Abstract
Kaempferol is a flavonoid that has been extensively investigated owing to its antitumor effects. Nevertheless, little is known about its underlying mechanisms of action. We aimed to explore the role of kaempferol in breast cancer (BC), and thus we investigated how kaempferol suppresses the growth of BC cells. The cells were treated with kaempferol, and the effects on multiple cancer-associated pathways were evaluated. The MTS assay was used to study the cell growth inhibition induced by kaempferol. The cell cycle was analyzed by flow cytometry. Western blotting was used to analyze cellular apoptosis and DNA damage. We found that the proliferation of the triple-negative BC (TNBC) MDA-MB-231 cells was suppressed effectively by kaempferol. Interestingly, the suppressive effect of kaempferol on cell proliferation was stronger in MDA-MB-231 cells than in the estrogen receptor-positive BT474 cell line. Furthermore, after the treatment with kaempferol for 48 h, the population of cells in the G1 phase was significantly reduced, from 85.48% to 51.35%, and the population of cells in the G2 phase increased markedly from 9.27% to 37.5%, which indicated that kaempferol contributed to the induction of G2/M arrest. Kaempferol also induced apoptosis and DNA damage in MDA-MB-231 cells. Kaempferol increased the expression levels of γH2AX, cleaved caspase 9, cleaved caspase 3, and p-ATM compared to those of the control group. Collectively, these results showed that kaempferol may be a potential drug for the effective treatment of TNBC.
Key words: Kaempferol, Cell proliferation, Cell cycle, Apoptosis, DNA damage, Breast cancer (BC)
INTRODUCTION
As one of the most common malignancies, breast cancer (BC) contributes to many deaths in women1,2. More than 1 million patients are diagnosed with advanced BC, over 400,000 of whom die each year3. In the US, approximately 200,000 patients with advanced BC and 50,000 with in situ breast carcinoma are diagnosed each year, and over 40,000 of these patients die4. Since 1990, BC-related mortality in the US has followed a decreasing trend, which is mainly attributed to early screening and refined therapies4. However, BC is still a predominant contributor to the deaths of women with cancer and accounts for 15% of them in the US5.
There are several subtypes of BC, and each has different prognosis and therapeutic indications6. Based on the markers such as progesterone receptor (PR) and HER-2/Neu amplification, BC is divided into several subtypes, such as hormone receptor-positive BC, HER-2/Neu amplified BC, and triple-negative BC (TNBC)7,8. TNBC accounts for approximately 10–15% of BCs and has poor prognosis compared with other subtypes9. It is therefore necessary to extend the available knowledge about TNBC and develop proper targets for treatment.
Kaempferol is a flavonoid that can be extracted from various fruits and vegetables10. In traditional medicine, it is used to treat many disorders, and its multiple biological effects, including antitumor, anti-inflammatory, and antioxidant activities, have attracted the attention of researchers11,12. However, little is known about the underlying mechanism of its antitumor action. In this study, we aimed to investigate the effects of kaempferol in TNBC cells.
MATERIALS AND METHODS
Cell Culture
The human BC cell lines BT474 and MDA-MB-231 were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). The cell lines were cultured in RPMI-1640 medium supplemented with FBS (10%) and penicillin/streptomycin (100 IU/ml). Kaempferol was obtained from Sigma-Aldrich (St. Louis, MO, USA).
MTS Assay
The cell lines mentioned above were seeded in 96-well plates and then incubated with different concentrations of kaempferol for 72 h. The cell proliferation was detected using an MTS assay kit (Promega, Durham, NC, USA) in accordance with the manufacturer’s guideline. A Wallac Victor 1420 Multilabel Counter (PerkinElmer, Akron, OH, USA) was employed for luminescence determination.
DAPI Staining
Nuclear morphology was observed by DAPI staining. FluoView FV10i confocal fluorescence microscopy (Olympus Corporation, Tokyo, Japan) was used to visualize the apoptotic cells.
Western Blotting
Western blotting of antibodies p-ATM, γH2AX, cleaved caspase 3, and cleaved caspase 9 was performed (Cell Signaling Technology, Danvers, MA, USA).
Cell Cycle
Different concentrations of kaempferol were treated to the cells, and the cell cycle distribution (CCD) was determined. After the cells were collected and fixed in 70% ethanol for 1 h at 4°C, they were stained with propidium iodide (PI) solution for 30 min at 4°C and then analyzed using a Becton Dickinson FACScan cytofluorimeter (Mansfield, TX, USA). ModFIT cell cycle analysis software (Becton Dickinson, Franklin Lakes, NJ, USA) was used for the calculation of CCD.
Apoptosis Assay
Hoechst 33258 (Invitrogen, Waltham, MA, USA) was used as a nuclear stain in the detection of apoptosis13–15. PI and annexin-Alexa 488 (Invitrogen) were used to perform annexin V/PI staining.
Statistical Analysis
Student’s t-test and one-way ANOVA were computed using GraphPad Prism V software for two group and multiple group comparisons, respectively. The data are presented as the mean ± SD and the values with p < 0.05 indicated significant differences.
RESULTS
Kaempferol Reduces BC Cell Proliferation
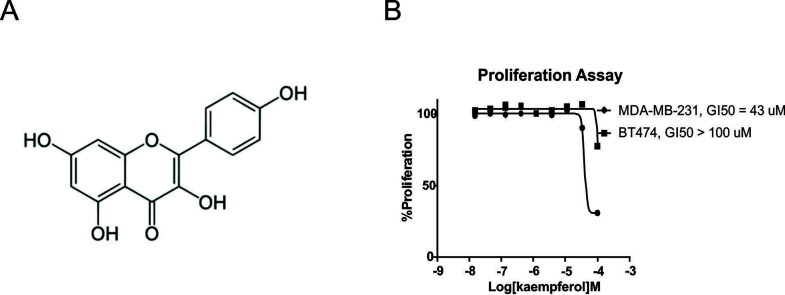
To investigate the antiproliferation activity of kaempferol (Fig. 1A) in BC cells, MDA-MB-231 cells were treated with different concentrations of kaempferol for 72 h and subjected to MTS assay. The assay showed that cell proliferation was suppressed by kaempferol in a dose-dependent manner, with an IC50 value of 43 μmol/L (Fig. 1B). In contrast, when the estrogen receptor-positive BT474 cell line was treated with kaempferol, the IC50 exceeded 100 μmol/L (Fig. 1B). This revealed that the suppressive effects of kaempferol on proliferation were strong in TNBC cells, which indicated that kaempferol might be an ideal drug for the treatment of TNBC.
Figure 1.
Kaempferol reduced MDA-MB-231 proliferation. (A) Structure of kaempferol. (B) MDA-MB-231 and BT474 cells were treated with different concentrations of kaempferol for 3 days. Cell proliferation was determined using the MTS assay. The data are presented as mean ± SD of three independent experiments.
Kaempferol Contributed to MDA-MB-231 CC Arrest
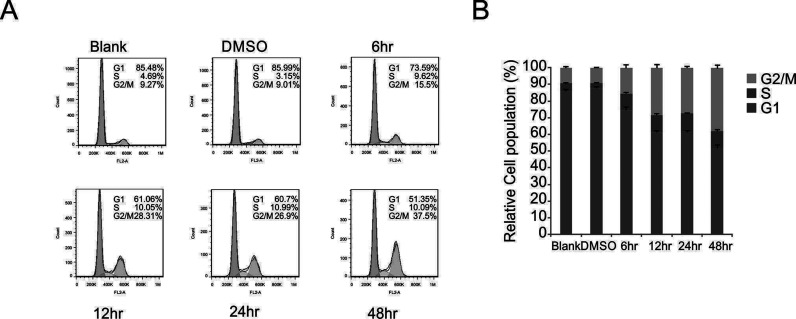
As the kaempferol treatment suppressed MDA-MB-231 cell proliferation, we investigated whether this occurred through the induction of cell cycle arrest. The role of kaempferol in MDA-MB-231 cell cycle was detected by flow cytometry using a cell cycle detection kit (Fig. 2A). The data indicated that kaempferol reduced the population of cells in the G1 phase and accordingly increased the population of cells in the G2 phase. After treatment with kaempferol for 48 h, the population of cells in the G1 phase was significantly reduced, from 85.48% to 51.35%, and the population of cells in the G2 phase was increased markedly from 9.27% to 37.5%, which indicated that kaempferol contributed to the induction of G2/M arrest (Fig. 2). These results show that kaempferol promoted the inhibition of MDA-MB-231 cells via the induction of cell cycle arrest.
Figure 2.
Kaempferol induced MDA-MB-231 cell arrest in the G2/M phase. (A) The cells were treated with 50 μmol/L kaempferol and analyzed by flow cytometry to determine the distribution of cells in different phases of the cell cycle. (B) Quantitation of the data in (A). The data are presented as mean ± SD of three independent experiments.
Kaempferol Promotes Apoptosis in MDA-MB-231 Cells
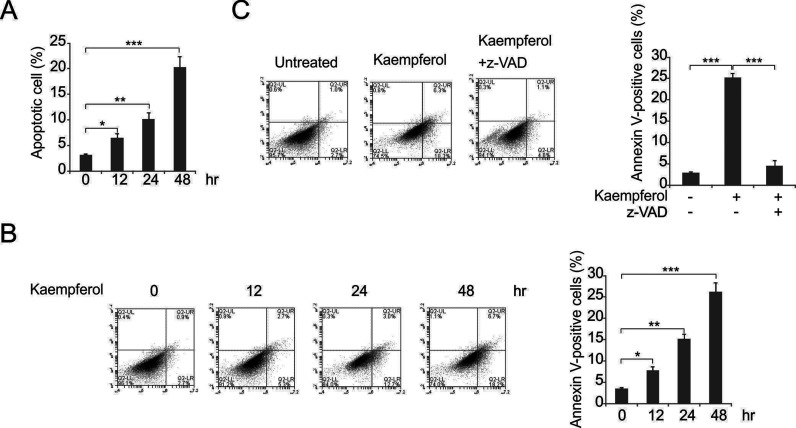
As kaempferol was cytotoxic to cells, we evaluated whether this was associated with the induction of apoptosis. The apoptotic assays showed that kaempferol treatment induced apoptosis in a time-dependent manner (Fig. 3A). Furthermore, kaempferol treatment increased the population of annexin V+ cells in MDA-MB-231 cells (Fig. 3B). As the induction of apoptosis was attenuated by the treatment with z-VAD-fmk (a pan-caspase inhibitor) (Fig. 3C), we concluded that the apoptotic response was mediated by caspases. These results indicated that kaempferol induced cell apoptosis.
Figure 3.
Kaempferol induced MDA-MB-231 cell apoptosis. (A) The cells were treated with kaempferol at the indicated concentrations for 3 days and subjected to nuclear fragmentation. (B) The MDA-MB-231 cells were treated with kaempferol at the indicated concentrations for 2 days and then analyzed by annexin V/propidium iodide (PI) staining and flow cytometry. (C) The MDA-MB-231 cells were treated with 50 μmol/L kaempferol in the presence or absence of z-VAD. The nuclear fragmentation assay was employed for analysis. The data presented in (A) to (C) are presented as mean ± SD of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
Kaempferol Induces DNA Damage in MDA-MB-231 Cells
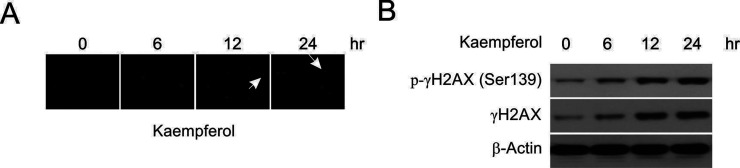
Next we attempted to confirm whether kaempferol induces MDA-MB-231 apoptosis. DAPI staining showed that kaempferol induced DNA condensation (Fig. 4A). The phosphorylation of histone H2AX always occurs after double-strand breaks (DSBs) that affect DNA13. Thus, the amount of phosphorylated H2AX (γH2AX) in cells treated with cytotoxic agents can be used to detect DNA DSBs. In MDA-MB-231 cells, kaempferol increased the phosphorylation of H2AX and the total levels of γH2AX compared to those of the control sample, as shown by Western blotting (Fig. 4B).
Figure 4.
Kaempferol induced DNA damage in MDA-MB-231 cells. (A) 4′,6-Diamidino-2-phenylindole (DAPI) staining of the cells after kaempferol treatment. (B) The cells were treated with 50 μmol/L kaempferol at the indicated time points, and the protein levels were analyzed by Western blotting.
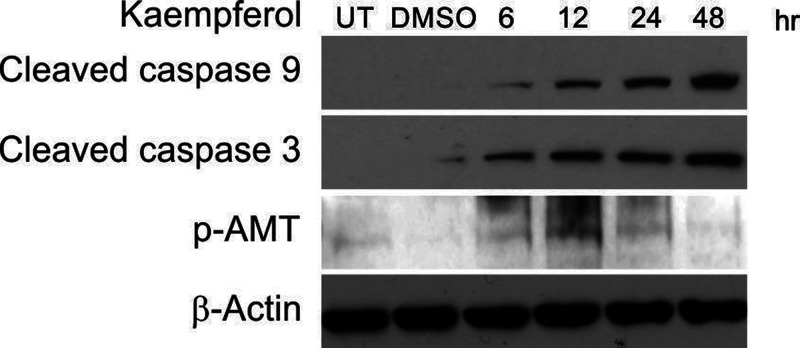
Kaempferol Induces the Expression of DNA Damage-Associated Proteins and Apoptotic Proteins
We next tested whether kaempferol induced the expressions of apoptotic proteins and DNA damage-associated proteins. The expression levels of the apoptotic proteins including cleaved caspase 9 and cleaved caspase 3, and the DNA damage-associated protein ATM, were analyzed (Fig. 5). The results showed that kaempferol increased the expression levels of cleaved caspase 9, cleaved caspase 3, and p-ATM compared to those of the control sample.
Figure 5.
Kaempferol affected apoptosis and DNA damage-associated proteins in MDA-MB-231 cells. The MDA-MB-231 cells were treated with 50 μmol/L kaempferol at the indicated time points, and the protein levels were analyzed by Western blotting.
DISCUSSION
Flavonoids are prevalent in various plants that represent an important proportion of our diet14. It has been reported that some flavonoids, such as quercetin and kaempferol, have antioxidant and antitumor properties12,15. The occurrence of kaempferol is widespread in nature; for example, it is found in Gingko biloba leaves and red wines15. The effects of kaempferol were concentration dependent, with a lower concentration attenuating the apoptosis of smooth muscle cells, and a higher concentration inducing the apoptosis of lung tumor cells, proteolytic caspase 7 activation, and PARP cleavage10,16. Nevertheless, the mechanism of the apoptosis-promoting activities is still unclear. The results of this study showed that kaempferol induced apoptosis in human BC cells, at least partly through the induction of DNA damage.
First, we evaluated the anticancer effects of kaempferol on BC cells. MDA-MB-231 and T47D cells were treated with kaempferol; the MTS results showed that the TNBC cell line MDA-MB-231 is more sensitive to kaempferol than T47D cells. Kaempferol has been well studied in ER+ BC cells17–19, but its effect on TNBC is poorly understood. Whereas a previous study reported that a low dose of kaempferol effectively retarded the migration of TNBC cells20, our results indicated that TNBC cells were more sensitive to kaempferol treatment.
Furthermore, we demonstrated that kaempferol inhibited cell viability by enhancing arrest in the G2/M phase. Apoptosis is responsible for the regulation of cell development, engaging many biological effects, such as cytochrome C release, membrane blebbing, DNA laddering, and chromosome condensation, which results in the removal of unnecessary cells21–23. Our results indicated that the MDA-MB-231 cell apoptosis was successfully induced by kaempferol via a mitochondria-dependent pathway. The cleavage of caspases 9 and 3 was observed after kaempferol treatment. Caspase 9 is closely correlated with the appearance of apoptotic cells, which indicated that caspase 9 activation was correlated with kaempferol-induced apoptosis. A previous study showed that kaempferol enhanced apoptosis through the PI3K/AKT and telomerase pathways in cervical cancer cells24. Kaempferol also induced the ATM/p53-mediated death receptor and mitochondrial apoptosis in human umbilical vein endothelial cells25. In this study, we found that z-VAD blocked kaempferol-induced apoptosis, which indicated that kaempferol induced caspase-dependent apoptosis.
Unrepaired DNA damage, including specific DNA lesions, single-strand breaks (SSBs), and DSBs, is known to cause cell death26,27. To maintain genomic stability and genetic integrity, DNA repair is required to counteract the abundance of daily DNA damage in the cells28. DSBs may induce apoptosis if they are not repaired in a timely manner28,29. ATM and/or ATR, responsible for the detection of DSBs, send signals to checkpoint kinases (Chk2 and Chk1) to initiate DNA repair30. It is known that ATM activation by DNA damage elicits various cellular processes to initiate DNA repair31. DNA damage in the cells may be regarded as the point of no return for the execution of cellular apoptotic pathway32. A previous study reported that kaempferol induces DNA damage and inhibits DNA repair-associated protein expressions in human promyelocytic leukemia HL-60 cells33. In the current study, we found that kaempferol increased the phosphorylation of H2AX and the total level of γH2AX in MDA-MB-231 cells. Furthermore, kaempferol treatment promoted AMT activation in the cells. Our results showed that kaempferol induced DNA damage in TNBC cells.
In conclusion, kaempferol effectively inhibited several cancer-associated pathways in the TNBC cells simultaneously, indicating that it may be an effective drug for the treatment of TNBC.
ACKNOWLEDGMENT
Authorship contributions: concept (Li Zhu); design (Li Zhu and Lijun Xue); supervision (Li Zhu and Lijun Xue); materials (Li Zhu and Lijun Xue); data collection and processing (Lijun Xue); analysis and interpretation (Li Zhu and Lijun Xue); literature search (Li Zhu); writing (Li Zhu); and critical review (Li Zhu and Lijun Xue).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Libson S, Lippman M. A review of clinical aspects of breast cancer. Int Rev Psychiatry 2014;26(1):4–15. [DOI] [PubMed] [Google Scholar]
- 2. Von Kleist S. Prognostic factors in breast cancer: Theoretical and clinical aspects (review). Anticancer Res. 1996;16(6C):3907–12. [PubMed] [Google Scholar]
- 3. Haynes B, Sarma A, Nangia-Makker P, Shekhar MP. Breast cancer complexity: Implications of intratumoral heterogeneity in clinical management. Cancer Metastasis Rev. 2017;36(3):547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12(7):381–94. [DOI] [PubMed] [Google Scholar]
- 5. Estrada SS. Review of the New American Cancer Society guidelines for breast cancer screening for women at average risk. J Adv Pract Oncol. 2016;7(5):563–6. [PMC free article] [PubMed] [Google Scholar]
- 6. Ieni A, Barresi V, Ricciardi GR, Adamo B, Adamo V, Tuccari G. Prognostic value of androgen receptor expression in triple negative breast carcinomas: Personal experience and comments on a review about “Triple-negative breast cancer: Treatment challenges and solutions” by Collignon et al. Breast Cancer (Dove Med Press) 2016;8:157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jeronimo AFA, Weller M. Differential association of the lifestyle-related risk factors smoking and obesity with triple negative breast cancer in a Brazilian population. Asian Pac J Cancer Prev. 2017;18(6):1585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun H, Zou J, Chen L, Zu X, Wen G, Zhong J. Triple-negative breast cancer and its association with obesity. Mol Clin Oncol. 2017;7(6):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138(4):2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devi KP, Malar DS, Nabavi SF, Sureda A, Xiao J, Nabavi SM, Daglia M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol Res. 2015;99:1–10. [DOI] [PubMed] [Google Scholar]
- 12. Rajendran P, Rengarajan T, Nandakumar N, Palaniswami R, Nishigaki Y, Nishigaki I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur J Med Chem. 2014;86:103–12. [DOI] [PubMed] [Google Scholar]
- 13. Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: A marker for DNA damage. Methods Mol Biol. 2012;920:613–26. [DOI] [PubMed] [Google Scholar]
- 14. Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74(4):418–25. [DOI] [PubMed] [Google Scholar]
- 15. Kim SH, Choi KC. Anti-cancer effect and underlying mechanism(s) of kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol Res. 2013;29(4):229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11(4):298–344. [DOI] [PubMed] [Google Scholar]
- 17. Kim SH, Hwang KA, Choi KC. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J Nutr Biochem. 2016;28:70–82. [DOI] [PubMed] [Google Scholar]
- 18. Oh SM, Kim YP, Chung KH. Biphasic effects of kaempferol on the estrogenicity in human breast cancer cells. Arch Pharm Res. 2006;29(5):354–62. [DOI] [PubMed] [Google Scholar]
- 19. Qiu W, Lin J, Zhu Y, Zhang J, Zeng L, Su M, Tian Y. Kaempferol modulates DNA methylation and downregulates DNMT3B in bladder cancer. Cell Physiol Biochem. 2017;41(4):1325–35. [DOI] [PubMed] [Google Scholar]
- 20. Li S, Yan T, Deng R, Jiang X, Xiong H, Wang Y, Yu Q, Wang X, Chen C, Zhu Y. Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. Onco Targets Ther. 2017;10:4809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tong J, Tan S, Nikolovska-Coleska Z, Yu J, Zou F, Zhang L. FBW7-dependent Mcl-1 degradation mediates the anticancer effect of Hsp90 inhibitors. Mol Cancer Ther. 2017;16(9):1979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kashafi E, Moradzadeh M, Mohamadkhani A, Erfanian S. Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed Pharmacother. 2017;89:573–7. [DOI] [PubMed] [Google Scholar]
- 25. Tu LY, Bai HH, Cai JY, Deng SP. The mechanism of kaempferol induced apoptosis and inhibited proliferation in human cervical cancer SiHa cell: From macro to nano. Scanning 2016;38(6):644–53. [DOI] [PubMed] [Google Scholar]
- 26. Roos WP, Kaina B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332(2):237–48. [DOI] [PubMed] [Google Scholar]
- 27. Tong J, Tan S, Zou F, Yu J, Zhang L. FBW7 mutations mediate resistance of colorectal cancer to targeted therapies by blocking Mcl-1 degradation. Oncogene 2017;36(6):787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12(9):440–50. [DOI] [PubMed] [Google Scholar]
- 29. Tong J, Wang P, Tan S, Chen D, Nikolovska-Coleska Z, Zou F, Yu J, Zhang L. Mcl-1 Degradation is required for targeted therapeutics to eradicate colon cancer cells. Cancer Res. 2017;77(9):2512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marechal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013;5(9)pii:a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ray A, Blevins C, Wani G, Wani AA. ATR- and ATM-mediated DNA damage response is dependent on excision repair assembly during G1 but not in S phase of cell cycle. PLoS One 2016;11(7):e0159344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Podhorecka M, Skladanowski A, Bozko P. H2AX phosphorylation: Its role in DNA Damage Response and Cancer Therapy. J Nucleic Acids 2010;2010:pii:920161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu LY, Lu HF, Chou YC, Shih YL, Bau DT, Chen JC, Hsu SC, Chung JG. Kaempferol induces DNA damage and inhibits DNA repair associated protein expressions in human promyelocytic leukemia HL-60 cells. Am J Chin Med. 2015;43(2):365–82. [DOI] [PubMed] [Google Scholar]