Abstract
Hepatocellular carcinoma (HCC) is one of the major malignant tumors that lead to death. Chronic hepatitis B virus infection is an important risk factor for HCC initiation. HBx protein, encoded by the HBV X gene, is a significant factor that promotes HBV-related HCC, although the exact molecular mechanism remains unclear. This article summarizes the pathological roles and related mechanisms of HBx in HCC. HBx plays a carcinogenic role by promoting cell proliferation, metastasis, and angiogenesis and inhibiting apoptosis in HCC. A detailed study of the biological functions of HBx will help to elucidate the mechanism of hepatocarcinogenesis and lead to the development of novel therapeutic targets for the treatment of HBV-related HCC.
Key words: Hepatocellular carcinoma (HCC), Hepatitis B virus (HBV), Carcinogenic mechanism, Hepatitis B virus X (HBx) protein
INTRODUCTION
Liver cancer is one of the most common fatal tumors worldwide. It ranks second in terms of cancer-related mortality and morbidity due to liver cancer has been ranked sixth among the major types of cancers1. GLOBOCAN 2012, published by the International Agency for Research on Cancer (IARC), reported approximately 782,500 new cases of liver cancer and 745,500 deaths in 2012 worldwide2. Hepatocellular carcinoma (HCC) accounted for 70%–90% of primary liver cancers3. Chronic hepatitis B virus (HBV) infection is a risk factor for HCC induction4. It is estimated that currently there are over 350 million HBV carriers globally5. However, the underlying mechanism of HBV-associated HCC remains unclear. Hepatitis B virus X (HBx) protein, encoded by the HBV X gene, is a multifunctional protein responsible for HBV-related hepatocarcinogenesis6. Numerous studies have thoroughly explored the roles that HBx played in the initiation and development of HCC and its molecular mechanisms. In this article, we comprehensively summarize the biological function of HBx in HCC by regulating a variety of biological processes, such as cell proliferation, cell invasion or metastasis, angiogenesis, and cell apoptosis.
THE HBV GENOME AND MOLECULAR MECHANISMS OF HBV INFECTION-RELATED HCC (HBV-HCC)
HBV, belonging to the Hepadnaviridae family, is a partially double-stranded and looped DNA virus. Its genome contains four overlapping open reading frames (ORFs) encoding four proteins. These are pre-S/S (virus surface protein), C (virus core protein), P (virus polymerase), and X (HBx protein).
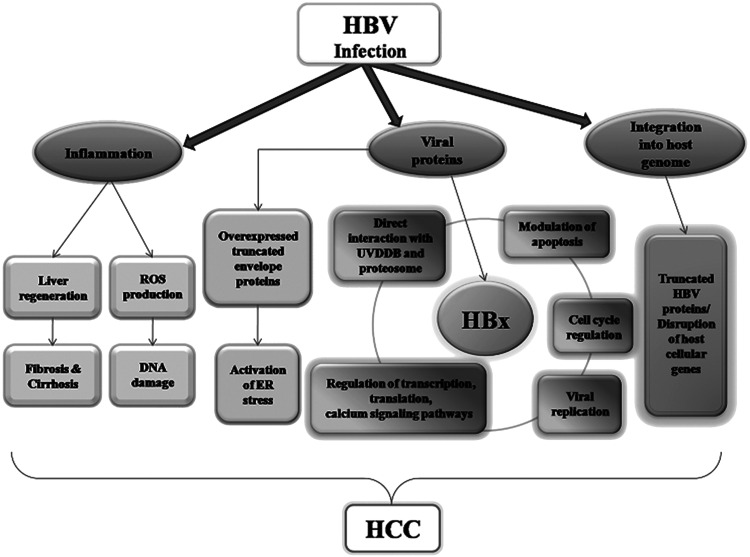
HBV infection is involved in the carcinogenesis of HCC by chronic inflammation, integration of DNA into the host genome and viral proteins (see Fig. 1), through the following concrete mechanisms. (1) Cytotoxic T lymphocytes, induced by immune system activation after HBV infection, attack HBV-infected hepatocytes. Necrosis of liver cells then causes inflammation, resulting in further liver cirrhosis by activating fibroblasts leading to liver fibrosis. This is consistent with the fact that over 80% of HCC patients may have liver fibrosis cirrhosis7. Additionally, inflammation and immune responses caused by HBV infection can generate oxidative stress and produce reactive oxygen species (ROS). It is well known that ROS causes oxidative damages to DNA by interrupting repair mechanisms8. The accumulation of DNA mutations also results in the development of HCC. (2) The HBV genome integrates into the host genome in 85% to 90% of HBV-HCC cases, whereas host genes in the HBV integration region can undergo further mutation9–11. This causes host cell genome instability and disorders of various intracellular signaling pathways, which are beneficial for promoting HCC development12. (3) Viral proteins encoded by the HBV genome also play carcinogenic roles. The surface protein is often localized on the endoplasmic reticulum with its truncated mutant, causing endoplasmic reticulum stress followed by the oxidative damage of DNA and host cell genome instability13,14. HBx has long been considered a key protein in malignant transformation and is an important factor in the onset of HBV-HCC15. Next, we summarize the biological function and carcinogenic mechanisms of HBx in HBV-HCC.
Figure 1.
Schematic representation of related carcinogenetic mechanisms of hepatitis B virus infection in hepatocellular carcinoma.
HBx PROTEIN
The X ORF of HBV genome encodes the HBx protein. HBx is a small, approximately 17-kDa multifunctional protein. A number of studies have shown HBx stimulated HBV replication and transcription16,17. Recent studies have concluded that HBx plays an important role in the carcinogenesis of HBV-HCC. Among the Hepadnaviridae family, only woodchuck hepatitis virus and HBV encode the HBx protein. Intriguingly, virus-associated liver cancer occurs only in hosts infected by these two viruses18. Transgenic mouse experiments have confirmed that HBx overexpression can induce HCC. Additionally, HBx can increase the rate of HCC formation in c-myc transfection mice and reduce the incubation period of HCC occurrence by diethylnitrosamine19,20. Although HBx is closely related to the onset and progression of HBV-HCC, the exact molecular mechanisms remain unknown. Here we will combine the current knowledge to elucidate the pathological function and related mechanisms of HBx in HCC from different perspectives.
HBx AND CELL PROLIFERATION
Recent studies have demonstrated that HBx plays critical roles in carcinogenesis through the promotion of HCC cell proliferation. Du et al. reported that lncRNA highly upregulated in liver cancer (HULC) promoted proliferation of hepatoma cells through suppression of p18 and HULC was upregulated by HBx21. Hu and colleagues reported that HBx upregulated lncRNA UCA1. UCA1 was physically associated with enhancer of zeste homolog 2 (EZH2), which suppresses p27Kip1 and results in the enhancement of cell proliferation22. Huang et al. found that HBx could upregulate the expression of DBH-AS1 and consequently promoted cell proliferation through activation of MAPK signaling in HCC23.
MicroRNAs are a new class of small noncoding RNAs that are 18 to 25 nucleotides in length. Recent work has revealed that expression of several miRNAs was altered in HBV-HCC; simultaneously aberrant expression of miRNAs played an important biological role by acting on downstream target genes. HBx plays critical roles in promoting cell proliferation through regulating miRNAs. HBx promotes aberrant HCC cell proliferation via the downregulation of miR-132, miR-429, miR-205, miR-15b, and miR-145 and the upregulation of miR-22124–29.
HBX AND CELL INVASION OR METASTASIS
HBx promotes HCC invasion and metastasis by multiple mechanisms. Liu et al. reported that HBx increases the expression of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) by activating the nuclear factor κB (NF-κB) pathway. The upregulation of VEGF and MMPs promotes HCC cell invasion and metastasis30. Han and colleagues found that the HBx protein is also localized to peroxisomes to increases the level of cellular ROS. The peroxisome-localized HBx increased the expressions of MMPs and decreased the expression of E-cadherin, which facilitated HCC cell invasion31. Zhang et al. reported that HBx promoted HCC cell migration through the upregulation of calpain small subunit 1 (Capn4)32.
HBx also plays a critical role in enhancing HCC cell invasion through regulating miRNAs and lncRNAs. Xu and colleagues found that HBx suppressed p53-mediated activation of miR-148a, which resulted in the upregulation of hematopoietic pre-B-cell leukemia transcription factor-interacting protein (HPIP). HPIP increased the expression of mTOR through the AKT/ERK/FOXO4/ATF5 pathway, which resulted in HCC cell invasion and metastasis33. Arzumanyan et al. reported that HBx suppressed miR-373, a positive regulator of E-cadherin expression. The downregulation of E-cadherin is associated with the enhancement of the cell invasion ability of HCC34. Kong et al. found that the upregulation of miR-29a by HBx promotes HCC cell by targeting PTEN35. A study by Zhao and colleagues reported that HBx elevates oncoprotein AEG-1 expression to promote HCC cell migration by downregulating miR-375 and miR-13636. Huang and colleagues discovered that the lncRNA Dreh was downregulated by HBx using chip sequencing technology and HBx transgenic mice. They also found that lncRNA Dreh inhibited metastasis via the downregulation of vimentin37.
HBx AND ANGIOGENESIS
HCC is a kind of highly vascularized solid tumor. Angiogenesis is important for the growth and metastasis of HCC. Previous studies showed that HBx stimulated angiogenesis during hepatocarcinogenesis. It was reported that HBx stimulated the transcription of VEGF, a known angiogenic factor38. Moon and colleagues found that HBx induced angiogenesis by increasing the transcriptional activity and protein level of hypoxia-inducible factor-1α (HIF-1α)39. A study by Lu et al. found that HBx upregulated endothelin 1 (END1) indirectly by suppressing miR-1. END1 plays a crucial role in promoting angiogenesis in HCC by activating the PI3K/AKT pathway40. Study from another group reported that HBx contributed to angiogenesis by downregulation of Lethal-7 through activating STAT341. Moreover, HBx increases the expression of MTA1 (metastasis-associated protein 1) coregulator via NF-κB signaling by the suppression of miR-661 to induce angiogenesis42. HBx induces the expression of miR-7, miR-107, and miR-21 and results in the downregulation of mammary serine protease inhibitor (Maspin). The repressed Maspin makes a contribution to angiogenesis in HCC43.
HBx AND CELL APOPTOSIS
HBx-induced apoptosis contributes to HCC carcinogenesis mainly because of the interaction between the HBx and p53 pathways. The p53 pathway, composed of the well-known tumor suppressor protein p53 and its downstream target genes including p21CIP/WAF1 44, Bax45, TGFα46, and EGFR47, is activated when the cell receives exogenous and endogenous stimulation. The main functions of p53 are to monitor DNA replication and cell division, block cell cycle progress, and induce apoptosis to maintain genomic integrity48. p53 drives progression of HCC. Previous studies have found that the p53 pathway is involved in the development of HCC49; additionally, interaction between p53 and HBx has been widely confirmed. At the transcriptional level, HBx can inhibit p53 promoter activity and significantly downregulate the mRNA level of p53 in HBx-transfected cells50. At the protein level, HBx can directly interact with p53 and inhibit p53 function51. p53 can no longer induce cell apoptosis after being inhibited by HBx—this may be crucial for the occurrence of early HCC52–54.
Upon DNA damage by carcinogens or radiation, p53 is activated and causes transcription activation of the downstream target genes including p21CIP/WAF1. p21CIP/WAF1 inhibits the cyclin–CDK complex that induces phosphorylation of pRb. Subsequently release of E2F1 is blocked, resulting in cell cycle arrest at the G1 phase, which can ultimately induce apoptosis44. A study on HBV-infected HCC tissues found that mRNA expression of p21CIP/WAF1 was reduced compared to that in the normal liver tissue55. The interaction between HBx and the p53 carboxy terminus may explain why the activation ofp21CIP/WAF1 transcription by p53 is suppressed, thus inhibiting the expression of p21CIP/WAF1 53. Similarly, p53 modulates apoptosis by interaction with ASPP1 and ASPP2. Nude mice experiments have confirmed that sensitivity of malignant cells to apoptotic stimuli decreased and tumor growth was promoted after knockdown of the ASPP1 and ASPP2 genes56. Zhao and coworkers found that, by analyzing 51 pairs of carcinoma samples and adjacent normal tissues, the expression of ASPP1 and ASPP2 was reduced due to promoter hypermethylation. Interestingly, HBx overexpression in hepatoma cells induced methylation of the ASPP2 promoter region57. Hence, HBx may inhibit p53-mediated apoptosis by downregulating ASPP2. In addition, HBx could inhibit Fas-mediated apoptosis by the upregulation of the SAPK/JNK pathway58 and prevent cell apoptosis by upregulating SATB1 and HURP (hepatoma upregulated protein) expression in HCC59.
HBx-induced apoptosis also contributes to chemotherapy drug resistance in HCC. As mentioned above, HBx can directly bind to p53 and cause inactivation of p5351, or it can inhibit p53 through regulating Cox-2, thus inhibiting p53-induced apoptosis60. Furthermore, Cheng and colleagues discovered that knockdown of HBx increased apoptosis induced by cisplatin61. MDR1 (multidrug resistance 1) is upregulated when tumor cells switch from chemotherapeutic drug sensitivity to resistance. HBx was found to activate HIF-1α that promotes expression of MDR1 in the H411E cell line62. Another study showed that in the Hep3B cell line, HBx increased antiapoptosis upon cisplatin treatment by upregulating HURP via the p38/MAPK pathway. In addition, HBx also promoted the expression of survivin, an antiapoptosis protein59. Huang and colleagues discovered that HBx caused resistance to bortezomib in HBV-HCC, and the resistance could be antagonized by an inhibitor of MEK signaling63.
CONCLUSION AND PROSPECTIVE
Multiple molecules and signaling pathways are involved in the formation and progression of HBV-HCC, and HBx is a key factor in hepatocarcinogenesis. We have summarized the various malignant biological functions of HBx in HCC, including regulation of cell proliferation, invasion and metastasis, apoptosis, and angiogenesis in the related molecular mechanisms.
However, the exact functions and molecular mechanisms of HBx in HCC have not yet been elucidated. Further study of the biological functions of HBx will help to elucidate the mechanism of hepatocarcinogenesis and promote the development of novel therapeutic targets for the treatment of HBV-HCC. In addition to present studies concentrating on trials of HBx in vitro, the functions of HBx must also be thoroughly explored in vivo.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- 3. Gelband H, Chen CJ, Chen W, Franceschi S, Hall SA, London WT, McGlynn KA, Wild CP. Liver Cancer. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Cancer: Disease control priorities, 3rd ed., Vol 3 Washington (DC): The World Bank; 2015. [Google Scholar]
- 4. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat Rev Cancer 2006;6(9):674–87. [DOI] [PubMed] [Google Scholar]
- 5. Yang HI, Yeh SH, Chen PJ, Iloeje UH, Jen CL, Su J, Wang LY, Lu SN, You SL, Chen DS, Liaw YF, Chen CJ; REVEAL-HBV Study Group. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(16):1134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin-Vilchez S, Lara-Pezzi E, Trapero-Marugan M, Moreno-Otero R, Sanz-Cameno P. The molecular and pathophysiological implications of hepatitis B X antigen in chronic hepatitis B virus infection. Rev Med Virol. 2011;21(5):315–29. [DOI] [PubMed] [Google Scholar]
- 7. Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32(1 Suppl):225–37. [DOI] [PubMed] [Google Scholar]
- 8. Higgs MR, Chouteau P, Lerat H. ‘Liver let die’: Oxidative DNA damage and hepatotropic viruses. J Gen Virol. 2014;95(Pt 5):991–1004. [DOI] [PubMed] [Google Scholar]
- 9. Matsubara K, Tokino T. Integration of hepatitis B virus DNA and its implications for hepatocarcinogenesis. Mol Biol Med. 1990;7(3):243–60. [PubMed] [Google Scholar]
- 10. Koshy R, Maupas P, Muller R, Hofschneider PH. Detection of hepatitis B virus-specific DNA in the genomes of human hepatocellular carcinoma and liver cirrhosis tissues. J Gen Virol. 1981;57(Pt 1):95–102. [DOI] [PubMed] [Google Scholar]
- 11. Shafritz DA, Shouval D, Sherman HI, Hadziyannis SJ, Kew MC. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981;305(18):1067–73. [DOI] [PubMed] [Google Scholar]
- 12. Sukowati CH, El-Khobar KE, Ie SI, Anfuso B, Muljono DH, Tiribelli C. Significance of hepatitis virus infection in the oncogenic initiation of hepatocellular carcinoma. World J Gastroenterol. 2016;22(4):1497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pollicino T, Cacciola I, Saffioti F, Raimondo G. Hepatitis B virus PreS/S gene variants: Pathobiology and clinical implications. J Hepatol. 2014;61(2):408–17. [DOI] [PubMed] [Google Scholar]
- 14. Hildt E, Urban S, Lauer U, Hofschneider PH, Kekule AS. ER-localization and functional expression of the HBV transactivator MHBst. Oncogene 1993;8(12):3359–67. [PubMed] [Google Scholar]
- 15. Xu C, Zhou W, Wang Y, Qiao L. Hepatitis B virus-induced hepatocellular carcinoma. Cancer Lett. 2014;345(2):216–22. [DOI] [PubMed] [Google Scholar]
- 16. Decorsiere A, Mueller H, van Breugel PC, Abdul F, Gerossier L, Beran RK, Livingston CM, Niu C, Fletcher SP, Hantz O, Strubin M. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature 2016;531(7594):386–9. [DOI] [PubMed] [Google Scholar]
- 17. Murphy CM, Xu Y, Li F, Nio K, Reszka-Blanco N, Li X, Wu Y, Yu Y, Xiong Y, Su L. Hepatitis B virus X protein promotes degradation of SMC5/6 to enhance HBV replication. Cell Rep. 2016;16(11):2846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feitelson MA. Hepatitis B virus in hepatocarcinogenesis. J Cell Physiol. 1999;181(2):188–202. [DOI] [PubMed] [Google Scholar]
- 19. Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature 1991;351(6324):317–20. [DOI] [PubMed] [Google Scholar]
- 20. Yu DY, Moon HB, Son JK, Jeong S, Yu SL, Yoon H, Han YM, Lee CS, Park JS, Lee CH, Hyun BH, Murakami S, Lee KK. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J Hepatol. 1999;31(1):123–32. [DOI] [PubMed] [Google Scholar]
- 21. Du Y, Kong G, You X, Zhang S, Zhang T, Gao Y, Ye L, Zhang X. Elevation of highly up-regulated in liver cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell proliferation via down-regulating p18. J Biol Chem. 2012;287(31):26302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu JJ, Song W, Zhang SD, Shen XH, Qiu XM, Wu HZ, Gong PH, Lu S, Zhao ZJ, He ML, Fan H. HBx-upregulated lncRNA UCA1 promotes cell growth and tumorigenesis by recruiting EZH2 and repressing p27Kip1/CDK2 signaling. Sci Rep. 2016;6:23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang JL, Ren TY, Cao SW, Zheng SH, Hu XM, Hu YW, Lin L, Chen J, Zheng L, Wang Q. HBx-related long non-coding RNA DBH-AS1 promotes cell proliferation and survival by activating MAPK signaling in hepatocellular carcinoma. Oncotarget 2015;6(32):33791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei X, Tan C, Tang C, Ren G, Xiang T, Qiu Z, Liu R, Wu Z. Epigenetic repression of miR-132 expression by the hepatitis B virus x protein in hepatitis B virus-related hepatocellular carcinoma. Cell Signal. 2013;25(5):1037–43. [DOI] [PubMed] [Google Scholar]
- 25. You X, Liu F, Zhang T, Li Y, Ye L, Zhang X. Hepatitis B virus X protein upregulates oncogene Rab18 to result in the dysregulation of lipogenesis and proliferation of hepatoma cells. Carcinogenesis 2013;34(7):1644–52. [DOI] [PubMed] [Google Scholar]
- 26. Zhang T, Zhang J, Cui M, Liu F, You X, Du Y, Gao Y, Zhang S, Lu Z, Ye L, Zhang X. Hepatitis B virus X protein inhibits tumor suppressor miR-205 through inducing hypermethylation of miR-205 promoter to enhance carcinogenesis. Neoplasia 2013;15(11):1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu CS, Yen CJ, Chou RH, Chen JN, Huang WC, Wu CY, Yu YL. Downregulation of microRNA-15b by hepatitis B virus X enhances hepatocellular carcinoma proliferation via fucosyltransferase 2-induced Globo H expression. Int J Cancer 2014;134(7):1638–47. [DOI] [PubMed] [Google Scholar]
- 28. Bandopadhyay M, Banerjee A, Sarkar N, Panigrahi R, Datta S, Pal A, Singh SP, Biswas A, Chakrabarti S, Chakravarty R. Tumor suppressor micro RNA miR-145 and onco micro RNAs miR-21 and miR-222 expressions are differentially modulated by hepatitis B virus X protein in malignant hepatocytes. BMC Cancer 2014;14:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen JJ, Tang YS, Huang SF, Ai JG, Wang HX, Zhang LP. HBx protein-induced upregulation of microRNA-221 promotes aberrant proliferation in HBV related hepatocellular carcinoma by targeting estrogen receptor-alpha. Oncol Rep. 2015;33(2):792–8. [DOI] [PubMed] [Google Scholar]
- 30. Liu LP, Liang HF, Chen XP, Zhang WG, Yang SL, Xu T, Ren L. The role of NF-kappaB in hepatitis b virus X protein-mediated upregulation of VEGF and MMPs. Cancer Invest. 2010;28(5):443–51. [DOI] [PubMed] [Google Scholar]
- 31. Han JM, Kang JA, Han MH, Chung KH, Lee CR, Song WK, Jun Y, Park SG. Peroxisome-localized hepatitis Bx protein increases the invasion property of hepatocellular carcinoma cells. Arch Virol. 2014;159(10):2549–57. [DOI] [PubMed] [Google Scholar]
- 32. Zhang F, Wang Q, Ye L, Feng Y, Zhang X. Hepatitis B virus X protein upregulates expression of calpain small subunit 1 via nuclear factor-kappaB/p65 in hepatoma cells. J Med Virol. 2010;82(6):920–8. [DOI] [PubMed] [Google Scholar]
- 33. Xu X, Fan Z, Kang L, Han J, Jiang C, Zheng X, Zhu Z, Jiao H, Lin J, Jiang K, Ding L, Zhang H, Cheng L, Fu H, Song Y, Jiang Y, Liu J, Wang R, Du N, Ye Q. Hepatitis B virus X protein represses miRNA-148a to enhance tumorigenesis. J Clin Invest. 2013;123(2):630–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arzumanyan A, Friedman T, Kotei E, Ng IO, Lian Z, Feitelson MA. Epigenetic repression of E-cadherin expression by hepatitis B virus x antigen in liver cancer. Oncogene 2012;31(5):563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kong G, Zhang J, Zhang S, Shan C, Ye L, Zhang X. Upregulated microRNA-29a by hepatitis B virus X protein enhances hepatoma cell migration by targeting PTEN in cell culture model. PLoS One 2011;6(5):e19518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao J, Wang W, Huang Y, Wu J, Chen M, Cui P, Zhang W, Zhang Y. HBx elevates oncoprotein AEG-1 expression to promote cell migration by downregulating miR-375 and miR-136 in malignant hepatocytes. DNA Cell Biol. 2014;33(10):715–22. [DOI] [PubMed] [Google Scholar]
- 37. Huang JF, Guo YJ, Zhao CX, Yuan SX, Wang Y, Tang GN, Zhou WP, Sun SH. Hepatitis B virus X protein (HBx)-related long noncoding RNA (lncRNA) down-regulated expression by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by targeting the intermediate filament protein vimentin. Hepatology 2013;57(5):1882–92. [DOI] [PubMed] [Google Scholar]
- 38. Lee SW, Lee YM, Bae SK, Murakami S, Yun Y, Kim KW. Human hepatitis B virus X protein is a possible mediator of hypoxia-induced angiogenesis in hepatocarcinogenesis. Biochem Biophys Res Commun. 2000;268(2):456–61. [DOI] [PubMed] [Google Scholar]
- 39. Moon EJ, Jeong CH, Jeong JW, Kim KR, Yu DY, Murakami S, Kim CW, Kim KW. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 2004;18(2):382–4. [DOI] [PubMed] [Google Scholar]
- 40. Lu JW, Liao CY, Yang WY, Lin YM, Jin SL, Wang HD, Yuh CH. Overexpression of endothelin 1 triggers hepatocarcinogenesis in zebrafish and promotes cell proliferation and migration through the AKT pathway. PLoS One 2014;9(1):e85318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Lu Y, Toh ST, Sung WK, Tan P, Chow P, Chung AY, Jooi LL, Lee CG. Lethal-7 is down-regulated by the hepatitis B virus x protein and targets signal transducer and activator of transcription 3. J Hepatol. 2010;53(1):57–66. [DOI] [PubMed] [Google Scholar]
- 42. Bui-Nguyen TM, Pakala SB, Sirigiri DR, Martin E, Murad F, Kumar R. Stimulation of inducible nitric oxide by hepatitis B virus transactivator protein HBx requires MTA1 coregulator. J Biol Chem. 2010;285(10):6980–6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Chen WS, Yen CJ, Chen YJ, Chen JY, Wang LY, Chiu SJ, Shih WL, Ho CY, Wei TT, Pan HL, Chien PH, Hung MC, Chen CC, Huang WC. miRNA-7/21/107 contribute to HBx-induced hepatocellular carcinoma progression through suppression of maspin. Oncotarget 2015;6(28):25962–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Michieli P, Chedid M, Lin D, Pierce JH, Mercer WE, Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994;54(13):3391–5. [PubMed] [Google Scholar]
- 45. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995;80(2):293–9. [DOI] [PubMed] [Google Scholar]
- 46. Shin TH, Paterson AJ, Kudlow JE. p53 stimulates transcription from the human transforming growth factor alpha promoter: A potential growth-stimulatory role for p53. Mol Cell Biol. 1995;15(9):4694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ludes-Meyers JH, Subler MA, Shivakumar CV, Munoz RM, Jiang P, Bigger JE, Brown DR, Deb SP, Deb S. Transcriptional activation of the human epidermal growth factor receptor promoter by human p53. Mol Cell Biol. 1996;16(11):6009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000;408(6810):307–10. [DOI] [PubMed] [Google Scholar]
- 49. Shibata T, Aburatani H. Exploration of liver cancer genomes. Nat Rev Gastroenterol Hepatol. 2014;11(6):340–9. [DOI] [PubMed] [Google Scholar]
- 50. Lee SG, Rho HM. Transcriptional repression of the human p53 gene by hepatitis B viral X protein. Oncogene 2000;19(3):468–71. [DOI] [PubMed] [Google Scholar]
- 51. Feitelson MA, Zhu M, Duan LX, London WT. Hepatitis B x antigen and p53 are associated in vitro and in liver tissues from patients with primary hepatocellular carcinoma. Oncogene 1993;8(5):1109–17. [PubMed] [Google Scholar]
- 52. Elmore LW, Hancock AR, Chang SF, Wang XW, Chang S, Callahan CP, Geller DA, Will H, Harris CC. Hepatitis B virus X protein and p53 tumor suppressor interactions in the modulation of apoptosis. Proc Natl Acad Sci USA 1997;94(26):14707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang XW, Gibson MK, Vermeulen W, Yeh H, Forrester K, Sturzbecher HW, Hoeijmakers JH, Harris CC. Abrogation of p53-induced apoptosis by the hepatitis B virus X gene. Cancer Res. 1995;55(24):6012–6. [PubMed] [Google Scholar]
- 54. Yun C, Lee JH, Park H, Jin YM, Park S, Park K, Cho H. Chemotherapeutic drug, adriamycin, restores the function of p53 protein in hepatitis B virus X (HBx) protein-expressing liver cells. Oncogene 2000;19(45):5163–72. [DOI] [PubMed] [Google Scholar]
- 55. Kobayashi S, Matsushita K, Saigo K, Urashima T, Asano T, Hayashi H, Ochiai T. P21WAF1/CIP1 messenger RNA expression in hepatitis B, C virus-infected human hepatocellular carcinoma tissues. Cancer 2001;91(11):2096–103. [DOI] [PubMed] [Google Scholar]
- 56. Samuels-Lev Y, O’Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S, Campargue I, Naumovski L, Crook T, Lu X. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 2001;8(4):781–94. [DOI] [PubMed] [Google Scholar]
- 57. Zhao J, Wu G, Bu F, Lu B, Liang A, Cao L, Tong X, Lu X, Wu M, Guo Y. Epigenetic silence of ankyrin-repeat-containing, SH3-domain-containing, and proline-rich-region- containing protein 1 (ASPP1) and ASPP2 genes promotes tumor growth in hepatitis B virus-positive hepatocellular carcinoma. Hepatology 2010;51(1):142–53. [DOI] [PubMed] [Google Scholar]
- 58. Diao J, Khine AA, Sarangi F, Hsu E, Iorio C, Tibbles LA, Woodgett JR, Penninger J, Richardson CD. X protein of hepatitis B virus inhibits Fas-mediated apoptosis and is associated with up-regulation of the SAPK/JNK pathway. J Biol Chem. 2001;276(11):8328–40. [DOI] [PubMed] [Google Scholar]
- 59. Kuo TC, Chao CC. Hepatitis B virus X protein prevents apoptosis of hepatocellular carcinoma cells by upregulating SATB1 and HURP expression. Biochem Pharmacol. 2010;80(7):1093–102. [DOI] [PubMed] [Google Scholar]
- 60. Cheng AS, Yu J, Lai PB, Chan HL, Sung JJ. COX-2 mediates hepatitis B virus X protein abrogation of p53-induced apoptosis. Biochem Biophys Res Commun. 2008;374(2):175–80. [DOI] [PubMed] [Google Scholar]
- 61. Cheng AS, Wong N, Tse AM, Chan KY, Chan KK, Sung JJ, Chan HL. RNA interference targeting HBx suppresses tumor growth and enhances cisplatin chemosensitivity in human hepatocellular carcinoma. Cancer Lett. 2007;253(1):43–52. [DOI] [PubMed] [Google Scholar]
- 62. Han HK, Han CY, Cheon EP, Lee J, Kang KW. Role of hypoxia-inducible factor-alpha in hepatitis-B-virus X protein-mediated MDR1 activation. Biochem Biophys Res Commun. 2007;357(2):567–73. [DOI] [PubMed] [Google Scholar]
- 63. Huang P, Zhuang B, Zhang H, Yan H, Xiao Z, Li W, Zhang J, Tang Q, Hu K, Koeffler HP, Wang J, Yin D. Hepatitis B virus X protein (HBx) is responsible for resistance to targeted therapies in hepatocellular carcinoma: Ex vivo culture evidence. Clin Cancer Res. 2015;21(19):4420–30. [DOI] [PubMed] [Google Scholar]