30 | 147 | 29 | 159 | .001 |
| (10.4‐87) | (43‐508) | (7.1‐122) | (47‐539) | A vs B, P = .01 | |
| C vs D, P = .01 | |||||
| Ang1‐5 (pg/mL) | 195 | 552 | 283 | 800 | .02 |
| (80‐474) | (148‐2064) | (106‐754) | (253‐2528) | A vs C, P = .004 | |
| AT3 | 10 | 47 | NA | NA | .67 |
| (2.6‐41) | (12‐186) | ||||
| AT4 | 3.7 | 20 | NA | NA | .48 |
| (1.1‐12) | (5.5‐73) | ||||
| PRA‐S | 375 | 1879 | 61 | 321 | <.001 |
| (331) | (1772) | (46) | (303) | A vs B, P = .007 | |
| A vs C, P < .001 | |||||
| C vs D, P = .007 | |||||
| B vs D, P < .001 | |||||
| ACE‐S | 3.4 | 3.3 | 0.034 | 0.009 | <.001 |
| (1.0) | (0.3) | (0.033) | (0.010) | A vs C, P < .001 | |
| B vs D, P < .001 | |||||
| ALT : TRAD | 0.88 | 0.62 | 7.1 | 6.1 | <.001 |
| (0.13) | (0.20) | (1.4) | (2.6) | A vs C, P < .001 | |
| B vs D, P = .005 |
Abbreviations: Ang1‐5, angiotensin 1‐5; Ang1‐7, angiotensin 1‐7; Ang1‐9, angiotensin 1‐9; AT1, angiotensin 1; AT2, angiotensin 2; AT3, angiotensin 3; AT4, angiotensin 4; LLOD, lower limit of detection.
All values less than the lower limit of detection, 2.0 pg/mL.
All values less than the lower limit of detection, 3.5 pg/mL.
FIGURE 4.
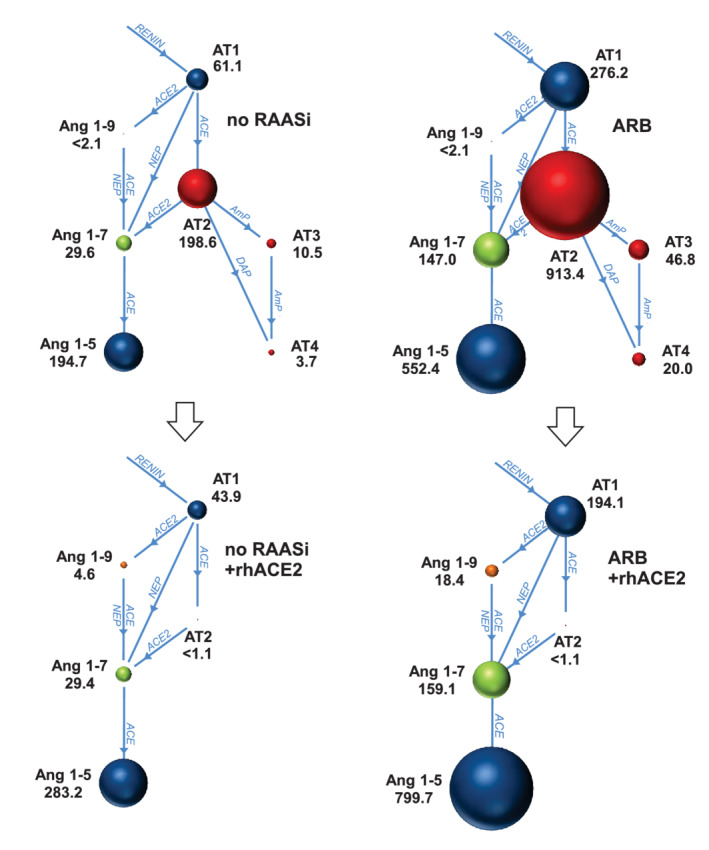
Plasma equilibrium concentrations and relationships of angiotensin peptides (APs) and angiotensin‐converting enzyme (ACE) and angiotensin‐converting enzyme‐2 (ACE2) pathways in 6 cats with cardiomyopathy absent any RAAS‐inhibiting drugs (no RAASi), during treatment with angiotensin receptor blocker (ARB), and after incubation with recombinant human ACE2 (rhACE2). The sizes of the circles are proportional to the geometric mean concentration (pg/mL) of each angiotensin peptide. AmP, aminopeptidase; Ang1‐5, angiotensin 1‐5; Ang1‐7, angiotensin 1‐7; Ang1‐9, angiotensin 1‐9; ARB, angiotensin receptor blocker; AT1, angiotensin 1; AT2, angiotensin 2; AT3, angiotensin 3; AT4, angiotensin 4; DAP, aspartyl aminopeptidase; NEP, neutral endopeptidase
4. DISCUSSION
Our main findings were the different AP profiles during no ACEI as compared with ARB treatment in cats with CM. Treatment of cardiomyopathic cats with ARB resulted in higher activity of the traditional AP pathway as evidenced by significantly higher AT1, AT2, and PRA‐S. Vasodilators, including ARBs, are potent stimuli of the RAAS, 27 and despite the absence of changes in systolic blood pressure during ARB treatment, local effects at the kidney might have led to more renin release. In theory, cats receiving ARB treatment would be protected against higher traditional AP system activity by blockade of AT2 receptors. The absolute or relative benefit of ACEI or ARB treatment in cats with CM is unknown, but a potential benefit unique to ARB treatment is the continued production of AT2, which instead of binding its receptor, can be diverted towards increasing salutary Ang1‐7. This effect might explain the significantly increased Ang1‐7 concentration observed during ARB treatment in our study.
An important hypothesis of our study was that in vitro incubation with rhACE2 would favorably rebalance equilibrium plasma concentrations of APs toward Ang1‐7 and related alternative RAAS APs, and our results indicated higher alternative APs and lower traditional APs compared to baseline. Specifically, compared to preincubation results, rhACE2 increased ALT : TRAD in both the CMnoRAASi and CMARB samples while decreasing PRA‐S and AT1. Activity of the traditional AP system was suppressed such that AT2 concentration decreased below the LLOD of the assay in all cats after rhACE2, regardless of whether or not they were receiving ARB. One pathway for AT2 depletion is its conversion to Ang1‐7 by rhACE2 and subsequent conversion of Ang1‐7 to Ang1‐5 by ACE. Plasma from CMARB cats with or without rhACE2 had significantly higher concentrations of Ang1‐7 as compared to CMnoRAASi samples. A plausible explanation for this finding is that ARB treatment provides AT2 substrate, and unlike the profile associated with ARB monotherapy where a large pool of AT2 existed, the combination of rhACE2 and ARB markedly decreased AT2 to the point of being undetectable. The AP system profile we consider most favorable (in which AT1, AT2, and PRA‐S are minimized and Ang1‐9, Ang1‐7, Ang1‐5, and ALT‐S are maximized) was achieved with the combination of ARB and rhACE2.
The AP profile of our limited cohort of CMnoRAASi cats was not different from that of healthy cats. Previous studies have measured ACE activity as a marker of RAAS activation by utilizing various methods. In a study 6 of cats with CM, ACE activity, as measured by colorimetric assay, was 45 U/mL and, in 2 previous studies of healthy cats, 28 , 29 ACE activity was approximately 35 U/mL and 13 U/mL as measured by radioimmunoassay and colorimetric assay, respectively. Comparison between our study and previous studies is made difficult by the different assay methods and standards used. One study 30 of 11 healthy cats reported AT1 and AT2 concentrations measured by radioimmunoassay, which cannot be directly compared to our equilibrium concentration results. Thus, the presence or extent of AP activation in cats with CM is unknown. In our study, a potential explanation for the lack of differences in cardiomyopathic cats compared to controls is that the majority of affected cats, despite having left atrial enlargement, were asymptomatic and not receiving diuretics, which are known to be potent RAAS activators. Other potential reasons include the reported importance of non‐RAAS systems, including the chymase system, which is capable of converting AT1 to AT2 independent of ACE, as well as the existence of tissue ACE, the activity of which might not necessarily be reflected by circulating APs. 31 In previous studies of healthy cats 29 and cats with experimentally induced pressure overload hypertrophy, 32 the chymase system comprised approximately 85% of all RAAS activity as compared to only 15% mediated by ACE. Chymase primarily is expressed in tissues, including mast cells, myocardial cells, and mesenchymal interstitial cells rather than being free in the circulation. 31 Previous studies in humans and animals have shown that local tissue AP production induces myocyte hypertrophy and myocardial fibrosis, and substantially contributes to morbidity and mortality. 1 , 33 Given that we only measured circulating APs, our results might underestimate the broader extent of RAAS activity, particularly within tissues, and APs and RAAS might still play an important role in the pathophysiology of CM in cats.
A related finding involving healthy cats in our study is that AT2 concentrations were substantially higher in comparison to concentrations in healthy dogs and humans. The AT2 equilibrium concentration in healthy cats was 6 to 12 times higher than that previously reported in healthy dogs 1 , 17 and 2.5 times higher than reported in healthy humans. 16 Higher constitutive AP system activity in the healthy cats is further suggested by PRA‐S and ACE‐S that were both an order of magnitude higher in healthy cats than in healthy dogs 1 , 17 A potential reason for this finding involves the evolutionary history of the domestic cat, which originates from the Near Eastern (African) wildcat (Felis silvestris lybica), a desert dwelling cat of ancient Egypt and the Fertile Crescent. 34 Similar to other mammals from arid habitats, where body water conservation is important to survival, 35 cats might have evolved a particularly robust AP system to meet the unique demands of their environment. 36 , 37 , 38
Our study had several important limitations. Two cats did not complete the study, which might have decreased the power to detect significant differences between some of the APs, but many potentially important differences between groups still were found. Treatment with telmisartan was not blinded or randomized. As previously stated, experience with ARBs in cats with heart disease is limited and we felt is was important to administer telmisartan in an open‐label fashion. In our study, 1 cat with HOCM experienced severe hypotension after the first 2 doses of ARB, which emphasizes the need for larger studies evaluating the safety and tolerability of ARB in cats with heart disease. The duration of ARB treatment was based on a previous study 12 in which telmisartan had an effect on blood pressure after 14 days of treatment in cats with systemic hypertension. Cats in our study were receiving a variety of cardiac medications, including diuretics and beta‐adrenergic blockers that might affect baseline AP concentrations, but the paired nature of the study permitted examination of direction and magnitude of change within each cat. Telmisartan is 1 of a number of ARBs, and because of pharmacologic and pharmacodynamic differences among various drugs in this class, 39 results from our study might not apply to other ARBs. We did not compare ARBs against other neurohormonal modulating drugs, such as ACEI, and the relative effects of ARBs and ACEIs in cats with heart disease require additional study. The AP system is only part of the broader RAAS, and additional studies of larger cohorts that specifically account for disease stage, variety of concomitant cardiac medications, and effect on other neurohormonal components of heart failure, such as aldosterone, should be performed to expand our findings.
In conclusion, ARB administration in cats with CM is associated with higher traditional AP system activity, as well as increased concentrations of the alternative AP, Ang1‐7 as compared with cats not receiving RAASi treatment. The combination of ARB plasma incubated with rhACE2 produced the most theoretically favorable balance between maladaptive and salutary APs. These findings suggest a novel therapeutic strategy in cats with CM and further studies are warranted.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
University of Pennsylvania IACUC approval, #806674, #803099. Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study. The authors thank the owners who volunteered their cats for this study.
Huh T, Larouche‐Lebel É, Loughran KA, Oyama MA. Effect of angiotensin receptor blockers and angiotensin‐converting enzyme 2 on plasma equilibrium angiotensin peptide concentrations in cats with heart disease. J Vet Intern Med. 2021;35:33–42. 10.1111/jvim.15948
REFERENCES
- 1. Ames MK, Atkins CE, Pitt B. The renin‐angiotensin‐aldosterone system and its suppression. J Vet Intern Med. 2019;33:363‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taugner FM. Stimulation of the renin‐angiotensin system in cats with hypertrophic cardiomyopathy. J Comp Pathol. 2001;125:122‐129. [DOI] [PubMed] [Google Scholar]
- 3. James R, Guillot E, Garelli‐Paar C, Huxley J, Grassi V, Cobb M. The SEISICAT study: a pilot study assessing efficacy and safety of spironolactone in cats with congestive heart failure secondary to cardiomyopathy. J Vet Cardiol. 2018;20:1‐12. [DOI] [PubMed] [Google Scholar]
- 4. King JN, Martin M, Chetboul V, et al. Evaluation of benazepril in cats with heart disease in a prospective, randomized, blinded, placebo‐controlled clinical trial. J Vet Intern Med. 2019;33:2559‐2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacDonald KA, Kittleson MD, Kass PH, White SD. Effect of spironolactone on diastolic function and left ventricular mass in Maine coon cats with familial hypertrophic cardiomyopathy. J Vet Intern Med. 2008;22:335‐341. [DOI] [PubMed] [Google Scholar]
- 6. MacDonald KA, Kittleson MD, Larson RF, et al. The effect of ramipril on left ventricular mass, myocardial fibrosis, diastolic function, and plasma neurohormones in Maine coon cats with familial hypertrophic cardiomyopathy without heart failure. J Vet Intern Med. 2006;20:1093‐1105. [DOI] [PubMed] [Google Scholar]
- 7. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1‐E9. [DOI] [PubMed] [Google Scholar]
- 8. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin‐converting enzyme. Cloning and functional expression as a captopril‐insensitive carboxypeptidase. J Biol Chem. 2000;275:33238‐33243. [DOI] [PubMed] [Google Scholar]
- 9. Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1‐7 axis of the renin‐angiotensin system in heart failure. Circ Res. 2016;118:1313‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810‐1852. [DOI] [PubMed] [Google Scholar]
- 11. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776‐803. [DOI] [PubMed] [Google Scholar]
- 12. Glaus TM, Elliott J, Herberich E, Zimmering T, Albrecht B. Efficacy of long‐term oral telmisartan treatment in cats with hypertension: results of a prospective European clinical trial. J Vet Intern Med. 2019;33:413‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sent U, Gossl R, Elliott J, et al. Comparison of efficacy of long‐term oral treatment with telmisartan and benazepril in cats with chronic kidney disease. J Vet Intern Med. 2015;29:1479‐1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tokmakova M, Solomon SD. Inhibiting the renin‐angiotensin system in myocardial infarction and heart failure: lessons from SAVE, VALIANT and CHARM, and other clinical trials. Curr Opin Cardiol. 2006;21:268‐272. [DOI] [PubMed] [Google Scholar]
- 15. Wong J, Patel RA, Kowey PR. The clinical use of angiotensin‐converting enzyme inhibitors. Prog Cardiovasc Dis. 2004;47:116‐130. [DOI] [PubMed] [Google Scholar]
- 16. Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH, Oudit GY. Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol. 2017;69:805‐819. [DOI] [PubMed] [Google Scholar]
- 17. Larouche‐Lebel E, Loughran KA, Oyama MA, et al. Plasma and tissue angiotensin‐converting enzyme 2 activity and plasma equilibrium concentrations of angiotensin peptides in dogs with heart disease. J Vet Intern Med. 2019;33:1571‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fox PR, Keene BW, Lamb K, et al. International collaborative study to assess cardiovascular risk and evaluate long‐term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: the REVEAL study. J Vet Intern Med. 2018;32:930‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Locatelli C, Pradelli D, Campo G, et al. Survival and prognostic factors in cats with restrictive cardiomyopathy: a review of 90 cases. J Feline Med Surg. 2018;20:1138‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeMadron E, Bonagura J, Herring DS. Two dimensional echocardiography in the normal cat. Vet Radiol Ultrasound. 1985;26:149‐158. [Google Scholar]
- 21. Abbott JA, MacLean HN. Two‐dimensional echocardiographic assessment of the feline left atrium. J Vet Intern Med. 2006;20:111‐119. [DOI] [PubMed] [Google Scholar]
- 22. Adin D, Atkins C, Domenig O, et al. Renin‐angiotensin aldosterone profile before and after angiotensin‐converting enzyme‐inhibitor administration in dogs with angiotensin‐converting enzyme gene polymorphism. J Vet Intern Med. 2020;34:600‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adin D, Kurtz K, Atkins C, Papich MG, Vaden S. Role of electrolyte concentrations and renin‐angiotensin‐aldosterone activation in the staging of canine heart disease. J Vet Intern Med. 2020;34:53‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Domenig O, Manzel A, Grobe N, et al. Neprilysin is a mediator of alternative renin‐angiotensin‐system activation in the murine and human kidney. Sci Rep. 2016;6:33678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keizer RJ, Jansen RS, Rosing H, et al. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect. 2015;3:e00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Binder C, Poglitsch M, Agibetov A, et al. Angs (angiotensins) of the alternative renin‐angiotensin system predict outcome in patients with heart failure and preserved ejection fraction. Hypertension. 2019;74:285‐294. [DOI] [PubMed] [Google Scholar]
- 27. Weber MA. Interrupting the renin‐angiotensin system: the role of angiotensin‐converting enzyme inhibitors and angiotensin II receptor antagonists in the treatment of hypertension. Am J Hypertens. 1999;12:189S‐194S. [DOI] [PubMed] [Google Scholar]
- 28. King JN, Humbert‐Droz E, Maurer M. Plasma angiotensin converting enzyme activity and pharmacokinetics of benazepril and benazeprilat in cats after single and repeated oral administration of benazepril.HCl. J Vet Pharmacol Ther. 1999;22:360‐367. [DOI] [PubMed] [Google Scholar]
- 29. Aramaki Y, Uechi M, Takase K. Angiotensin converting enzyme and chymase activity in the feline heart and serum. J Vet Med Sci. 2003;65:1115‐1118. [DOI] [PubMed] [Google Scholar]
- 30. Mishina M, Watanabe T, Fujii K, et al. Non‐invasive blood pressure measurements in cats: clinical significance of hypertension associated with chronic renal failure. J Vet Med Sci. 1998;60:805‐808. [DOI] [PubMed] [Google Scholar]
- 31. Urata H, Boehm KD, Philip A, et al. Cellular localization and regional distribution of an angiotensin II‐forming chymase in the heart. J Clin Invest. 1993;91:1269‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uechi M, Tanaka Y, Aramaki Y, et al. Evaluation of the renin‐angiotensin system in cardiac tissues of cats with pressure‐overload cardiac hypertrophy. Am J Vet Res. 2008;69:343‐348. [DOI] [PubMed] [Google Scholar]
- 33. De Mello WC, Frohlich ED. Clinical perspectives and fundamental aspects of local cardiovascular and renal renin‐angiotensin systems. Front Endocrinol. 2014;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Driscoll CA, Menotti‐Raymond M, Roca AL, et al. The near eastern origin of cat domestication. Science. 2007;317:519‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riad F, Ben Goumi M, Giry J, et al. Renin‐aldosterone axis and arginine‐vasopressin responses to sodium depletion in camels. Gen Comp Endocrinol. 1994;95:240‐247. [DOI] [PubMed] [Google Scholar]
- 36. Fournier D, Luft FC, Bader M, Ganten D, Andrade‐Navarro MA. Emergence and evolution of the renin‐angiotensin‐aldosterone system. J Mol Med. 2012;90:495‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beuchat CA. Structure and concentrating ability of the mammalian kidney: correlations with habitat. Am J Physiol. 1996;271:R157‐R179. [DOI] [PubMed] [Google Scholar]
- 38. Mbassa GK. Mammalian renal modifications in dry environments. Vet Res Commun. 1988;12:1‐18. [DOI] [PubMed] [Google Scholar]
- 39. Abraham HM, White CM, White WB. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015;38:33‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]


