Abstract
In previous investigations, we reported that peroxisome proliferator-activated receptor β/δ (PPARβ/δ) activation by GW501516 inhibits proliferation and promotes apoptosis in the undifferentiated C666-1 nasopharyngeal carcinoma (NPC) cells by modulating caspase-dependent apoptotic pathway. In the present study, the mechanism by which GW501516 induces apoptosis was explored from the perspective of microRNA (miRNA) expression. Among the assayed miRNAs that were involved in regulating the expression of antiapoptotic protein Bcl-2, miR-206 was increased significantly and specifically by GW501516 in C666-1 cells at both the in vitro level and at the in vivo xenograft samples. The induction on miR-206 expression caused by GW501516 was capable of being antagonized by the PPARβ/δ antagonist GSK3787 and AMPK antagonist dorsomorphin in C666-1 cells. GW501516’s suppression on the growth and apoptosis of C666-1 cells was found to be dependent on the presence of miR-206. miR-206 overexpression resulted in suppressed proliferation and colony formation ability, and further triggered increased apoptosis in C666-1 cells in a caspase-dependent manner. The expression of cleaved caspase 3 and caspase 9, and the ratio of Bax to Bcl-2 were elevated remarkably by miR-206. Consistent with the in vitro result, miR-206 was corroborated to suppress the ectopic NPC xenograft tumorigenesis that derived from the C666-1 cells in BALB/c nu/nu mice. Taken together, the current data demonstrated that miR-206 plays a critical role in the direct apoptosis-promoting effect induced by GW501516 in C666-1 cells. Furthermore, the emphasized tumor-suppressive role of miR-206 in the C666-1 cells indicates that it has the potential to provide a new therapeutic approach for the undifferentiated NPC.
Key words: Nasopharyngeal carcinoma (NPC), miRNAs, GW501516, Apoptosis, PPARβ/δ
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is an epithelium of the nasopharyngeal recess-deprived malignant carcinoma and has been a major health threat to humans, particularly to those living in the Southeast Asian countries and North Africa1. Local invasion, early distant metastasis, and chemotherapy resistance are still a conundrum for NPC, and particularly the most malignant undifferentiated type NPC2, although concurrent chemo/radiotherapy is a relatively effective therapy. Novel targets or approaches based on molecular targeted therapy undoubtedly will share new lights in discovering new chemotherapy agents for the management of NPC.
MicroRNAs (miRNAs) are a family of highly conserved short (18–25 nt) nonprotein-coding RNA molecules. They regulate gene expression and function by base pairing with the 3′-untranslated region (3′-UTR) of target protein-coding mRNAs3,4. An increasing body of evidence has demonstrated that miRNAs play an extremely vital role in NPC tumorigenesis5–7. Deregulated expression of miRNAs and its deregulation has been demonstrated to be involved in multiple stages of NPC tumorigenesis8,9. As either tumor suppressors or oncogenes, miRNAs could suppress or promote the growth, proliferation, invasion, and metastasis of NPC cells (for detailed summary refer to recent reviews5,6). Among them, the onco-miRNAs such as miRNA-21 (miR-21) and miR-10b have been shown to facilitate carcinogenesis of NPC6,10,11. In contrast, lowered expression of tumor-suppressing miRNAs including miR-15a and miR-29c was found to contribute to the malignant behavior of NPC cells12–14. Recent studies revealed that miR-206 is closely related to various tumors including breast, lung, liver, and cervical cancer, etc.15–18. miR-206 overexpression could inhibit cell proliferation and migration, activate apoptosis, and induces cell cycle arrest17,19. However, the role of miR-206 on NPC is still ambiguous.
Previously we had found that the expression of PPARβ/δ, one isotope of the nuclear hormone receptor peroxisome proliferator-activated receptors (PPARs), is in reverse correlation with the differentiation degree of the NPC cell lines, and a strikingly reduced expression of PPARβ/δ was revealed in the EBV + undifferentiated NPC cell line C666-120. PPARβ/δ activation by GW501516 could effectively suppress the growth of C666-1 cells and inhibit its tumorigenesis in nude mice by promoting apoptosis through downregulating the expression of apoptotic-associated proteins, particularly the antiapoptotic protein B-cell lymphoma 2 (Bcl-2)20. However, the precise molecular mechanism and pathway by which PPARβ/δ activation connects to increased NPC cell apoptosis was still unclear. In view of the key roles of miRNAs in NPC’s tumorigenesis, progression, and metastasis we explored the impact of GW501516 on miRNAs that might orchestrate the PPARβ/δ-dependent apoptosis. The possible association between PPARβ/δ activation and miR-206, and several other miRNAs that were supposed to exert critical roles in regulating expression of Bcl-2, was assayed in C666-1 cells at both in vitro and in vivo levels. Furthermore, the role of miR-206 in GW501516’s suppression on the growth of C666-1 cells was systematically investigated.
MATERIALS AND METHODS
Compounds
PPARβ/δ selective agonist GW501516 and PPARβ/δ antagonist GSK3787 were purchased from MedChemExpress (Monmouth Junction, NJ, USA). The AMPK agonist AICAR and inhibitor dorsomorphin (compound C) were bought from Selleck Chemical (Houston, TX, USA).
Cell Cultures and Reagents
CNE-1 cells were obtained from Institute of Virology, Chinese Academy of Preventive Medicine; NP-69 (the SV40 large T immortalized nasopharyngeal epithelial cell line) and CNE-2 cells were purchased from the Shanghai Institute of Cell Biology (Shanghai, P.R. China). The EBV+ C666-1 cells were from the cell bank of Xiangya Central Laboratory (Central South University, Changsha, P.R. China). Cells were maintained in RPMI-1640 or DMEM/F12 (1:1) medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) containing streptomycin, penicillin, and supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific) and cultured at 37°C in a humidified incubator with 5% CO2. For functional analysis, C666-1 cells grown to 80%–90% confluence were transfected with the miR-206 mimic, the miR-206 inhibitor, or scrambled miRNA as a control (Genepharma Company, Suzhou, P.R. China) using Lipofectamine RNAimax (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). Cells were harvested 48–72 h after transfection for further experiments. Lentiviral transduction of C666-1 cells was carried out with lentivirus carrying miR-206 precursor and its control according to the manufacturer’s protocol (Genechem Company, Shanghai, P.R. China). Stable miR-206-overexpressing cells and the control cells were then selected using puromycin.
PPARβ/δ Overexpression in Cells
As reported in our previous study20, PPARβ/δ overexpression in NP-69, CNE-1, CNE-2, and C666-1 cells was respectively performed by recombinant adenoviruses that were produced by Genechem (Shanghai, P.R. China). In brief, cells seeded in a six-well plate were infected by adenoviruses PPARβ/δ (Ad-PPARβ/δ; 6 × 1010 pfu/ml) containing PPARβ/δ cDNA; the adenovirus containing green fluorescent protein (GFP; Ad-GFP; 4 × 1010 pfu/ml) was used as a control. The impact of PPARβ/δ overexpression on the expression of miR-206 and miR-21 was assayed in these cells by real-time quantitative PCR (RT-qPCR).
Cell Proliferation Assay
The impact of miR-206 overexpression on NPC cell viability was analyzed using MTT spectrophotometric dye assay as previously reported20. Briefly, cells seeded into a 96-well plate (at a density of 3 × 104 cells/well) were treated with miR-206 mimic, scrambled miRNA, or transfection reagent. Then cell proliferation rate was examined on days 1–5. For assay, the cells were incubated for 3 h at 37°C with sterile MTT labeling dye (0.5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA). After removal of the culture medium and addition of dimethyl sulfoxide, the absorbance was measured at 550 nm with a scanning multiwell spectrophotometer (ELISA reader; Perkin Elmer, Waltham, MA, USA). All experiments were performed in triplicates.
Colony Formation Assay
For the colony formation assay, miR-206-transfected C666-1 cells were examined by seeding an equal number of cells in six-well plates with 2 ml of complete medium, with the medium being refreshed every 3 days. After incubation for 14 days, cells were fixed with 4% formaldehyde and stained with 0.5% crystal violet. Finally, images were taken, visible cell colonies were counted manually, and the results were shown as the fold change relative to that of vehicle control group.
Cell Apoptosis Assay
Cell apoptosis assay was performed with the Annexin-V–fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Detection kit (BD Biosciences, San Diego, CA, USA) as previously reported20. In brief, C666-1 cells seeded at a density of 8 × 104 cells/ml in six-well plates were cultured overnight and then transfected with miR-206 mimics or scrambled miRNA control. At the end of incubation, the cells were harvested via centrifugation and stained with Annexin-V and PI according to the manufacturer’s protocol, and then measured within 1 h of staining with FACSCalibur Cytometer (BD Biosciences). According to the extent of staining, the cells were classified as “survival,” “early apoptosis,” “late apoptosis,” and “necrosis.”
RNA Extraction and RT-qPCR
Total RNA from cultured cells and xenografted tumors was extracted using the mirVana isolation kit (Life Technologies, Saint-Aubin, France) according to the procedure for separation of small RNAs. For detection of miRNA expression, miRNAs were reverse-transcribed with the Exiqon miRCURY LNA™ Universal RT microRNA kit. RT-qPCR was performed with SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) on an ABI 7500 Real-time PCR system (Applied Biosystems, Foster City, CA, USA). The primers used for PCR are shown in Table 1. Relative expression of miRNA was evaluated by comparative CT method (2−ΔΔC) and normalized to the expression of U6.
Table 1.
The Primer Sequences Used for qPCR
| Genes | Forward Primers | Reverse Primers |
|---|---|---|
| miR-15a | 5′-ACGTGCTGCTAAGGCACTGCT-3′ | 5′-TGCAGGCCATATTGTGCTGCCT-3′ |
| miR-16 | 5′-GCGGCAACCCGTAGATCCGAA-3′ | 5′-GTGCAGGGTCCGAGGT-3′ |
| miR-29c | 5′-GGCTTCGAGGCTGCTGCTTT-3′ | 5′-TCGAGGTGCAGACCCTGGGAG-3′ |
| miR-184 | 5′-TACGACTATGTGACCTGCCTG-3′ | 5′-TGGTTCAACTCTCCTTTCCA-3′ |
| miR-206 | 5′-GAGCACAGGTTTGGTGACCT-3′ | 5′-CTCAAGAGGGGGAGATAGGG-3′ |
| miR-21 | 5′-GCCCGCTAGCTTATCAGACTGAT-3′ | 5′-GTGCAGGGTCCGAGGT-3′ |
| U6 | 5′-CTCGCTTCGGCAGCACA-3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
| ILK | 5′-ATGAAGACCCTGCAAAGCGA-3′ | 5′-GAGTTTGGGCAAGGACCTGA-3′ |
| β-Actin | 5′-TAAAGACCTCTATGCCAACACAG-3′ | 5′-CACGATGGAGGGGCCGGACT-3′ |
Cell Protein Extraction and Western Blotting
Proteins were extracted with RIPA lysis buffer containing a protease inhibitor cocktail. After quantification with BCA Protein Assay Kit (Pierce Biotechnology, Waltham, MA, USA), protein expression was measured by Western blotting. Briefly, equal amounts of protein (30 μg) were resolved by 10% SDS-PAGE, and then transferred onto polyvinylidene difluoride (PVDF) membrane. After blocking with 5% nonfat milk in Tris-buffered saline at room temperature, the membrane was incubated with the primary antibodies overnight at 4°C, followed by horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Signals were developed using Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA) according to the manufacturer’s instruction. Antibodies against Bcl-2, Bcl-2-associated X protein (Bax), caspase 3, and caspase 9 were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Antibody against GAPDH was purchased from Santa Cruz Biotechnology.
Subcutaneous Xenograft Mice Model
Six-week-old female BALB/c nu/nu athymic mice (Shanghai Slac Experimental Animals Co., Shanghai, P.R. China) were used for experimental tumorigenesis assay. During the study, the mice were maintained in pathogen-free conditions under controlled temperature and humidity with 12-h light/dark cycle, and supplied with standard rodent food and water ad libitum. For the xenograft tumor growth assay, equivalent amount of miR-206-overexpressing C666-1 cells (approximately 1 × 107) and their control cells were inoculated subcutaneously into the axilla of the nude mice. Tumor volume during the experiment was measured weekly with slide calipers, and volumes were estimated according to the standard formula V = (length × width2)/2. After 3 weeks, the mice were euthanized, and the tumors were exercised and weighed. The 4% paraformaldehyde-fixed tumor tissues were embedded in paraffin, then sectioned at 5-μm thickness, and used for immunohistochemistry for Ki-67 or caspase 3, and reaction was developed with a diaminobenzidine (DAB) substrate kit (Vector Laboratories, Inc., Burlingame, CA, USA). All experimental protocols for animal study were approved by the Ethics Committee for Animal Research at the Fudan University.
Statistical Analysis
Data were expressed as means ± SE. Statistical analysis was performed using the SPSS 19.0 software (SSPS Inc., New York, NY, USA). Statistical significance was assessed by Student’s t-test or one-way ANOVA followed by Tukey’s multiple comparison tests. A significance level of the tests was taken at a value of p < 0.05.
RESULTS
GW501516 Increased miR-206 Expression in C666-1 Cells at Both the In Vitro and the In Vivo Level
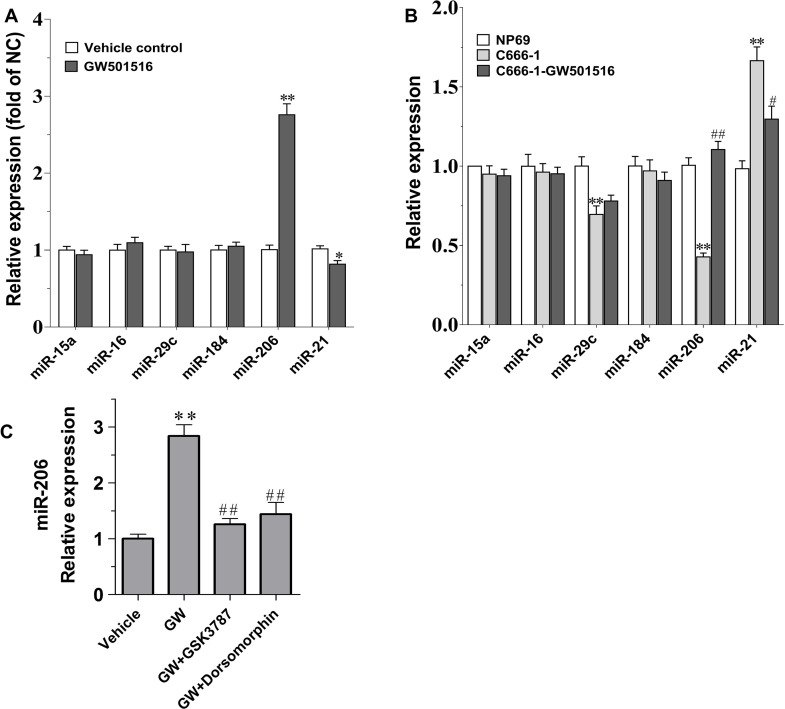
We previously revealed that PPARβ/δ activation by GW501516 could inhibit the growth of C666-1 cells by promoting apoptosis through downregulating the expression of the key antiapoptotic protein Bcl-220. The impact of GW501516 on the expression of miR-206 and other miRNAs that exert vital roles in regulating expression of Bcl-2 were thus examined in GW501516-treated C666-1 cells at both the in vivo and in vitro level. Interestingly, among the several miRNAs assayed, the expression of miR-206 was found to be upregulated dramatically (p < 0.01), while the onco-miRNA miR-21’s expression was slightly downregulated in GW501516-treated xenograft samples (p < 0.05) (Fig. 1A). To further corroborate this, we compared the expression level of these miRNAs in GW501516-treated C666-1 cells at the in vitro level. Figure 1B indicates, when compared to that of the control NP69 cells, that the content of miR-206 and miR-29c in the C666-1 NPC cells were diminished prominently (p < 0.01 for both), whereas miR-21’s expression was markedly higher than that in the NP69 cells (p < 0.01) (Fig. 1B). Consistent with the result from xenograft samples, GW501516 treatment was found to be associated with an apparent elevation on the expression of miR-206, while the elevated miR-21’s expression was also partially suppressed simultaneously (Fig. 1B). Correspondingly, we found that the promoting effect of GW501516 on the expression of miR-206 could be antagonized by the PPARβ/δ selective antagonist GSK378721, signifying the specificity of PPARβ/δ activation on the suppression of miR-206 (Fig. 1C).
Figure 1.
The impact of GW501516 on miRNA expression in C666-1 xenograft samples and C666-1 cells. (A) MicroRNA (miRNA) expression in C666-1 cell subcutaneous xenograft tumors. Mice were treated with 30 mg/kg GW501516 for 4 weeks, and then the samples were exercised to determine gene expression (n = 8). Gene expression results were normalized to β-actin and then expressed as relative expression compared with that in the control group. *p < 0.05, **p < 0.01 versus the control group. (B) miRNA expression in C666-1 cells. C666-1 cells were treated with 30-μM GW501516 for 48 h, and then miRNA expression was assayed. Gene expression was normalized to β-actin and then expressed as relative expression compared with that in the NP-69 cells (n = 3). (C) Peroxisome proliferator-activated receptor β/δ (PPARβ/δ) antagonist GSK3787 and AMPKα inhibitor dorsomorphin-antagonized GW501516 (GW) induced upregulation of miR-206 in C666-1 cells. GSK3787 (1 μM) or dorsomorphin (10 μM) was coadministered with GW501516 (30 μM) for 48 h, and then the expression of miR-206 was determined by real-time quantitative (RT-qPCR) (n = 3). Values are means ± SE, **p < 0.01 versus the vehicle control group or NP-69 group. #p < 0.05, ##p < 0.01 versus GW or C666-1 group.
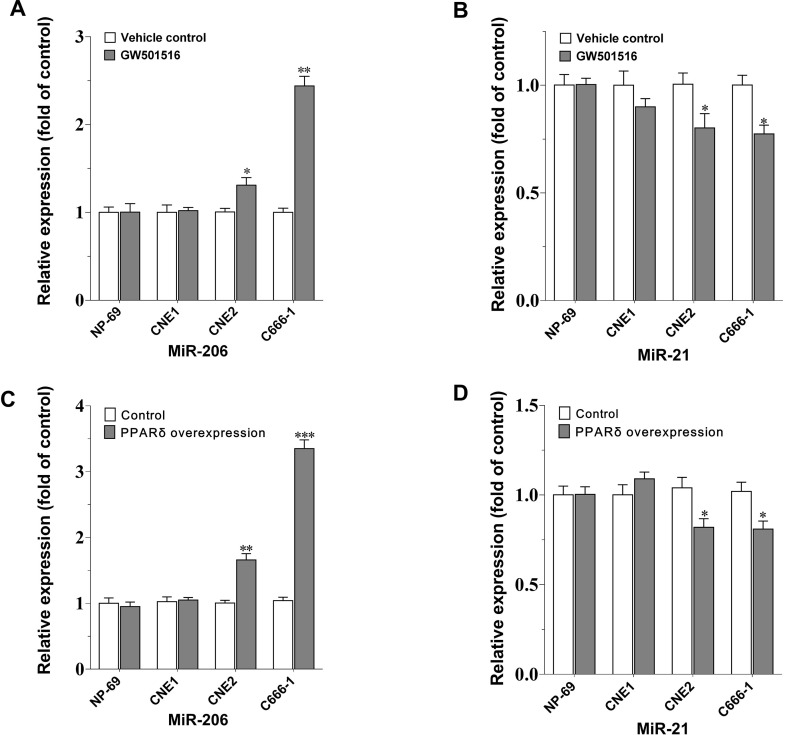
To determine if the regulation of GW501516 on the expression of miR-206 and miR-21 is specific to C666-1 cells, and if it is associated with the differentiation degree of NPC cells, we further examined and compared the expression of these two miRNAs in several human NPC cell lines with different degrees of differentiation after GW501516 treatment, including the well-differentiated CNE-1 cells, the poorly differentiated CNE-2 cells, and the undifferentiated C666-1 NPC cells. Figure 2 shows that, compared to the vehicle control, miR-206 expression was upregulated most apparently in the C666-1 cells by GW501516 (p < 0.01), and followed by a weaker but significant effect on CNE-2 cells (p < 0.05). However, similar potency on the suppression of miR-21’s expression was observed in the undifferentiated and poorly differentiated NPC cells (CNE-2 and C666-1 cells) (Fig. 2B). No impact on these two miRNAs’ expression was observed in the normal NP69 cells and the well-differentiated CNE-1 cells under such condition. Additionally, we observed the impact of PPARβ/δ overexpression on the regulation of these miRNAs. Consistent with the effect of PPARβ/δ agonism, PPARβ/δ overexpression led to a similar, but relatively more significant, upregulation on miR-206’s expression, but equivalent suppression on miR-21’s expression in C666-1 and CNE-2 cells (Fig. 2C and D). These results signified that PPARβ/δ activation or overexpression could increase the expression of miR-206 specifically in C666-1 cells and also explained why the CNE-1 cells are refractory to GW501516’s antiproliferation effect.
Figure 2.
The impact of GW501516 or PPARβ/δ overexpression on the expression of miR-206 and miR-21 in different nasopharyngeal carcinoma (NPC) cell lines. (A, B) The expression of miR-206 and miR-21 in different NPC cells after GW501516 treatment. Cells were treated with 30-μM GW501516 for 48 h, and then miRNA expression was assayed. (C, D) The expression of miR-206 and miR-21 in PPARβ/δ-overexpressed NPC cells. Gene expression results were normalized to β-actin within group and then expressed as relative expression compared with the control group (n = 3). Values are means ± SE. *p < 0.05, **p < 0.01, ***p < 0.001 versus the corresponding control group.
miR-206 Inhibitor Antagonized GW501516’s Suppression on the Growth of C666-1 Cells
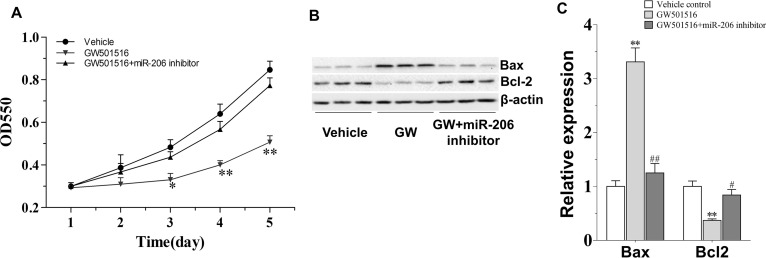
To further confirm the effectiveness of miR-206 on GW501516’s antiproliferation effect in C666-1 cells, the effect of GW501516 was comparatively observed in normal and miR-206 knockdown cells. As expected, the undifferentiated EBV+ C666-1 NPC cells were sensitive to administration of GW501516 (30 μM). They showed considerable slower growth rate than the vehicle control cells from 3 days on (p < 0.05), and the effect was more apparent at days 4 and 5 (p < 0.01) (Fig. 3A). However, the growth suppression effect of GW501516 was found almost totally antagonized by combining with miR-206 inhibitor. Corresponding with this, miR-206 inhibition also reversed the regulation of GW501516 on the expression of apoptotic-associated proteins, particularly Bax and Bcl-2 (Fig. 3B and C). The significantly enhanced ratio of Bax to Bcl-2 induced by GW501516 was diminished to the level of vehicle-treated condition. This result confirmed that miR-206 played a vital role in GW501516’s suppression on the growth of C666-1 cells.
Figure 3.
The antagonism effect of miR-206 inhibitor to GW501516 on the growth and expression of apoptotic proteins in C666-1 cells. (A) miR-206 inhibitor reverses the growth inhibition effect of GW501516 in C666-1 cells. C666-1 cells were treated by 30 μM GW501516 with or without miR-206 inhibitor for 1–5 days, and then cell proliferation rate was examined. (B) miR-206 inhibitor antagonizes the impact of GW501516 on apoptotic protein expression in C666-1 cells. (C) The quantification result of (B) (n = 3). Values are means ± SE. *p < 0.05, **p < 0.01 versus control group; #p < 0.05, ##p < 0.01 versus GW501516 group.
miR-206 Inhibits the Growth of C666-1 NPC Cells
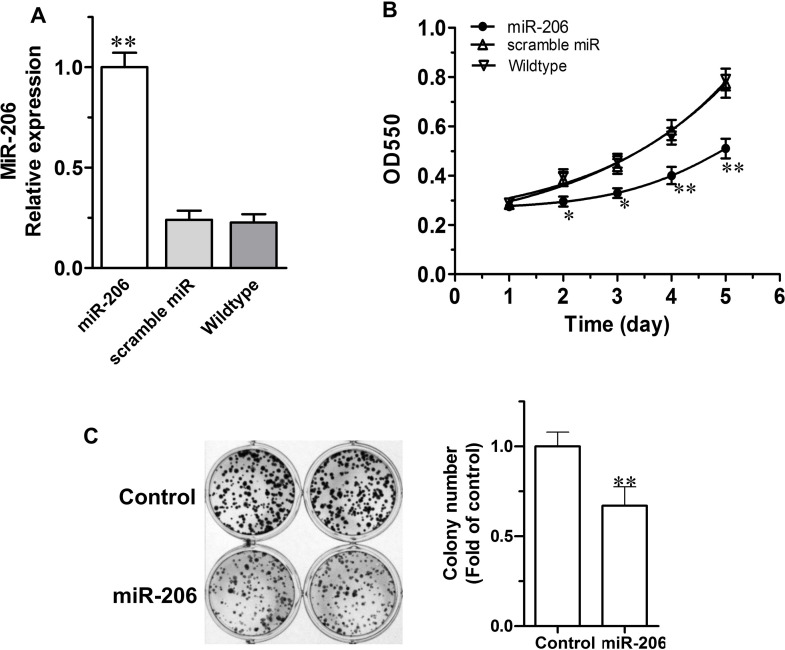
To explore the impact of miR-206 overexpression on the proliferation of C666-1 NPC cells, we next mimicked miR-206 upregulation by transfecting human NPC cells with a miR-206 mimic oligonucleotide sequence, using a scrambled miRNA as a control. As shown in Figure 4A, the expression of miR-206 increased more than four times after transfection when compared to the wild-type and scramble miRNA control cells. This led to a dramatically suppressed growth of the C666-1 cells as observed from day 2 until day 5, while the control cells (C666-1 + scramble miRNA or C666-1 wild type) proliferated continuously until day 5 (Fig. 4B). Correspondingly, a striking decrease in the clonogenic capability in miR-206-overexpressed C666-1 cells was also noted in contrast to the control cells (Fig. 4C).
Figure 4.
miR-206 inhibits proliferation in C666-1 cells. (A) Compared to control cells (scramble and wild type), miR-206 overexpression led to a significantly higher expression of miR-206 in C666-1 cells. (B) miR-206 overexpression inhibits C666-1 cell proliferation. C666-1 cells were stably transfected by vectors expressing miR-206 or scramble miRNA or nontransfected. (C) miR-206 overexpression decreased colony formation in C666-1 cells (n = 3). Values are means ± SE. *p < 0.05, **p < 0.01 versus the control group.
miR-206 Promotes Apoptosis in C666-1 NPC Cells
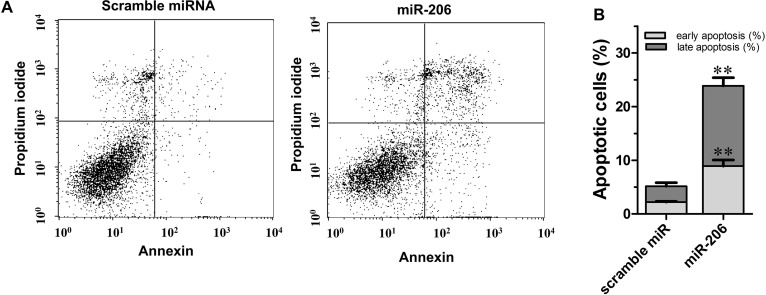
To further examine the impact of miR-206 on apoptosis, annexin V–FITC/PI double staining was performed on miR-206 or scramble miRNA transfected cells with FCM. The results in Figure 5 indicated that miR-206 overexpression caused remarkable higher apoptosis in C666-1 cells. The rate of early and late apoptotic cells increased to 8.92% and 14.96%, respectively (p < 0.01 for both), which is significantly higher than that of the control cells (2.21% and 2.94%, respectively), suggesting that miR-206 inhibits C666-1 NPC cell growth by promoting apoptosis.
Figure 5.
miR-206 overexpression promotes apoptosis in C666-1 cells. (A) Cells were transfected with miR-206 or scramble miRNA control. Apoptosis was then assayed with flow cytometry after 72 h. (B) The bar graph shows a significant increase in the apoptosis rate in miR-206 overexpressed C666-1 cells (n = 3). Values are means ± SE. **p < 0.01 versus the control group.
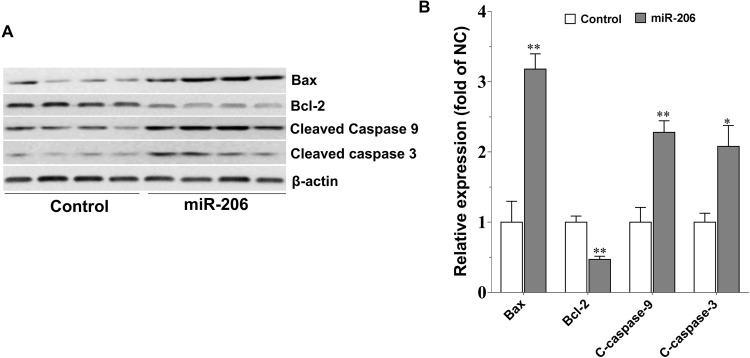
Consistent with the above result, compared with the scramble miRNA control, miR-206 overexpression dramatically increased the expression of cleaved caspase 3 and caspase 9 (p < 0.01 for both) (Fig. 6). Bax and Bcl-2, the two typical proteins of the Bcl-2 family that play critical roles in caspase-dependent apoptosis, were downregulated and upregulated, respectively, by miR-206, and thus an elevated ratio of Bax/Bcl-2 was induced by miR-206 (p < 0.01) (Fig. 6), which is similar to the effect produced by GW50151620.
Figure 6.
miR-206 overexpression promotes the expression of apoptosis-associated protein in C666-1 cells. (A) Western blotting analysis of the protein expression of caspase and B-cell lymphoma 2 (Bcl-2) family members in response to miR-206 overexpression. After transfection for 72 h, cells were harvested, lysed, and processed for Western blotting assay as described in Materials and Methods. (B) The quantification result of (A) (n = 3). Values are means ± SE. *p < 0.05, **p < 0.01 versus the control group.
miR-206 Suppresses Tumorigenesis of C666-1 Cells in Nude Mice
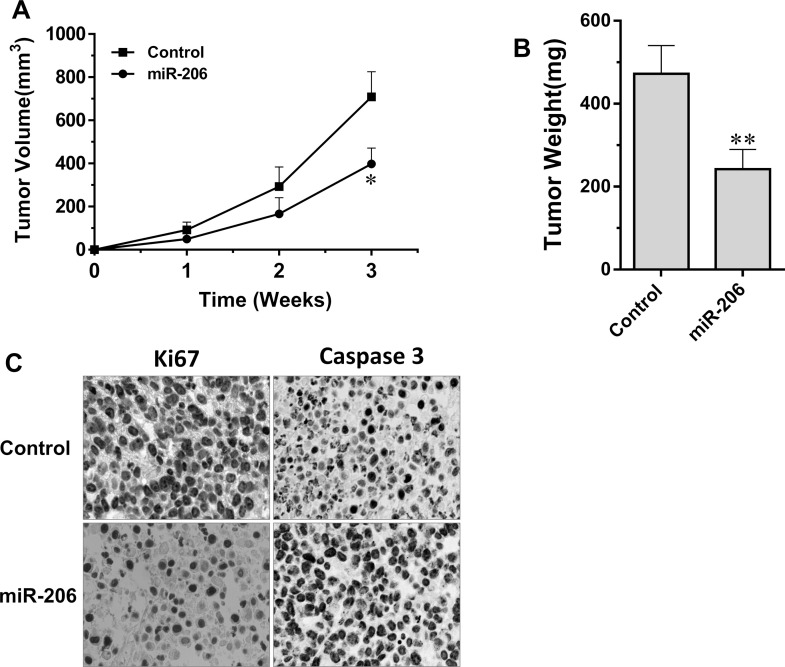
To investigate the in vivo antiproliferation effect of miR-206, the mouse tumorigenesis assay was performed. Figure 7 shows that miR-206 overexpression strikingly suppressed the growth and the weight of subcutaneous xenograft tumors, when comparing with that of the control mice. At week 3, a 43.8% inhibition rate on tumor volume was obtained. The average tumor volume reached 398 mm3 in miR-206-overexpressed C666-1 xenograft samples, in contrast to that of 700 mm3 in the control mice (p < 0.05) (Fig. 7A). The weight of the subcutaneous xenograft tumors was only about half of that of the control mice (Fig. 7B). In addition, the immunoassay of cell proliferation and apoptosis was performed by detection of the expression of Ki-67 and caspase 3 on the xenograft samples, respectively. Corresponding with the in vitro result, the brown-stained caspase 3 was markedly increased, while the expression of Ki-67 was reduced apparently in miR-206-overexpressed C666-1 xenograft tissues (Fig. 7C). These results demonstrated that miR-206 could suppress the growth of C666-1 NPC cells and inhibit NPC tumor formation at the in vivo level, which is associated with induced apoptosis and inhibited proliferation.
Figure 7.
miR-206 suppresses tumorigenesis of C666-1 cells in nude mice. (A) Growth curves of miR-206-overexpressing C666-1 cells and their control (n = 7). (B) Tumor weight measured at the end of the experiment (n = 7). (C) Immunohistochemical detection of the expression and distribution of caspase 3 and Ki-67 in C666-1 xenograft tumor. Values are means ± SD. *p < 0.05, **p < 0.01 versus the control group.
DISCUSSION
Based on our previous work, we get further insights into the mechanism on the underlying antitumorigenic effect of GW501516 in C666-1 NPC cells in the current study. We found that miRNAs, particularly miR-206, might connect PPARβ/δ activation to increase cell apoptosis and suppress cell growth in C666-1 NPC cells. Similar to the condition observed in other types of cancer19,22, the expression of miR-206 was reduced in C666-1 cells when compared with that of the normal NP69 cells. However, its expression was significantly and specifically upregulated by GW501516 in C666-1 cells at both the in vitro cellular level and the in vivo xenograft samples. Moreover, we confirmed that miR-206 plays a critical role in GW501516’s suppression on the growth of C666-1 cells and on regulating the expression of apoptotic-associated proteins. Most importantly, the in vivo antitumor effect of miR-206 was confirmed in the C666-1 subcutaneous xenograft mice.
Despite increasing evidence that pointed to a role for miR-206 as a tumor suppressor, the antitumor effect of miR-206 in the undifferentiated NPC cells has never been examined. To the best of our knowledge, the present study is the first to explore the function and probable underlying mechanism of action of miR-206 in the undifferentiated NPC. Here we found in all the assayed miRNAs that only the expression of miR-206 was upregulated specifically by GW501516 in C666-1 cells. Although the onco-miRNA miR-21 was also slightly repressed under the same condition, it seems not specific to the C666-1 cells, as the CNE-2 cells, the relatively less-sensitive cells to GW501516 treatment, also displayed equivalent reduction in the expression of miR-21 compared to that in C666-1 cells20. We also found that miR-21 inhibitor showed no significant impact to GW501516’s antiproliferation effect in C666-1 cells (data not shown). miR-21 previously had been demonstrated to promote proliferation and migration in many kinds of cancers, including the NPC, by promoting the expression of antiapoptosis protein Bcl-211. In contrast, miR-206 inhibitor was able to effectively reverse GW501516’s suppression on the growth of C666-1 cells by antagonizing the attenuation of antiapoptotic protein Bcl-2 and the elevation of apoptosis-promoting protein Bax induced by GW501516. The ninefold elevation on the ratio of Bax/Bcl-2 in GW501516-treated cells was almost reversed to the level of vehicle-treated group; the caspase-dependent apoptosis was thus avoided by the presence of miR-206 inhibitor. Promoting apoptosis is a vital way for chemotherapy of solid tumors including NPC. Upon initiation from the activation of caspase 9, the apoptosis cascade can be triggered in the C666-1 cells by upregulating intracellular miR-206 content after activation of PPARβ/δ, and the executioner caspase 3 and its direct executant PARP were activated subsequently20. During this process, the enhanced ratio of Bax to Bcl-2 will facilitate this caspase-dependent apoptosis. The finding in the C666-1 cells is consistent with previous reports that miR-206 directly targets and regulates Bcl-2 expression in lung and hepatocellular tumor cells19,22.
In addition, the tumor suppressor miRNAs that previously had been shown to take part in modulating the expression of Bcl-2, including miR-16, miR-29c, miR-15a, and miR-184, were assayed simultaneously in GW501516-treated C666-1 cells. Their expression was usually attenuated in NPC cells, and this led to accelerated tumorigenesis of the NPC cells8,23. The cell proliferation, migration, and invasion of the NPC cells could be suppressed by forced expression of these miRNAs, which was found to be achieved majorly through targeting apoptotic-associated proteins8. As a tumor suppressor in several kinds of human cancers, miR-16 has been identified to inhibit CNE-2 cell proliferation and invasion, and increase apoptosis and chemo- or radiosensitivity by repressing its downstream target Bcl-224,25. miR-184 is reported to directly suppress Bcl-2 and C-MYC, and participate in programmed cell death 4 (PDCD4)-mediated suppression of cell proliferation and survival in NPC cells26. miR-29c substantially enhances the sensitivity of NPC cells to ionizing radiation (IR) and cisplatin treatment through repressing the expression of antiapoptotic factors myeloid cell leukemia 1 (MCL-1) and Bcl-2 in NPC tissues and cell lines14. miR-15a has been shown to produce antitumor roles at in vitro and in vivo levels in the well-differentiated HONE and HONE1 NPC cells via downregulating its downstream proteins including Bcl-227,28. miR-15a overexpression inhibited cell growth by arresting cells at G1/S phase27, in contrast to the G2/M phase of cell cycle arrest induced by GW501516 in the C666-1 NPC cells and T24 bladder cancer cells20,29. Therefore, although miR-15a has been implicated in regulating the expression of Bcl-2 and caspases, it was not the key miRNA modulated by GW501516. This differential regulation might underpin the specific antitumorigenesis effect of GW501516 in the undifferentiated NPC. GW501516 also displayed no impact on the expression of miR-16, miR-29c, and miR-184 in the C666-1 cells, which suggested that the regulation of PPARβ/δ activation by GW501516 on expression of miRNA is NPC-type dependent.
Furthermore, miR-206 overexpression was demonstrated to be capable of mimicking the antitumor effect of PPARβ/δ activation by GW501516 in C666-1 cells. We found that the expression of cleaved caspase 3 and caspase 9 was elevated by miR-206, together with an augmented ratio of Bax to Bcl-2. This paved the basis for promoted apoptosis, lowered growth rate, and reduced colony formation capability in miR-206-treated C666-1 cells, and further revealed that miR-206 might directly regulate the content of these apoptotic-associated proteins in tumor cells. More importantly, here we further corroborated that miR-206 could apparently suppress the tumorigenesis of C666-1 cells in the BALB/c nu/nu mice xenograft model, which is manifested as a reduced tumor volume and weight. Thus, our current result is in well accordance with the tumor suppressor properties of miR-206 produced in other human cancers, where the expression level of miR-206 was found to be significantly reduced18,19,22. However, the proliferation and invasion of these tumor cells was strikingly inhibited by overexpression of miR-206. This action is presumed to be realized through controlling the Bcl-2 induced mitochondria-directed apoptosis pathway by miR-20619,22. Therefore, based on current results, it is reasonable to speculate that miR-206 underpinned GW501516’s regulation on the expression of apoptotic-associated proteins. However, it is worth further exploring if other miRNAs also take part in this process.
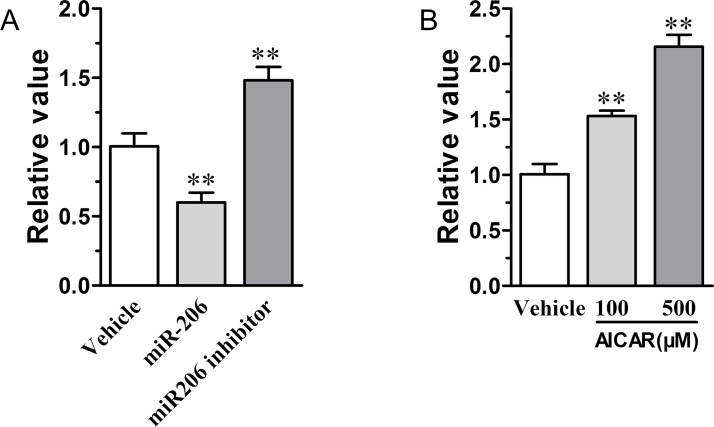
As a PPARβ/δ agonist with AMPKα activation property, GW501516 inhibiting tumorigenesis of the undifferentiated C666-1 NPC cells had been identified by us to be greatly associated with its modulation on the expression of integrin-linked kinase (ILK), a vital cancer cell survival-promoting protein20,29,30. We thus analyzed the relation between miR-206 and ILK, and found that miR-206 overexpression could suppress ILK gene expression within the C666-1 cells. In contrast, ILK’s expression was further promoted in the miR-206 inhibitor-treated cells (Fig. 8A). Furthermore, we found that an increased miR-206 expression induced by GW501516 also could be antagonized by the AMPKα antagonist dorsomorphin (Fig. 1C). This information suggested that miR-206 is an upstream regulator of ILK expression that is directly under the control of AMPKα activation. Indeed, the AMPKα agonist AICAR also could dose-dependently induce miR-206 expression (Fig. 8B). This is consistent with the findings that AICAR treatment inhibited proliferation and anchorage-independent growth, and induced apoptosis of C666-1 NPC cells by activating caspase 3 and altering the Bax/Bcl-2 apoptotic signaling31,32.
Figure 8.
miR-206 regulates the expression of integrin-linked kinase (ILK), and AMPKα agonist upregulates the expression of miR-206 in C666-1 cells. (A) miR-206 regulates the expression of ILK in C666-1 cells. (B) AMPKα agonist AICAR increases the expression of miR-206 in C666-1 cells (n = 3). Values are means ± SD. **p < 0.01 versus vehicle group.
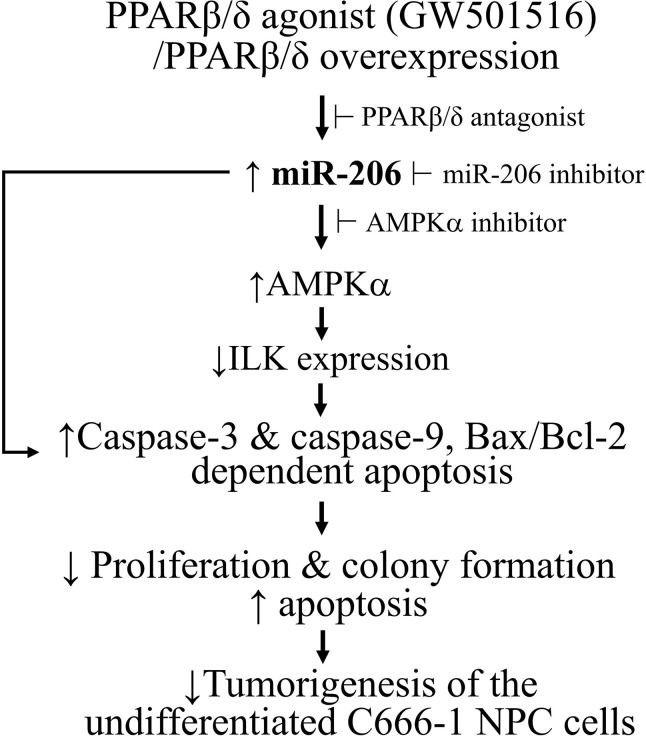
In summary, our current results confirmed a reduced expression of miR-206 in the undifferentiated C666-1 NPC cells compared to the nasopharyngeal epithelial cells. The direct apoptosis-promoting effect induced by GW501516 in C666-1 cells is presumed to be associated with its upregulation on the expression of miR-206, which then promoted the caspase-dependent apoptosis (Fig. 9). In addition, our data also suggested that upregulation of miR-206 may have the potential to provide a fundamentally new approach for gene therapy of the undifferentiated NPC.
Figure 9.
The scheme of miR-206 connecting PPARβ/δ activation and apoptosis in undifferentiated nasopharyngeal carcinoma C666-1 cells.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Chien SY, Hsu CH, Lin CC, Chuang YC, Lo YS, Hsi YT, Hsieh MJ, Chen MK. Nimbolide induces apoptosis in human nasopharyngeal cancer cells. Environ Toxicol. 2017;32(8):2085–92. [DOI] [PubMed] [Google Scholar]
- 2. Bruce JP, Yip K, Bratman SV, Ito E, Liu FF. Nasopharyngeal cancer: Molecular landscape. J Clin Oncol. 2015;33(29):3346–55. [DOI] [PubMed] [Google Scholar]
- 3. Zhang B, Wang Q, Pan X. MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol. 2007;210(2):279–89. [DOI] [PubMed] [Google Scholar]
- 4. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 5. Bruce JP, Liu FF. MicroRNAs in nasopharyngeal carcinoma. Chin J Cancer 2014;33(11):539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spence T, Bruce J, Yip KW, Liu FF. MicroRNAs in nasopharyngeal carcinoma. Chin Clin Oncol. 2016;5(2):17. [DOI] [PubMed] [Google Scholar]
- 7. Lee KT, Tan JK, Lam AK, Gan SY. MicroRNAs serving as potential biomarkers and therapeutic targets in nasopharyngeal carcinoma: A critical review. Crit Rev Oncol Hematol. 2016;103:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Deng B, Su F, Xie R, Tang W. miR-371-5p suppresses the proliferative and migratory capacity of human nasopharyngeal carcinoma by targeting BCL2. Oncol Lett. 2018;15(6):9209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo Z, Zhang L, Li Z, Li X, Li G, Yu H, Jiang C, Dai Y, Guo X, Xiang J, Li G. An in silico analysis of dynamic changes in microRNA expression profiles in stepwise development of nasopharyngeal carcinoma. BMC Med Genomics 2012;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun XJ, Liu H, Zhang P, Zhang XD, Jiang ZW, Jiang CC. miR-10b promotes migration and invasion in nasopharyngeal carcinoma cells. Asian Pac J Cancer Prev. 2013;14(9):5533–7. [DOI] [PubMed] [Google Scholar]
- 11. Li Y, Yan L, Zhang W, Wang H, Chen W, Hu N, Ou H. miR-21 inhibitor suppresses proliferation and migration of nasopharyngeal carcinoma cells through down-regulation of BCL2 expression. Int J Clin Exp Pathol. 2014;7(6):3478–87. [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Z, Cheng C, Luo X, Xia Q, Zhang Y, Long X, Jiang Q, Fang W. CDK4 and miR-15a comprise an abnormal automodulatory feedback loop stimulating the pathogenesis and inducing chemotherapy resistance in nasopharyngeal carcinoma. BMC Cancer 2016;16:238. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Zhang C, Fang X, Li W, Shi Q, Wu L, Chen X, Huang Z, Wu P, Wang Z, Liao Z. Influence of recombinant lentiviral vector encoding miR-15a/16-1 in biological features of human nasopharyngeal carcinoma CNE-2Z cells. Cancer Biother Radiopharm. 2014;29(10):422–7. [DOI] [PubMed] [Google Scholar]
- 14. Zhang JX, Qian D, Wang FW, Liao DZ, Wei JH, Tong ZT, Fu J, Huang XX, Liao YJ, Deng HX, Zeng YX, Xie D, Mai SJ. MicroRNA-29c enhances the sensitivities of human nasopharyngeal carcinoma to cisplatin-based chemotherapy and radiotherapy. Cancer Lett. 2013;329(1):91–8. [DOI] [PubMed] [Google Scholar]
- 15. Ren XL, He GY, Li XM, Men H, Yi LZ, Lu GF, Xin SN, Wu PX, Li YL, Liao WT Ding YQ, Liang L. MicroRNA-206 functions as a tumor suppressor in colorectal cancer by targeting FMNL2. J Cancer Res Clin Oncol. 2016;142(3):581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Ling C, Bai Y, Zhao J. MicroRNA-206 is associated with invasion and metastasis of lung cancer. Anat Rec. (Hoboken) 2011;294(1):88–92. [DOI] [PubMed] [Google Scholar]
- 17. Yunqiao L, Vanke H, Jun X, Tangmeng G. MicroRNA-206, down-regulated in hepatocellular carcinoma, suppresses cell proliferation and promotes apoptosis. Hepatogastroenterology 2014;61(133):1302–7. [PubMed] [Google Scholar]
- 18. Ling S, Ruiqin M, Guohong Z, Bing S, Yanshan C. Decreased microRNA-206 and its function in cervical cancer. Eur J Gynaecol Oncol. 2015;36(6):716–21. [PubMed] [Google Scholar]
- 19. Liu W, Xu C, Wan H, Liu C, Wen C, Lu H, Wan F. MicroRNA-206 overexpression promotes apoptosis, induces cell cycle arrest and inhibits the migration of human hepatocellular carcinoma HepG2 cells. Int J Mol Med. 2014;34(2):420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ji Y, Li H, Wang F, Gu L. PPARbeta/delta agonist GW501516 inhibits tumorigenicity of undifferentiated nasopharyngeal carcinoma in C666-1 cells by promoting apoptosis. Front Pharmacol. 2018;9:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shearer BG, Wiethe RW, Ashe A, Billin AN, Way JM, Stanley TB, Wagner CD, Xu RX, Leesnitzer LM, Merrihew RV, Shearer TW, Jeune MR, Ulrich JC, Willson TM. Identification and characterization of 4-chloro-N-(2-{[5-trifluoromethyl)-2-pyridyl]sulfonyl}ethyl)benzamide (GSK3787), a selective and irreversible peroxisome proliferator-activated receptor delta (PPARdelta) antagonist. J Med Chem. 2010;53(4):1857–61. [DOI] [PubMed] [Google Scholar]
- 22. Sun C, Liu Z, Li S, Yang C, Xue R, Xi Y, Wang L, Wang S, He Q, Huang J, Xie S, Jiang W, Li D. Down-regulation of c-Met and Bcl2 by microRNA-206, activates apoptosis, and inhibits tumor cell proliferation, migration and colony formation. Oncotarget 2015;6(28):25533–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He Q, Ren X, Chen J, Li Y, Tang X, Wen X, Yang X, Zhang J, Wang Y, Ma J, Liu N. miR-16 targets fibroblast growth factor 2 to inhibit NPC cell proliferation and invasion via PI3K/AKT and MAPK signaling pathways. Oncotarget 2016;7(3):3047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuan TZ, Zhang HH, Lin XL, Yu JX, Yang QX, Liang Y, Deng J, Huang LJ, Zhang XP. microRNA-125b reverses the multidrug resistance of nasopharyngeal carcinoma cells via targeting of Bcl-2. Mol Med Rep. 2017;15(4):2223–8. [DOI] [PubMed] [Google Scholar]
- 25. Cheng JZ, Chen JJ, Wang ZG, Yu D. MicroRNA-185 inhibits cell proliferation while promoting apoptosis and autophagy through negative regulation of TGF-beta1/mTOR axis and HOXC6 in nasopharyngeal carcinoma. Cancer Biomark. 2018;23(1):107–23. [DOI] [PubMed] [Google Scholar]
- 26. Zhen Y, Liu Z, Yang H, Yu X, Wu Q, Hua S, Long X, Jiang Q, Song Y, Cheng C, Wang H, Zhao M, Fu Q, Lyu X, Chen Y, Fan Y, Liu Y, Li X, Fang W. Tumor suppressor PDCD4 modulates miR-184-mediated direct suppression of C-MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell Death Dis. 2013;4:e872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang C, Cheng Y, Liu H, Xu Y, Peng H, Lang J, Liao J, Liu H, Liu H, Fan J. Pectolinarigenin suppresses the tumor growth in nasopharyngeal carcinoma. Cell Physiol Biochem. 2016;39(5):1795–803. [DOI] [PubMed] [Google Scholar]
- 28. Zhu K, He Y, Xia C, Yan J, Hou J, Kong D, Yang Y, Zheng G. MicroRNA-15a inhibits proliferation and induces apoptosis in CNE1 nasopharyngeal carcinoma cells. Oncol Res. 2016;24(3):145–51. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Pechery A, Fauconnet S, Bittard H, Lascombe I. Apoptotic effect of the selective PPARbeta/delta agonist GW501516 in invasive bladder cancer cells. Tumour Biol. 2016;37(11):14789–802. [DOI] [PubMed] [Google Scholar]
- 30. McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase—Essential roles in physiology and cancer biology. J Cell Sci. 2008;121(Pt 19):3121–32. [DOI] [PubMed] [Google Scholar]
- 31. Lo AK, Lo KW, Ko CW, Young LS, Dawson CW. Inhibition of the LKB1-AMPK pathway by the Epstein-Barr virus-encoded LMP1 promotes proliferation and transformation of human nasopharyngeal epithelial cells. J Pathol. 2013;230(3):336–46. [DOI] [PubMed] [Google Scholar]
- 32. Cai Y, Zhao L, Qin Y, Zhang M, He Y. Resveratrol inhibits proliferation and induces apoptosis of nasopharyngeal carcinoma cell line C666-1 through AMPK activation. Pharmazie 2015;70(6):399–403. [PubMed] [Google Scholar]