Abstract
Apatinib is an oral TKI with antiangiogenic properties, and it is currently approved for the treatment of advanced gastric cancer in China. This agent has also been tested in other human solid tumors, including non-small cell lung cancer (NSCLC). Since the combination of chemotherapy and an antiangiogenic agent has been shown to be a feasible strategy in NSCLC, it is conceivable that a similar approach combining apatinib with chemotherapy may yield clinical activity. With this in mind, we investigated the efficiency of apatinib in combination with pemetrexed or docetaxel in advanced NSCLC. We treated a total of 20 patients with metastatic NSCLC adenocarcinoma with apatinib in combination with either pemetrexed or docetaxel from January 2016 to March 2017. The performance status of these patients was 0 or 1. All of these patients had been previously treated with two or more lines of treatment and had experienced disease progression prior to study enrollment. The overall objective response rate (ORR) was 30%, with 6 patients who had partial response (PR), 10 patients who had stable disease (SD), and 4 patients who had progressive disease (PD). The main adverse events were skin rash, hypertension, palmar–plantar erythrodysesthesia syndrome, diarrhea, and fatigue. Nearly 30% of patients required interruption of treatment as a result of toxicity. Our study demonstrated that apatinib combined with systemic cytotoxic chemotherapy has clinical efficacy in patients with disease-refractory metastatic NSCLC and provides evidence for further studies investigating apatinib-based combination regimens.
Key words: Antiangiogenesis therapy, Apatinib, Non-small cell lung cancer (NSCLC), Cytotoxic chemotherapy, Adenocarcinoma
INTRODUCTION
Lung cancer is the most common malignancy with the highest incidence worldwide, and non-small cell lung cancer (NSCLC) remains the most common histopathologic subtype. Although advanced NSCLC remains incurable, significant progress has been made over the past 10–15 years to improve clinical efficacy with improvements in progression-free survival (PFS) and overall survival (OS). The rapid development of molecular targeted therapy and immunotherapy highlights the significant advances that have been made. Currently, it is commonly recognized that in the era of precision medicine, a portion of patients who harbor specific genetic abnormalities may benefit from targeted therapies. However, there remain a significant number of patients whose tumors harbor no specific target, and in this setting chemotherapy remains the only treatment option1. With modern platinum-based chemotherapy, the median OS is only approximately 10 months. Fortunately, further trials proved that the combination of chemotherapy and antiangiogenesis, such as nintedanib2, ramucirumab3, and others4–6, is able to further improve the treatment efficacy, which provide evidence for the tremendous potential of the combination of antiangiogenic agents and chemotherapy in the future.
Apatinib is a small molecule that targets the vascular endothelial growth factor receptor 2 (VEGFR-2)-associated tyrosine kinase, and it is the first oral tyrosine kinase inhibitor (TKI) that has been approved for gastric cancer in China7,8. Preclinical studies have demonstrated that apatinib also displays anticancer activity against several other human cancers, including NSCLC9–11. In addition, apatinib has demonstrated clinical activity in patients with NSCLC, albeit in small case reports12–14. With this in mind, we retrospectively analyzed the clinical efficacy of advanced NSCLC patients who had received apatinib in combination with systemic chemotherapy after two or more lines of systemic treatment. The goal of this study was to investigate the clinical activity and safety profile of the combination of apatinib and chemotherapy in advanced NSCLC.
MATERIALS AND METHODS
Inclusion and Exclusion Criteria
Patients between 18 and 70 years of age with histologically confirmed advanced NSCLC were eligible for study enrollment. Enrollment criteria included prior lack of response or intolerance to at least two systemic chemotherapy regimens (including both doublet platinum regimen), and confirmation of drug resistance was necessary for patients who have accepted EGFR TKI therapy previously. Before disease progression, no patient had accepted immune checkpoint therapy such as anti-PD-1 or anti-CTLA-4. The criteria for disease progression on systemic chemotherapy or targeted therapy were based on computed tomography (CT) and magnetic resonance imaging (MRI) evaluation. The study allowed recruitment of patients who were intolerant to second-line chemotherapy, as there are no alternative therapeutic options for these patients. Additional enrollment criteria were as follows: at least one measurable lesion as defined by RECIST; an Eastern Cooperative Oncology Group performance status of 0 or 1; and acceptable hematologic, hepatic, and renal function. Exclusion criteria included history of hemoptysis (one-half teaspoon of bright red blood in the 3 months before enrollment), tumor invading major blood vessels, asymptomatic central nervous system (CNS) metastases, and uncontrolled hypertension. Current or recent (within 10 days of first apatinib dose) use of full-dose anticoagulants or a thrombolytic agent for therapeutic purposes was not permitted, but prophylactic use of anticoagulants was allowed.
Molecular Analysis
All patients underwent CT-guided needle aspiration for diagnosis. Once the diagnosis was confirmed pathologically, part of the sample was used for multiple gene detection, such as EGFR, ALK, c-Met, and K-RAS. Molecular analysis was performed using well-established sequencing methods.
Drug Administration
All patients had previously received two or more lines of systemic chemotherapy. Once disease progression or drug resistance was confirmed, systemic chemotherapy was administered using standard drug doses of pemetrexed and docetaxel, which were 500 and 75 mg/m2, respectively. The recommended dosage of apatinib was 500 mg PO given on a daily basis. Drug interruption or drug discontinuation can be performed once grade 3 or 4 toxicity occurs; the period for drug interruption was not more than 1 week, and for drug reduction the dosage was gradually reduced from 500 to 250 mg. The minimum dosage for apatinib was 250 mg PO once a day. Apatinib was given simultaneously with chemotherapy. Each cycle period was 28 days.
With regard to the concrete drug combination, 10 patients accepted the combination therapy of pemetrexed and apatinib, while docetaxel plus apatinib was administrated to six patients. The objective response rates (ORRs) were 40% and 33% for each subgroup, with 4 and 2 patients with partial response (PR), respectively. Although the efficiency brought about by the former regimen seems to be relatively higher than the latter, because of the limitation of sample size it is still too early to draw a positive conclusion.
Evaluation of Clinical Activity
To be considered evaluable for clinical activity, all patients had to have received at least one cycle of therapy. Either chest CT or chest MRI scans were performed every 4 weeks. The main criterion was RECIST 1.0. The ORR was the ratio of complete response (CR) plus PR patients and total patients, and the disease control rate (DCR) was the ratio of nonprogressive disease (PD) patients and total patients.
Statistical Analysis
The progression-free survival (PFS) and overall survival (OS) analysis was performed by using SPSS 13.0, and the corresponding figures were drawn by using GraphPad Prism 5.0. A value of p ≤ 0.05 was regarded as statistically significant.
RESULTS
Patient Characteristics
In our study, a total of 20 advanced NSCLC patients with adenocarcinoma were enrolled. All of them had previously received two or more lines of systemic treatment. The performance status of these patients was 0 or 1. In this 20-patient cohort, 9 patients were female and 11 were male, and the mean age was 61 years. Most of these patients had EGFR wild type; six patients with EGFR mutation had disease progression after first-line TKI treatment. Most of the female patients were nonsmokers, while all of the male patients were heavy smokers (see Table 1).
Table 1.
Patient Demographics and Characteristics
| Patient No. | Gender | Age | Stage | Metastasis Status | Gene Profile | PFS (Months) | Response |
|---|---|---|---|---|---|---|---|
| 1 | Male | 68 | IV | Pleural | c-Met (+,15%) | 1 | PD |
| 2 | Female | 45 | IV | Brain | c-Met (+,25%) | 4 | PR |
| 3 | Male | 56 | IV | Pleural | c-Met (+,40%) | 3 | SD |
| 4 | Male | 48 | IV | Liver | EGFR wt | 6 | PR |
| 5 | Female | 64 | IV | Liver | EGFR del19 | 4 | SD |
| 6 | Female | 61 | IV | Pleural | EGFR wt | 6 | PR |
| 7 | Male | 58 | IV | Lung | EGFR L858R | 3 | SD |
| 8 | Male | 65 | IV | Liver | EGFR L858R | 5 | PR |
| 9 | Male | 57 | IV | Liver | c-Met (+,40%) | 1 | PD |
| 10 | Male | 63 | IV | Liver | c-Met (+,35%) | 1 | PD |
| 11 | Female | 53 | IV | Lung | EGFR wt | 1 | PD |
| 12 | Female | 36 | IV | Pleural and bone | EGFR wt | 7 | PR |
| 13 | Male | 61 | IV | Lung and pleural | EGFR wt | 3 | SD |
| 14 | Male | 70 | IV | Pleural | EGFR wt | 2 | SD |
| 15 | Female | 63 | IV | Pleural | EGFR L858R | 5 | PR |
| 16 | Female | 48 | IV | Lung and bone | EGFR wt | 2 | SD |
| 17 | Male | 62 | IV | Bone | EGFR L858R | 3 | SD |
| 18 | Male | 65 | IV | Brain | c-Met (+,30%) | 2 | SD |
| 19 | Female | 61 | IV | Bone | EGFR L858R | 3 | SD |
| 20 | Female | 56 | IV | Brain and bone | EGFR wt | 2 | SD |
PFS, progression free survival; EGFR, epithelial growth factor receptor; wt, wild type; SD, stable disease; PR, partial response; PD, progressive disease.
Previous Treatment
Most of these patients with EGFR wild type had previously received two or more lines of treatment of systemic chemotherapy. The most common first-line treatment regimen was pemetrexed plus platinum, and the main agents used in the second-line setting were docetaxel and gemcitabine. All of the EGFR mutated patients had previously received first-line TKI treatment, and the mutation profile of these patients included exon 19 Del (one patient), exon 21 L858R mutation (five patients), and c-Met amplification (six patients). The targeted therapies used for the first-line treatment of these patients were icotinib, erlotinib, or gefitinib. After disease progression, all of these patients underwent subsequent molecular testing. The patients who had T790M mutation accepted AZD9291 treatment directly, and the other patients chose chemotherapy. The regimens were basically similar to those of patients with EGFR wild type (see Table 1).
Clinical Activity
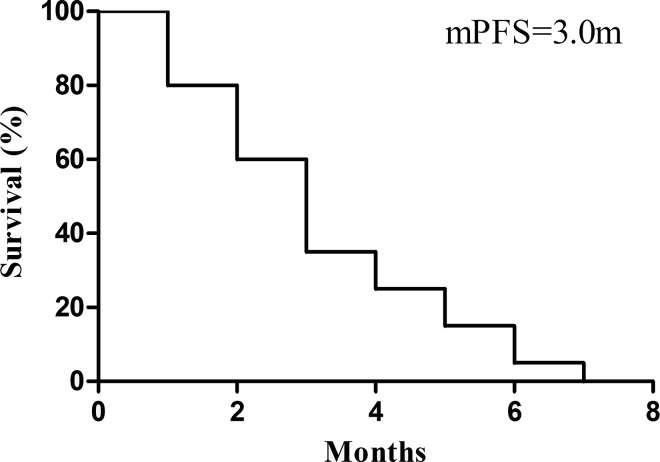
All patients had accepted at least one cycle treatment; the mean treatment line of apatinib was three. Our efficiency analysis indicated that no patient had CR, 6 patients had PR, 10 patients had SD, and 4 had disease progression. The ORR was 30%, the mean PFS was 3.0 months, and the disease control ratio was nearly 80%. The OS data are not yet matured (see Table 2 and Fig. 1).
Table 2.
Clinical Activity of Apatinib Plus Systemic Chemotherapy
| Patient No. | % | |
|---|---|---|
| Complete response | 0 | 0 |
| Partial response | 6 | 30% |
| Stable disease | 10 | 50% |
| Progressive disease | 4 | 20% |
| Objective response rate | 30% | |
| Median progression-free survival | 3.0 months | |
| Disease control rate | 80% |
Figure 1.
Progression-free survival (PSF).
Toxicity
In general, the combination of apatinib plus systemic chemotherapy was relatively well tolerated. The most common treatment-related hematologic side effects were leucopenia, neutropenia, anemia, and thrombocytopenia. The nonhematologic toxicities were fatigue, proteinuria, hypertension, hand–foot syndrome (HFS), abdominal pain, decreased appetite, diarrhea, and elevated transaminases. In the large majority of cases, these were low-grade toxicities, and the incidence of grade 3/4 toxicity was extremely low. The mean dose of apatinib that was administered in this study was 500 mg PO once a day. Fifty percent of patients required drug interruption as a result of toxicity, and 30% of patients had the drug discontinued. As seen in Table 3, the major adverse events (AEs) were leukopenia, neutropenia, HFS, proteinuria, hypertension, and stomatitis. In the large majority of cases, the AEs were prevented and/or reduced by implementing supportive care therapies.
Table 3.
Analysis of Adverse Events
| Adverse Event | Apatinib Plus Chemotherapy [n (%)] | |
|---|---|---|
| Any Grade | Grade 3 or 4 | |
| Hematological | ||
| Leukopenia | 8 (40%) | 1 (5%) |
| Neutropenia | 6 (30%) | 1 (5%) |
| Anemia | 4 (20%) | 0 (0) |
| Thrombocytopenia | 3 (15%) | 0 (0) |
| Nonhematologic | ||
| Proteinuria | 4 (20%) | 1 (5%) |
| Hypertension | 9 (45%) | 1 (10%) |
| Hand-foot syndrome | 5 (25%) | 2 (10%) |
| Elevated transaminase | 3 (15%) | 1 (5%) |
| Hyperbilirubinemia | 2 (10%) | 0 (0) |
| Bleeding | 2 (10%) | 0 (0) |
| Fatigue | 11 (55%) | 0 (0) |
| ALP increased | 1 (5%) | 0 (0) |
| Elevated GGT | 1 (5%) | 0 (0) |
| Abdominal pain | 4 (20%) | 0 (0) |
| Decreased appetite | 6 (30%) | 0 (0) |
| Hypoproteinemia | 1 (5%) | 0 (0) |
| Diarrhea | 4 (20%) | 0 (0) |
| Elevated LDH | 1 (5%) | 0 (0) |
| Oral ulcer | 1 (5%) | 0 (0) |
| Stomatitis | 1 (5%) | 1 (5%) |
| Dysphagia | 1 (5%) | 0 (0) |
| Dysphonia | 2 (10%) | 0 (0) |
| Rash | 2 (10%) | 0 (0) |
Adverse events are listed if they were reported in at least 5% of patients in either treatment group. ALP, alkaline phosphatase; GGT, g-glutamyl transferase; LDH, lactate dehydrogenase.
Molecular Biomarkers
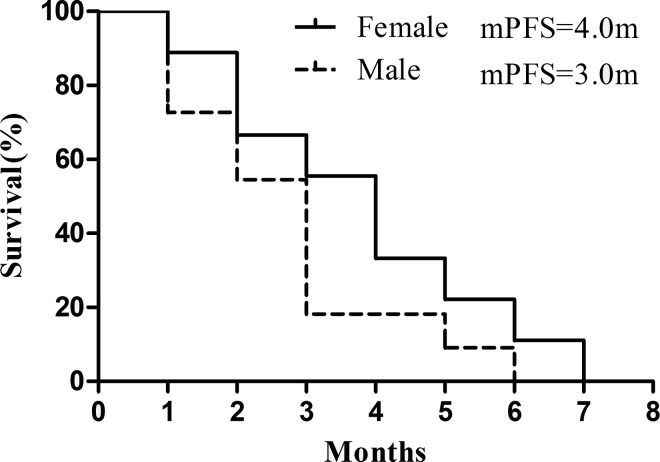
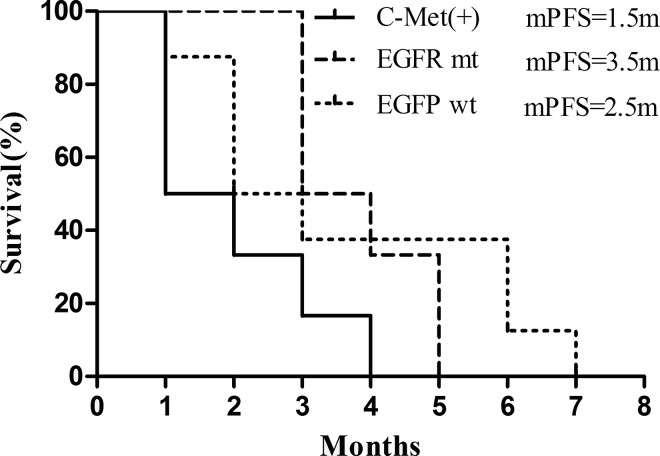
Most of these patients had accepted multiple gene detection, such as EGFR, ALK, BRAF, c-Met, ROS1, and RET. Besides EGFR mutation, c-Met amplification was the main molecular alteration observed. Six patients had c-Met amplification, with varied amplification ratio from 15% to 40%; subgroup analysis indicated no PFS difference existed between male and female patients (see Fig. 2). Surprisingly, patients with c-Met amplifications showed a lower response rate toward apatinib, with the median PFS (mPFS) being only 1.5 months. In contrast, a higher clinical activity was observed for patients with EGFR mutations (mPFS = 3.5 months), suggesting that the underlying gene mutation profile may predict the clinical efficacy of apatinib treatment (see Fig. 3). However, the conclusions above need to be further proven in large, prospective trials. Moreover, most of the six patients who had PR experienced serious skin reactions, such as rash and oral ulcer, indicating that the severe skin reaction might be utilized as a potential pharmacodynamic biomarker to predict clinical efficacy.
Figure 2.
Comparison of PFS in female and male patients.
Figure 3.
Comparison of PFS in patients with different gene profiles.
DISCUSSION
With the increasing focus on precision medicine and the continued expansion on the number of targeted agents, targeted therapies have become an important treatment option for patients with a wide range of human cancers1. However, to date, there remain only a small handful of human cancers for which there are potentially actionable mutations, and these cancer types include NSCLC, melanoma, breast cancer, and ovarian cancer. The application of drugs targeting so-called oncogenic driver mutations has greatly changed the landscape of cancer treatment. It is now widely accepted that TKIs are the first choice for NSCLC patients with EGFR mutations, ALK translocation, and ROS1 amplification15.
The process of angiogenesis has been viewed as important for the growth of primary tumors as well as metastatic disease, and significant efforts have focused on developing either antibodies or small molecules that inhibit key angiogenic signaling pathways. The VEGF signaling pathway has been most intensely studied to date, and there are now several anti-VEGF agents that are used to treat a wide range of human cancers, including colorectal cancer (CRC)5, NSCLC3, ovarian cancer, and renal cell carcinoma. These anti-VEGF agents are used either alone (apatinib for gastric cancer, sorafenib for RCC) or in combination with other systemic therapies (bevacizumab, aflibercept, and ramucirumab combined with FOLFIRI chemotherapy for metastatic CRC). From the perspective of drug application, antiangiogenesis therapies now account for nearly one third of the targeted therapies that have been approved by the Food and Drug Administration (FDA) since 200516.
With respect to NSCLC, antiangiogenesis therapies are used in both the first- and second-line treatment settings. There are three antiangiogenic drugs that are currently approved in NSCLC by the FDA, and they include bevacizumab4, ramucirumab3, and nintedanib2. An antiangiogenesis strategy may be especially useful for patients with no specific driver mutations or for those who have disease progression on TKI treatment13. Moreover, for NSCLC and CRC, there is compelling evidence for the enhanced efficacy of combination regimens that include systemic chemotherapy plus an antiangiogenic agent. Although these strategies have been shown to be effective in the clinic, further work needs to be done with respect to drug selection, optimal drug combination, and dose and sequencing of agents to further improve the treatment efficacy16.
Apatinib is an interesting small molecule that targets the VEGFR-2-associated tyrosine kinase17. It was first approved by the Chinese FDA for the treatment of gastric cancer7,8 and is being tested in other human cancers, including breast cancer and hepatocellular cancer. While apatinib has demonstrated clinical activity in these various cancers, most of these studies are small case reports. Our own group discovered that the combination of apatinib and pemetrexed/docetaxel had clinical activity in advanced NSCLC in the second- and third-line treatment settings. With a PFS of 2.8 months, apatinib has shown a clinical efficacy equivalent to ramucirumab and nintedanib2,3. However, apatinib is associated with a much higher ORR than these other anti-VEGF agents. In other clinical studies testing apatinib in gastric cancer, glioma, or ovarian cancer, the ORR has been reported to be less than 10%, yet the mPFS is comparable to what we report herein. This finding suggests that PFS may be the better parameter to judge clinical efficacy, which is similar to what is now being used to assess clinical efficacy of anti-PD-1/L1 treatment18.
One of the potential shortcomings of our study was the relatively small sample size (only 20 patients were included). It is possible that with the size increase of similar studies, the PFS of the combination of apatinib and chemotherapy would be improved obviously. Although our study was retrospective in nature, we had carefully analyzed the patient characteristics, and there were no significant patient imbalances that might have skewed the study results. Our patients experience the typical AEs associated with antiangiogenic drugs, such as hypertension, proteinuria, HFS, and gastrointestinal (GI) toxicity. Although about 30% of our patients required an interruption in drug administration, most of these common AEs were well tolerated, and the appropriate management of these AEs ensures the continued treatment of these patients.
The identification of key predictive biomarkers is critical for the development of targeted therapies. With this in mind, we attempted to explore the potential association between apatinib clinical efficacy and various mutational events, such as EGFR, c-Met, RET, and others. While our molecular analysis identified higher c-Met amplification in our patients, we were unable to detect an association between clinical efficacy of apatinib and the expression level of c-Met amplification. As it is now well established that other antiangiogenic inhibitors, such as sorafenib, sunitinib, and cabozantinib19, target a wide range of signaling pathways beyond VEGF, it is certainly conceivable that apatinib may also target additional signaling pathways beyond VEGFR-2. Moreover, as in the case of bevacizumab and ramucirumab, apatinib may enhance the clinical activity of systemic chemotherapy by enhancing tumor blood flow delivery.
In addition to NSCLC, the combination of apatinib and capecitabine has demonstrated clinical activity in the maintenance therapy of ovarian cancer20. Currently, the combination of apatinib and chemotherapy is being tested in 30 different clinical trials in various tumor types. With respect to NSCLC, low-dose apatinib is being combined with the fluoropyrimidine S-1 in patients with advanced NSCLC (NCT02829385), and apatinib at a dose of 500 mg is being studied in combination with docetaxel (60 mg/m2) in advanced NSCLC (NCT02780778).
One observation resulting from our study relates to the fact that most of the patients who were found to have had a PR to therapy experience severe skin reactions. This finding suggests that the intensity of the skin reaction has the potential to serve as a pharmacodynamic biomarker to predict the clinical activity of apatinib21,22. However, because of the limited size of our study, further work is required to explore the therapeutic potential of apatinib in combination with systemic chemotherapy and to identify potential molecular and/or pharmacodynamic biomarkers that can be used to identify the subset of patients who would respond to apatinib-based regimens23,24.
REFERENCES
- 1. Li F, Liao Z, Zhao J, Zhao G, Li X, Du X, Yang Y, Yang J. Efficacy and safety of Apatinib in stage IV sarcomas: Experience of a major sarcoma center in China. Oncotarget 2017;8(38):64471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, Gann CN, Barrueco J, Gaschler-Markefski B, Novello S. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143–55. [DOI] [PubMed] [Google Scholar]
- 3. Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, Czyzewicz G, Orlov SV, Lewanski CR, Thomas M, Bidoli P, Dakhil S, Gans S, Kim JH, Grigorescu A, Karaseva N, Reck M, Cappuzzo F, Alexandris E, Sashegyi A, Yurasov S, Pérol M. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384(9944):665–73. [DOI] [PubMed] [Google Scholar]
- 4. Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, Feng J, He J, Han B, Wang J, Jiang G, Hu C, Zhang H, Cheng G, Song X, Lu Y, Pan H, Zheng W. BEYOND: A randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small cell lung cancer. J Clin Oncol. 2015;33(19):2197–204. [DOI] [PubMed] [Google Scholar]
- 5. Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, Prausová J, Garcia-Alfonso P, Yamazaki K, Clingan PR, Lonardi S, Kim TW, Simms L, Chang SC, Nasroulah F; RAISE Study Investigators. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. [DOI] [PubMed] [Google Scholar]
- 6. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A; RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35. [DOI] [PubMed] [Google Scholar]
- 7. Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, Cheng Y, Wang Z, Zheng L, Tao M, Zhu X, Ji D, Liu X, Yu H. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: Results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31(26):3219–25. [DOI] [PubMed] [Google Scholar]
- 8. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–54. [DOI] [PubMed] [Google Scholar]
- 9. Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, Ma XX, To KK, Ambudkar SV, Chen ZS, Fu LW. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70(20):7981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin C, Wang S, Xie W, Zheng R, Gan Y, Chang J. Apatinib inhibits cellular invasion and migration by fusion kinase KIF5B-RET via suppressing RET/Src signaling pathway. Oncotarget 2016;7(37):59236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scott AJ, Messersmith WA, Jimeno A. Apatinib: A promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc) 2015;51(4):223–9. [DOI] [PubMed] [Google Scholar]
- 12. Ding L, Li QJ, You KY, Jiang ZM, Yao HR. The use of apatinib in treating nonsmall-cell lung cancer: Case report and review of literature. Medicine (Baltimore) 2016;95(20):e3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langer CJ, Mok T, Postmus PE. Targeted agents in the third-/fourth-line treatment of patients with advanced (stage III/IV) non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2013;39(3):252–60. [DOI] [PubMed] [Google Scholar]
- 14. Fang SC, Zhang HT, Zhang YM, Xie WP. Apatinib as post second-line therapy in EGFR wild-type and ALK-negative advanced lung adenocarcinoma. Onco Targets Ther. 2017;10:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Y, Zhang W, Tang F, Luo Y, Min L, Zhang W, Shi R, Duan H, Tu C. A case report of apatinib in treating osteosarcoma with pulmonary metastases. Medicine (Baltimore) 2017;96(15):e6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song Z, Yu X, Lou G, Shi X, Zhang Y. Salvage treatment with apatinib for advanced non-small-cell lung cancer. Onco Targets Ther. 2017;10:1821–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther. 2015;9:6075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33(31):3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K, Roth BJ, Bjarnason GA, Géczi L, Keam B, Maroto P, Heng DY, Schmidinger M, Kantoff PW, Borgman-Hagey A, Hessel C, Scheffold C, Schwab GM, Tannir NM, Motzer RJ; METEOR Investigators. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deng L, Wang Y, Lu W, Liu Q, Wu J, Jin J. Apatinib treatment combined with chemotherapy for advanced epithelial ovarian cancer: A case report. Onco Targets Ther. 2017;10:1521–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fornaro L, Vasile E, Falcone A. Apatinib in advanced gastric cancer: A doubtful step forward. J Clin Oncol. 2016;34(31):3822–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee HJ, Moon JY, Baek SW. Is treatment-emergent toxicity a biomarker of efficacy of apatinib in gastric cancer? J Clin Oncol. 2016;34(31):3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang S. Problematic analysis and inadequate toxicity cata in phase III apatinib trial in gastric cancer. J Clin Oncol. 2016;34(31):3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hellmann MD, Sturm I, Trnkova ZJ, Lettieri J, Diefenbach K, Rizvi NA, Gettinger SN. Preliminary safety, pharmacokinetics, and efficacy of regorafenib, cisplatin, and pemetrexed in patients with advanced nonsquamous non-small-cell lung cancers. Clin Lung Cancer 2015;16(6):514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]