Abstract
Background
Breed predispositions, survival, and prognostic factors have not been evaluated in dogs with nonregenerative immune‐mediated anemia (nrIMA).
Hypothesis/Objectives
To describe clinicopathologic variables, evaluate their associations with survival, and determine breed predispositions for dogs with nrIMA.
Animals
Fifty‐nine client‐owned dogs with nrIMA.
Methods
Referral hospital records were reviewed retrospectively for dogs with primary nrIMA (PCV ≤30%, corrected reticulocyte percentage (CR%) ≤1.0, bone marrow sampling with evidence of immune‐mediated destruction, and underlying causes excluded). Breed predispositions were evaluated by calculation of odds ratios in a case control study; prognostic factors by logistic regression in a cohort study.
Results
Fifty‐nine dogs with nrIMA had a median PCV of 12% (interquartile range [IQR]: 10%‐17%) and CR% 0.1 (0%‐0.2%). At least ≥1 ACVIM IMHA diagnostic criteria were met by 35 dogs (59%). Whippets, Lurchers, and miniature Dachshunds were predisposed to nrIMA. Median survival time was 277 days (IQR: 37‐1925), with 3‐ and 12‐month survival rates 61% and 43%, respectively. Erythroid regeneration and remission were achieved by 88% and 62% of dogs, respectively. Corrected reticulocyte percentage >0.2 was associated with improved survival.
Conclusion and Clinical Importance
Although there is overlap of clinical features between dogs with IMHA and nrIMA, the prognosis for those with nrIMA depends predominantly on the severity of reticulocytopenia.
Keywords: bone marrow, CHAOS, IMHA, PIMA, rubriphagocytosis
Abbreviations
- ACVIM
American College of Veterinary Internal Medicine
- BM
bone marrow
- CHAOS
canine hemolytic anemia objective score
- CR%
corrected reticulocyte percentage
- IMHA
immune‐mediated hemolytic anemia
- nrIMA
nonregenerative immune‐mediated anemia
- PIMA
precursor‐targeted immune‐mediated anemia
- ROC
receiver‐operating characteristic
1. INTRODUCTION
The diagnosis of immune‐mediated hemolytic anemia (IMHA) can be made confidently in dogs that fulfill the criteria outlined recently in the American College of Veterinary Internal Medicine (ACVIM) consensus statement. 1 Within 5 days of becoming anemic, dogs with IMHA typically develop erythroid regeneration 2 ; however, 30% to 55% remain nonregenerative with a corrected reticulocyte percentage (CR%) ≤1.0, which has been ascribed to destruction of erythroid progenitors by phagocytes in the bone marrow (BM) (rubriphagocytosis). 1 , 3 , 4 , 5 , 6 , 7 Similar rubriphagocytosis occurs also in dogs with nonregenerative anemia that lack evidence of peripheral immune‐mediated erythroid destruction or hemolysis, recently described as precursor‐targeted immune‐mediated anemia (PIMA). 3 , 8 However, in our experience, dogs meeting the criteria for PIMA frequently have features of peripheral erythrocyte destruction and hemolysis, and dogs with PIMA and IMHA both respond to immunosuppressive treatment. These findings suggested to us that PIMA and nonregenerative IMHA are not separate syndromes but represent different parts of a clinical spectrum attributable to immune‐mediated destruction of some combination of erythroid progenitors, immature stages, and mature red blood cells (RBCs) sufficient to cause nonregenerative anemia. To investigate this, we searched for cases in our medical database that fulfilled similar clinical and diagnostic criteria as described for PIMA. However, commensurate with our hypothesis, we refer to these dogs as having nonregenerative immune‐mediated anemia (nrIMA) because it remains unclear whether precursor destruction is the only or most important cause of anemia. 3 , 7 , 8
In dogs with IMHA, several clinicopathologic variables measured at diagnosis have been associated with mortality, singly or when combined into the composite canine hemolytic anemia objective score (CHAOS), 9 , 10 , 11 , 12 , 13 which encompasses serum albumin and bilirubin concentrations, age, body temperature, and presence of RBC agglutination. Conversely, in dogs with nrIMA/PIMA, 3 , 6 , 7 , 8 , 14 only development of a thromboembolic event 3 or presence of BM erythroid hyperplasia with concurrent neutropenia and thrombocytopenia have been associated with shorter survival times. 7 We considered that confirmation of previously identified prognostic factors, or identification of additional abnormalities that could predict survival in dogs with nrIMA would be useful clinically for 2 reasons. First, because they might be helpful for clinicians and owners making decisions about treatment of dogs with nrIMA, which frequently require long periods of immunosuppressive treatment and repeated blood transfusion. 3 Second, because overlap between the prognostic factors identified in dogs with IMHA and nrIMA might indicate whether these diseases belong to different parts of the same spectrum.
The aims of this study were (a) to describe the clinical presentation, clinicopathologic abnormalities, BM findings, treatment, and survival of dogs with primary nrIMA, and to compare these findings with previous descriptions of PIMA and nonregenerative IMHA; (b) to identify breeds significantly predisposed to development of nrIMA among dogs presenting to a referral hospital; and (c) to evaluate associations between clinicopathologic variables and survival. We hypothesized that dogs with nrIMA would have clinicopathological features overlapping with (nonregenerative) IMHA, and that nrIMA prognostic factors would be similar to those for dogs with IMHA.
2. MATERIALS AND METHODS
2.1. Study design
The study consisted of 2 parts: a retrospective case control study comparing the frequency of different breeds among dogs with nrIMA with frequencies among a population of control dogs and a retrospective cohort study to assess prognostic factors for death at 3 and 12 months after diagnosis in dogs with nrIMA.
2.2. Data collection
The electronic medical record system of a tertiary referral institution was searched from January 2007 to July 2019 to identify dogs diagnosed with primary nrIMA that had undergone BM sampling. Dogs were included if they fulfilled all of the following criteria:
Anemic on presentation with a PCV of ≤30% (or ≤35% in sighthound breeds).
Maintained a CR% of ≤1.0 or absolute reticulocyte concentration of ≤60 000/μL for ≥5 days after diagnosis of anemia.
-
BM cytology or histopathology of adequate diagnostic quality showed evidence of immune‐mediated erythroid precursor destruction, either by:
the presence of rubriphagocytosis, or
erythroid hypoplasia with unappreciable rubriphagocytosis, with or without marked fibrosis, which responded to immunosuppressive medication resulting in resolution of anemia.
Complete blood count, serum biochemical profile, and thoracic and abdominal imaging were performed, revealing no underlying cause for nrIMA.
Additional investigations were performed at the discretion of the attending clinician to exclude underlying causes of immune‐mediated diseases. Because infectious diseases causing nonregenerative anemia are not prevalent in the location of the study, tests for infectious agents were performed only if there was a history of travel to another area.
The following data were recorded for each dog: signalment; clinical signs and duration of signs; treatment before referral; results of tests including CR%, in‐saline agglutination, direct antiglobulin test (DAT) titer, CBC, serum biochemistry profile, urinalysis, and BM cytology and histopathology; and treatment administered. All BM reports were reviewed by 3 of the authors (Vanessa L. Woolhead, Barbara Glanemann, and Balazs Szladovits), including a board‐certified clinical pathologist (Balazs Szladovits). In BM samples, the stage of erythroid precursor subject to rubriphagocytosis (early, mid, or late stage) and the extent of fibrosis (mild, moderate, or severe) were graded subjectively as described previously. 3 Additionally, the number of polychromatophils observed in monolayer‐type areas of aspirate samples were subjectively graded as absent, low, moderate, or high. The CHAOS score 10 , 13 was calculated for each dog using their age (≥7 years, score 2), rectal temperature (≥102.0 F or 38.9°C, score 1), presence of agglutination (score 1), serum albumin (≤3.0 g/dL, score 1), and total bilirubin concentration (≥5.0 mg/dL, score 2).
Referring veterinarians were contacted by telephone to obtain follow‐up data for each dog, including repeat CBC results, duration of immunosuppressive treatment, date of any relapse, and date of euthanasia or death. Dogs were considered to have developed evidence of regeneration if the absolute reticulocyte concentration increased to ≥60 000/μL, CR% ≥1.0, or their PCV spontaneously increased by ≥5% compared to previous evaluation. Dogs were considered to be in remission if their PCV was maintained at ≥30% once all immunosuppressive medication was stopped, with no clinical deterioration reported on follow‐up assessments. A relapse was defined as a drop in PCV necessitating increase or reintroduction of immunosuppressive treatment. The study was approved by the Royal Veterinary College ethical review committee (URN SR2019‐0262 and 2015/T25).
2.3. Statistical analysis
All analyses were performed using statistical software (IBM SPSS Statistics for Windows, Version 26.0, IBM Corp, Armonk, New York; GraphPad Prism version 7.0 for Mac, GraphPad Software, La Jolla, California). Odds ratios were calculated to assess breeds predisposed to nrIMA, by comparing the proportion of dogs with nrIMA to the proportion of control dogs for the same breed (MedCalc 2020, https://www.medcalc.org/calc/odds_ratio.php, May 29, 2020). Bonferroni correction was applied to the P value to account for the effect of multiple comparisons. A Sankey diagram demonstrating outcome was produced using online software (Sankey Diagram Generator, Acquire Procurement Services, Brisbane, http://sankey-diagram-generator.acquireprocure.com/, October 4, 2020). Variables were assessed for normality using Shapiro‐Wilks tests, and presented as either mean (±SD) or median (interquartile range [IQR]) according to their distribution. Eight variables were selected as potential prognostic factors based on prior importance 4 , 5 , 7 , 8 , 9 , 10 , 11 , 15 , 16 and to decrease redundancy and correlation among variables. Any variable where data were highly skewed was dichotomized around the median. Logistic regression analysis was used to assess possible prognostic factors for death at 3 and 12 months after diagnosis. Variables were evaluated by univariable analysis and all those with a P < .1 were carried into the multivariable model. Model discrimination was determined by calculating the area under the receiver‐operating characteristic (ROC) curve. Model accuracy was determined using 2 × 2 classification tables. 10 Model calibration was assessed by Hosmer‐Lemeshow test (rejected if P < .05), and model utility was assessed using Nagelkerke's R2. Survival and time to demonstrate erythroid regeneration was assessed by Kaplan‐Meier product limit estimates, and the log rank test was used to assess differences between groups.
3. RESULTS
3.1. Population characteristics
Fifty‐nine dogs were eligible for inclusion in this study. The mean age was 7 years 7 months (SD 3 years 3 months), and the sample included 37 females (63%, 7 intact and 30 neutered) and 22 males (37%, 7 entire and 15 neutered). Dogs weighed a median of 19.2 kg (IQR: 9.7‐26.8), with a median body condition score of 4.5/9 (n = 46, IQR: 4‐5). There were 26 different breeds represented, with the most frequent being mixed breed (n = 10, 17%), Boxer (5, 8%), Jack Russell Terrier (4, 7%), Labrador (4, 7%), Lurcher (4, 7%), miniature Dachshund (3, 5%), Border Collie (3, 5%), English Springer Spaniel (3, 5%), and Whippet (3, 5%).
During the same time period over which the dogs with nrIMA were treated, 61 176 individual dogs were presented to the institution for other reasons. Comparison of the frequencies of breeds among nrIMA and control groups revealed that several were overrepresented among the dogs with nrIMA (Table 1), including Whippets (odds ratio [OR], 8.69; 95% confidence interval [CI], 2.71‐27.87), Lurchers (OR, 8.08; 95% CI, 2.92‐22.36), miniature Dachshunds (OR, 3.76; 95% CI, 1.71‐17.59), and Boxers (OR, 3.76; 95% CI, 1.50‐9.41). However, breeds with known predispositions to IMHA, including Cocker and Springer spaniels, 16 were not predisposed to nrIMA.
TABLE 1.
Breed predispositions for dogs with nonregenerative immune‐mediated anemia (nrIMA) compared to control dogs. Bold results indicate values with P value ≤.006, which indicates significance at 0.05 level after Bonferroni correction
| Breed | Number of dogs with nrIMA (%) | Number of control dogs (%) | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|---|---|
| Mixed breed | 10 (17) | 5571 (9.1) | 2.04 | 1.03‐4.02 | .04 |
| Boxer | 5 (8) | 1471 (2.4) | 3.76 | 1.50‐9.41 | .005 |
| Jack Russell Terrier | 4 (7) | 2103 (3.4) | 2.04 | 0.74‐5.64 | .17 |
| Labrador | 4 (7) | 5718 (9.3) | 0.71 | 0.26‐1.95 | .50 |
| Lurcher | 4 (7) | 546 (0.9) | 8.08 | 2.92‐22.36 | .0001 |
| Miniature Dachshund | 3 (5) | 591 (1.0) | 5.49 | 1.71‐17.59 | .004 |
| Border Collie | 3 (5) | 1335 (2.2) | 2.40 | 0.75‐7.68 | .14 |
| English Springer Spaniel | 3 (5) | 1203 (2.0) | 2.67 | 0.83‐8.54 | .09 |
| Whippet | 3 (5) | 375 (0.6) | 8.69 | 2.71‐27.87 | .0003 |
| N | 59 | 61 176 |
Dogs with nrIMA were referred after showing clinical signs for a median of 21 days (IQR: 9‐28). The most common presenting complaints were lethargy (n = 50, 85%), weakness or syncope (n = 31, 53%), inappetence (n = 30, 51%), weight loss (n = 17, 29%), polydipsia and polyuria (n = 13, 22%), and pica (n = 7, 12%). At the time of admission, 16 dogs (27%) had received prednisolone for a median of 7 days (IQR: 4‐10 days) at a median dosage of 2 mg/kg/day (n = 15, IQR: 1.2‐2.1); the dosage was unknown in 1 dog. Three dogs in this group (5%) were also receiving azathioprine, cyclosporine, or cyclophosphamide (n = 1 of each).
3.2. Clinicopathological data
Data from CBC and serum biochemical tests are summarized in Table 2. Dogs with nrIMA were presented with severe nonregenerative anemia: the median PCV was 12% (IQR: 10‐17) and CR% 0.1 (IQR: 0‐0.2). There was persistent agglutination of erythrocytes after dilution in saline in 29/54 dogs (54%) including 6 that were already receiving prednisolone. A DAT (n = 12) was positive at a titer of >1 : 16 in 1 dog (8%). Blood smear evaluation revealed spherocytosis in 11 dogs (19%) and ghost cells in 3 dogs (5%). Complete blood count revealed a concurrent cytopenia in 13 dogs. Pancytopenia was evident in 1 dog (2%) with mild neutropenia and thrombocytopenia. Thrombocytopenia was evident in an additional 2 dogs (3%), neutropenia in a further 7 (12%) with no evidence of toxicity or left shift (6 mild, 1 moderate according to published criteria 17 ), and 3 with lymphopenia (5%).
TABLE 2.
Selected results from CBC and serum biochemical tests from dogs with nrIMA at the time of hospital admission
| Variable (units) | Reference interval (RI) | Median (IQR) | Number of dogs with values below RI (%) | Number of dogs with values above RI (%) |
|---|---|---|---|---|
| PCV (%) | 37‐55 | 12 (10‐17) | 59 (100) | 0 (0) |
| CR% (%) | ≤1.0 | 0.7 (0‐0.22) | 59 (100) | 0 (0) |
| Platelets (×103/μL) | 150‐900 | 310 (200‐560) | 4 (7) | 4 (7) |
| Neutrophils (×103/μL) | 3.0‐11.5 | 6.9 (4.4‐12.4) | 7 (12) | 16 (27) |
| Lymphocytes (×103/μL) | 1.0‐4.8 | 1.4 (0.9‐1.9) | 3 (5) | 3 (5) |
| Albumin (g/dL; mean, ±SD) | 2.6‐3.8 | 3.1 (±0.4) | 10 (17) | 1 (20) |
| Bilirubin (mg/dL) | ≤0.26 | 0.2 (0.1‐3.0) | 0 (0) | 14 (24) |
Abbreviations: CR%, corrected reticulocyte percentage; nrIMA, nonregenerative immune‐mediated anemia; RI, reference interval.
Serum biochemistry results revealed 10 dogs (17%) had mild hypoalbuminemia (mean for all dogs 3.1 g/dL, ±0.4 g/dL), whereas hyperbilirubinemia (median for all dogs, 0.2 mg/dL, IQR: 0.1‐3.0 mg/dL) was found in 14 dogs (24%) and was particularly severe (>5.0 mg/dL) in only 1 dog (2%).
In accordance with the ACVIM consensus statement, 1 clinicopathological results were “diagnostic” for IMHA in 9 dogs (15%), “supportive” in 21 dogs (36%), and “suggestive” for IMHA in 5 dogs (8%). Twenty‐four dogs (41%) had no evidence to support a diagnosis of IMHA at any level. The median CHAOS was 3 (n = 54, IQR: 2‐4).
3.3. Bone marrow evaluation
Paired BM aspirates and core biopsies were available for 55 dogs (93%); 2 dogs had only an aspirate and 2 only a core biopsy available. The quality of either the aspirate or core biopsy sample was inadequate in 11 dogs (19%); however, the alternative sample was of appropriate quality in all such dogs.
Rubriphagocytosis was detected in BM samples in 53 dogs (90%) affecting the late‐stage precursors in 35 (66%), mid in 3 (6%), and early in 15 dogs (28%). Thirty‐three dogs (56%) had erythroid hyperplasia, 21 (36%) hypoplasia, 4 (7%) normoplasia, and 1 dog (2%) had myelofibrosis that was too severe to assess erythroid status. Bone marrow polychromatophils were present in 69% of samples (in low and moderate numbers for 24 and 17 dogs, respectively) despite the dogs having persistent nonregenerative anemia, suggesting ongoing destruction of the erythroid lineage at this stage. Evaluation of core biopsies of adequate quality (n = 52) revealed fibrosis affecting 19 dogs (37%), which was graded as mild, moderate or severe in 11, 4 and 4 dogs, respectively. Myelofibrosis was present in 12/33 dogs with erythroid hyperplasia (36%), 5/21 hypoplasia (24%), and 1/4 normoplasia (25%); the myelofibrosis was too severe in 1 dog to determine erythroid cellularity.
3.4. Treatment
Forty‐nine dogs (83%) required a median of 1 unit of packed RBCs (IQR: 1.0‐1.5) during their initial hospitalization. Twenty‐eight dogs (47%) required at least 1 further unit a median of 19 days after discharge (IQR: 10‐43).
Fifty‐six dogs (95%) survived their initial hospitalization period and were discharged a median of 5 days (IQR: 4‐6) after admission. All dogs were discharged with prednisolone (median 3 mg/kg/day, n = 54, IQR: 2.4‐4.0), except 1 dog with concurrent diabetes mellitus that received cyclosporine (8 mg/kg/d) instead; the prednisolone dosage was unknown in 1 dog. Prednisolone was the sole immunosuppressive agent used in 11 dogs (20%), but 5 were later prescribed a second drug. At the time of discharge, 32 dogs (57%) were also receiving azathioprine (median 1 mg/kg/d, IQR: 0.9‐1.2) and 13 (23%) cyclosporine (median 10 mg/kg/d, IQR: 8‐10). All 3 of these immunosuppressive medications were prescribed at the time of discharge in 2 dogs (4%).
Antiplatelet medications were prescribed to 32 dogs (57%), including aspirin (n = 20, 36%), clopidogrel (n = 11, 20%) or both drugs (n = 1, 2%). Low molecular weight heparin was also prescribed in addition to aspirin and clopidogrel in 2 dogs. These 2 dogs experienced either a pulmonary or neurological thromboembolic event before introduction of treatment; no additional dogs had thromboembolic events reported. Doxycycline was prescribed for 3 dogs (5%) pending infectious disease test results, but no dogs were ultimately found to have an infectious agent. Additional medications commonly prescribed included omeprazole (n = 20, 36%), amoxicillin‐clavulanate (n = 8, 14%), sucralfate (n = 4, 7%), cyanocobalamin (n = 4, 7%), ferrous sulfate (n = 1, 2%), and lithium carbonate (n = 1, 2%).
3.5. Outcome
The median survival time for all 59 dogs was 277 days (IQR: 37‐1925) (Figure 1). Three dogs (5%) did not survive to discharge and were euthanized owing to development of pancytopenia (n = 1), pancreatitis (1), or aspiration pneumonia occurring after neurologic deterioration that was thought to be attributable to thromboembolism (1).
FIGURE 1.
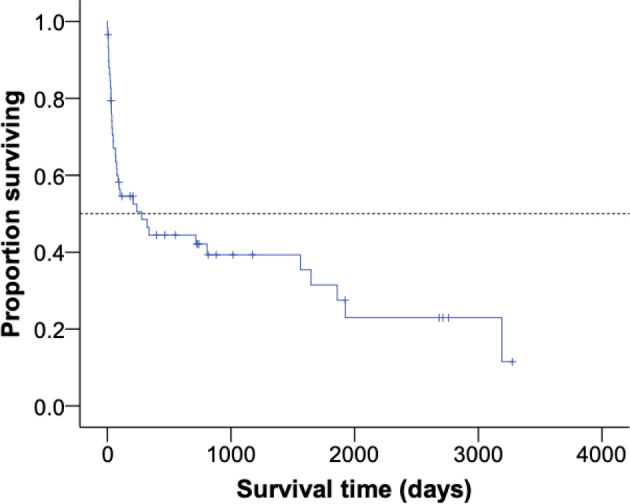
Kaplan‐Meier curve showing survival times in dogs with nonregenerative immune‐mediated anemia (nrIMA). Tick marks indicate censored cases. Dotted line indicates median value
The median follow‐up time for the 56 dogs discharged from the hospital was 1016 days (IQR: 551‐2714), with 2 dogs (4%) lost to follow‐up immediately after discharge, and 11 (20%) still alive on November 1, 2019. Of the dogs with complete follow‐up available for at least 3 and 12 months, 33/54 (61%; 95% CI, 48‐73) and 22/51 (43%; 95% CI, 31‐57) dogs survived, respectively (Figure 2).
FIGURE 2.

Bar chart demonstrating the percentage (with 95% confidence intervals) of dogs surviving at 3 (A) and 12 months (B) after diagnosis
Of the 33 dogs alive 3 months after discharge, 29 (88%) had evidence of erythroid regeneration a median of 31 days after diagnosis (IQR: 16‐38), with time to regeneration for all cases shown in Figure 3. Immunosuppressive medications were stopped in 18/29 dogs (62%) a median of 7 months after diagnosis (n = 17, IQR: 5.5‐11.0); the duration of immunosuppression was unknown in 1 dog. Nine of the 29 dogs (31%) experienced a relapse of their nrIMA, necessitating re‐introduction of immunosuppressive treatment (n = 4) or an increased dosage (n = 5) a median of 19.5 months after diagnosis (IQR: 14‐23). Fourteen of 29 dogs (48%) remained in remission. Of the 51 dogs followed for up to 12 months after diagnosis, 21 (41%) had no evidence of erythroid regeneration on serial CBCs and were euthanized a median of 34 days after diagnosis (IQR: 22‐65). The different outcomes for the 59 dogs with nrIMA are shown in Figure 4.
FIGURE 3.
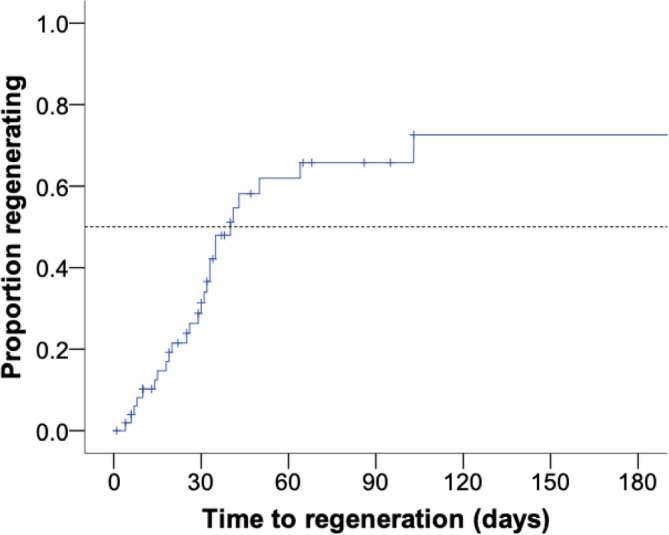
Kaplan‐Meier curve showing the time taken to demonstrate erythroid regeneration in dogs with nonregenerative immune‐mediated anemia (nrIMA) over the first 6 months after diagnosis. Tick marks indicate cases that were euthanized without displaying a regenerative response. Dotted line indicates median values
FIGURE 4.

Sankey diagrams illustrating the outcome of 59 dogs diagnosed with nonregenerative immune‐mediated anemia (nrIMA), in terms of survival (A) and treatment and relapse (B). Outcomes are written horizontally in each node with the height representing the number of dogs affected. Numbers of dogs for each outcome are written vertically in each node
3.6. Prognostic factors
Eight variables were evaluated as possible prognostic factors for survival in dogs with nrIMA based on previous data suggesting they were important in IMHA and/or nrIMA, 4 , 5 , 7 , 8 , 9 , 10 , 11 , 15 , 16 including PCV, CR%, serum albumin, bilirubin, and BUN concentrations, CHAOS, severity of BM fibrosis, degree of BM polychromasia, and stage of erythroid precursor affected by rubriphagocytosis. In the univariable analysis, only a CR% >0.2 was associated with decreased mortality at 3 months (OR, 0.16; 95% CI, 0.05‐0.57; P = .005) and 12 months (OR, 0.27; 95% CI, 0.09‐0.82; P = .02). Kaplan‐Meier survival curves for dogs with a CR% ≤0.2 and >0.2 are shown in Figure 5, with a significant difference in survival time between the 2 groups (log rank test, P = .04). To compare the performance of a validated prognostic score for IMHA (CHAOS) or of variables previously associated with survival in dogs with IMHA (total bilirubin and BUN) with CR% in this population of dogs, both were entered into separate multivariable logistic regression models, with survival at 3 months as the outcome. This showed that CR% was a significant predictor of survival to 3 months, but this was not true for either the CHAOS score (Figure 6A; Supplementary Table 1) or the combination of bilirubin and BUN (Figure 6B; Supplementary Table 1) when considered alongside CR%. Both models had good predictive capability, with an area under the ROC curves of 0.772 (CHAOS with CR%) and 0.736 (bilirubin and BUN with CR%) for survival to 3 months (Supplementary Figure 1A,B).
FIGURE 5.
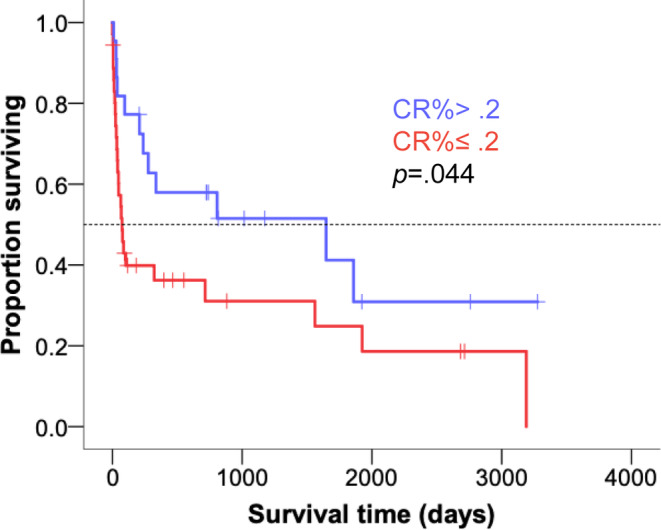
Kaplan‐Meier curve to compare survival times in dogs with nonregenerative immune‐mediated anemia (nrIMA) with a corrected reticulocyte percentage (CR%) ≤0.2 (red line) and >0.2 (blue line). Tick marks indicate censored cases. Dotted line indicates median values. Groups compared by log rank test
FIGURE 6.

Forest plots showing results of multivariable logistic regression models for death at 3 months after diagnosis, including corrected reticulocyte percentage (CR%) with either canine hemolytic anemia objective score (CHAOS) score (A) or bilirubin and BUN concentrations at presentation (B). Graphs show odds ratio with 95% confidence intervals and P values for multivariable models. Model assessment parameters are shown below
4. DISCUSSION
In this retrospective study, we describe a large cohort of dogs diagnosed with primary nrIMA and evaluate the prognostic potential of their clinicopathologic and BM findings. Almost 60% of dogs with nrIMA had features that were at least “suggestive” of IMHA according to the recent ACVIM consensus statement. Erythroid hyperplasia and rubriphagocytosis targeting late‐stage erythroid precursors were most commonly identified on BM evaluation. Immunosuppressive treatment resulted in almost two thirds of dogs surviving at least 3 months after diagnosis, with most survivors developing a regenerative response within 1 month of diagnosis. Using logistic regression, we identified that a CR% >0.2 at presentation was associated with longer survival times, but the CHAOS score and other variables predictive of outcome in IMHA were not associated with outcome for dogs with nrIMA.
Hyperbilirubinemia and signs of immune‐mediated peripheral erythrocyte destruction were observed commonly in dogs with nrIMA: 59% fulfilled at least part of the published ACVIM diagnostic criteria for IMHA 1 and almost a quarter had an increased serum bilirubin concentration. This contrasts with previous studies that have identified these features rarely, 3 , 6 , 8 , 14 leading to the use of the term “PIMA” in preference to “nonregenerative IMHA.” 8 , 14 The greater number of dogs fulfilling IMHA diagnostic criteria in our study could represent a genuine difference in case presentation at different institutions or could be a result of variations in clinical approach to BM sampling in dogs with features of IMHA but persistent nonregenerative anemia. Specifically, if BM sampling were not performed in dogs with nonregenerative anemia and signs of peripheral erythrocyte destruction in previous studies, it could give the false impression that IMHA and “PIMA” were distinct disease processes. Because study enrollment required a CR% <1.0 for >5 days, it is likely that few, if any, dogs in our study were presented during the “preregenerative” phase of IMHA. 1 , 4 , 5 These findings support the hypothesis that nrIMA and IMHA overlap on a spectrum of disorders caused by immune‐mediated destruction of the erythroid lineage. Consequently, we prefer the term “nrIMA” over “PIMA” to describe the clinical presentation outlined in this and other studies because our findings suggest destruction of precursors is unlikely to be the only cause of anemia in a substantial proportion of cases. Confirmation of this hypothesis will require testing for antibodies directed against different stages in the erythroid lineage to see which are being targeted and identification of the surface antigens of erythroid cells that are being targeted in the immune response. This approach has been undertaken in dogs with IMHA, indicating that immunodominant antigens vary among individual cases. 18 , 19 Accordingly, we reiterate our earlier thesis 20 that the regeneration status of patients with IMHA/nrIMA will be determined by the pattern of expression of the targeted antigen on different maturation stages in the erythroid lineage, with some immune responses directed at antigens only expressed in the BM and others directed at antigens shared by mature and immature stages.
Although peripheral destruction of RBCs was observed frequently in dogs with nrIMA, other features suggest this syndrome has a distinct natural history compared to IMHA, which might be attributable to differences in the underlying immune response or differences in its management by clinicians. First, the breeds that were predisposed to nrIMA in our hospital differed from those reported to be at greatest risk of developing IMHA, 5 , 16 suggesting underlying genetic risk factors might differ. Second, the major predictor of outcome was the extent of the regenerative response and not the serum bilirubin concentration, which is normally taken as an indicator of the rate of ongoing hemolysis and has been identified consistently as a prognostic factor for dogs with IMHA in several independent samples of dogs. 10 , 16 , 21 , 22 However, this finding should be interpreted with caution because we suspect many owners and clinicians would use the regeneration status as an important piece of information to guide their decision to pursue further treatment or opt for euthanasia, potentially creating a self‐fulfilling prophecy that would confound the association between CR% and survival. Indeed, many dogs were euthanized within 2 weeks of diagnosis in our study, suggesting willingness to pursue treatment could have been an important factor. Unlike other studies, we did not detect associations between survival and BM findings. 7 , 8 In particular, the presence of myelofibrosis, observed in 37% of dogs, did not affect survival, supporting previous recommendations to monitor the response of affected dogs to immunosuppressive treatment 23 rather than assume a poor outcome. The absence of a standardized approach to classifying BM pathology affects clinical interpretation and limits the ability to make meaningful comparisons between different populations of dogs. Although BM biopsies permit exclusion of alternative diagnoses, the lack of prognostic information derived from them in our study should encourage clinicians to question the rationale for performing the procedure on a case‐by‐case basis if dogs already have evidence of peripheral RBC destruction.
Rubriphagocytosis was observed in >90% of dogs with nrIMA, predominantly targeting late‐stage precursors (66%) and commonly occurring in conjunction with BM erythroid hyperplasia (56%). Interestingly, BM polychromasia was also detected in two thirds of dogs with nrIMA and was evident in a third of dogs where rubriphagocytosis could not be identified. Because identification of rubriphagocytosis is challenging in some dogs, particularly when trying to classify erythroid precursor stages being phagocytosed by macrophages, 3 the presence of BM polychromasia in the face of peripheral nonregenerative anemia might assist in making a diagnosis of nrIMA, though it does not directly demonstrate immune‐mediated destruction of erythroid cells.
Sixty percent of dogs survived at least 3 months, with most of those cases demonstrating erythroid regeneration within 1 month. Previous studies report that 50% to 85% of dogs respond to immunosuppressive treatment within 1 month; however, some dogs only developed erythroid regeneration after being treated for 15 weeks. 3 , 6 , 8 , 14 Because 41% of dogs in our study were euthanized after a median of 34 days of treatment, it is possible some might have gone on to develop a regenerative response later. Among those dogs surviving 3 months, erythroid regeneration was documented in 88%, which closely parallels previously published data, and almost two‐thirds of these dogs ultimately achieved remission after ~7.5 months of immunosuppressive treatment. On the basis of this information, we suggest that owners and veterinarians should be prepared for an initial period of immunosuppressive treatment of 2 to 3 months, even if no regenerative response is apparent within the first few weeks. However, prospective studies would be required to determine whether this recommendation would actually improve outcome compared to the results we report.
This study had several limitations, including retrospective collection of data over a long period of time, during which approaches to treatment and supportive care could have changed. The retrospective study design also means that some associations detected in our study are likely to have been confounded by the impact of client and clinician decisions. Dogs were required to have BM evaluation to be enrolled, meaning those that could not be anesthetized safely or that were owned by clients who declined the procedure would have been excluded. Using similar inclusion criteria to other studies, 4 dogs without evidence of BM rubriphagocytosis or polychromasia were still included in this study because they had a favorable response to immunosuppressive treatment. However, we believe nrIMA remained the most plausible diagnosis for these dogs. Although dogs were euthanized owing to their presumed poor response to immunosuppressive treatment, postmortem examinations were not performed; therefore, secondary nrIMA could not be definitively excluded in cases that did not respond to treatment, even though all dogs had a consistent minimum database to exclude the most likely underlying causes. Sixteen dogs received immunosuppressive treatment before hospital admission, which might have affected the time reported to demonstrate erythroid regeneration, remission, and overall survival. Although this outcome data were generated based on the date of diagnosis at our institution, the overall impact of this limitation is probably small because these dogs had only received a maximum of 7 days of treatment before referral. In addition, the effect of treatment regimen and tapering protocol was not taken into account in investigation of prognostic factors owing to the variation in these regimens among individual dogs. Finally, complete follow‐up data were not always available, meaning that some cases had to be censored in our survival analysis. Nevertheless, this study includes the largest number of dogs with nrIMA that has yet been analyzed.
5. CONCLUSIONS
Dogs with nrIMA most commonly exhibit BM erythroid hyperplasia with late‐stage precursor rubriphagocytosis, and the identification of BM polychromasia might facilitate diagnosis. A favorable response to immunosuppressive treatment occurred in the majority of dogs, with ~60% surviving to 3 months after diagnosis. More than half of dogs diagnosed with nrIMA demonstrated some evidence of peripheral RBC destruction, supporting the theory that nrIMA and IMHA form part of a single clinical spectrum. Despite this, established IMHA prognostic factors could not be extrapolated to dogs with nrIMA. Whilst findings from BM samples did not provide prognostic information, a CR% >0.2 on presentation was predictive of survival to 3 months.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Royal Veterinary College Ethics Committee (URN SR2019‐0262 and 2015/T25).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplementary Figure 1 Receiver operator characteristic (ROC) curves discriminating dogs with nrIMA that survived or died at 3 months using the multivariable logistic regression models shown in Figure 6, for corrected reticulocyte percentage (CR%) with CHAOS score (A) or CR% with bilirubin and BUN (B). Figure show area under the curve (AUC) with 95% confidence intervals (CIs).
Supplementary Table 1 Model parameters for logistic regression models predicting survival to 3 months after diagnosis.
ACKNOWLEDGMENTS
No funding was received for this study. The authors acknowledge all veterinary surgeons for the provision of follow‐up data.
Woolhead VL, Szladovits B, Chan A, Swann JW, Glanemann B. Breed predispositions, clinical findings, and prognostic factors for death in dogs with nonregenerative immune‐mediated anemia. J Vet Intern Med. 2021;35:252–260. 10.1111/jvim.15986
James W. Swann and Barbara Glanemann contributed equally to this study.
REFERENCES
- 1. Garden OA, Kidd L, Mexas AM, et al. ACVIM consensus statement on the diagnosis of immune‐mediated hemolytic anemia in dogs and cats. J Vet Intern Med. 2019;33:313‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cowgill ES, Neel JA, Grindem CB. Clinical application of reticulocyte counts in dogs and cats. Vet Clin North Am Small Anim Pract. 2003;33:1223‐1244. v. [DOI] [PubMed] [Google Scholar]
- 3. Assenmacher TD, Jutkowitz LA, Koenigshof AM, de Lucidi CA, Scott MA. Clinical features of precursor‐targeted immune‐mediated anemia in dogs: 66 cases (2004‐2013). J Am Vet Med Assoc. 2019;255:366‐376. [DOI] [PubMed] [Google Scholar]
- 4. Klag AR, Giger U, Shofer FS. Idiopathic immune‐mediated hemolytic anemia in dogs: 42 cases (1986‐1990). J Am Vet Med Assoc. 1993;202:783‐788. [PubMed] [Google Scholar]
- 5. Piek CJ. Canine idiopathic immune‐mediated haemolytic anaemia: a review with recommendations for future research. Vet Q. 2011;31:129‐141. [DOI] [PubMed] [Google Scholar]
- 6. Stokol T, Blue JT, French TW. Idiopathic pure red cell aplasia and nonregenerative immune‐mediated anemia in dogs: 43 cases (1988‐1999). J Am Vet Med Assoc. 2000;216:1429‐1436. [DOI] [PubMed] [Google Scholar]
- 7. Weiss DJ. Bone marrow pathology in dogs and cats with non‐regenerative immune‐mediated haemolytic anaemia and pure red cell aplasia. J Comp Pathol. 2008;138:46‐53. [DOI] [PubMed] [Google Scholar]
- 8. Lucidi CA, de Rezende CLE, Jutkowitz LA, et al. Histologic and cytologic bone marrow findings in dogs with suspected precursor‐targeted immune‐mediated anemia and associated phagocytosis of erythroid precursors. Vet Clin Pathol. 2017;46:401‐415. [DOI] [PubMed] [Google Scholar]
- 9. Carr AP, Panciera DL, Kidd L. Prognostic factors for mortality and thromboembolism in canine immune‐mediated hemolytic anemia: a retrospective study of 72 dogs. J Vet Intern Med. 2002;16:504‐509. [DOI] [PubMed] [Google Scholar]
- 10. Goggs R, Dennis SG, Di Bella A, et al. Predicting outcome in dogs with primary immune‐mediated hemolytic anemia: results of a multicenter case registry. J Vet Intern Med. 2015;29:1603‐1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swann JW, Skelly BJ. Systematic review of prognostic factors for mortality in dogs with immune‐mediated hemolytic anemia. J Vet Intern Med. 2015;29:7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weinkle TK, Center SA, Randolph JF, Warner KL, Barr SC, Erb HN. Evaluation of prognostic factors, survival rates, and treatment protocols for immune‐mediated hemolytic anemia in dogs: 151 cases (1993‐2002). J Am Vet Med Assoc. 2005;226:1869‐1880. [DOI] [PubMed] [Google Scholar]
- 13. Whelan MFRE, O'Toole TE, et al. Use of the canine hemolytic anemia objective score (CHAOS) to predict survival in dogs with immune mediated hemolytic anemia. J Vet Intern Med. 2006;20:714‐715. [Google Scholar]
- 14. Weiss DJ. Primary pure red cell aplasia in dogs: 13 cases (1996‐2000). J Am Vet Med Assoc. 2002;221:93‐95. [DOI] [PubMed] [Google Scholar]
- 15. Ishihara M, Fujino Y, Setoguchi A, et al. Evaluation of prognostic factors and establishment of a prognostic scoring system for canine primary immune‐mediated hemolytic anemia. J Vet Med Sci. 2010;72:465‐470. [DOI] [PubMed] [Google Scholar]
- 16. Swann JW, Skelly BJ. Evaluation of immunosuppressive regimens for immune‐mediated haemolytic anaemia: a retrospective study of 42 dogs. J Small Anim Pract. 2011;52:353‐358. [DOI] [PubMed] [Google Scholar]
- 17. DeClue AE, Spann DR. Leukopenia, leukocytosis In: Ettinger SJ, Feldman EC, Cote E, eds. Textbook of Veterinary Internal Medicine. 8th ed. St Louis, MO: Elsevier; 2017:235‐237. [Google Scholar]
- 18. Barker RN, Gruffydd‐Jones TJ, Stokes CR, Elson CJ. Identification of autoantigens in canine autoimmune haemolytic anaemia. Clin Exp Immunol. 1991;85:33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corato A, Shen CR, Mazza G, Barker RN, Day MJ. Proliferative responses of peripheral blood mononuclear cells from normal dogs and dogs with autoimmune haemolytic anaemia to red blood cell antigens. Vet Immunol Immunopathol. 1997;59:191‐204. [DOI] [PubMed] [Google Scholar]
- 20. Swann JW, Skelly BJ. Canine autoimmune hemolytic anemia: management challenges. Vet Med (Auckl). 2016;7:101‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piek CJ, van Spil WE, Junius G, Dekker A. Lack of evidence of a beneficial effect of azathioprine in dogs treated with prednisolone for idiopathic immune‐mediated hemolytic anemia: a retrospective cohort study. BMC Vet Res. 2011;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reimer ME, Troy GC, Warnick LD. Immune‐mediated hemolytic anemia: 70 cases (1988‐1996). J Am Anim Hosp Assoc. 1999;35:384‐391. [DOI] [PubMed] [Google Scholar]
- 23. Weiss DJ, Smith SA. A retrospective study of 19 cases of canine myelofibrosis. J Vet Intern Med. 2002;16:174‐178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Receiver operator characteristic (ROC) curves discriminating dogs with nrIMA that survived or died at 3 months using the multivariable logistic regression models shown in Figure 6, for corrected reticulocyte percentage (CR%) with CHAOS score (A) or CR% with bilirubin and BUN (B). Figure show area under the curve (AUC) with 95% confidence intervals (CIs).
Supplementary Table 1 Model parameters for logistic regression models predicting survival to 3 months after diagnosis.


