Abstract
Emerging evidence suggests that 17β-estradiol (E2) and estrogen receptor (ER) signaling are protective against hepatocellular carcinoma (HCC). In our previous study, we showed that E2 suppressed the carcinogenesis and progression of HCC by targeting NLRP3 inflammasome activation, whereas the molecular mechanism by which the NLRP3 inflammasome initiated cancer cell death was not elucidated. The present study aimed to investigate the effect of NLRP3 inflammasome activation on cell death pathways and autophagy of HCC cells. First, we observed an increasing mortality in E2-treated HCC cells, and then apoptotic and pyroptotic cell death were both detected. The mortality of HCC cells was largely reversed by the caspase 1 antagonist, YVAD-cmk, suggesting that E2-induced cell death was associated with caspase 1-dependent pyroptosis. Second, the key role of the NLRP3 inflammasome in autophagy of HCC cells was assessed by E2-induced activation of the NLRP3 inflammasome, and we demonstrated that autophagy was inhibited by the NLRP3 inflammasome via the E2/ERβ/AMPK/mTOR pathway. Last, the interaction of pyroptosis and autophagy was confirmed by flow cytometry methods. We observed that E2-induced pyroptosis was dramatically increased by 3-methyladenine (3-MA) treatment, which was abolished by YVAD-cmk treatment, suggesting that caspase 1-dependent pyroptosis was negatively regulated by autophagy. In conclusion, E2-induced activation of the NLRP3 inflammasome may serve as a suppressor in HCC progression, as it triggers pyroptotic cell death and inhibits protective autophagy.
Key words: Hepatocellular carcinoma (HCC), NLRP3 inflammasome, Pyroptosis, Autophagy
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant cancers and the third leading cause of cancer-related deaths worldwide. Men are two to seven times more likely to develop HCC than women, despite equal exposure to major risk factors, such as infection to hepatitis B or smoking1. Epidemiologic evidence has shown that 17β-estradiol (E2) may have a protective role in repressing HCC growth in females2,3. In our previous study, we found that the NLRP3 inflammasome was activated by E2/estrogen receptor (ER) β signaling, which could inhibit HCC progression, whereas the mechanism of NLRP3 inflammasome-initiated cancer cell death was not elucidated4,5. Hence, a deep exploration of the effect of the NLRP3 inflammasome activation mediated by E2 on HCC cells is needed to provide mechanistic insights.
The NLRP3 inflammasome is an intracellular multiprotein complex consisting of NLRP3, ASC, and procaspase 1. Activation of the NLRP3 inflammasome can regulate the release of the proinflammatory cytokines interleukin (IL)-1β and IL-186,7. Despite leading to inflammatory responses, another important role of NLRP3 inflammasome activation is to induce pyroptosis, a proinflammatory form of programmed cell death (PCD). Pyroptosis is dependent on caspase 1 release, which features rapid plasma membrane rupture and release of proinflammatory intracellular contents8,9. Pyroptosis was discovered only recently and has complemented research on the intensively studied pathways of apoptosis, necroptosis, and autophagy10. Cancer cells can acquire the ability to resist apoptosis and survive under stress, known as anoikis resistance11,12. Therefore, it would be of interest to determine which cell death pathway is primarily responsible for E2-induced mortality in HCC cells.
Autophagy is a homeostatic degradative process that removes damaged organelles or turns over cytoplasmic constituents via lysosomal compartments in eukaryotic cells. Although autophagy was initially identified to enhance cell survival, increasing evidence shows that a high level of autophagy is involved in cell death13,14. Recent observations have demonstrated an inverse relationship between autophagy induction and maturation of the NLRP3 inflammasome15,16. However, it remains unclear whether activation of the NLRP3 inflammasome will have an effect on autophagy, and we investigate the issue in this study.
Given the above information, the aim of this study was to investigate whether NLRP3 inflammasome activation induced by E2 interferes with cellular signaling pathways influencing pyroptosis and autophagy in HCC cells. In addition, we investigate the relationship between autophagy and pyroptosis. Our results reveal a regulatory effect of autophagy inhibition on pyroptosis induction, which provides a scientific basis for the suppressive effect of E2-induced NLRP3 inflammasome activation on HCC development.
MATERIALS AND METHODS
Cell Culture and Treatment
The human HepG2 hepatoma cell line was purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, P.R. China). Cells were maintained in RPMI-1640 medium (Gibco by Invitrogen, Carlsbad, CA, USA), supplemented with 10% FBS at 37°C and 5% CO2 conditions. Cells were treated with E2 for 24 h.
Reagents and Antibodies
All reagents were commercially obtained and of analytical grade. E2, PHTPP, YVAD-cmk, 3-methyladenine (3-MA), and rapamycin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies used for Western blot were as follows: caspase 3, caspase 8, caspase 9, LC3-b, beclin 1, P62, phosphor-mTOR (Ser2448), phosphor-S6K1 (Thr389), phosphor-AMPKα (Thr172), and β-actin. Antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). All antibodies were used in a dilution of 1:1,000.
Trypan Staining, CCK-8, and Lactate Dehydrogenase (LDH) Release Experiments
Cell viability was measured with trypan staining and a CCK-8 assay (Dojindo, Kumamoto, Japan). Trypan blue solution (0.1%) was added into the cell suspension for 3–5 min. Then the number of dead cells (stained with blue) and living cells (without blue color) out of the total cells (counting 1,000 cells per group) were counted. The cells viability rate = number of living cells/(number of dead cells + living cells) × 100%. The CCK-8 assay was performed according to the manufacturer’s protocol. Briefly, 10 μl of CCK-8 solution was added to each well of a 96-well plate and incubated at 37°C for 4 h. The optical density was measured at an absorption wavelength of 450 nm. Results were normalized to control levels.
Cell death was measured by an LDH release assay. LDH is released from cells into a culture medium upon cell lysis. Cells were plated in 24-well plates. At 12 h after E2 exposure, the supernatant was collected, and LDH activity was assessed by determining the amount of NADH generated in a reaction between NAD+ and lactate (L-type LD. J; Wako, Osaka, Japan).
Western Blotting
Total proteins were extracted after digesting the cells with lysis buffer. Then 60 μg of protein was resolved on a 12% SDS-PAGE gel and transferred onto PVDF membranes. Membranes were blocked with a 5% skimmed milk solution and incubated overnight at 4°C with the primary antibodies beclin 1, LC3-b, P62, p-AMPKα, p-mTOR, p-S6K1, or β-actin diluted in TBST. The membranes were then washed and incubated with secondary antibody for 1 h. Immunoreactivity was detected with Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL, USA). ImageJ software (Rawak Software, Inc., Germany) was used to quantify the optical density of each band.
RNA Analysis
The total RNA samples were extracted from cells or tumor tissues using TRIzol reagent (Invitrogen) according to the manufacturer’s directions. Total RNA for 2 μg was reverse transcribed to cDNA using the Reverse Transcription Master Kit (Toyobo, Osaka, Japan).
Equal amounts of cDNA were subjected to PCR with the condition including an initial denaturation at 94°C for 5 min, followed by 40 cycles of 94°C for 30 s, 54°C for 30 s, 72°C for 30 s, and a terminal extension at 72°C for 5 min. After the reaction, 10 μl of the PCR products was used for running in agarose gels, stained with ethidium bromide, and visualized using UV transillumination.
Real-time (RT)-PCR was performed on ABI 7500 fast system (Applied Biosystems, Foster City, CA, USA) using SYBR Green I (Toyobo). Each sample was examined in triplicate, and β-actin was used as the internal control. The relative quantification of gene expression was determined using the 2−ΔΔCT method. GraphPad Prism v5 (GraphPad Software, San Diego, CA, USA) was used to create scatter plots.
Quantification of Cellular Autophagy Using Confocal Fluorescence Microscopy
The cellular autophagic level of HCC cells was examined with the Cyto-ID® Green Autophagy Detection Kit. In brief, cells were seeded in an eight-well chamber slide at about 40% confluence. The cells were treated with E2 at 100 nM for 24 h. In some experiments, cells were pretreated with PHTPP or YVAD-cmk for 0.5 h, and then 100 nM of E2 was added. After incubation, the cells were washed twice with 1× assay buffer and stained with Cyto-ID® detection agent according to the manufacturer’s instructions. The cells were examined on an inverted fluorescence microscope.
Flow Cytometry
Pyroptosis was assessed by double-positive staining of activated caspase 1 and propidium iodide (PI) in cells using FAM-FLICA Caspase-1 Assay Kit (ImmunoChemistry Technologies, LCC, Bloomington, MN, USA) according to the manufacturer’s instructions. Stained cells were then analyzed by flow cytometry (BD FACSCalibur, Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Statistical Analyses
Data were analyzed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and expressed as the mean ± standard deviation (SD). Statistical significance was examined by two-tailed Student’s t-test and one-way ANOVAs. For all of the analyses, values of p < 0.05 were considered statistically significant.
RESULTS
E2-Initiated NLRP3 Inflammasome Activation Induces HCC Cell Death
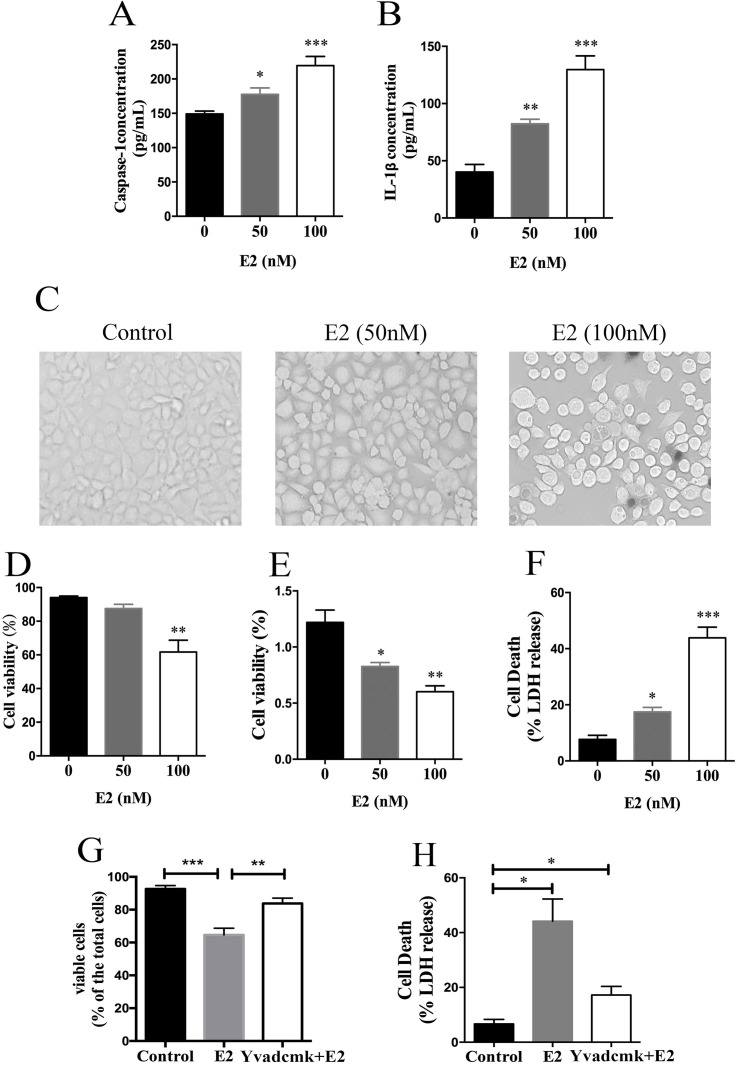
As shown in our previous work, the NLRP3 inflammasome was activated by E2 treatment in HCC cells. The expression of caspase 1 and IL-1β was first measured using ELISA in the present study to confirm our previously published result. Caspase 1 and IL-1β expressions were significantly upregulated in E2-treated HCC cells in a dose-dependent manner, suggesting that activation of the NLRP3 inflammasome was induced by E2 (Fig. 1A and B). Then we observed that cells turned round in shape and appeared to swell after treatment with E2, suggesting that E2 caused cell death of HepG2 cells (Fig. 1C). The cytotoxic effect of E2 on HepG2 cells was assessed using a trypan blue staining assay, CCK-8 test, and LDH release assay. As shown in Figure 1D–F, cell viability was gradually decreased and mortality rate was increased under the treatment of E2 in a dose-dependent manner.
Figure 1.
17β-Estradiol (E2)-initiated NLRP3 inflammasome activation induces hepatocellular carcinoma (HCC) cell death. (A, B) HepG2 cells were treated with E2 at concentrations of 50 nM and 100 nM for 24 h, and then caspase 1 and IL-1β expressions were detected by ELISA. (C) HepG2 cells were treated with 100 nM of E2, and the morphology of the cells was observed with a phase-contrast microscope. Scale bars: 100 μm. (D–F) HepG2 cells were treated with E2 at concentrations of 50 nM and 100 nM for 24 h, and then cell viability was detected by trypan blue stain assay, CCK-8 test, and lactate dehydrogenase (LDH) release assay. (G, H) HepG2 cells were pretreated with YVAD-cmk (50 μM) before E2 (100 nM) treatment, and cell viability and mortality were detected by trypan blue and LDH release assays. *p < 0.05, **p < 0.01, ***p < 0.001.
To confirm whether the cytotoxic effect of E2 on HepG2 cells was mediated by the NLRP3 inflammasome, we pretreated cells with caspase 1-specific inhibitor YVAD-cmk before E2 treatment, and cell viability and mortality were detected. YVAD-cmk treatment markedly reversed the E2-induced growth inhibitory effect on HepG2 cells, as shown in Figure 1G and H.
NLRP3 Inflammasome Activation Results in Caspase 1-Dependent Pyroptosis
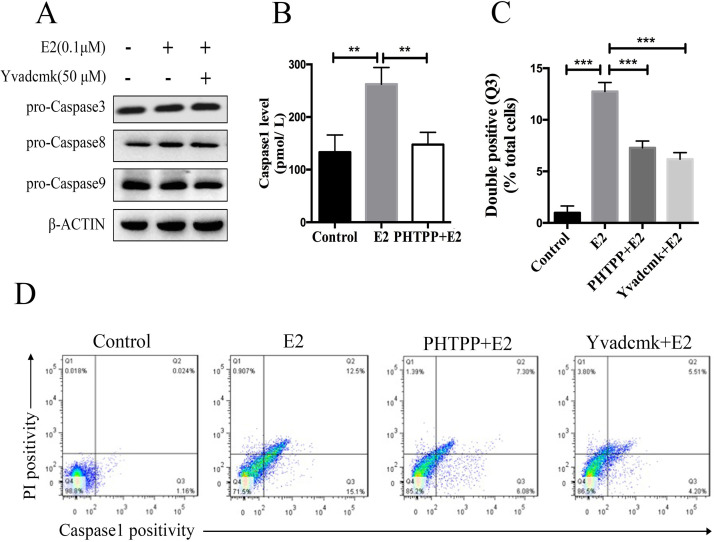
Several forms of cell death were acknowledged, including apoptosis, necroptosis, and autophagic cell death. Pyroptosis, a term coined by Cookson and Brannan8 a decade ago, is a distinct form of PCD that differs from apoptosis and is characterized by its dependence on caspase 1 activation. To further identify the major cell death pathway induced by NLRP3 inflammasome activation, we detected both apoptotic and pyroptotic levels in E2-treated HCC cells. First, we analyzed the activation of caspases 3, 8, and 9, which represented critical enzymes in apoptotic pathway by Western blot. As shown in Figure 2A, there was no apparent difference of probands of caspases 3, 8, and 9 among the control group, E2 group, and YVAD-cmk plus E2 group. In addition, cleaved bands of the three molecules were all negative. Then pyroptotic cell death was assessed by measuring the caspase 1 level by ELISA and double-positive staining of activated caspase 1 and PI. ELISA analysis revealed that E2 treatment significantly promoted caspase 1 production in supernatant compared to controls, while E2/ERβ signaling blockade by PHTPP abolished the caspase 1 induction by E2 treatment, indicating that the NLRP3 inflammasome activation resulted in pyroptotic cell death in E2-treated HCC cells (Fig. 2B). To further confirm the notion, we exposed HCC cells to E2 and evaluated pyroptotic cells using double-positive staining of activated caspase 1 and PI by flow cytometry. As shown in Figure 2C and D, the proportion of double-positive cells (Q3) significantly increased in the E2 group in comparison with the control group, which was significantly decreased by PHTPP or YVAD-cmk treatment (12.50%, 7.30%, and 5.51%, p < 0.001).
Figure 2.
NLRP3 inflammasome activation results in caspase 1-dependent pyroptosis. (A) HepG2 cells were pretreated with YVAD-cmk (50 μM) before E2 (100 nM) treatment, and expressions of caspases 3, 8, and 9 were detected by Western blot. (B) HepG2 cells were pretreated with YVAD-cmk (50 μM) before E2 (100 nM) treatment, and caspase 1 expression was tested by ELISA. (C) Detected double positivity of caspase 1 fluorescent inhibitor probe (FAM-YVAD-FMK) and PI (Q3) using flow cytometry after 24 h. The proportions of Q3 were then presented with a histogram. (D) Representative flow cytometry scatter plots. Bars represent mean ± SEM. **p < 0.01, ***p < 0.001.
NLRP3 Inflammasome Activation Inhibits Autophagy in HCC Cells
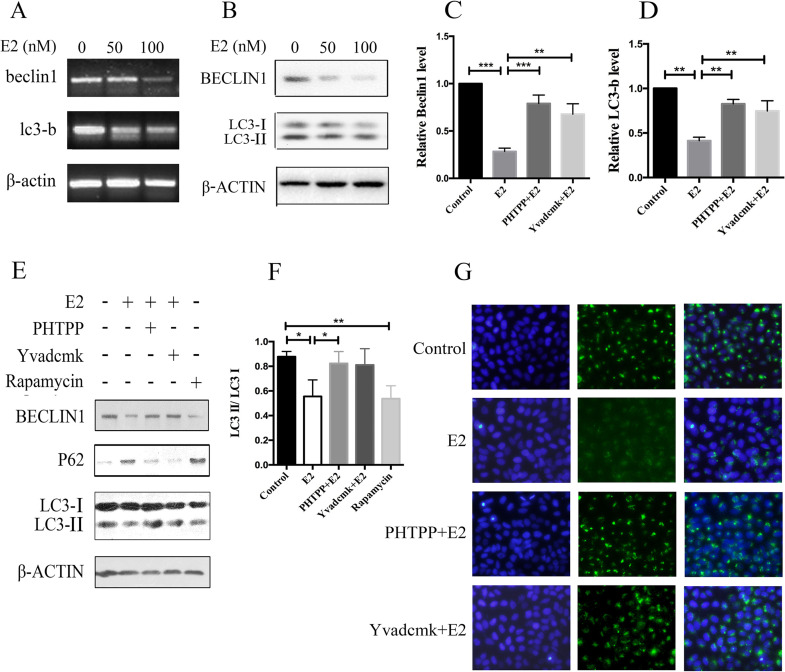
Emerging evidence suggests that autophagy dysfunction plays an essential role in NLRP3 inflammasome activation. However, not many studies have been performed concerning the effect of the NLRP3 inflammasome on autophagic activity. We observed that the expression of autophagy-related markers in the groups exposed to E2 (50 and 100 nM) illustrated a dose-dependent decrease at both mRNA and protein levels (Fig. 3A and B). As shown in Figure 3C–F, the protein expression of beclin 1 and LC3 II/I ratio markedly (p < 0.05) decreased after E2 treatment at 100 nM. Moreover, E2 treatment significantly increased the level of P62 compared to the control cells. In addition, the Cyto-ID® Green reagent staining also demonstrated decreased autophagic vacuole formation in E2-treated cells compared with the control cells, which was restored under THTPP or YVAD-cmk treatment, suggesting that E2-induced inhibition of autophagy was mediated by NLRP3 inflammasome activation in HCC cells (Fig. 3G).
Figure 3.
NLRP3 inflammasome activation inhibits autophagy in HCC cells. HepG2 cells were treated with different concentrations of E2 (0, 50 nM, and 100 nM) for 24 h. mRNA expressions of beclin 1 and LC3-b were detected by real-time (RT)-PCR (A), and protein levels of beclin 1 and LC3 were tested by Western blot (B). HepG2 cells were pretreated with PHTPP (1 μM) or YVAD-cmk (50 μM) before E2 (100 nM) treatment. mRNA expressions of beclin1 and LC3-b were detected by real-time PCR (C, D), and protein levels of beclin 1, LC3, and P62 were tested by Western blot (E, F). (G) Cytoplasmic expression of LC3-b in HepG2 cells. Cells were treated with 100 nM of E2 alone or in combination with 1 μM of PHTPP or 50 μM of YVAD-cmk and incubated for 24 h. The level of autophagy was evaluated using a Cyto-ID® detection agent on an inverted fluorescence microscope. Scale bar: 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001.
AMPK/mTOR Pathway Is Required for E2-Induced Autophagy Inhibition
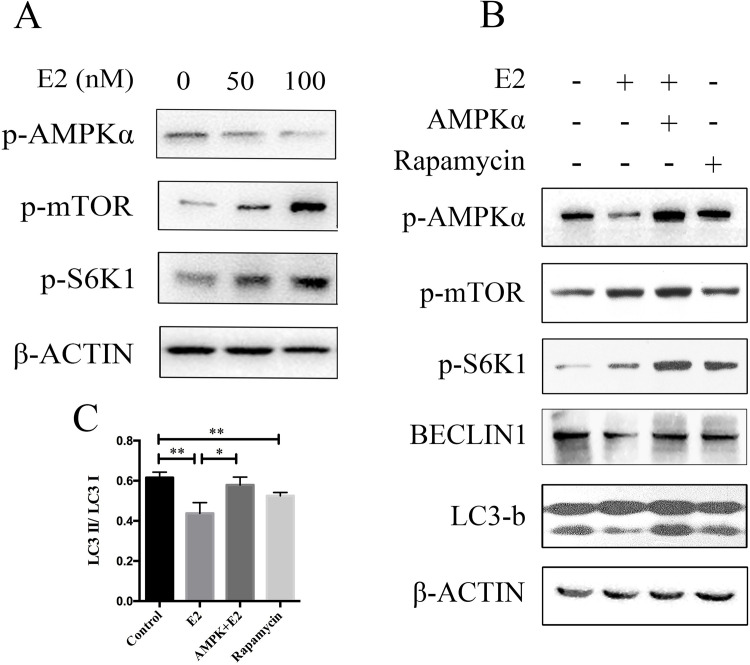
Abundant evidence has shown that the AMP-activated protein kinase (AMPK)/mTOR signaling pathway is essential in mediating autophagy. To determine whether AMPK/mTOR signaling was involved in E2-induced inhibition of autophagy, HepG2 cells were treated with increasing doses of E2, and phosphorylation of key enzymes in the AMPK/mTOR pathway was detected by Western blotting. As shown in Figure 4A, AMPK phosphorylation was gradually decreased, while the phosphorylation of mTOR and S6K1 was increased in an E2 dose-dependent manner. To confirm that the AMPK/mTOR pathway plays a key role in autophagic regulation, cells were transfected with AMPK or incubated with rapamycin (100 nM, an mTOR inhibitor) in the absence or presence of 100 nM of E2. We found that E2-induced beclin 1 and LC3-b degradation were reversed in AMPK-overexpressed cells and mTOR-deficient cells (Fig. 4B and C). Therefore, these results suggest that the AMPK/mTOR signaling pathway is involved in E2-induced autophagy inhibition.
Figure 4.
AMP-activated protein kinase (AMPK)/mTOR pathway is required for E2-induced autophagy inhibition. (A) HepG2 cells were treated with different concentrations of E2 (0, 50 nM, and 100 nM) for 24 h, and phosphorylation of key enzymes in AMPK/mTOR pathway were tested by Western blot. (B) HepG2 cells were pretreated with pEGFP-C1-AMPK or rapamycin (100 nM) before E2 (100 nM) treatment, and phosphorylation of key enzymes in AMPK/mTOR pathway were tested by Western blot. (C) The intensity of the LC3 band was quantified by LC3 II/I ratio using the densitometry analysis. *p < 0.05, **p < 0.01.
Autophagy Deficiency Promotes Pyroptosis in HCC Cells
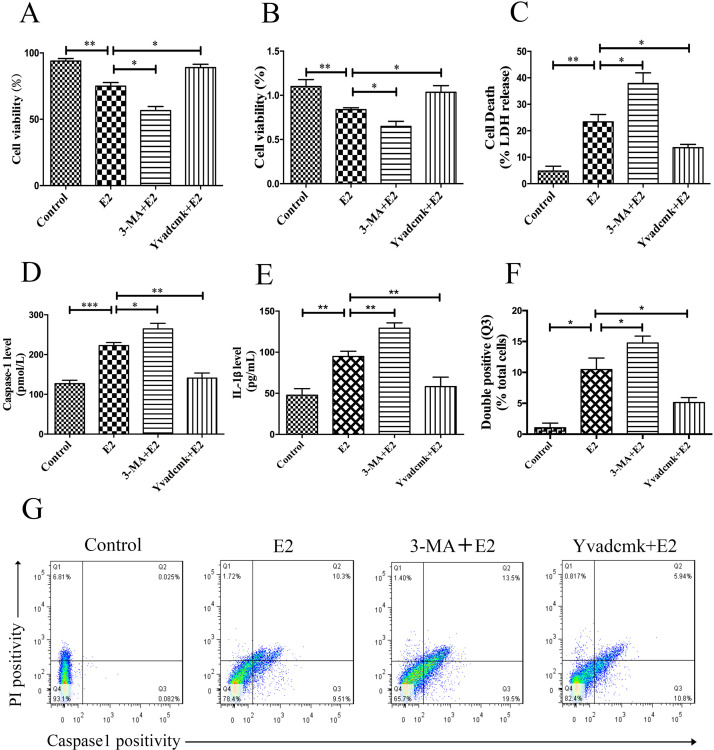
According to the results above, we considered that pyroptosis and autophagy may be involved in the regulation of the cell fate of HCC cells. We sought to determine whether autophagy benefits survival or accelerates death of HCC cells. Furthermore, we investigated the relationship of pyroptosis and autophagy in E2-treated cells. The cell mortality was much higher when autophagy was inhibited by 3-MA, while YVAD-cmk could protect cells from cell death (Fig. 5A–C).
Figure 5.
Autophagy deficiency promotes pyroptosis in HCC cells. (A–C) HepG2 cells were pretreated with 3-MA (50 μM) or YVAD-cmk (50 μM) before E2 (100 nM) treatment, and cell viability and mortality were detected by trypan blue, CCK-8, and LDH release assays. (D, E) HepG2 cells were pretreated with 3-MA (50 μM) or YVAD-cmk (50 μM) before E2 (100 nM) treatment. Caspase 1 and IL-1β expressions were tested by ELISA. (F) Detected double positivity of caspase 1 fluorescent inhibitor probe (FAM-YVAD-FMK) and PI (Q3) using flow cytometry after 24 h. The proportions of Q3 were then presented with a histogram. (G) Representative flow cytometry scatter plots. Bars represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
To investigate if the increase in cell mortality occurred by pyroptotic cell death, we executed a caspase 1 release test and double-positive staining of PI and caspase 1. Compared to the control, the E2-treated group had a trend toward elevated caspase 1 and IL-1β levels, whereas 3-MA treatment further upregulated these expressions (Fig. 5D and E). Moreover, the caspase 1 inhibitor YVAD-cmk could abolish the induction of caspase 1 and IL-1β by E2 treatment. Similarly, the proportion of double-positive (activated caspase 1 and PI) cells significantly increased in the E2 group (10.3%) and 3-MA plus E2 group (13.5%), while this proportion dramatically decreased in the YVAD-cmk plus E2 group (5.94%; p < 0.05) (Fig. 5F and G).
DISCUSSION
E2 has been suggested to have a protective role in attenuating HCC development for a few decades. In a previous study, we have clarified the protective role of the E2/ERβ pathway and its downstream targeting of the NLRP3 inflammasome in HCC progression4,5. However, the molecular mechanism by which the NLRP3 inflammasome initiated cancer cell death was not well documented. In the present study, we investigated whether the cytotoxicity induced by E2 could be mediated by pyroptotic cell death in HCC cells. Here we have demonstrated that E2 can induce NLRP3 inflammasome activation and caspase 1 release, which triggers pyroptosis. In addition, our results show that inhibition of autophagy was mediated by NLRP3 inflammasome activation under E2 treatment in HCC cells. Therefore, both of these events decide the fate of HCC cells.
Pyroptosis is a newly discovered pathway of PCD, which is dependent on caspase 1 activation. It is involved in the pathologic process of several kinds of cancers, and its beneficial effect against HCC has been reported17–19. The NLRP3 inflammasome could be either beneficial or detrimental depending on different pathological conditions20,21. It is a key signaling platform that detects pathogenic microorganisms and activates the proinflammatory cytokines IL-1β and IL-18, which is critically correlated with inflammation and cancer development22. On the other hand, the potential of NLRP3 inflammasome activation in inducing cell death against cancer cells exerts beneficial effects23. Our data show that E2 restored the abnormal downregulation of the NLRP3 inflammasome in HCC cells and that the downstream cell death was mediated by pyroptosis, but not apoptosis. The effect was attenuated by the caspase 1 inhibitor, YVAD-cmk. These findings suggest that the anticancer efficacy of E2 could be ascribed, at least partially, to its ability for triggering pyroptosis via activation of the NLRP3 inflammasome. Chu et al.17 reported that pyroptosis is inhibited in HCC tissues and cells, and that activation of caspase 1-dependent pyroptosis offers a therapeutic potential against HCC, which is consistent with our findings.
It has been widely accepted that autophagy, a cellular waste removal and rejuvenation process, serves an important role as a negative regulator of the NLRP3 inflammasome. We assume that there is a crosstalk between them, and we would like to investigate the role of the NLRP3 inflammasome in modulating the autophagic response in E2-treated HCC cells. Our investigation shows that activation of the NLRP3 inflammasome, induced by E2, decreased autophagic levels, and the effect was reversed by the caspase 1 inhibitor, YVAD-cmk. Consistent with this finding, Deng et al.24 also observed that overexpression of the NLRP3 inflammasome core molecules or treatment with IL-1β had the regulatory role of autophagy in Pseudomonas aeruginosa-infected macrophages. These findings indicate that the relationship between autophagy and the NLRP3 inflammasome is intimately linked and intertwined. To the best of our knowledge, this is the first report showing that autophagy could be regulated by the NLRP3 inflammasome in tumor cells.
As it has been reported that autophagy is activated by AMPK and inhibition of mTOR, we evaluated the AMPK/mTOR signaling after E2 treatment in our present study25,26. Of note, autophagy was inhibited by decreasing the phosphorylation of AMPK and activating mTOR and S6K1. Furthermore, enhancing AMPK expression or inhibiting mTOR by rapamycin reversed autophagic level. These results suggest that E2 decreased autophagy through the AMPK/mTOR signaling pathway.
Regarding cancer, autophagy functions as a double-edged sword. It not only protects tumor cells from nutrient deprivation and other cellular stresses, functioning as a tumor-suppressing mechanism, but autophagy also promotes the malignant progression according to different cancer cell types and pathological stages27,28. The present study shows that autophagy deficiency promotes pyroptosis in HCC cells, which provides a new insight for the treatment of HCC via modulation of the extent of autophagy.
In summary, our study demonstrates that E2-induced activation of the NLRP3 inflammasome leads to caspase 1-dependent pyroptosis and has unraveled a novel role of the NLRP3 inflammasome in modulating the autophagic response. Furthermore, we show that inhibition of autophagy promotes pyroptosis in HCC cells. Our study reveals a novel molecular mechanism by which E2-induced NLRP3 inflammasome activation suppresses HCC progression, suggesting that pyroptosis and inhibiting autophagy may be a potential neoplastic target for the treatment of HCC.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81501813, No. 81702062). M.L. and Q.W. conceived and designed the study. Q.W., R.Z., J.Y., and R.P. performed the experiments. Q.W. and J.Y. wrote the manuscript. J.Y. and R.P. reviewed and revised the manuscript. All authors read and approved the manuscript and agreed to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142(6):1264–73.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: The roles of sex hormones. Oncology 2010;78(Suppl 1):172–9. [DOI] [PubMed] [Google Scholar]
- 3. Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007;317(5834):121–4. [DOI] [PubMed] [Google Scholar]
- 4. Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X, Zhao W, Huai W, Guo P, Han L. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest. 2014;94(1):52–62. [DOI] [PubMed] [Google Scholar]
- 5. Wei Q, Guo P, Mu K, Zhang Y, Zhao W, Huai W, Qiu Y, Li T, Ma X, Liu Y, Han L. Estrogen suppresses hepatocellular carcinoma cells through ERbeta-mediated upregulation of the NLRP3 inflammasome. Lab Invest. 2015;95(7):804–16. [DOI] [PubMed] [Google Scholar]
- 6. Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265(1):6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity 2017;47(1):15–31. [DOI] [PubMed] [Google Scholar]
- 8. Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–4. [DOI] [PubMed] [Google Scholar]
- 9. Tsai YM, Chiang KH, Hung JY, Chang WA, Lin HP, Shieh JM, Chong IW, Hsu YL. Der f1 induces pyroptosis in human bronchial epithelia via the NLRP3 inflammasome. Int J Mol Med. 2018;41(2):757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Awad F, Assrawi E, Louvrier C, Jumeau C, Georgin-Lavialle S, Grateau G, Amselem S, Giurgea I, Karabina SA. Inflammasome biology, molecular pathology and therapeutic implications. Pharmacol Ther. 2018;187:133–49. [DOI] [PubMed] [Google Scholar]
- 11. Lee S, Lee M, Kim JB, Jo A, Cho EJ, Yu SJ, Lee JH, Yoon JH, Kim YJ. 17beta-estradiol exerts anticancer effects in anoikis-resistant hepatocellular carcinoma cell lines by targeting IL-6/STAT3 signaling. Biochem Biophys Res Commun. 2016;473(4):1247–54. [DOI] [PubMed] [Google Scholar]
- 12. Cao Z, Livas T, Kyprianou N. Anoikis and EMT: Lethal “liaisons” during cancer progression. Crit Rev Oncog. 2016;21(3–4):155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryter SW, Mizumura K, Choi AM. The impact of autophagy on cell death modalities. Int J Cell Biol. 2014;2014:502676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu S, Wang Y, Jing L, Claret FX, Li Q, Tian T, Liang X, Ruan Z, Jiang L, Yao Y, Nan K, Lv Y, Guo H. Autophagy in the “inflammation-carcinogenesis” pathway of liver and HCC immunotherapy. Cancer Lett. 2017;411:82–9. [DOI] [PubMed] [Google Scholar]
- 15. Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, NLRP3 inflammasome and auto-inflammatory/immune diseases. Clin Exp Rheumatol. 2016;34(4 Suppl 98):12–6. [PubMed] [Google Scholar]
- 16. Spalinger MR, Lang S, Gottier C, Dai X, Rawlings DJ, Chan AC, Rogler G, Scharl M. PTPN22 regulates NLRP3-mediated IL1B secretion in an autophagy-dependent manner. Autophagy 2017;13(9):1590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chu Q, Jiang Y, Zhang W, Xu C, Du W, Tuguzbaeva G, Qin Y, Li A, Zhang L, Sun G, Cai Y, Feng Q, Li G, Li Y, Du Z, Bai Y, Yang B. Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma. Oncotarget 2016;7(51):84658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pizato N, Luzete BC, Kiffer L, Correa LH, de Oliveira Santos I, Assumpcao JAF, Ito MK, Magalhaes KG. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep. 2018;8(1):1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang F, Liu W, Ning J, Wang J, Lang Y, Jin X, Zhu K, Wang X, Li X, Yang F, Ma J, Xu S. Simvastatin suppresses proliferation and migration in non-small cell lung cancer via pyroptosis. Int J Biol Sci. 2018;14(4):406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: A double-edged sword. Protein Cell 2014;5(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kantono M, Guo B. Inflammasomes and cancer: The dynamic role of the inflammasome in tumor development. Front Immunol. 2017;8:1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karki R, Man SM, Kanneganti TD. Inflammasomes and cancer. Cancer Immunol Res. 2017;5(2):94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Z, Zhong S, Shen Z. Targeting the inflammatory pathways to enhance chemotherapy of cancer. Cancer Biol Ther. 2011;12(2):95–105. [DOI] [PubMed] [Google Scholar]
- 24. Deng Q, Wang Y, Zhang Y, Li M, Li D, Huang X, Wu Y, Pu J, Wu M. Pseudomonas aeruginosa triggers macrophage autophagy to escape intracellular killing by activation of the NLRP3 inflammasome. Infect Immun. 2016;84(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun J, Mu Y, Jiang Y, Song R, Yi J, Zhou J, Sun J, Jiao X, Prinz RA, Li Y, Xu X. Inhibition of p70 S6 kinase activity by A77 1726 induces autophagy and enhances the degradation of superoxide dismutase 1 (SOD1) protein aggregates. Cell Death Dis. 2018;9(3):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell 2014;159(6):1263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gills JJ, Lopiccolo J, Dennis PA. Nelfinavir, a new anti-cancer drug with pleiotropic effects and many paths to autophagy. Autophagy 2008;4(1):107–9. [DOI] [PubMed] [Google Scholar]
- 28. Saleh T, Cuttino L, Gewirtz DA. Autophagy is not uniformly cytoprotective: A personalized medicine approach for autophagy inhibition as a therapeutic strategy in non-small cell lung cancer. Biochim Biophys Acta 2016;1860(10):2130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]