Abstract
MicroRNAs (miRNAs) have been reported to be involved in many human cancers and tumor progression. The dysregulation of miR-449a is found in many types of malignancies and is associated with tumor growth, migration, and invasion. However, its expression and function in non-small cell lung cancer (NSCLC) still remains unclear. In our study, miR-449a was found to be downregulated in both NSCLC tissues and cell lines, and low miR-449a expression was obviously associated with tumor differentiation, TMN stage, and poor overall survival (OS). Moreover, we demonstrated that miR-449a could inhibit tumor proliferation, migration, and invasion in NSCLC. We also confirmed that HMGB1 was a direct target gene of miR-449a in NSCLC with dual-luciferase reporter assay, and upregulation of HMGB1 could reverse the miR-449a-induced suppression of growth, migration, and invasion in NSCLC cells. Last, we found that miR-449a suppressed tumor initiation and development through the NF-κB signaling pathway. These results indicate that miR-449a functions as a tumor suppressor in NSCLC by targeting the HMGB1-mediated NF-κB signaling pathway in NSCLC.
Key words: miR-449a, Non-small cell lung cancer (NSCLC), High-mobility group box 1 (HMGB1), NF-κB
INTRODUCTION
Lung cancer is one of the most common cancers that arises from lung tissues and is the leading cause of cancer-related death in the world1. About 80% of lung cancers are non-small cell lung cancer (NSCLC), with a high mortality and low survival rate after initial diagnosis2. Although advancements in treatments for NSCLC, including surgery, chemotherapy, radiotherapy, immune therapy, and genetic therapy, play vital roles in NSCLC treatment3,4, the 5-year overall survival (OS) rate for NSCLC patients still remains poor. More than 70% of patients with NSCLC are diagnosed at an advanced stage, and therefore there is an urgent need to explore the molecular mechanisms of NSCLC5.
MicroRNAs (miRNAs), a type of endogenous small noncoding RNA (18–25 nucleotides in length), can functionally carry out biological effects through direct binding to 3′-untranslated regions (3′-UTR) of their target mRNAs by inducing mRNA degradation and/or translational repression6. A number of studies have demonstrated that dysregulated miRNAs play a vital role in the pathogenetic processes of NSCLC, such as at the epigenetic, transcriptional, and posttranscriptional levels7,8.
miR-449a, located on chromosome 5q11, has been reported to be dysregulated and acts as a tumor suppressor in various cancers, such as colorectal cancer9, lung cancer10, prostate cancer11, nasopharyngeal carcinoma12, hepatocellular carcinoma13, gastric cancer14, and glioblastoma15. However, the function of miR-449a in NSCLC progression, especially with regard to proliferation, migration, and invasion, remains unclear. Here we attempted to assess the underlying roles and mechanisms of miR-449a in NSCLC tumorigenesis. In our study, we found that miR-449a was obviously downregulated in NSCLC cell lines and tissue specimens when compared to a normal human bronchial epithelial cell line and adjacent normal tissue (ANT) specimens, respectively. The downregulation of miR-449a in NSCLC tissues predicted an unfavorable prognosis and was associated with differentiation degree and TNM stage. Furthermore, we demonstrated that miR-449a could regulate cell proliferation, migration, and invasion through targeting high-mobility group box 1 (HMGB1), which has been found to be overexpressed and involved in the pathogenesis of varieties of human cancers16 and to be upregulated both in NSCLC cells and tissues.
MATERIALS AND METHODS
Tissue Samples
Fifty paired NSCLC and corresponding ANT samples were obtained from NSCLC patients from The Second Affiliated Hospital of Nantong University and were stored at −80°C until RNA extraction. The Clinical Research Ethics Committee of The Second Affiliated Hospital of Nantong University approved the research protocols. Written informed consent was obtained from each individual participant in the study.
Cell Culture and Transfection
Three human NSCLC cell lines (A549, H1299, and H460) and a normal human lung epithelial cell line (BEAS-2B) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, P.R. China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Los Angeles, CA, USA) containing 10% fetal bovine serum (FBS; HyClone) at 37°C with 5% CO2.
The miR-449a mimic, pcDNA3.1-HMGB1, and their corresponding negative control (miR-NC, blank vector) were purchased from GenePharma (Shanghai, P.R. China). The miRNA or HMGB1 transfection was performed using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Additionally, cells without transfection were treated with a NF-κB inhibitor JSH-23 (Abcam, Cambridge, MA, USA) at a concentration of 40 μM.
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
Total RNA from cells and tissues was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. The relative expression of miR-449a was detected by a Hairpin-it™ MicroRNAs Quantitation PCR Assay Kit (GenePharma), and U6 small nuclear RNA was used as an internal control. The cDNAs were reverse transcribed from total RNA using the PrimeScript RT-PCR Kit (Takara, Dalian, P.R. China), and the relative expression of HMGB1 mRNA was assessed by RT-qPCR using the SYBR Green method (SYBR Premix Ex Taq; Takara) according to the manufacturer’s protocol on an ABI 7500 system (Applied Biosystem, Foster City, CA, USA). β-Actin was used as internal reference. The specific primers for HMGB1 mRNA are listed as follows: 5′-TGCTCAGAGAGGTGGAAGACCA-3′ (forward) and 5′-TTGGGCGATACTCAGAGCAGAA-3′ (reverse). The relative expression was calculated by the 2−ΔΔCT method.
Plasmid Constructs and Luciferase Reporter Assay
The potential target genes of miR-449a were predicted by MicroRNA.org (http://www.microrna.org). The wild-type (WT) HMGB1-3′-UTR and mutant (Mut) HMGB1-3′-UTR containing the putative miR binding site were chemically synthesized and cloned into the pMIR report vector (Ambion, Austin, TX, USA). The recombination vector was transfected along with mimic or miR-NC using Lipofectamine 2000 (Invitrogen). Luciferase activity levels were detected using the Dual-Luciferase Reporter Assay System (Promega, Madison WI, USA) 24 h after transfection according to the manufacturer’s information.
Western Blotting
The expression of protein extracted from cells was analyzed by Western blot. Thirty micrograms of protein was separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking with Tris-buffered saline with Tween 20 (TBST) containing 5% nonfat milk for 1 h, the membranes were incubated with primary antibody rabbit against human HMGB1, p65, phosphorylated p65 (p-p65; 1:1,000; Cell Signaling Technology, Danvers, MA, USA), and β-actin (1:10,000; Cell Signaling Technology) overnight at 4°C. Then the membranes were incubated with secondary antibody for 1 h after being washed fully. The protein bands were detected with the enhanced chemiluminescence system (ECL) reagent (KeyGEN BioTECH, Nanjing, P.R. China).
Cell Proliferation Assay
Cell proliferation was measured by a Cell Counting Kit-8 (CCK-8; KeyGEN BioTECH) according to the manufacturer’s instructions. Briefly, cells were plated into a 96-well plate at a density of 1.0 × 103 cells/well and incubated for 12, 24, 48, and 72 h. The absorbance was measured at 450 nm on a microplate reader (Infinite M200 PRO; TECAN, Switzerland).
Cell Migration and Invasion Assay
Cell migration and invasion assays were performed using 24-well Transwell chambers. For the migration assay, 6 × 104 (A549) cells were cultured in 200 μl of serum-free medium in the upper chamber without Matrigel (BD Biosciences, San Jose, CA, USA). For the invasion assay, the upper chamber was precoated with Matrigel (BD Biosciences), and 100 μl of serum-free medium containing 3 × 105 (A549) cells was added. Then 500 μl of DMEM containing 10% FBS was added into the lower chamber. After incubation for 24 h, the cells were fixed with 90% methanol and stained by 0.05% crystal violet, and then counted under an inverted microscope (Olympus, Tokyo, Japan).
Statistical Analysis
All the data were expressed as means ± SD from three independent experiments and analyzed by the Student’s t-test or one-way analysis of variance (ANOVA) using SPSS 22.0 (IBM, Chicago, IL, USA). Pearson’s Mann–Whitney U-test or chi-square test was used to analyze the relationship between the expression of miR-449a and clinicopathological features. The Kaplan–Meier method with the log-rank test was used to calculate OS rates for comparisons. The Cox proportional hazards model was used in the multivariate and univariate analyses, and a value of p < 0.05 was considered to be statistically significant.
RESULTS
miR-449a Expression Is Downregulated in NSCLC Tissues and Cell Lines
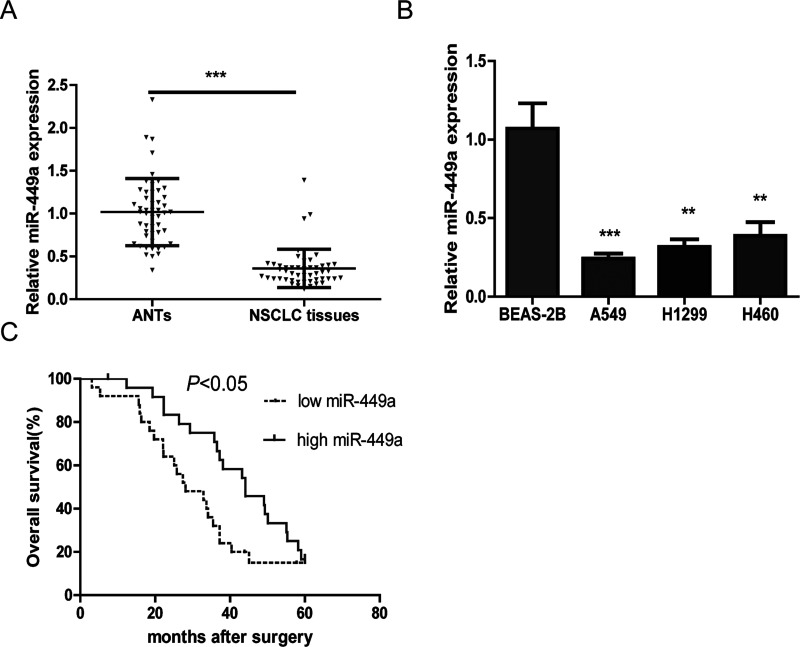
First, the expression of miR-449a was detected in 50 NSCLC tissues and corresponding matched ANTs. The results showed that miR-449a was markedly downregulated in NSCLC tissues compared with that in matched ANTs (Fig. 1A).
Figure 1.
A) The relative expression of microRNA-449a (miR-449a) in non-small cell lung cancer (NSCLC) tissue samples is compared with that in adjacent normal tissues (ANTs). U6 was used as a control. The results were obtained from three independent experiments, mean ± SD. (B) The relative expression levels of miR-449a in the A549, H1299, H460, and BEAS-2B cell lines were analyzed by quantitative real-time PCR (qRT-PCR). (C) Survival analysis indicated that the NSCLC patients with low miR-449a levels had shorter overall survival (OS) time compared with those with high miR-449a levels. **p < 0.01, ***p < 0.001.
Moreover, the expression of miR-449a in the NSCLC cell lines was also assessed, and it was found that miR-449a expression was significantly reduced in NSCLC cell lines compared with that in the normal human lung epithelial cell line (Fig. 1B).
Downregulation of miR-449a Is Associated With Adverse Clinicopathological Features and Poor Prognosis
miR-449a downregulation was associated with differentiation degree (p = 0.008) and TNM stage (p = 0.004). However, there was no significant difference between age, gender, smoking history, histologic type, lymph node metastasis, and tumor size. Kaplan–Meier survival analysis indicated that NSCLC patients with low miR-449a had a significantly poorer OS than those with high miR-449a expression (p < 0.05) (Fig. 1C). Furthermore, a univariate analysis showed that differentiation degree (p = 0.017), lymph node metastasis (p = 0.005), TNM stage (p < 0.001), and miR-449a (p = 0.012) were significantly associated with OS in NSCLC patients. A multivariate analysis also indicated that miR-449a was an independent prognostic indicator for OS (p = 0.019).
miR-449a Suppresses NSCLC Cell Proliferation
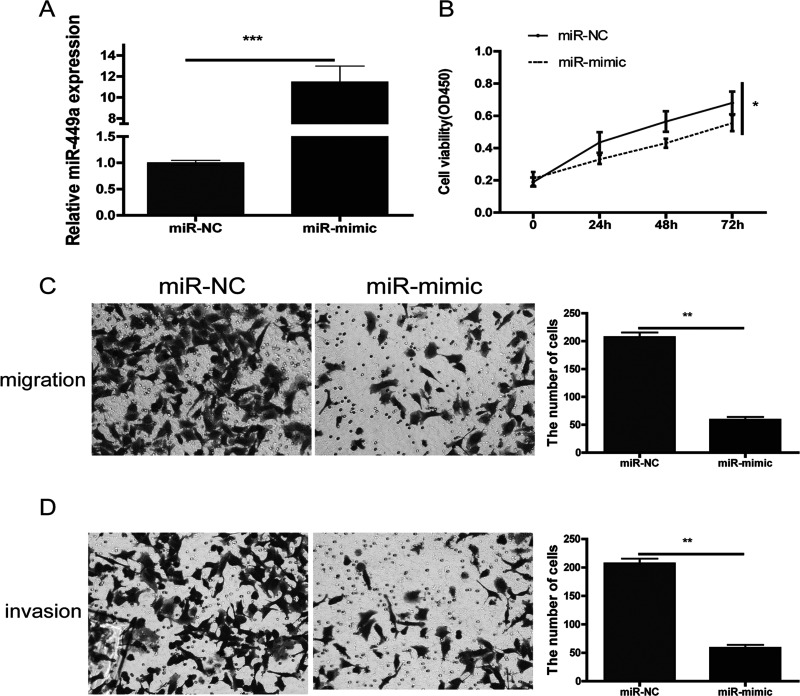
To explore the potential role of miR-449a in NSCLC pathogenesis, A549 cells were transfected with miR-449a mimic or miR-NC. The efficiency of transfection was confirmed by RT-qPCR (Fig. 2A). CCK-8 data showed that upregulation of miR-449a by transfection with mimic significantly impaired cell proliferation of A549 cells (Fig. 2B).
Figure 2.
(A) Relative miR-449a levels were assessed in A549 cells after transfection with mimic or miR-NC. miR-449a inhibits proliferation, migration, and invasion in NSCLC cells. Overexpression of miR-449a significantly decreased proliferation (B), migration (C), and invasion (D) in A549 cells. *p < 0.05, **p < 0.01, ***p < 0.001.
miR-449a Inhibits Cell Migration and Invasion
In order to detect the effect of miR-449a on the migrative capacity and invasion ability of NSCLC cells, we upregulated miR-449a in A549 cells as described above, and the Transwell assay exhibited a marked decrease in cell migration and invasion compared with that in the NC group (Fig. 2C and D).
HMGB1 Is a Direct of miR-449a in NSCLC
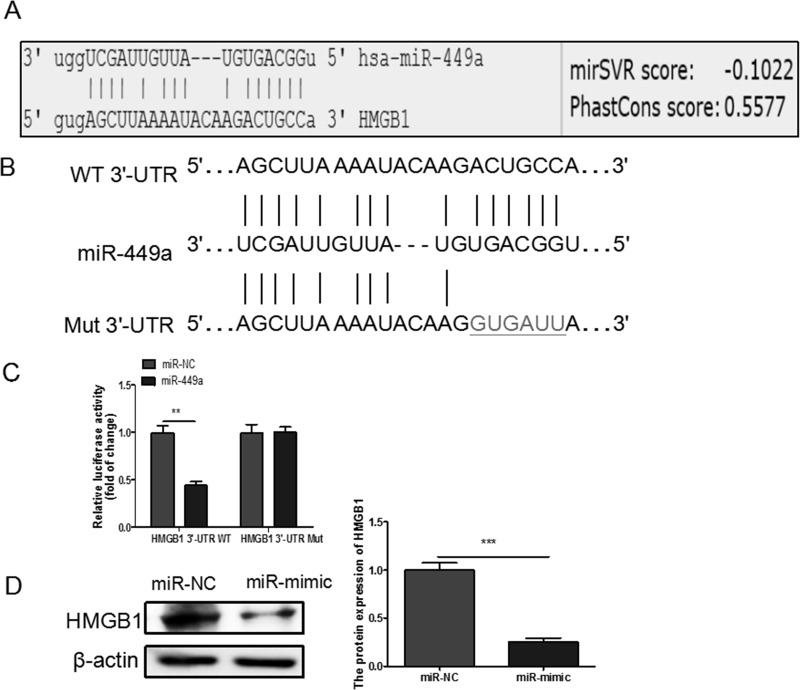
To explore the potential molecular mechanisms underlying miR-449a-induced regulation of NSCLC biology, the bioinformatics database MicroRNA.org was searched to identify the potential target gene of miR-449a, and we found that HMGB1 was a potential target gene of miR-449a (Fig. 3A), which was subsequently confirmed by the dual-luciferase reporter assay. The WT and Mut 3′-UTR of HMGB1 were constructed as shown (Fig. 3B). Our results showed that the luciferase activity of HMGB1 3′-UTR-WT, but not HMGB1 3′-UTR-Mut, was decreased by transfecting with mimic when compared to the NC, verifying that miR-449a could directly bind to the 3′-UTR of HMGB1 (Fig. 3C). Moreover, we also demonstrated that miR-449a overexpression significantly decreased HMGB1 protein levels by Western blotting assay (Fig. 3D). These data indicated that HMGB1 is a direct target of miR-449a.
Figure 3.
(A) Predicted miR-449a target sequence in high-mobility group box 1 (HMGB1) 3′-untranslated region (3′-UTR) is shown. (B) The predicted binding sites for miR-449a in the 3′-UTR of HMGB1 and the mutations in the binding sites are shown. (C) A549 cells were cotransfected with mimic or miR-NC and wild-type (WT) or mutant (Mut) HMGB1 3′-UTR reporter plasmid. Luciferase activity was measured 48 h after transfection. (D) HMGB1 protein expression was downregulated after the transfection of miR-449a mimic in A549 cells. **p < 0.01, ***p < 0.001.
miR-449a Suppresses NSCLC Malignant Progression by Targeting HMGB1
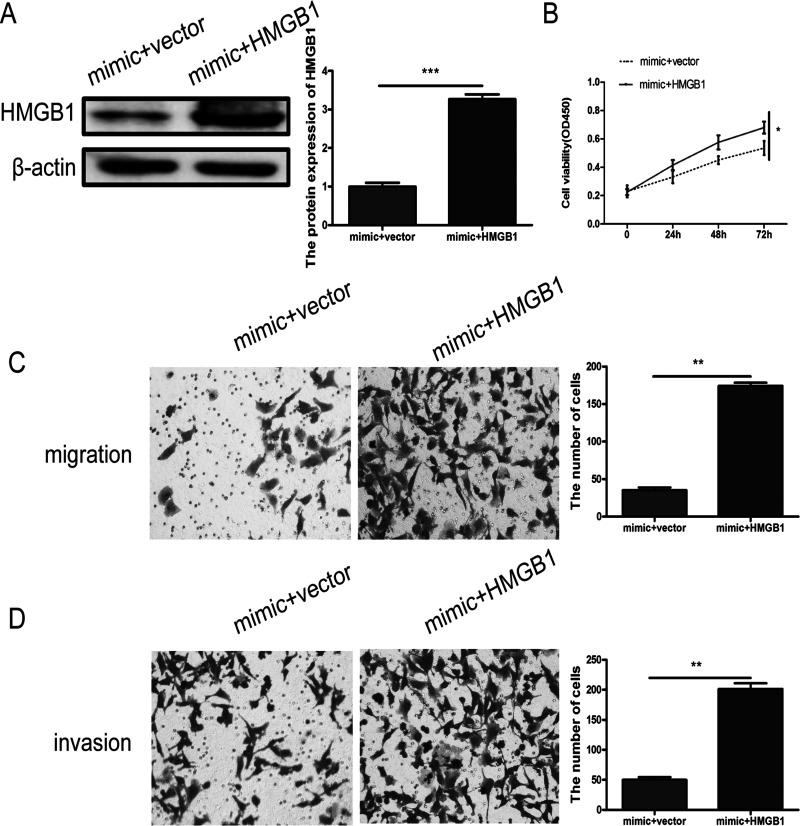
To verify that miR-449a suppresses NSCLC malignant progression through HMGB1, we cotransfected A549 cells with mimic and pcDNA3.1-HMGB1 or vector, and the efficiency of transfection was evaluated by Western blotting (Fig. 4A). The results showed that the proliferation (Fig. 4B), migration (Fig. 4C), and invasion (Fig. 4D) abilities of A549 cells in the mimic + HMGB1 group could be partially restored compared with the mimic + vector group.
Figure 4.
(A) HMGB1 protein expression levels were measured in A549 cells after cotransfection with mimic and vector or HMGB1 by Western blot, respectively. Cell proliferation (B), migration (C), and invasion (D) were evaluated after upregulation of HMGB1. *p < 0.05, **p < 0.01, ***p < 0.001.
miR-449a Suppresses NSCLC Malignant Progression Through HMGB1-Mediated NF-κB Signaling Pathway
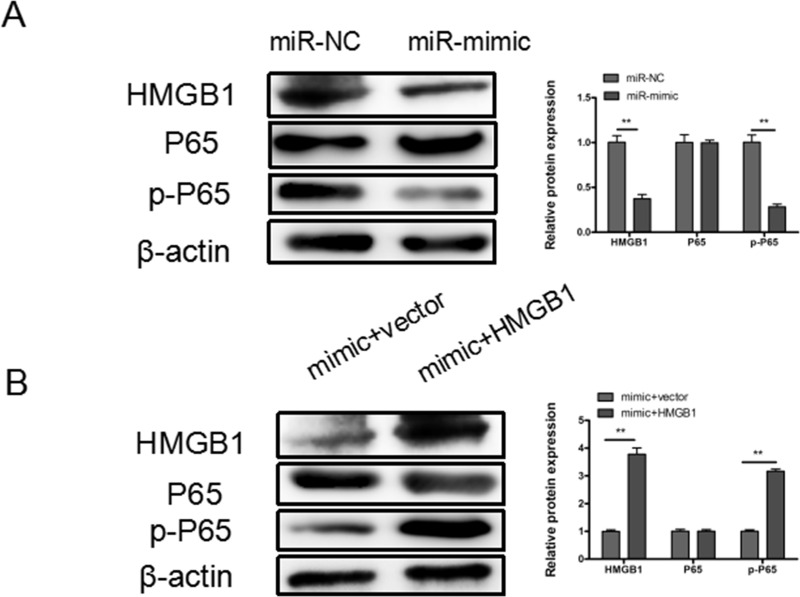
Mounting evidence has demonstrated that HMGB1 promoted malignant progression through the NF-κB signaling pathway in many types of cancers. Here we found that overexpression of miR-449a markedly decreased the expression of HMGB1 and prevented the phosphorylation of p65 (p-NF-κB) in A549 cells (Fig. 5A), and upregulation of HMGB1 could partly restore the expression of p-p65 (Fig. 5B).
Figure 5.
miR-449a inhibits NSCLC through HMGB1-mediated NF-κB signaling pathway. (A) Representative Western blotting results for HMGB1, p65, and p-p65 protein expression after transfection with miR-449a mimic or miR-NC. (B) Representative Western blotting results for HMGB1, p65, and p-p65 protein expression after cotransfection with mimic and pcDNA3.1-HMGB1 or blank vector. β-Actin was used as internal control. **p < 0.01.
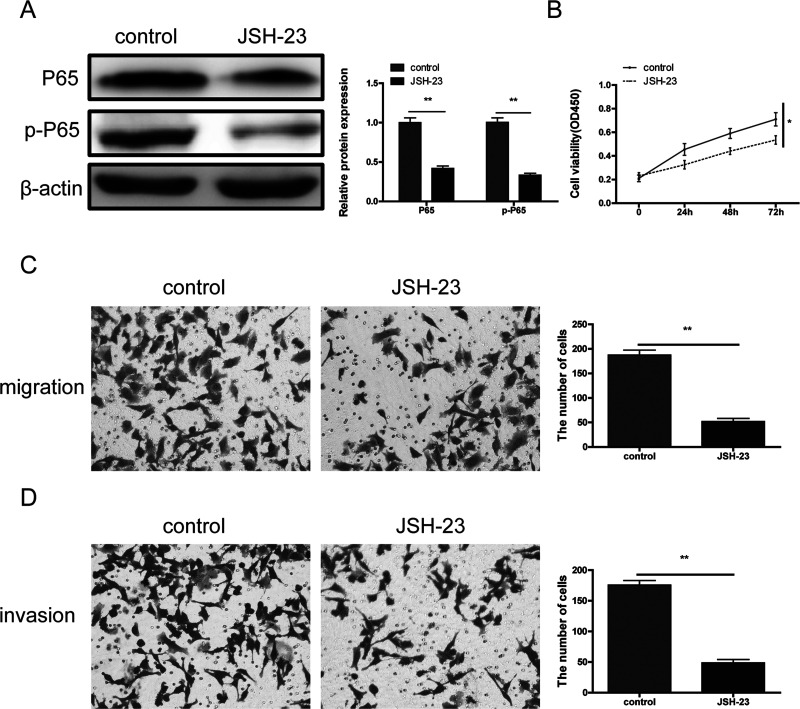
In addition, to further verify that miR-449a inhibited progression of NSCLC through the NF-κB signaling pathway, a p65 inhibitor JSH-23 (40 μM) was used. The results showed that JSH-23 significantly downregulated the expression of p65 and p-p65 (Fig. 6A) and markedly suppressed NSCLC cell proliferation (Fig. 6B), migration (Fig. 6C), and invasion (Fig. 6D).
Figure 6.
The effects of NF-κB inhibitor JSH-23 on NSCLC cell proliferation, migration, and invasion. (A) Compared with the blank control group, JSH-23 significantly decreased the expression of p65 and p-p65. JSH-23 obviously inhibited NSCLC cell proliferation (B), migration (C), and invasion (D). *p < 0.05,**p < 0.01.
Based on these results, we could conclude that miR-449a suppresses NSCLC malignant progression, at least partially through the HMGB1-mediated NF-κB signaling pathway.
DISCUSSION
Increasingly, miRNAs, acting as either oncomiRs or anti-oncomiRs, have been reported to be involved in the development and progression of many types of cancers17–20. miR-449a has been reported to act as a suppressor in many types of cancers by targeting c-Met, WISP2, cyclin D1, HDAC1, Sirt1, CDC25A, CDK6, and SATB29,11,21–23. However, the biological functions of miR-449a in the progression of NSCLC are largely unknown. Here we are the first to report that miR-449a inhibited proliferation, migration, and invasion of NSCLC by targeting HMGB1.
In our study, we found that the expression of miR-449a was significantly downregulated both in NSCLC tissues or cell lines compared with that in matched ANTs or the normal human lung epithelial cell line BEAS-2B, respectively. Moreover, clinicopathological correlation analysis indicated that miR-449a was markedly associated with differentiation, TNM stage, and poor OS. Furthermore, we also showed that downregulated miR-449a obviously suppressed proliferation, migration, and invasion of NSCLC cells. Taken together, we could conclude that miR-449a might function as a tumor suppressor in NSCLC.
HMGB1, acting as an oncogene, has been reported to be a target gene of several miRNAs24,25. We verified that HMGB1 was a target gene of miR-449a by dual-luciferase reporter assay. miR-449a was found to inhibit HMGB1 expression in NSCLC cells. Additionally, we demonstrated that miR-449a suppressed proliferation, migration, and invasion of NSCLC cells by targeting HMGB1. JAK/STAT and NF-κB signaling were previously reported to be involved in the functional role of HMGB126–28. NF-κB signaling is vital for the growth and survival of various cancers29–31. To investigate whether downregulated miR-449a promoted progression and development in NSCLC through the HMGB1-mediated NF-κB signaling pathway, the upregulation of miR-449a resulted in the downregulation of HMGB1 and p-p65, and upregulation of HMGB1 could rescue the expression of p-p65. Moreover, a NF-κB inhibitor could markedly inhibit NSCLC cell proliferation, migration, and invasion. Based on the above results, we could conclude that miR-449a inhibits proliferation, migration, and invasion by targeting HMGB1 via the NF-κB signaling pathway in NSCLC.
In conclusion, our data showed that miR-449a was frequently downregulated both in NSCLC tissues and cell lines and is an independent predictor for NSCLC patient survival. Moreover, miR-449a overexpression inhibits NSCLC progression and development by suppressing the HMGB1-mediated NF-κB signaling pathway. Our findings may provide new insight into the underlying mechanisms of NSCLC progression and highlight miR-449a as a potential prognostic biomarker and therapeutic target gene in NSCLC.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Heist RS, Engelman JA. SnapShot: Non-small cell lung cancer. Cancer Cell 2012;21(3):442–8. [DOI] [PubMed] [Google Scholar]
- 3. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. [DOI] [PubMed] [Google Scholar]
- 4. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vansteenkiste J, De Ruysscher D, Eberhardt WE, Lim E, Senan S, Felip E, Peters S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):i89–i98. [DOI] [PubMed] [Google Scholar]
- 6. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–79. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe K, Amano Y, Ishikawa R, Sunohara M, Kage H, Ichinose J, Sano A, Nakajima J, Fukayama M, Yatomi Y, Nagase T, Ohishi N, Takai D. Histone methylation-mediated silencing of miR-139 enhances invasion of non-small-cell lung cancer. Cancer Med. 2015;4(10):1573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun CC, Li SJ, Zhang F, Zhang YD, Zuo ZY, Xi YY, Wang L, Li DJ. The novel miR-9600 suppresses tumor progression and promotes paclitaxel sensitivity in non-small-cell lung cancer through altering STAT3 expression. Mol Ther Nucleic Acids 2016;5(11):e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun X, Liu S, Chen P, Fu D, Hou Y, Hu J, Liu Z, Jiang Y, Cao X, Cheng C, Chen X, Tao Y, Li C, Hu Y, Liu Z, Zhan Y, Mao J, Wang Q, Ma Y, Cong X, Sun R, Shi Y, Wang M, Zhang X. miR-449a inhibits colorectal cancer progression by targeting SATB2. Oncotarget 2016;8(60):100975–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. You J, Zhang Y, Li Y, Fang N, Liu B, Zu L, Zhou Q. miR-449a suppresses cell invasion by inhibiting MAP2K1 in non-small cell lung cancer. Am J Cancer Res. 2015;5(9):2730–44. [PMC free article] [PubMed] [Google Scholar]
- 11. Noonan EJ, Place RF, Pookot D, Basak S, Whitson JM, Hirata H, Giardina C, Dahiya R. miR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene 2009;28(14):1714–24. [DOI] [PubMed] [Google Scholar]
- 12. Li H, Li X, Ge X, Jia L, Zhang Z, Fang R, Yang J, Liu J, Peng S, Zhou M, Xiang J, Zeng Z, Zhou W, Xiong W, Xiao G, Fang L, Li GY, Li Z. miR-34b-3 and miR-449a inhibit malignant progression of nasopharyngeal carcinoma by targeting lactate dehydrogenase A. Oncotarget 2016;7(34):54838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu S, Liu K, Zhang W, Wang Y, Jin Z, Jia B, Liu Y. miR-449a inhibits proliferation and invasion by regulating ADAM10 in hepatocellular carcinoma. Am J Transl Res. 2016;8(6):2609–19. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Bou KT, Futoma-Kazmierczak E, Jacobsen A, Krogh A, Bardram L, Hother C, Gronbaek K, Federspiel B, Lund AH, Friis-Hansen L. miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol Cancer 2011;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yao Y, Ma J, Xue Y, Wang P, Li Z, Li Z, Hu Y, Shang X, Liu Y. miR-449a exerts tumor-suppressive functions in human glioblastoma by targeting myc-associated zinc-finger protein. Mol Oncol. 2015;9(3):640–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan XG, Yan Z, Sun X, Wang H, Wang Q, Tsung A, Billiar TR, Zeh HR, Lotze MT, Tang D. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peng Y, Zhang X, Ma Q, Yan R, Qin Y, Zhao Y, Cheng Y, Yang M, Wang Q, Feng X, Huang Y, Huang W, Zhao Z, Wang L, Wei Y, He Z, Fan X, Li S, Jin Z, Meltzer SJ. miRNA-194 activates the Wnt/beta-catenin signaling pathway in gastric cancer by targeting the negative Wnt regulator, SUFU. Cancer Lett. 2017;385:117–27. [DOI] [PubMed] [Google Scholar]
- 18. Liu L, Bi N, Wu L, Ding X, Men Y, Zhou W, Li L, Zhang W, Shi S, Song Y, Wang L. microRNA-29c functions as a tumor suppressor by targeting VEGFA in lung adenocarcinoma. Mol Cancer 2017;16(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu F, Li J, Guo N, Wang XH, Liao YQ. miRNA-27a promotes the proliferation and invasion of human gastric cancer MGC803 cells by targeting SFRP1 via Wnt/beta-catenin signaling pathway. Am J Cancer Res. 2017;7(3):405–16. [PMC free article] [PubMed] [Google Scholar]
- 20. Cao Y, Zhao D, Li P, Wang L, Qiao B, Qin X, Li L, Wang Y. MicroRNA-181a-5p impedes IL-17-induced nonsmall cell lung cancer proliferation and migration through targeting VCAM-1. Cell Physiol Biochem. 2017;42(1):346–56. [DOI] [PubMed] [Google Scholar]
- 21. Noonan EJ, Place RF, Basak S, Pookot D, Li LC. miR-449a causes Rb-dependent cell cycle arrest and senescence in prostate cancer cells. Oncotarget 2010;1(5):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang X, Feng M, Jiang X, Wu Z, Li Z, Aau M, Yu Q. miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb-E2F1 activity through a feedback loop by targeting CDK6 and CDC25A. Genes Dev. 2009;23(20):2388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, Libutti SK, Nakakura EK, Golub TR, Hanahan D. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009;23(18):2152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu L, Ren W, Chen K. miR-34a promotes apoptosis and inhibits autophagy by targeting HMGB1 in acute myeloid leukemia cells. Cell Physiol Biochem. 2017;41(5):1981–92. [DOI] [PubMed] [Google Scholar]
- 25. Jiang D, Wang H, Li Z, Li Z, Chen X, Cai H. miR-142 inhibits the development of cervical cancer by targeting HMGB1. Oncotarget 2017;8(3):4001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang D, Kang R, Zeh HR, Lotze MT. High-mobility group boxc1 and Cancer. Biochim Biophys Acta 2010;1799(1–2):131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu B, Antoine DJ, Kwan K, Lundback P, Wahamaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, Erlandsson-Harris H, Chavan SS, Wang H, Andersson U, Tracey KJ. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci USA 2014;111(8):3068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, He J, Zhong D, Li J, Liang H. High-mobility group box 1 protein activating nuclear factor-kappaB to upregulate vascular endothelial growth factor C is involved in lymphangiogenesis and lymphatic node metastasis in colon cancer. J Int Med Res. 2015;43(4):494–505. [DOI] [PubMed] [Google Scholar]
- 29. Ren D, Yang Q, Dai Y, Guo W, Du H, Song L, Peng X. Oncogenic miR-210-3p promotes prostate cancer cell EMT and bone metastasis via NF-kappaB signaling pathway. Mol Cancer 2017;16(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng Z, Qu JQ, Yi HM, Ye X, Huang W, Xiao T, Li JY, Wang YY, Feng J, Zhu JF, Lu SS, Yi H, Xiao ZQ. miR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-kappaB signaling pathway. Cell Death Dis. 2017;8(6):e2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang F, Liu X, Liu Y, Liu Y, Zhang C, Wang Z, Jiang T, Wang Y. miR-181d/MALT1 regulatory axis attenuates mesenchymal phenotype through NF-kappaB pathways in glioblastoma. Cancer Lett. 2017;396:1–9. [DOI] [PubMed] [Google Scholar]