Abstract
Cysteine oxidation occurs at the active site of deubiquitinases (DUBs) during many biologic signaling cascades. Here we report that hepatocellular carcinoma cells (HCCs) generated higher levels of endogenous reactive oxygen species (ROS). This elevated ROS production was inhibited by NADPH oxidase inhibitor diphenylene iodonium (DPI) and mitochondria electron chain inhibitor rotenone in HCC cells. Moreover, we found that H2O2 could activate NF-κB-dependent inflammatory effect through increased induction of matrix metalloproteinase 2 (MMP2), MMP9, and intercellular adhesion molecule 1 (ICAM1) expression levels. In addition, we found that H2O2 could prolong NF-κB activation by suppressing the negative regulatory functions of Cezanne in HCC cells. Ubiquitin-derived thiol-reactive probe (HA-UbVME) assay and biotin-tagged 1,3-cyclohexadione derivative (DCP-Bio1) assay showed that H2O2 has the capacity to inhibit the catalytic activity of Cezanne, and the reducing agent, DTT, could reactivate the Cezanne deubiquitinating enzyme activity. Taken all together, these findings demonstrated an important role for oxidation of Cezanne by ROS in regulation of the inflammatory effect of hepatocellular carcinoma.
Key words: Cezanne, Cysteine oxidation, Hepatocellular carcinoma (HCC)
INTRODUCTION
Reactive oxygen species (ROS) are naturally produced by cells through aerobic metabolism, and high levels of cellular ROS are associated with many diseases, including cancer1,2. High levels of ROS were spontaneously produced by ovarian and prostate cancer cells, and ROS regulated hypoxia inducible factor 1 (HIF-1) and vascular endothelial growth factor (VEGF) expression in ovarian cancer cells3. Some growth factors such as epidermal growth factor (EGF), insulin, and angiopoietin-1 could increase ROS production in the cells for regulating cell migration and proliferation4–7, and ROS could induce the activation of mitogen-activated protein kinase (MAPK), nuclear factor κB (NF-κB), and activator protein 1 (AP1), which are known to be associated with cancer development8–10. In addition, high levels of ROS in some cancer cells are considered to induce DNA damage leading to genomic instability and tumor initiation11,12. However, the direct roles of ROS in tumor development remain elusive.
Reversible oxidation of amino acid residues can directly regulate the activity of cytosol enzymes in cells. Some cysteine residues could be reversibly oxidized to alter the protein activity in response to prevailing conditions, in a manner akin to protein regulation by phosphorylation. The most notable examples of enzymes affected by such oxidation have been the tyrosine phosphatases, several of which were directly inhibited by ROS13. Recently, Kulathu et al.14 and Lee et al.15 showed that cysteine oxidation occurs at the active site of deubiquitinases (DUBs). The reversible oxidation of cysteine residues in these enzymes was mediated by ROS chemically reactive molecules that were formed during normal cellular metabolism and that were involved in many signaling pathways. ROS-mediated oxidation requires deprotonation (loss of a hydrogen ion) of the cysteine residue, and so those residues with a lower acid dissociation constant will be especially susceptible. This condition was usually accompanied by cysteines in their active site of hydrolytic enzymes.
Cezanne, a member of the A20 family of deubiquitinating enzymes16, has emerged as a negative regulator that balances the strength and duration of NF-κB signaling through feedback mechanisms16,17. However, how these serial feedback loops are simultaneously disrupted in liver cancer remains unclear. Although numerous studies have identified a role for ROS in regulating signaling to NF-κB pathway18,19, their potential effects on negative regulators of NF-κB have received little attention. Nevertheless, emerging reports suggest that ROS may enhance cellular activation by suppressing the activity of anti-inflammatory enzymes. Here we demonstrate that H2O2 can suppress the negative regulatory functions of Cezanne by oxidation of its catalytic cysteine residual, thus prolonging NF-κB pathway activation and proinflammatory transcriptional responses in hepatocellular carcinoma (HCC).
MATERIALS AND METHODS
The study was performed in accordance with relevant guidelines and regulations, following the approval of the licensing committee of Tongji Medical College, Huazhong University of Science and Technology. This work received approval from the institution ethics committee and conformed to the tenets of the Declaration of Helsinki.
Cell Culture and Transfection
Human normal hepatocyte THLE-2 cells, human hepatocarcinoma HepG2, and SK-HEP-1 cell lines were provided by the Cell Bank, Shanghai Institute of Biochemistry and Cell Biology, Shanghai Institutes of Biological Sciences, Chinese Academy of Science. HepG2 and SK-HEP-1 cells were cultured in the DMEM media supplemented with 2 mM l-glutamine and 10% fetal calf serum (FBS; Life Technologies, Carlsbad, CA, USA) at 37°C in a humidified atmosphere with 5% CO2. pcDNA 3.1 Cezanne WT and C209S plasmids were introduced into cells with the Lipofectamine 2000 (Life Technologies). Cell extracts were also collected and subjected to immunoblot with antibody against Cezanne (Abcam, San Francisco, CA, USA).
Measurement of Intracellular ROS Production
Cells were treated and harvested between 50% and 80% visible confluency. Cells were treated with hydrogen peroxide (H2O2; Sigma-Aldrich, St. Louis, MO, USA) with the concentrations and for the amount of time indicated. To measure ROS generation in the cells, cells were preincubated with Hoechst 33342 for 30 min (final concentration 5 mg/ml); cells were then washed, trypsinzed, and collected. Cells were then incubated with 5-(and-6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCF-DA; final concentration 10 mM; Life Technologies), and Hoechst 33342 (final concentration 5 mg/ml) for 30 min at 37°C in the dark. Cells were immediately placed on ice and analyzed by fluorescence-activated cell sorting (FACS). Flow cytometry analysis was performed using LSRII and analyzed using FACS DIVA software and FlowJ 8.7. Diphenylene iodonium (DPI) and rotenone were from Sigma-Aldrich.
Isolation of Tumorous and Nontumorous Primary Cells
Human primary hepatocellular carcinoma cells were obtained from HCC patients undergoing surgical treatment, with informed consent. Hepatocellular carcinoma cells were selectively retrieved from fresh tumor samples with PALM microlaser technology (PALM) under microscopic control as previously described20.
Expression Analysis
Total RNAs were extracted from HepG2 cells using the QIAamp RNA isolation kit (Qiagen, Amtsgericht, Dusseldorf, Germany). Reverse transcription was performed using the SuperScript III First-Strand Synthesis kit (Life Technologies). Quantitative real-time RT-PCR was performed with specific primers for MMP2 and MMP9 transcripts applying SYBR Green Supermix (Applied Biosystems, Foster City, CA, USA) using the thermocycler. Melting curve analysis was done at the end of the reaction to assess the quality of the final PCR products. The threshold cycle C(t) values were calculated by fixing the basal fluorescence at 0.05 U. Three replicates were used for each sample, and the average C(t) value was calculated. The ΔC(t) values were calculated as C(t) sample − C(t) tubulin. The N-fold increase or decrease in expression was calculated by the ΔΔCt method using the C(t) value as the reference point.
Assay of NF-κB Transcriptional Activity
NF-κB transcriptional activity was measured using an NF-κB reporter (pGL3) as described previously21. Cells were cotransfected with pNF-κB-Luc and pRL-TK (encoding Renilla luciferase to normalize transfection efficiency) using Lipofectamine 2000 and incubated for 16 h. Cells were then treated with TNF-α (10 ng/ml) for 16 h before measurement of NF-κB activity. Firefly and Renilla luciferase activity was assessed using the Dual-Luciferase reporter assay kit (Promega, Madison, WI, USA) and luminescence counter (Topcount microplate scintillation; Packard, Palo Alto, CA, USA).
Western blot, Coimmunoprecipitation, Protein Binding, and Antibodies
Western blots were performed with whole-cell extracts prepared in SDS sampling buffer [0.1 M Tris, pH 6.8, 2% (w/v) SDS, and 12% (v/v) β-mercaptoethanol]. For immunoprecipitation (IP) and biotin–streptavidin pull-down studies, cells were lysed in low IP buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.2% NP40) prior to IP or incubated with streptavidin agarose (Millipore, Billerica, MA, USA). Protein extracts were separated on Nupage 3%–8% Tris-acetate or 4%–12% Bis–Tris gels (Life Technologies). The supernatants were subjected to SDS-PAGE. The proteins were then transferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA), blotted with primary antibodies and horseradish peroxidase-conjugated secondary antibodies followed by chemiluminescent detection. The antibodies (Abcam) used for Western blot analysis were the following: polyclonal anti-Cezanne (1:1,000), anti-ICAM1 (1:1,500), anti-Flag (1:1,500), anti-HA (1:1,000), anti-β-actin (1:5,000), and anti-ubiquitin (1:2,000).
Cezanne DUB Activity Assay for In Vivo Measurements
The catalytic activity of Cezanne was assessed by measuring its capacity to bind to a ubiquitin-derived probe (HA-UbVME; Boston Biochem, Cambridge, MA, USA), which contained a thiol-reactive vinylmethyl ester group at the C terminus. UbVME DUB activity assay was performed according to Borodovsky et al.22 with several modifications. The probe is tagged with HA-epitope to facilitate detection and is ∼10 kDa in size. Cytosolic lysates were made from cells expressing Flag-tagged Cezanne using 50 mM Tris (pH 7.6), 0.2% Nonidet P-40, 150 mM NaCl, 0.5 mM EDTA, and 0.5 mM 4-(2-aminoethyl) benzenesulfonyl fluoride. Supernatant from cell lysates was collected after 10-min spin at 10,000 rpm at 4°C. Cell extracts and 1–1.5 mg of UbVME probe were incubated at 25°C for 60 min in DUB reaction buffer (50 mM Tris, 50 mM NaCl, 10% glycerol, and 1 mM EDTA). Reactions were terminated with 250 mM Tris (pH 6.8), 2% SDS, 20% glycerol, and 200 mM 2-mercaptoethanol, boiled for 10 min. Probe–Cezanne conjugates were detected by Western blotting.
Deubiquitinase Assays
Cezanne were diluted to 2× final concentration in 150 mM NaCl, 25 mM Tris (pH 7.5), and 10 mM DTT and used directly, or activated at 23°C for 10 min. Subsequently, 10 ml of diluted enzyme were mixed with 1–2 mg of di-Ub and 2 ml of 10× DUB buffer [500 mM NaCl, 500 mM Tris (pH 7.5), and 50 mM DTT] in a 20-ml reaction. For oxidation studies, Cezanne was preactivated with 10 mM DTT, and subsequently dialyzed against degassed reaction buffer lacking DTT. Protein was incubated with indicated concentrations of H2O2 for 15 min, and either used in a DUB reaction, or again DTT-treated (10 mM, 15 min) and used in a DUB reaction.
Statistical Analysis
Statistical differences were determined using Student’s t-test. Values of p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 10.0 (SPSS Inc. Chicago, IL, USA).
RESULTS
Hepatocellular Carcinoma Cells Generated High Levels of Endogenous ROS
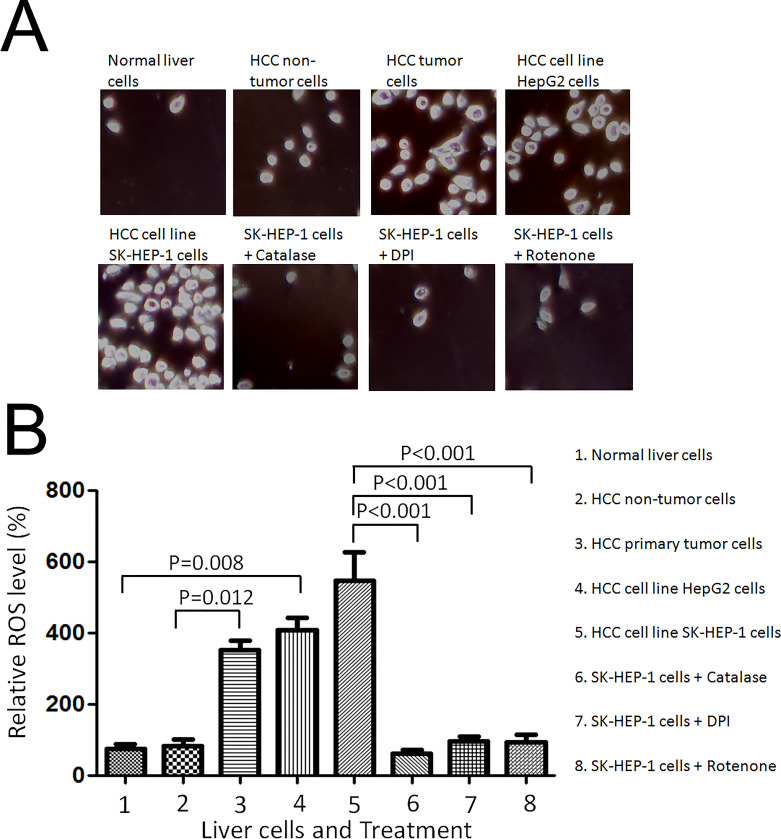
We first used intracellular CM2-DCFHDA staining method to measure the endogenous ROS levels in the cells and found that the ROS levels in both HepG2 cells and SK-HEP-1 cells were fivefold and sixfold higher, respectively, than those in normal liver cells (Fig. 1A and B). The fluorescent signal was completely inhibited by the addition of catalase to indicate the specificity of ROS staining. To further test the ROS levels in other hepatocellular carcinoma cells, hepatocellular primary tumor cells were used. As shown in Figure 1A and B, the ROS levels in the hepatocellular tumor primary cells were significantly higher (3.5-fold) than that in HCC nontumor cells, indicating that ROS were also spontaneously overproduced in hepatocellular carcinoma. The endogenous ROS production was inhibited by DPI, NADPH-dependent oxidase inhibitor, and rotenone, the mitochondria complex I inhibitor (Fig. 1A and B). These results suggested that NADPH oxidase and the mitochondria respiratory chain were required for inducing ROS production in HCC cells.
Figure 1.
Hepatocellular cancer cells generated higher levels of endogenous reactive oxygen species (ROS). (A) Normal liver cells THLE-2 cells, hepatocellular carcinoma cells (HCC) nontumor cells, HCC primary tumor cells, and HCC carcinoma cell lines, HepG2 and SK-HEP-1 cells were seeded onto a glass coverslip in a six-well plate at 1 × 105 cells per well for 24 h. CM2-DCFHDA (5 mmol/L) was added into the cell culture medium and incubated for 30 min. For the catalase treatment, catalase (750 U/ml) was added to SK-HEP-1 cells 30 min before the addition of CM2-DCFHDA. The cells were washed three times with 1× PBS and fixed with 10% buffered formalin. The representative images were captured with a confocal fluorescence microscope (at excitation wavelength, 485 nm; emission wavelength, 530 nm). (B) The mean value of DCF fluorescence intensity was obtained from 1 × 105 cells at 485 nm excitation and 540 nm emission settings using a flow cytometer (Becton Dickinson FACSort). SK-HEP-1 cells were treated with solvent, 5 mmol/L DPI, 5 mmol/L rotenone for 30 min, and then stained with 5 mmol/L CM2-DCFHDA for 15 min. The relative fluorescence intensity was analyzed by flow cytometry and normalized to that of normal liver cells. Significant difference was presented when the value of treatment was compared with that of the control.
H2O2 Enhanced Induction of MMP2, MMP9, and ICAM1 Expression in Response to TNF-α
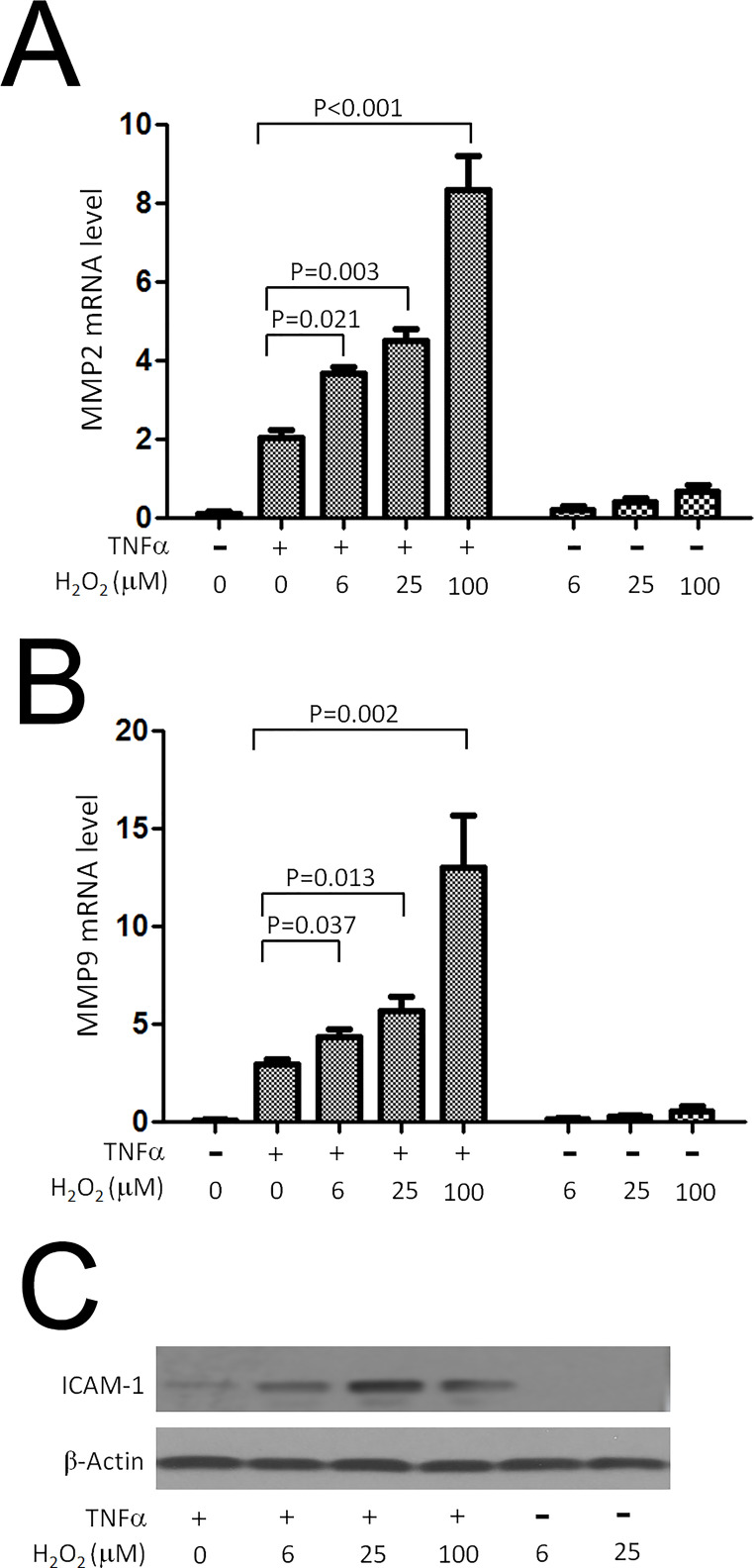
It was reported that MMP2, MMP9, and ICAM1 levels were regulated by Cezanne2 through modulation of NF-kB signaling cascade23. To investigate the ROS effect on NF-κB-dependent inflammatory activation, we examined whether reactive oxygen species such as H2O2 could regulate proinflammatory activation in HepG2 cells, either alone or in combination with TNF-α. We observed by comparative real-time RT-PCR that the application of H2O2 alone (6–100 mM) had little or no effect on MMP2 and MMP9 mRNA expression levels in HepG2 cells (Fig. 2A and B). However, H2O2 significantly enhanced the production of MMP2 and MMP9 expression levels in a concentration-dependent manner in cells that were cotreated with TNF-α (Fig. 2A and B) without affecting cell proliferation or survival (data not shown). Furthermore, we observed upregulation of ICAM1 protein level response to TNF-α stimulation with H2O2 treatment at varying concentrations (Fig. 2C). These data indicated that H2O2 could activate inflammatory effect through increased induction of MMP2, MMP9, and ICAM1 expression.
Figure 2.
H2O2 enhanced induction of metalloproteinase 2 (MMP2), MMP9, and intercellular adhesion molecule 1 (ICAM1) expression in response to TNF-α. HepG2 cells were stimulated with TNF-α (10 ng/ml) for 4 h either in the absence or in the presence of varying concentrations of H2O2. (A, B) MMP2 and MMP9 mRNA transcript levels were quantified by comparative real-time PCR and were normalized by measuring β-actin. (C) Expression of ICAM1 was compared by Western blot between HepG2 cells treated with TNF-α stimulation and varying concentrations of H2O2.
Oxidation Could Promote NF-κB Signaling Cascade by Inhibiting Cezanne DUB Activity
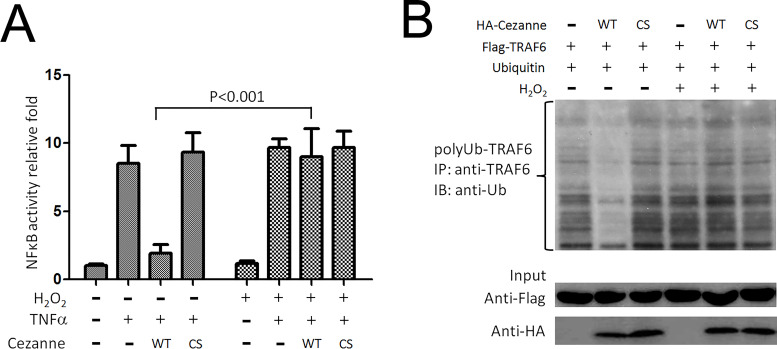
Given the exquisite sensitivity of cysteine proteases to oxidative stress, we reasoned that H2O2 may prolong NF-κB signaling activation by inhibiting the catalytic activity of Cezanne. First, we observed by reporter gene assay that H2O2 significantly reduced the capacity of overexpressed Cezanne to suppress NF-κB transcriptional activity in response to TNF-α (Fig. 3A). TRAF6 was a target candidate of Cezanne2 substrates and further regulated the NF-kB signaling pathway23. Western blotting revealed that overexpression of Cezanne significantly reduced the buildup of polyubiquitinated TRAF6 in HepG2 cells treated with TNF-α (Fig. 3B) but had little or no effect in HepG2 cells that were cotreated with TNF-α and H2O2. These data indicated that H2O2 could suppress the capacity of Cezanne to inhibit TRAF6 signaling in HCC cells.
Figure 3.
Oxidation inhibits NF-κB signaling cascade by inhibiting Cezanne. (A) Reporter gene assays were performed to assess the effects of H2O2 on NF-κB suppression by Cezanne. HepG2 cells were cotransfected with pcDNA3.1-Cezanne (or empty vector), pGL3 basic vector (NF-κB reporter), and pRL-TK (Renilla luciferase control). After 16 h, cells were stimulated with TNF-α (10 ng/ml) for 16 h either in the presence or absence of 100 mM H2O2. Cell lysates were analyzed, and the ratio of firefly/Renilla luciferase activity was calculated, which is a measure of NF-κB activity normalized for transfection efficiencies. Mean values of NF-κB activity calculated from triplicate wells were pooled from three experiments and are shown with standard deviations. (B) TRAF6 polyubiquitination was measured from HepG2 cells transfected with an expression vector encoding Cezanne (pcDNA3.1-Cez) or remained untransfected when treatment with TNF-α. Streptavidin-coated beads were then used to precipitate TRAF6 complexes, which were tested by Western blot using anti-TRAF6 antibody. Cytosolic lysates were tested by Western blot using anti-TRAF6 or anti-Cezanne antibodies. Levels of polyubiquitinated TRAF6 (polyUb-TRAF6) were quantified by densitometry. Mean values calculated from triplicate measurements are shown with standard deviations.
Oxidation of Cezanne Is Dependent on its Catalytic Competency
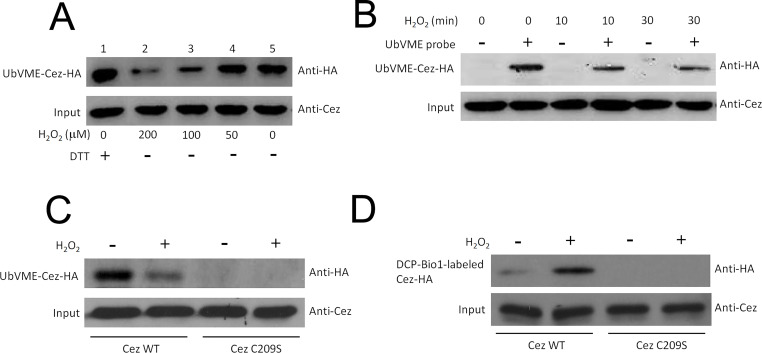
Next, we tested whether Cezanne could be a direct target of oxidative stress and whether this could affect its catalytic activity. Cys oxidation by H2O2 results in the formation of sulfenic (–SOH), sulfinic (–SO2H), or sulfonic (–SO3H) acid, which represents the addition of one, two, or three oxygen molecules, respectively24. To assess the catalytic activity of the modified Cezanne by measuring total active site thiol content, we assessed the effects of H2O2 on the feature of Cezanne using an assay employing a ubiquitin-derived thiol-reactive probe (HA-UbVME), which is known to bind specifically to the catalytic cysteine of deubiquitinating enzymes22. Incubation of probe with lysates from HepG2 cells transfected with flag-tagged Cezanne revealed a prominent band corresponding to ubiquitin-modified Cezanne (Fig. 4A and B). We confirmed that probe binding observed in these experiments was due to Cezanne sequences by demonstrating that it was not observed using lysates from untransfected cells (data not shown). We observed that probe binding to Cezanne was not influenced by treatment of cells with TNF-α alone but was reduced by cotreatment of HepG2 cells with TNF-α and H2O2. In addition, probe binding to Cezanne was suppressed by the addition of H2O2 to reaction mixtures in vitro in a concentration-dependent manner (Fig. 4A and B). To determine whether the labeling, and therefore oxidation, of Cezanne was dependent on its catalytic competency, we tested labeling of Cezanne mutated in the active site Cys domain. This mutant (C209S) compromises the nucleophilic ability of the catalytic Cys residue because of loss of the active site thiol25, and, when expressed, it was not labeled by the HA-UbVME probe in an oxidative stress-dependent manner (Fig. 4C). These findings indicated that the catalytic activity of Cezanne could be inhibited by H2O2.
Figure 4.
Oxidation of Cezanne catalytic Cys to the sulfenic acid intermediates. A thiol-reactive probe (HA-UbVME) was used to assess the effects of H2O2 on the catalytic activity of Cezanne. Since this is an irreversible reagent it measures total active site thiol content, not activity. To measure its activity, excess reagent was used and that labeling was measured over the first several percent of the reaction. (A) Cytosolic lysates made from cells expressing Flag-tagged Cezanne were incubated with HA-UbVME probe in the presence of varying concentrations of H2O2. Alternatively, lysates were incubated with probe in the presence of a reducing agent, DTT. Cezanne linked covalently to probe was detected by Western blotting using anti-HA epitope antibodies. Results are representative of two independent experiments. (B, C) HepG2 cells expressing Flag-tagged Cezanne were treated with TNF-α (10 ng/ml) either in the presence or absence of 100 mM H2O2 or remained untreated as a control. Cytosolic lysates were incubated with HA-UbVME probe or were incubated in the absence of probe as a control. Cezanne linked covalently to probe was detected by Western blotting using anti-HA epitope antibodies. Levels of Cezanne in cytosolic lysates were normalized by Western blot using anti-Cezanne antibodies. (D) Oxidation of Cezanne catalytic Cys to the sulfenic acid intermediate can be captured by DCP-Bio1 probe. HepG2 cells were transfected for 48 h with Cezanne-Flag WT or C209S mutant. Cells were then treated with H2O2 (final concentration 0.3 mM) for 30 min. Extracts were made and divided for two separate reactions: labeling with DCP-Bio1 probe or with the UbVME DUB activity probe. Input represents 30% of extracts used for the DCP-Bio1 reaction.
Furthermore, we used a biotin-tagged 1,3-cyclohexadione derivative, DCP-Bio1, to investigate the oxidation status of Cezanne in response to oxidative stress. Because the analysis of the endogenous Cezanne oxidation state was difficult to assess due to the low abundance of active Cezanne pool in cells, exogenous Cezanne was forced expressed in HepG2 cells to ensure higher levels of active DUB. Upon H2O2 treatment, we found that exogenously expressed C-terminally epitope-tagged human Cezanne (Cezanne-Flag) was labeled by DCP-Bio1 in cell lysates (Fig. 4D), suggesting that Cezanne was oxidized to the sulfenic acid intermediate in response to oxidative stress. Taken all together, we conclude that H2O2 has capacity to inhibit the catalytic activity of Cezanne.
Reducing Agents Activate the Cezanne DUB
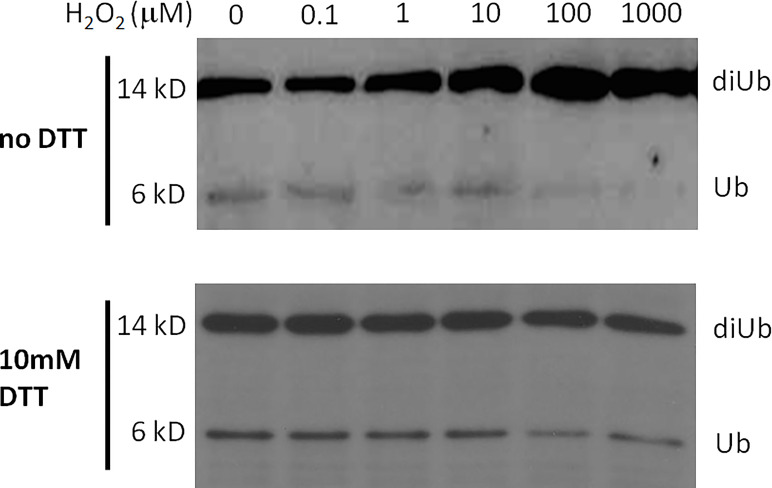
The effect of reducing agents on Cezanne activity suggested that the enzyme is susceptible to regulation by ROS. Dithiothreitol (DTT) is a reducing agent. To test this hypothesis, we first treated 5 mM Cezanne with increasing amounts of H2O2 in vitro for 15 min. Catalase was added to rapidly deplete H2O2 and stop oxidation. Samples were then split and to one half 10 mM DTT was added and incubated for 15 min at room temperature. The proteins were used in DUB assays against Lys48-linked diUb (Fig. 5). This experiment revealed that H2O2 concentrations exceeding 10 mM significantly reduced Cezanne DUB activity. Importantly, this inhibition was completely reversed by DTT up to a H2O2 concentration of 100 mM, suggesting fully reversible inhibition of Cezanne DUB activity in the physiologically important range of 10–100 mM H2O2. Incubation with higher concentrations of H2O2 (1 mM) led to irreversible inhibition that could not be reversed by subsequent DTT treatment. Overall, these results indicated that ROS-mediated oxidation of Cezanne could be reversed by DTT treatment, allowing for reversible regulation of enzyme activity.
Figure 5.
Reversible Cezanne oxidation in vitro. Cezanne was first incubated with indicated concentrations of H2O2 for 15 min at 23°C, and 100 U of catalase was added to quench the H2O2. To one-half of the sample, DTT was added to a final concentration of 10 mM and incubated at room temperature for 15 min. The activity of the treated Cezanne was tested in a DUB assay using Lys48-linked diUb as substrate.
DISCUSSION
There are growing interests of ROS signaling in carcinogenesis. However, the direct roles and mechanism of ROS in tumor pathophysiology remain to be elucidated. In this study, we found that reactive oxygen species (ROS) such as H2O2 may enhance NF-kB activation via suppressing deubiquitinase Cezanne in liver cancer cell lines. Generation of ROS in cells constitutes an important signaling event. However, its transient nature, high reactivity, and high diffusion rate pose challenges for detecting the roles and targets of ROS. To date, protein tyrosine phosphatases are the best studied enzyme class regulated by ROS, and the impact of ROS production in phosphorylation cascades is unquestioned26,27. Phosphatases were also instrumental to illuminate the different mechanisms of reversible inhibition of enzyme activity by oxidation28. Given the sensitivity of cysteine proteases to oxidative stress, we reasoned that H2O2 may prolong signaling to NF-κB by inhibiting the activity of Cezanne. Consistent with this hypothesis, we observed that the catalytic activity of Cezanne and its ability to suppress TRAF6 polyubiquitination and NF-κB transcriptional activity were inhibited by H2O2. We concluded that the ability of H2O2 to prolong NF-κB signaling could potentially be explained by its inhibitory effects on Cezanne activity. A previous study by Xu et al. demonstrated that Snail1-mediated suppression of Cezanne2 may play a key role in hepatocellular carcinoma malignancy20. However, Cezanne, which has 46% homology to Cezanne2, maintained comparatively stable expression in HCC cells and tumorous tissues. Because both of them may share similar biologic function in the downstream signaling cascades, there may be a possibility that some inhibitory effect would exist in Cezanne protein via posttranslational level but not on transcriptional level. Therefore, results from the current study for the first time confirmed the hypothesis that endogenous Cezanne in hepatocellular carcinoma was oxidated by ROS in its catalytic cysteine residual to inactivate tumor suppressor function in HCC.
The regulation of ubiquitin-dependent signaling pathways by ROS was established29 in a study which showed that treating cells with H2O2 enhances an inflammatory response involving the NF-κB signaling pathway. Recently, three distinct works have shown that Cys oxidation can modulate DUB activity. Cotto-Rios et al.30 reported the transient sulfenylation of catalytic Cys for several members of the Ub-specific protease (USP) family. In particular, the authors established that USP1, a DUB involved in DNA damage response pathways, is reversibly inactivated following the induction of oxidative stress in cells. Additionally, studies from Kulathu et al.14 demonstrate that many members of the ovarian tumor DUBs also undergo Cys oxidation upon H2O2 treatment, including the tumor suppressor A20. Crystal structure analysis of oxidized A20 showed that transient RSOH can be stabilized by the formation of hydrogen bonds with the highly conserved residues located in the loop preceding catalytic Cys. Both works noted that each DUB family member exhibits a distinct level of sensitivity to oxidation. Differences in behavior can reflect various ranges of catalytic activation in which the conformational inactive enzyme could be less susceptible to oxidation. Lee et al.15 confirmed this hypothesis by showing that preincubation of USP7 with Ub, which behaves as an allosteric activator, increases USP7 sensitivity to ROS. Collectively, these articles14,15,30 highlight the ubiquity of ROS sensitivity across the main cysteine–protease families of the DUBs. These discoveries coincided with ROS-dependent inhibition of the DUB enzyme Cezanne in our current study.
Elevated levels of ROS have been found in many disease states, notably cancer, diabetes, and conditions of chronic inflammation31. Given the role of deregulation of ubiquitination in disease pathogenesis32, it is possible that oxidative stress exerts its pathological functions, at least in part, also via deregulation of DUBs. The realization that DUBs are a target of ROS may lead to reevaluation of cell signaling processes in oxidative stress-induced conditions. Studies of cultured cells have revealed that ROS have profound effects on numerous physiological activities including proinflammatory activation, which can be altered by ROS through poorly defined mechanisms. Numerous reports have indicated that NF-κB-dependent transcription is a redox-sensitive process. However, the effects of oxidative stress on cellular activation are complex and vary between particular types of cells, ROS, and coactivating stimuli. For example, H2O2 has been shown to be a direct inducer of NF-κB transcriptional activity and proinflammatory activation in some cell types (e.g., T cells and MCF-7 cells). In addition, H2O2 can enhance or prolong cellular activation in response to proinflammatory cytokines in several cell types. NF-κB induces other negative regulators of proinflammatory signaling including A20 and its sister molecule Cezanne, which belong to the OTU family of deubiquitinating cysteine proteases that can cleave ubiquitin monomers from modified proteins. The next challenge will be to define cellular situations in which OTU oxidation occurs. Interestingly, combined treatment of cells with TNF-α and H2O2 leads to prolonged changes in the kinetics of NF-κB activation, and sustained polyubiquitination of TRAF6 substrates, suggesting that Cezanne may be affected. Our further study demonstrated that oxidative stress could inactivate its DUB activity by oxidation of the catalytic Cysteine residual in Cezanne. While our studies implicated that Cezanne is involved in this process, it could also be other OTU family member that mediates the reported effects.
In summary, we described a novel mechanism for the proinflammatory effects of reactive oxygen species, which caused oxidation of Cezanne cysteine in HCC. Further, oxidation of Cezanne results in a prolonged, or increased, response to TNF and that hepatocellular carcinoma is particularly sensitive to this regulation. Therefore, our findings provide strong evidence that endogenous ROS may play an important role for pathophysiology in HCC, and these results may be useful to develop new therapeutic strategy by targeting ROS signaling in human hepatocellular carcinoma in the future.
ACKNOWLEDGMENTS
This study is supported by the Health Commission of Hubei Province scientific research project (Grant No. WJ2017M114) and the Fundamental Research Funds for the Central Universities (Grant No. 2017KFYXJJ240). This project is supported by the Natural Science Foundation of Hubei Province, China (Grant No. 2018CFB590). Z.Y.Y., L.Y., F.W., and J.S.F. performed the experiments and data analysis. J.J.X., Y.J., and G.H.Y. collected the subject’s samples and clinical data. All the authors contributed to the manuscript writing and revision.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Diplock AT, Rice-Evans CA, Burdon RH. Is there a significant role for lipid peroxidation in the causation of malignancy and for antioxidants in cancer prevention? Cancer Res. 1994;54(7 Suppl):1952s–6s. [PubMed] [Google Scholar]
- 2. Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11(1):1–14. [DOI] [PubMed] [Google Scholar]
- 3. Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67(22):10823–30. [DOI] [PubMed] [Google Scholar]
- 4. Harfouche R, Malak NA, Brandes RP, Karsan A, Irani K, Hussain SN. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB J. 2005;19(12):1728–30. [DOI] [PubMed] [Google Scholar]
- 5. Liu LZ, Hu XW, Xia C, He J, Zhou Q, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1alpha expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic Biol Med. 2006;41(10):1521–33. [DOI] [PubMed] [Google Scholar]
- 6. Zhou Q, Liu LZ, Fu B, Hu X, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate insulin-induced VEGF and HIF-1alpha expression through the activation of p70S6K1 in human prostate cancer cells. Carcinogenesis 2007;28(1):28–37. [DOI] [PubMed] [Google Scholar]
- 7. Kim YM, Kim KE, Koh GY, Ho YS, Lee KJ. Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Cancer Res. 2006;66(12):6167–74. [DOI] [PubMed] [Google Scholar]
- 8. Puri PL, Avantaggiati ML, Burgio VL, Chirillo P, Collepardo D, Natoli G, Balsano C, Levrero M. Reactive oxygen intermediates mediate angiotensin II-induced c-Jun.c-Fos heterodimer DNA binding activity and proliferative hypertrophic responses in myogenic cells. J Biol Chem. 1995;270(38):22129–34. [DOI] [PubMed] [Google Scholar]
- 9. Stevenson MA, Pollock SS, Coleman CN, Calderwood SK. X-irradiation, phorbol esters, and H2O2 stimulate mitogen-activated protein kinase activity in NIH-3T3 cells through the formation of reactive oxygen intermediates. Cancer Res. 1994;54(1):12–5. [PubMed] [Google Scholar]
- 10. Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175(5):1181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bianchi NO, Bianchi MS, Richard SM. Mitochondrial genome instability in human cancers. Mutat Res. 2001;488(1):9–23. [DOI] [PubMed] [Google Scholar]
- 12. Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477(1–2):7–21. [DOI] [PubMed] [Google Scholar]
- 13. Tonks NK. Redox redux: Revisiting PTPs and the control of cell signaling. Cell 2005;121(5):667–70. [DOI] [PubMed] [Google Scholar]
- 14. Kulathu Y, Garcia FJ, Mevissen TE, Busch M, Arnaudo N, Carroll KS, Barford D, Komander D. Regulation of A20 and other OTU deubiquitinases by reversible oxidation. Nat Commun. 2013;4:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee JG, Baek K, Soetandyo N, Ye Y. Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells. Nat Commun. 2013;4:1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Evans PC, Smith TS, Lai MJ, Williams MG, Burke DF, Heyninck K, Kreike MM, Beyaert R, Blundell TL, Kilshaw PJ. A novel type of deubiquitinating enzyme. J Biol Chem. 2003;278(25):23180–6. [DOI] [PubMed] [Google Scholar]
- 17. Enesa K, Zakkar M, Chaudhury H, Luong le A, Rawlinson L, Mason JC, Haskard DO, Dean JL, Evans PC. NF-kappaB suppression by the deubiquitinating enzyme Cezanne: A novel negative feedback loop in pro-inflammatory signaling. J Biol Chem. 2008;283(11):7036–45. [DOI] [PubMed] [Google Scholar]
- 18. Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem. 2005;280(17):17497–506. [DOI] [PubMed] [Google Scholar]
- 19. Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21(1):103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu Z, Pei L, Wang L, Zhang F, Hu X, Gui Y. Snail1-dependent transcriptional repression of Cezanne2 in hepatocellular carcinoma. Oncogene 2014;33(22):2836–45. [DOI] [PubMed] [Google Scholar]
- 21. Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 2003;424(6950):797–801. [DOI] [PubMed] [Google Scholar]
- 22. Borodovsky A, Ovaa H, Kolli N, Gan-Erdene T, Wilkinson KD, Ploegh HL, Kessler BM. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem Biol. 2002;9(10):1149–59. [DOI] [PubMed] [Google Scholar]
- 23. Xu Z, Pei L, Wang L, Zhang F, Hu X, Gui Y. Snail1-dependent transcriptional repression of Cezanne2 in hepatocellular carcinoma. Oncogene 2014;33(22):2836–45. [DOI] [PubMed] [Google Scholar]
- 24. Claiborne A, Yeh JI, Mallett TC, Luba J, Crane EJ 3rd, Charrier V, Parsonage D. Protein-sulfenic acids: Diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry 1999;38(47):15407–16. [DOI] [PubMed] [Google Scholar]
- 25. Rawlings ND, Barrett AJ, Bateman A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2012;40(Database issue):D343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature 2003;423(6941):773–7. [DOI] [PubMed] [Google Scholar]
- 27. Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 2003;423(6941):769–73. [DOI] [PubMed] [Google Scholar]
- 28. Tanner JJ, Parsons ZD, Cummings AH, Zhou H, Gates KS. Redox regulation of protein tyrosine phosphatases: Structural and chemical aspects. Antioxid Redox Signal. 2011;15(1):77–97. [DOI] [PubMed] [Google Scholar]
- 29. Enesa K, Ito K, Luong le A, Thorbjornsen I, Phua C, To Y, Dean J, Haskard DO, Boyle J, Adcock I, Evans PC. Hydrogen peroxide prolongs nuclear localization of NF-kappaB in activated cells by suppressing negative regulatory mechanisms. J Biol Chem. 2008;283(27):18582–90. [DOI] [PubMed] [Google Scholar]
- 30. Cotto-Rios XM, Bekes M, Chapman J, Ueberheide B, Huang TT. Deubiquitinases as a signaling target of oxidative stress. Cell Rep. 2012;2(6):1475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lambeth JD. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43(3):332–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen P, Tcherpakov M. Will the ubiquitin system furnish as many drug targets as protein kinases? Cell 2010;143(5):686–93. [DOI] [PubMed] [Google Scholar]