Abstract
Glioma is the most common malignant tumor of the central nervous system, and it is characterized by high relapse and fatality rates and poor prognosis. Bufalin is one of the main ingredients of Chan-su, a traditional Chinese medicine (TCM) extracted from toad venom. Previous studies revealed that bufalin exerted inhibitory effects on a variety of tumor cells. To demonstrate the inhibitory effect of bufalin on glioma cells and glioma stem-like cells (GSCs) and discuss the underlying mechanism, the proliferation of glioma cells was detected by MTT and colony formation assays following treatment with bufalin. In addition, we investigated whether bufalin inhibits or kills GSCs using flow cytometry, Western blotting, and reverse transcription polymerase chain reaction analysis (RT-PCR). Finally, we investigated whether bufalin could improve the therapeutic effect of temozolomide (TMZ) and discussed the underlying mechanism. Taken together, our data demonstrated that bufalin inhibits glioma cell growth and proliferation, inhibits GSC proliferation, and kills GSCs. Bufalin was found to induce the apoptosis of GSCs by upregulating the expression of the apoptotic proteins cleaved caspase 3 and poly(ADP-ribose) polymerase (PARP) and by downregulating the expression of human telomerase reverse transcriptase, which is a marker of telomerase activity. Bufalin also improved the inhibitory effect of TMZ on GSCs by activating the mitochondrial apoptotic pathway. These results suggest that bufalin damages GSCs, induces apoptosis, and enhances the sensitivity of GSCs to TMZ.
Key words: Bufalin, Glioma, Cancer stem-like cells, Apoptosis, Telomerase, Temozolomide (TMZ)
INTRODUCTION
Glioma has the highest incidence among primary malignancies of the nervous system, accounting for 42.6% of malignant tumors in the brain1. Because of the characteristics of gliomas, particularly glioblastoma, including invasive growth, high degree of malignancy, and unclear boundaries between the tumor tissue and the surrounding brain tissue, complete surgical resection of the tumor is difficult2. Although glioma is one of the deadliest malignancies, the effectiveness of traditional treatments is not satisfactory3. Thus, novel effective treatment methods and drugs for glioma are urgently needed.
Cancer stem cells (CSCs) are responsible for the metastasis of tumor cells and development of resistance to chemotherapeutic drugs4. Although certain malignant tumors may regress after traditional treatment, the majority relapse, which is attributed to the self-regeneration and differentiation capacities of CSCs5,6. Therefore, novel chemotherapeutic drugs that exert a clear inhibitory effect on glioma stem cells are required.
Bufalin, an extract of traditional Chinese medicine (TCM) Chan-su, exerts a clear inhibitory effect on several types of tumor cells, such as prostate, lung, liver, ovarian, and colon cancer cells7–12. Furthermore, early studies revealed that bufalin induces glioma cell apoptosis and autophagy through endoplasmic reticulum stress13, and miR-203, a regulatory factor for cancer progression, plays a key role in the ability of bufalin to inhibit the growth and development of glioma stem-like cells (GSCs)14.
Human telomerase reverse transcriptase (hTERT), a catalytic subgroup in human telomerase, is highly expressed in most malignant tumor cells and is not expressed in normal tissues, and its expression is closely associated with the occurrence and development of glioma15–17. It was previously reported that the expression of hTERT is associated with cell apoptosis and telomerase activity in human hepatocellular carcinoma cells (SMMC-7721) and basal-type breast cancer cells18,19. Therefore, we investigated whether bufalin can affect the telomerase activity of GSCs and induce apoptosis.
Temozolomide (TMZ), a first-line oral alkylating agent used in the clinical treatment of glioma20, can activate the DNA damage pathway and lead to cell cycle arrest at the G2/M phase to induce glioma cell death21–23. TMZ can easily cross the blood–brain barrier to inhibit the proliferation and induce apoptosis of glioma cells by increasing the Bax/B-cell lymphoma-2 (Bcl-2) expression ratio, reducing the expression of O 6-methylguanine-DNA methyltransferase (MGMT) and Bcl-xL24–26. However, whether bufalin affects the efficacy of TMZ in glioma cells or GSCs has not yet been reported. Therefore, we herein aimed to investigate the effect of bufalin on sensitivity of glioma cells and GSCs to TMZ and elucidate the underlying mechanism.
MATERIALS AND METHODS
Materials
Bufalin was purchased from Sigma-Aldrich (St. Louis, MO, USA). The antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was purchased from PeproTech (Rocky Hill, NJ, USA). The following antibodies were obtained from Cell Signaling Technology (Boston, MA, USA): β-actin rabbit mAb [horseradish peroxidase (HRP) conjugated], caspase 3, Bcl-2, Bcl-xL, caspase 9, and poly(ADP-ribose) polymerase (PARP). The antibodies against Bad and p-Bad were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibodies against SOX2, Nanog, Oct4, and hTERT anti-mouse IgG-HRP and anti-rabbit IgG-HRP were purchased from Abcam (San Antonio, TX, USA). RNAiso Plus kits, PrimeScript Reverse Transcriptase kits, and SYBR Premix Ex Taq II kits were purchased from Takara (Tokyo, Japan). The hTERT and β-actin gene primers were synthesized by Sangon Biotech Co. Ltd. (Shanghai, P.R. China). Recombinant human epidermal growth factor (EGF; Invitrogen, Carlsbad, CA, USA), basic fibroblast growth factor (bFGF; EMD Millipore, Billerica, MA, USA), growth factor B-27 (Gibco, Grand Island, NY, USA), growth factor N2 (Gibco), and heparin (Selleck, Houston, TX, USA) were used for spheroid culture.
Cell Lines and Cell Culture
The human glioma cell lines U87MG and LN-229 and LO2 hepatocytes were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). U87MG cells were cultured in modified Eagle’s medium (MEM) (HyClone, South Logan, UT, USA) with 10% fetal bovine serum (FBS; Sigma-Aldrich). LN-229 cells were cultured in Dulbecco’s MEM (DMEM) (Gibco) with 5% FBS. The LO2 hepatocytes were cultured in RPMI-1640 medium (Gibco) with 10% FBS. Penicillin (100 U/ml) and streptomycin (100 μg/ml) (PS) (Gibco) were also added to the three types of media. Primary glioma cells were isolated from fresh glioma tissues obtained from 20 patients undergoing surgical resection (Department of Neurosurgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, P.R. China), with a confirmed diagnosis of glioma following postoperative pathological examination. The tissue acquisition was approved by the ethics committee of the hospital, and all the patients signed informed consent forms. All the cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C.
Cell Viability Assay
U87MG, LN-229, LO2, and primary glioma cells were seeded in three 96-well plates at a density of 5 × 103 cells/well and cultured with various concentrations of bufalin or 1% dimethyl sulfoxide (DMSO) for solvent control in 100 μl of medium for 24, 48, or 72 h. After treatment, MTT solution [0.5 mg/ml in 10 μl of phosphate-buffered saline (PBS)] was added to the wells for 4 h in the original environment. Subsequently, the supernatant was discarded and 200 μl of DMSO was added to each well for 15 min to fully dissolve the crystals. Cell viability was assessed using the optical density (OD) value at 490 nm with enzyme-linked immunoassay assay. The viability of LO2 hepatocytes was considered to indirectly reflect the toxicity of bufalin against normal glial cells, and the viability of primary glioma cells suggested the effect of bufalin on glioma patients. The experiments were repeated three times independently.
Colony Formation
U87MG and LN-229 cells were seeded (1 × 103/well) in six-well plates and incubated overnight at 37°C, followed by the addition of various concentrations of bufalin to the wells and culture for 14 days. The colonies were fixed with 4% paraformaldehyde (2 ml/well for 20 min) and stained with 2 ml/well crystal violet (0.2% in anhydrous ethanol) for 10 min at room temperature. The colonies were then washed slowly with water and naturally air dried. The colonies were counted and photographed. The results were calculated as the mean from three separate experiments using the Image-Pro Plus 6.0 software.
Spheroid Formation and Cell Death Assay
U87MG and LN-229 cells were seeded (1 × 103/well in 96-well plates and 1 × 104/well in 6-well plates) in low-attachment plates and suspended in serum-free DMEM/F12 (Gibco) containing 20 ng/ml EGF, 20 ng/ml bFGF, 8 μg/ml heparin, 2% B-2727,28, 1% N2, and 1% PS29. Subsequently, the GSCs were treated with various concentrations of bufalin, and the morphological changes of the spheroids were observed using an electron microscope. In addition, the spheroids of bufalin-containing cultures were stained with 10 μg/ml propidium iodide (PI)30. Subsequently, the cells were observed by fluorescence microscopy, and dead cells were identified by red fluorescent PI staining.
Flow Cytometry Analysis
Cells were inoculated in six-well low-attachment plates for 10 days, and the formed spheroids (GSCs) were treated with different concentrations of bufalin for 48 or 72 h. The supernatant was discarded after centrifugation, and StemPro Accutase was used for disaggregation to single cells. The cell suspension was washed twice with precooled PBS by centrifugation, and the cells were stained with annexin V-FITC/PI (AV/PI) in the dark for 15 min. Finally, the single-cell suspension was examined using flow cytometry. The analyzed apoptotic data were obtained from three separate experiments.
Western Blot
Following treatment with bufalin for 48 h, GSCs were lysed with lysis buffer and the mixture was gently shaken for 30 min on ice. Total cellular protein was extracted and the protein concentration was quantified using the BCA method. The same amount of protein was subjected to 10%–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to blotting membranes, and blocked in TBST with 5% nonfat milk at room temperature for 1.5 h. Subsequently, the membranes were incubated with the primary antibodies overnight on a shaking table at a slow speed at 4°C. After washing five times with precooled TBST, the membranes were incubated with the corresponding secondary antibodies at room temperature with gentle shaking for 1 h, and then washed with TBST. The objective protein bands were observed using a gel imaging system and chemiluminescence.
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
GSCs were treated with different concentrations of bufalin for 24 h, and total RNA was extracted according to the RNAiso Plus reagent instructions. Subsequently, the RNA concentration was detected by spectrophotometry, and first-strand cDNA was synthesized using PrimeScript Reverse Transcriptase. The primers for hTERT were as follows: 5′-CGGAAGAGTGTCTGGAGCAA-3′ (forward) and 5′-GGATGAAGCGGAGTCTGGA-3′ (reverse). PCR amplification was performed with SYBR Premix Ex Taq II kit using the iQ5 real-time fluorescence qPCR system (iQ5; Bio-Rad).
Drug Combination Index (CI) Analysis
Different concentrations of the two drugs (bufalin and TMZ) and the effects of individual and combined applications were entered into the CompuSyn software, and the corresponding CI values were obtained. CI > 1.1 indicated antagonism; CI = 0.9–1.1, an additive effect; and CI < 0.9, synergism.
Statistical Analysis
The data are expressed as mean ± standard deviation (SD). The t-test was used for comparisons between two groups, and comparison of multiple groups was performed by one-way analysis of variance. All statistical analyses were performed using the GraphPad Prism 5 software, and a value of p ≤ 0.05 was considered to indicate statistically significant differences.
RESULTS
Bufalin Inhibits the Proliferation of Glioma Cells
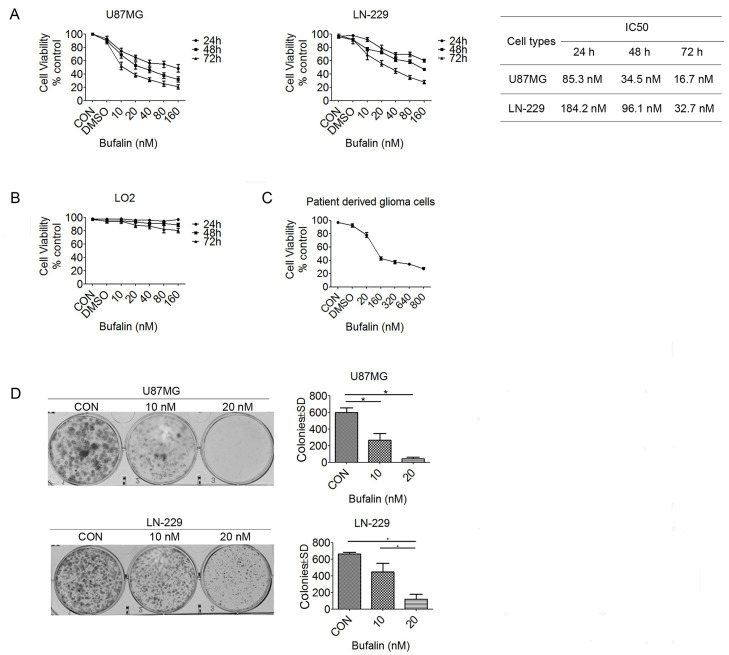
The MTT assay was used to demonstrate the effect of bufalin on glioma cell proliferation, and the results demonstrated that bufalin treatment significantly decreased the viability of U87MG and LN-229 cells in a dose- and time-dependent manner. The half-maximal inhibitory concentration (IC50) values in U87MG cells were 85.3, 34.5, and 16.7 nM for 24, 48, and 72 h of treatment, respectively. Similarly, the IC50 values in LN-229 cells were 184.2, 96.1, and 32.7 nM for 24, 48, and 72 h of treatment, respectively (Fig. 1A). Different concentrations of bufalin were used in LO2 hepatocytes, and the results demonstrated that bufalin exerted almost no effect after 24 h; with increasing concentrations of bufalin, the inhibition rate of LO2 cells was ∼20%–30% after 48 and 72 h (Fig. 1B). We then investigated whether bufalin exerted antitumor effects on human primary glioma cells. A total of 20 glioma tissue samples were obtained during surgery, and the glioma cells were treated by bufalin at different concentrations for 24 h. As shown in Figure 1C, the inhibition rate was increased significantly at concentrations >160 nM, and the IC50 was ∼120 nM. The colony formation assay revealed that the clonogenic survival of U87MG and LN-229 cells was significantly reduced following the addition of bufalin (Fig. 1D), and it was significantly lower with 20 nM bufalin treatment compared with 10 nM. These data indicate that bufalin inhibits the growth and proliferation of U87MG and LN-229 cells; furthermore, bufalin also leads to minor damage of normal cells and exerts obvious inhibitory effects on primary glioma cells.
Figure 1.
Bufalin suppresses the growth of glioma cells. (A) The U87MG, LN-229, and (B) normal liver LO2 cells were treated with bufalin (0, 10, 20, 40, 80, and 160 nM) for 24, 48, and 72 h, and cell viability was evaluated using an MTT assay. The data are presented as the mean ± standard deviation (SD). (C) Human primary glioma cells were treated with bufalin (0, 20, 160, 320, 640, and 800 nM) for 24 h, and an MTT assay was used to assess cell viability. (D) U87MG and LN-229 cells were treated with bufalin (0, 10, and 20 nM) for 14 days, after which a colony formation assay was performed. The number of colonies was counted, and the results are presented as the mean ± SD. All experiments were repeated three times. *p < 0.05.
Bufalin Suppresses and Damages GSCs
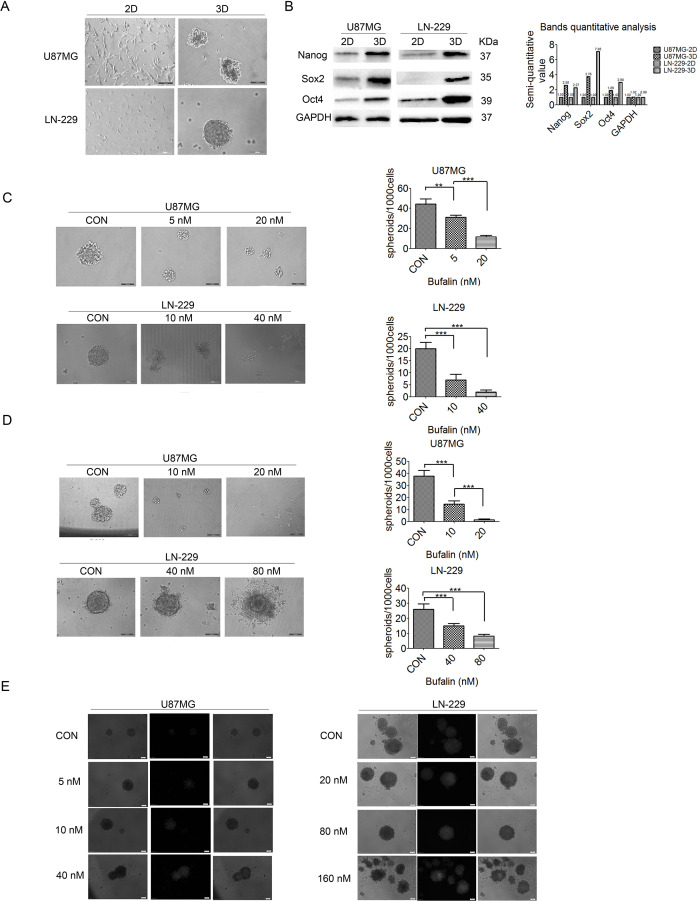
To elucidate whether bufalin exerts inhibitory effects on GSCs, the growth ability of the GSCs from U87MG and LN-229 cells was examined after a 10-day culture using an electron microscope, and GSCs formed three-dimensional (3D) spheroids suspended in medium (Fig. 2A). The expression levels of the stem cell markers Nanog, SOX2, and Oct4 were markedly increased in the 3D spheroids compared with those in normally cultured 2D U87MG and LN-229 cells (Fig. 2B), suggesting that the 3D spheroids display the characteristics of CSCs. When U87MG and LN-229 cells were cultured in serum-free DMEM/F12 containing different concentrations of bufalin for 7 days, the 3D spheroids were significantly smaller compared with those observed in the nonbufalin group in U87MG cells, whereas in LN-229 cells GSCs were unable to form spheroids under a bufalin concentration of 40 nM (Fig. 2C). These findings indicate that GSC activity was inhibited by bufalin. In addition, GSCs derived from U87MG cells were significantly smaller compared with those in the control group, and the spheroids derived from LN-229 cells were looser and surrounded by cell debris following treatment with bufalin for 3 days (Fig. 2D). PI staining revealed that the cell death rate increased significantly with the increase in bufalin concentration (Fig. 2E). As shown in Figure 2D and E, GSCs were damaged or killed by bufalin to a certain extent.
Figure 2.
Bufalin affects cell activity in glioma spheroid cultures. (A) U87MG and LN-229 cells were cultured in two-dimensional (2D) and 3D (spheroid) cultures. The cells formed spheroids following a 10-day culture in serum-free medium. Scale bars: 100 μm. (B) The human embryonic stem cell markers Nanog, SOX2, and Oct4 were upregulated in 3D cell cultures compared with those in 2D cell cultures. (C) The cells formed spheroids following a 7-day culture in serum-free medium with bufalin (0, 5, and 20 nM in U87MG and 0, 10, and 40 nM in LN-229 cells). Scale bars: 100 μm. **p < 0.01, ***p < 0.001. (D) Cell spheroids were treated with bufalin (0, 10, and 20 nM in U87MG and 0, 40, and 80 nM in LN-229 cells) for 5 days. The number of spheroids (diameter ≥100 μm) was counted, and the results are expressed as the mean ± SD. Scale bars: 100 μm. ***p < 0.001. (E) U87MG and LN-229 cell spheroids were treated with bufalin (0, 5, 10, and 40 nM in U87MG and 0, 20, 80, and 160 nM in LN-229 cells) for 3 days and then stained with propidium iodide. All experiments were repeated three times. Scale bars: 50 μm.
Bufalin Induces Apoptosis and Reduces Telomerase Activity in GSCs
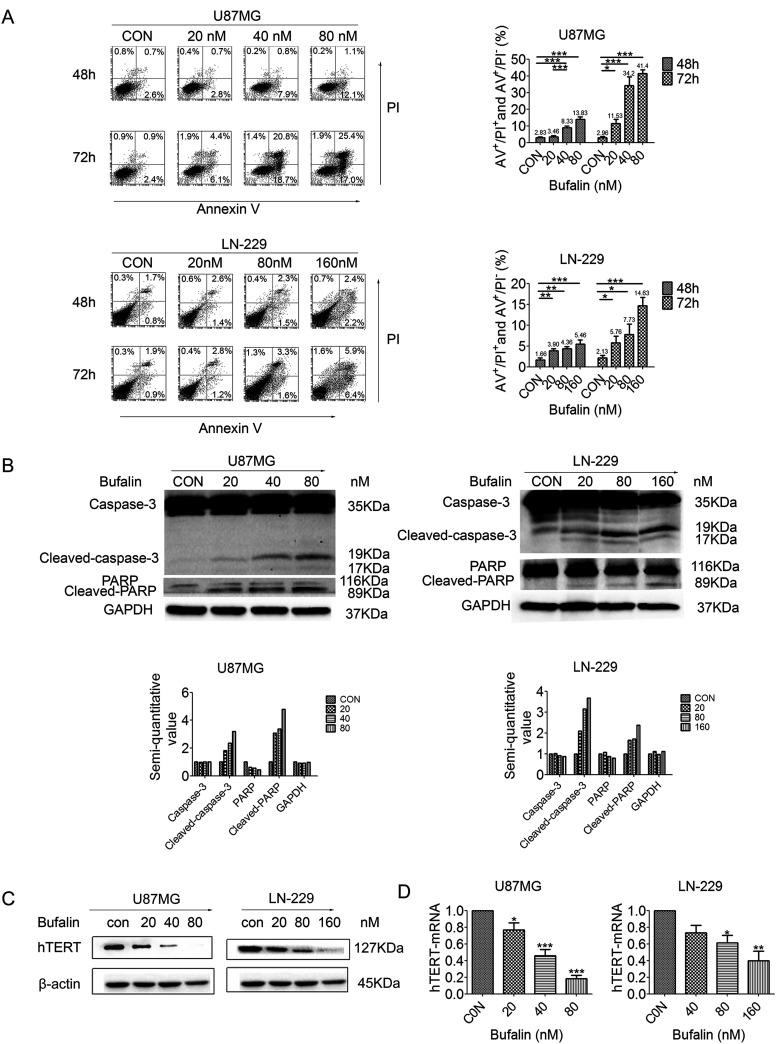
To determine whether bufalin induces GSC apoptosis, GSCs from U87MG and LN-229 cells were analyzed by flow cytometry with AV/PI staining. The cell apoptosis ratio increased significantly from 3.46% to 13.83% following treatment with 20–80 nM bufalin for 48 h and from 11.53% to 41.40% after treatment for 72 h in U87MG GSCs. In GSCs derived from LN-229 cells, the apoptosis ratio increased from 3.9% to 5.46% at 48 h and from 5.76% to 14.63% at 72 h after treatment with 20–160 nM bufalin (Fig. 3A). The apoptosis ratio was increased by bufalin in a dose- and time-dependent manner. In addition, Western blotting detected the cleavage of apoptotic proteins caspase 3 and PARP after 48 h of treatment with bufalin in U87MG and LN-229 cells (Fig. 3B). In summary, apoptosis was induced by bufalin in U87MG and LN-229 cells.
Figure 3.
Bufalin induces apoptosis and reduces telomerase activity in GSCs. (A) GSCs were treated with bufalin (0, 20, 40, and 80 nM in U87MG and 0, 20, 80, and 160 nM in LN-229 cells) for 48 and 72 h. Cell apoptosis was analyzed using flow cytometry. The apoptotic rate is presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. (B) Western blot analysis for caspase3, PARP, and GAPDH. (C) Western blot analysis for hTERT and β-actin. (D) Expression of hTERT-mRNA in GSCs induced by bufalin (0, 20, 40, and 60 nM in U87MG and 0, 40, 80, and 160 nM in LN-229 cells) for 24 h. All experiments were repeated three times. *p < 0.05, **p < 0.01, ***p < 0.001. GSCs, glioma stem-like cells; PARP, poly(ADP-ribose) polymerase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; hTERT, human telomerase reverse transcriptase.
To determine the effect of bufalin on telomerase activity, hTERT was detected by Western blot analysis, which revealed that the expression of the hTERT protein decreased significantly in U87MG GSCs and gradually decreased in LN-229 GSCs, in direct association with the increase in the concentration of bufalin (Fig. 3C). The expression levels of hTERT mRNA were significantly reduced compared with the control group after the GSCs were treated with different concentrations of bufalin (Fig. 3D). These data indicate that bufalin effectively inhibits telomerase activity in GSCs derived from U87MG and LN-229 cells.
Bufalin Increases the Sensitivity of GSCs to TMZ
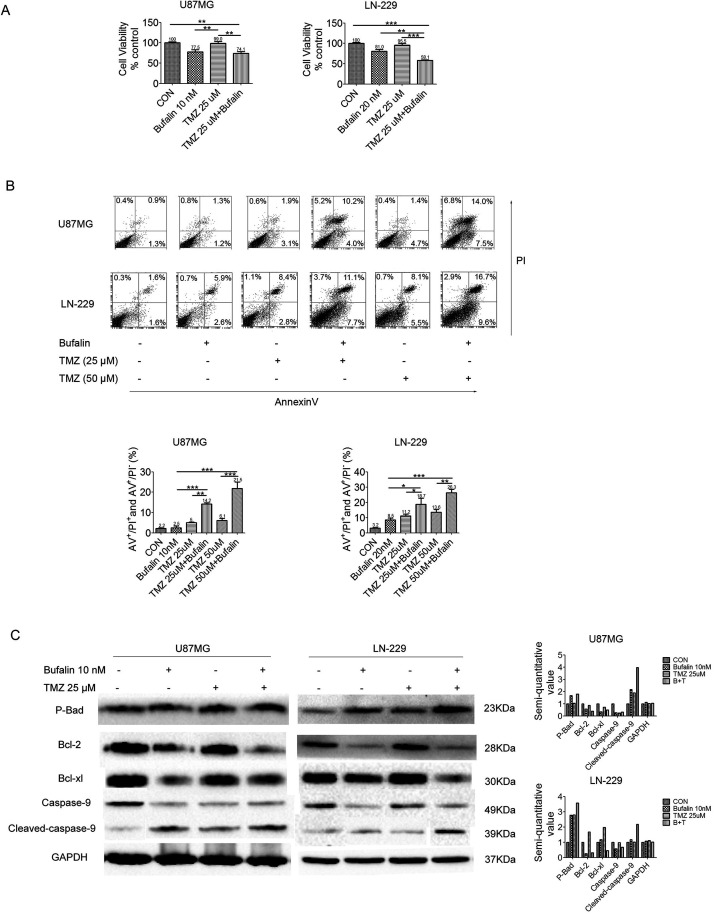
Previous studies demonstrated that the IC50 of TMZ is >200 μM in U87MG and LN-229 cells31,32; therefore, these two cell types were selected as TMZ resistant, and a lower dose (25 or 50 μM) of TMZ was used to conduct the experiment in order to determine whether bufalin can improve the sensitivity of GSCs to TMZ. MTT analysis was performed with a combination of bufalin and TMZ (74.1% in U87MG and 58.1% in LN-229 cells), and the combination significantly reduced cell viability compared with TMZ alone (103.7% in U87MG and 95.5% in LN-229 cells) after treatment for 48 h of GSCs from U87MG and LN-229 cells (Fig. 4A). The cell apoptosis ratio was assessed by flow cytometry, and the results revealed that the apoptosis ratio of TMZ 25 μM combined with bufalin (14.2% in U87MG and 18.7% in LN-229 cells) was higher compared with that of TMZ 25 μM alone (5% in U87MG and 11.2% in LN-229 cells). Similarly, the apoptosis ratio of TMZ 50 μM combined with bufalin (21.5% in U87MG and 6.3% in LN-229 cells) was significantly higher compared with that of TMZ 50 μM alone (6.1% in U87MG and 13.6% in LN-229 cells) in GSCs (Fig. 4B). To investigate the mechanism underlying the improvement of sensitivity to TMZ by bufalin, Western blotting was performed and the results revealed that the expression of the phosphorylated apoptotic protein Bad (p-Bad) in the mitochondrial apoptosis pathway was significantly increased and the inhibition of apoptotic proteins Bcl-2 and Bcl-xL was significantly reduced when bufalin was combined with TMZ, compared with TMZ alone (Fig. 4C), in U87MG and LN-229 GSCs. The aforementioned experimental results suggest that bufalin enhances the effect of TMZ on GSCs. The underlying mechanism is that bufalin induces apoptosis by TMZ via upregulation of p-Bad and cleaved caspase 9, and downregulation of Bcl-2 and Bcl-xL in the mitochondrial apoptosis pathway. These results suggest that bufalin increases sensitivity to TMZ and enhances its effect by inducing mitochondrial apoptosis.
Figure 4.
Bufalin increases the sensitivity of GSCs to TMZ. (A) GSCs derived from U87MG and LN-229 cells were treated with bufalin (0, 10, or 20 nM), TMZ (25 μM), or combination of bufalin and TMZ for 48 h, and the cell viability was assessed by MTT assay. **p < 0.01, ***p < 0.001. (B) GSCs derived from U87MG and LN-229 cells were treated with bufalin (0, 10, or 20 nM), TMZ (25 or 50 μM), and a combination of bufalin and TMZ for 72 h. Cell apoptosis was detected by flow cytometry, and the proportion of apoptotic cells is presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. (C) Western blot analysis for p-Bad, Bcl-2, Bcl-xL, caspase 9, and GAPDH. All experiments were repeated three times. TMZ, temozolomide; Bcl-2, B-cell lymphoma-2.
Drug CI Analysis
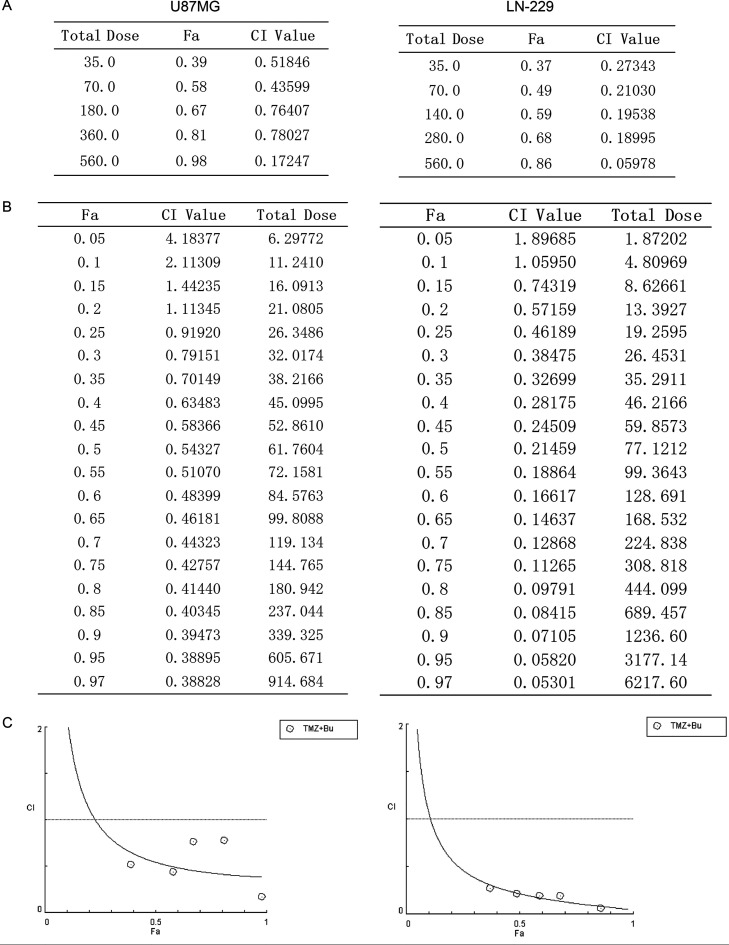
We analyzed the effect of bufalin in enhancing the sensitivity to TMZ using CompuSyn software to determine the CI. The results revealed that the CI values of U87MG and LN-229 GSCs were between 0.1 and 0.8 and between 0.1 and 0.3, respectively, after the combined application of bufalin and TMZ, indicating a synergistic effect between the two agents (Fig. 5A). The linear analysis results demonstrated that there may be an additive effect or antagonism at very low doses of the two drugs in U87MG and LN-229 cells (Fig. 5B and C).
Figure 5.
Drug combination index (CI) analysis. (A) GSCs derived from U87MG and LN-229 cells were treated with bufalin (10, 20, 40, 80, and 160 nM) combined with TMZ (25, 50, 100, 200, and 400 μM) for 48 h, and CI values were obtained using CompuSyn software. (B, C) The results and the graphs of the linear analysis of the drug combinations were obtained from CompuSyn using the five combined doses indicated above.
DISCUSSION
Complete surgical resection of glioma is difficult in clinical practice, and the tumors are often insensitive to radiotherapy, with the additional risk of injury to normal nervous system tissues1. In addition, due to the presence of CSCs, most tumors relapse after traditional therapy5,6. Therefore, safer and more effective anti-glioma or anti-GSC drugs are urgently needed to improve the outcome of chemotherapy for glioma patients.
In recent years, TCM has made progress in the treatment of tumors, and various agents extracted from TCM substances have been verified to inhibit glioma cell growth33–36. In particular, the combination of TCM and Western medicine has a unique advantage in improving the survival rate and reducing the toxicity of chemoradiotherapy37. Bufalin, a major component of the TCM Chan-Su13, has been found to exert a clear inhibitory effect on various tumors9,10,12,38. Studies have shown that the Na-K-ATP enzyme is likely one of the targets for the inhibition of proliferation of hepatocellular carcinoma cells by bufalin39. Furthermore, bufalin facilitates apoptosis of prostatic carcinoma cells by inhibiting the AKT/Bcl pathway40, and it inhibits the proliferation and migration of esophageal squamous cell carcinoma cells by arresting the cell cycle at the S and G/M phases and by blocking the Raf/MEK/ERK signaling pathway41,42. In glioma, bufalin has been shown to induce glioma cell apoptosis and autophagy via upregulating the protein expression of cleaved caspase 3, cleaved PARP, and Bax; downregulating the expression of Bcl-2; and promoting the release of cytochrome c. These mechanisms are closely associated with endoplasmic reticulum stress13. However, there has been no report to date on whether bufalin damages normal glial cells. As glioma is a central nervous system malignant tumor, therapeutic interventions aim to preserve normal brain tissue function to the greatest extent possible in order to ensure quality of life after surgery. Normal glial cells are difficult to obtain in clinical practice. Thus, normal liver cells were selected instead to indirectly reflect the toxicity of bufalin against normal cells. In addition, bufalin was used to treat primary glioma cells obtained from patients during surgery to explore its effect on cell activity, which may represent a step forward in the future clinical application of bufalin.
Recent studies reported that bufalin inhibits the proliferation of CSCs from osteosarcoma (OS) by regulating miR-148a and miR-148a, further regulating DNMT1 and p27 to control the stemness of OS cells43,44. Bufalin also suppressed pancreatic CSCs in gemcitabine-resistant MIAPaCa-2 cells, and the Hedgehog (Hh) signaling pathway, which is key to the therapeutic targeting of CSCs in pancreatic cancer, may be involved in this process45–47. In addition, bufalin inhibits cancer stem-like cell phenotypes by upregulating the expression of miR-203, a tumor-suppressive microRNA, to affect the proliferation, invasion, and drug resistance of glioma cells and GSCs48–51. In the present study, bufalin inhibited GSC activity and induced GSC apoptosis and death. hTERT has been shown to promote the activation and function of telomerase, and changes in its expression level are closely associated with telomerase activity52–54. It has been reported that hTERT is associated not only with telomerase activity but also with apoptosis. When hTERT is highly expressed, cell apoptosis is inhibited55–58. Therefore, we investigated the effect of bufalin on the expression of hTERT to determine whether bufalin can reduce the expression of hTERT, decrease the activity of telomerase, and promote apoptosis. We observed that bufalin induced GSC apoptosis, and the underlying mechanism may be associated with the reduction of telomerase activity by downregulating the expression of hTERT.
TMZ is the preferred chemotherapeutic drug for the treatment of high-grade glioma26,59–61; however, it is expensive for patients and many patients have varying degrees of interruption or dose reduction in the application process of a clinical setting. If bufalin can improve the efficacy of TMZ or reduce TMZ dosage without compromising its efficacy, it may prove valuable for the future treatment of glioma patients. In this study, bufalin enhanced the sensitivity of GSCs to TMZ and enhanced the efficacy of TMZ by inducing GSC apoptosis via upregulating p-Bad and downregulating Bcl-2 and Bcl-xL in the mitochondrial apoptosis pathway, as well as upregulating the apoptotic protein cleaved caspase 9. However, whether the specific effect of bufalin in enhancing the efficacy of TMZ is achieved by activating the mitochondrial apoptosis pathway and whether other proteins of the apoptotic pathway are involved require further investigation. CI analysis further proved that bufalin acts synergistically with TMZ in U87MG and LN-229 cells. This may represent a novel approach to the chemotherapy of glioma in the future.
Upon summarizing all the experimental results, we found that bufalin exerts a clear inhibitory effect on the growth and proliferation of glioma cells, causes minimum damage to normal liver cells, and exerts an obvious inhibitory effect on the proliferation of human primary glioma cells. Furthermore, bufalin inhibits and kills GSCs and induces GSC apoptosis by upregulating the expression of apoptotic proteins cleaved caspase 3 and cleaved PARP and by downregulating the expression of hTERT, which may be associated with reduced telomerase activity and induction of apoptosis. It has been demonstrated that the activity of telomerase is closely linked to the occurrence and development of glioma15–17. Bufalin was shown to inhibit the telomerase activity of GSCs, and this finding may provide a novel therapeutic target for the clinical treatment of glioma. Finally, we observed that bufalin enhanced the sensitivity of GSCs to TMZ, and the underlying mechanism may be through enhancing the ability of TMZ to induce apoptosis of GSCs through the mitochondrial apoptosis pathway. In the result of the drug CI analysis, we found that the combination of bufalin and TMZ in effective concentrations can be synergistic. If more drug concentration gradients were set in future studies, it would be very meaningful to find the respective medicinal concentrations of bufalin and TMZ for optimal therapeutic effect. There will be a new breakthrough to reduce the economic burden of patients in the treatment of glioma.
There were certain limitations to the present study. Thus far, the effects of bufalin have only been studied on glioma cells and GSCs, and the mechanism related to telomerase activity has not yet been extensively investigated. In future studies, the association between bufalin-induced apoptosis and telomerase activity, and the specific molecular targeted mechanisms should be further elucidated. It also should be confirmed whether bufalin is effective or has toxic side effects in animal models. If bufalin is effective in animal models, we have no reason not to conduct clinical testing of the combination of bufalin and TMZ to contribute to the treatment of glioma patients.
ACKNOWLEDGMENTS
The authors thank Professor Songshu Meng, Institute of Cancer Stem Cell, Dalian Medical University, for the academic discussion and guidance. The present study was supported by the Central Laboratory, Liaoning Cancer Hospital & Institute and the construction project fund of the Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC-B04-2015) and the Guiding funds for the development of local science and technology by the Central government (grant No. 2017106014).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17(Suppl 4):iv1–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de la Iglesia N1, Puram SV, Bonni A. STAT3 regulation of glioblastoma pathogenesis. Curr Mol Med. 2009;9(5):580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li C, Lee CJ, Simeone DM. Identification of human pancreatic cancer stem cells. Methods Mol Biol. 2009;568:161–73. [DOI] [PubMed] [Google Scholar]
- 5. Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, Olive D. Human acute myeloid leukemia CD34+/CD38- progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60(16):4403–11. [PubMed] [Google Scholar]
- 6. Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5(4):275–84. [DOI] [PubMed] [Google Scholar]
- 7. Liang Y, Liu AH, Qin S, Sun JH, Yang M, Li P, Guo DA. Simultaneous determination and pharmacokinetics of five bufadienolides in rat plasma after oral administration of Chansu extract by SPE-HPLC method. J Pharm Biomed Anal. 2008;46(3):442–8. [DOI] [PubMed] [Google Scholar]
- 8. Yeh JY, Huang WJ, Kan SF, Wang PS. Effects of bufalin and cinobufagin on the proliferation of androgen dependent and independent prostate cancer cells. Prostate 2003;54(2):112–24. [DOI] [PubMed] [Google Scholar]
- 9. Jiang Y, Zhang Y, Luan J, Duan H, Zhang F, Yagasaki K, Zhang G. Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology 2010;62(6):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qi F, Inagaki Y, Gao B, Cui X, Xu H, Kokudo N, Li A, Tang W. Bufalin and cinobufagin induce apoptosis of human hepatocellular carcinoma cells via Fas- and mitochondria-mediated pathways. Cancer Sci. 2011;102(5):951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takai N, Ueda T, Nishida M, Nasu K, Narahara H. Bufalin induces growth inhibition, cell cycle arrest and apoptosis in human endometrial and ovarian cancer cells. Int J Mol Med. 2008;21(5):637–43. [PubMed] [Google Scholar]
- 12. Wang J, Chen C, Wang S, Zhang Y, Yin P, Gao Z, Xu J, Feng D, Zuo Q, Zhao R, Chen T. Bufalin inhibits HCT116 colon cancer cells and its orthotopic xenograft tumor in mice model through genes related to apoptotic and PTEN/AKT pathways. Gastroenterol Res Pract. 2015;2015:457193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen S, Zhang Y, Wang Z, Liu R, Gong X. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int J Biol Sci. 2014;10(2):212–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu T, Wu C, Weng G, Zhao Z, He X, Fu C, Sui Z, Huang SX. Bufalin inhibits cellular proliferation and cancer stem cell-like phenotypes via upregulation of MiR-203 in glioma. Cell Physiol Biochem. 2017;44(2):671–81. [DOI] [PubMed] [Google Scholar]
- 15. Wang T, Xue Y, Wang M, Sun Q. Silencing of the hTERT gene through RNA interference induces apoptosis via bax/bcl-2 in human glioma cells. Oncol Rep. 2012;28(4):1153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khaw AK, Hande MP, Kalthur G, Hande MP. Curcumin inhibits telomerase and induces telomere shortening and apoptosis in brain tumour cells. J Cell Biochem. 2013;114(6):1257–70. [DOI] [PubMed] [Google Scholar]
- 17. Yang Y, Chen Y, Zhang C, Huang H, Weissman SM. Nucleolar localization of hTERT protein is associated with telomerase function. Exp Cell Res. 2002;277(2):201–9. [DOI] [PubMed] [Google Scholar]
- 18. Cheng X, Shi JB, Liu H, Chen LZ, Wang Y, Tang WJ, Liu XH. Discovery of (4-bromophenyl)(3-hydroxy-4-methoxyphenyl)methanone through upregulating hTERT induces cell apoptosis and ERS. Cell Death Dis. 2017;8(8):e3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dinami R, Buemi V, Sestito R, Zappone A, Ciani Y, Mano M, Petti E, Sacconi A, Blandino G, Giacca M, Piazza S, Benetti R, Schoeftner S. Epigenetic silencing of miR-296 and miR-512 ensures hTERT dependent apoptosis protection and telomere maintenance in basal-type breast cancer cells. Oncotarget 2017;8(56):95674–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang JT, Lee IN, Lu FJ, Chung CY, Lee MH, Cheng YC, Chen KT, Chen CH. Propyl gallate exerts an antimigration effect on temozolomide-treated malignant glioma cells through inhibition of ROS and the NF-κB pathway. J Immunol Res. 2017;2017:9489383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene 2007;26(2):186–97. [DOI] [PubMed] [Google Scholar]
- 22. Knizhnik AV, Roos WP, Nikolova T, Quiros S, Tomaszowski KH, Christmann M, Kaina B. Survival and death strategies in glioma cells: Autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS One 2013;8(1):e55665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito M, Ohba S, Gaensler K, Ronen SM, Mukherjee J, Pieper RO. Early Chk1 phosphorylation is driven by temozolomide-induced, DNA double strand break- and mismatch repair-independent DNA damage. PLoS One 2013;8(5):e62351.23667469 [Google Scholar]
- 24. Shi L, Zhang S, Feng K, Wu F, Wan Y, Wang Z, Zhang J, Wang Y, Yan W, Fu Z, You Y. MicroRNA-125b-2 confers human glioblastoma stem cells resistance to temozolomide through the mitochondrial pathway of apoptosis. Int J Oncol. 2012;40(1):119–29. [DOI] [PubMed] [Google Scholar]
- 25. Tang JH, Huang GH, Mou KJ, Zhang EE, Li N, Du L, Zhu XP, Chen L, Yang H, Zhang KB, Lv SQ. Pyrrolidine dithiocarbamate sensitizes U251 brain glioma cells to temozolomide via downregulation of MGMT and BCL-XL. Oncol Lett. 2017;14(5):5135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weyhenmeyer BC, Noonan J, Würstle ML, Lincoln FA, Johnston G, Rehm M, Murphy BM. Predicting the cell death responsiveness and sensitization of glioma cells to TRAIL and temozolomide. Oncotarget 2016;7(38):61295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen DG, Zhu B, Lv SQ, Zhu H, Tang J, Huang C, Li Q, Zhou P, Wang DL, Li GH. Inhibition of EGR1 inhibits glioma proliferation by targeting CCND1 promoter. J Exp Clin Cancer Res. 2017;36(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okada M, Takeda H, Sakaki H, Kuramoto K, Suzuki S, Sanomachi T, Togashi K, Seino S, Kitanaka C. Repositioning CEP-1347, a chemical agent originally developed for the treatment of Parkinson’s disease, as an anti-cancer stem cell drug. Oncotarget 2017;8(55):94872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu T, Wang Y, Hu Q, Wu W, Wu Y, Wei W, Han D, You Y, Lin N, Liu N. The EZH2 inhibitor GSK343 suppresses cancer stem-like phenotypes and reverses mesenchymal transition in glioma cells. Oncotarget 2017;8(58):98348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu L, Sun S, Wang T, Li Y, Jiang K, Lin G, Ma Y, Barr MP, Song F, Zhang G, Meng S. Oncolytic newcastle disease virus triggers cell death of lung cancer spheroids and is enhanced by pharmacological inhibition of autophagy. Am J Cancer Res. 2015;5(12):3612–23. [PMC free article] [PubMed] [Google Scholar]
- 31. Akbarnejad Z, Eskandary H, Dini L, Vergallo C, Nematollahi-Mahani SN, Farsinejad A, Abadi MFS, Ahmadi M. Cytotoxicity of temozolomide on human glioblastoma cells is enhanced by the concomitant exposure to an extremely low-frequency electromagnetic field (100Hz, 100G). Biomed Pharmacother. 2017;92:254–64. [DOI] [PubMed] [Google Scholar]
- 32. Fukai J, Koizumi F, Nakao N. Enhanced anti-tumor effect of zoledronic acid combined with temozolomide against human malignant glioma cell expressing O6-methylguanine DNA methyltransferase. PLoS One 2014;9(8):e104538. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Zhang WF, Yang Y, Li X, Xu DY, Yan YL, Gao Q, Jia AL, Duan MH. Angelica polysaccharides inhibit the growth and promote the apoptosis of U251 glioma cells in vitro and in vivo. Phytomedicine 2017;33:21–7. [DOI] [PubMed] [Google Scholar]
- 34. Wang YB, Hu Y, Li Z, Wang P, Xue YX, Yao YL, Yu B, Liu YH. Artemether combined with shRNA interference of vascular cell adhesion molecule-1 significantly inhibited the malignant biological behavior of human glioma cells. PLoS One 2013;8(4):e60834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hai J, Lin Q, Lu Y, Yi J, Zhang H. Growth inhibition and induction of differentiation by panaxydol in rat C6 glioma cells. Neurol Res. 2008;30(1):99–105. [DOI] [PubMed] [Google Scholar]
- 36. Shao J, Zheng D, Jiang Z, Xu H, Hu Y, Li X, Lu X. Curcumin delivery by methoxy polyethylene glycol-poly(caprolactone) nanoparticles inhibits the growth of C6 glioma cells. Acta Biochim Biophys Sin. (Shanghai) 2011;43(4):267–74. [DOI] [PubMed] [Google Scholar]
- 37. Liu EY, Zhang X, Zhang JN, Liu WP, Jiang XF. ING4 expression in human gliomas and therapeutic role of curcumin on gliomas. Chinese J Neurosurg Dis Res. 2007;6(3):233–6. [Google Scholar]
- 38. Wang HY, Zhang CY, Xu LT, Zang K, Ning ZY, Jiang F, Chi HY, Zhu XY, Meng ZQ. Bufalin suppresses hepatocellular carcinoma invasion and metastasis by targeting HIF-1α via the PI3K/AKT/mTOR pathway. Oncotarget 2016;7(15):20193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li H, Wang P, Gao Y, Zhu X, Liu L, Cohen L, Meng Z, Yang P. Na+/K+-ATPase α3 mediates sensitivity of hepatocellular carcinoma cells to bufalin. Oncol Rep. 2011;25(3):825–30. [DOI] [PubMed] [Google Scholar]
- 40. Li F, Ma ZF. The effects and mechanisms of bufalin on the proliferation and apoptosis of PC3 cell line. Chin J Clin Med. 2011;5(22):6594–8. [Google Scholar]
- 41. Tian X, Luo Y, Yan YB, Sui CG, Meng FD, Liu YP. Effect of bufalin on cellular proliferation and apoptosis in human esophageal squamous carcinoma EC9706 cells. J Chin Acad Med Sci. 2012;34(06):556–62. [DOI] [PubMed] [Google Scholar]
- 42. Ding Y, Wang XL, Deng HY, Su LH, Zhang P, Liu YP. Mechanism of bufalin affecting the proliferation and migration of human esophageal carcinoma cells through inhibiting the activity of Raf/MEK/ERK pathway. Chin Gen Pract. 2015;18(21):2535–41. [Google Scholar]
- 43. Chang Y, Zhao Y, Zhan H, Wei X, Liu T, Zheng B. Bufalin inhibits the differentiation and proliferation of human osteosarcoma cell line hMG63-derived cancer stem cells. Tumour Biol. 2014;35(2):1075–82. [DOI] [PubMed] [Google Scholar]
- 44. Chang Y, Zhao Y, Gu W, Cao Y, Wang S, Pang J, Shi Y. Bufalin inhibits the differentiation and proliferation of cancer stem cells derived from primary osteosarcoma cells through Mir-148a. Cell Physiol Biochem. 2015;36(3):1186–96. [DOI] [PubMed] [Google Scholar]
- 45. Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 2003;425(6960):846–51. [DOI] [PubMed] [Google Scholar]
- 46. Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324(5933):1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang H, Ning Z, Li Y, Zhu X, Meng Z. Bufalin suppresses cancer stem-like cells in gemcitabine-resistant pancreatic cancer cells via Hedgehog signaling. Mol Med Rep. 2016;14(3):1907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liao H, Bai Y, Qiu S, Zheng L, Huang L, Liu T, Wang X, Liu Y, Xu N, Yan X, Guo H. MiR-203 downregulation is responsible for chemoresistance in human glioblastoma by promoting epithelial-mesenchymal transition via SNAI2. Oncotarget 2015;6(11):8914–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen Z, Li D, Cheng Q, Ma Z, Jiang B, Peng R, Chen R, Cao Y, Wan X. MicroRNA-203 inhibits the proliferation and invasion of U251 glioblastoma cells by directly targeting PLD2. Mol Med Rep. 2014;9(2):503–8. [DOI] [PubMed] [Google Scholar]
- 50. Noguchi S, Mori T, Otsuka Y, Yamada N, Yasui Y, Iwasaki J, Kumazaki M, Maruo K, Akao Y. Anti-oncogenic microRNA-203 induces senescence by targeting E2F3 protein in human melanoma cells. J Biol Chem. 2012;287(15):11769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu T, Wu C, Weng G, Zhao Z, He X, Fu C, Sui Z, Huang SX. Bufalin inhibits cellular proliferation and cancer stem cell-like phenotypes via upregulation of MiR-203 in glioma. Cell Physiol Biochem. 2017;44(2):671–81. [DOI] [PubMed] [Google Scholar]
- 52. Yang Y, Chen Y, Zhang C, Huang H, Weissman SM. Nucleolar localization of hTERT protein is associated with telomerase function. Exp Cell Res. 2002;277(2):201–9. [DOI] [PubMed] [Google Scholar]
- 53. Wang F, Diao Y, Xu RA. Application of human telomerase reverse transcriptase (hTERT) promoter in cancer gene therapy. Chin J Health Med. 2012;33(5):686–9. [Google Scholar]
- 54. Gollahon LS, Holt SE. Alternative methods of extracting telomerase activity from human tumor samples. Cancer Lett. 2000;159(2):141–9. [DOI] [PubMed] [Google Scholar]
- 55. Li Y, Tergaonkar V. Noncanonical functions of telomerase: Implications in telomerase-targeted cancer therapies. Cancer Res. 2014;74(6):1639–44. [DOI] [PubMed] [Google Scholar]
- 56. Rubis B, Holysz H, Gladych M, Toton E, Paszel A, Lisiak N, Kaczmarek M, Hofmann J, Rybczynska M. Telomerase downregulation induces proapoptotic genes expression and initializes breast cancer cells apoptosis followed by DNA fragmentation in a cell type dependent manner. Mol Biol Rep. 2013;40(8):4995–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Indran IR, Hande MP, Pervaiz S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res. 2011;71(1):266–76. [DOI] [PubMed] [Google Scholar]
- 58. Singhapol C, Pal D, Czapiewski R, Porika M, Nelson G, Saretzki GC. Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS One 2013;8(1):e52989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Patil VM, Tonse R, Kothari R, Chandrasekaran A, Pande N, Epari S, Gupta T, Jalali R. Rechallenge temozolomide in glioma: A case series from India. Indian J Cancer 2017;54(1):368–71. [DOI] [PubMed] [Google Scholar]
- 60. Li M, Liang RF, Wang X, Mao Q, Liu YH. BKM120 sensitizes C6 glioma cells to temozolomide via suppression of the PI3K/Akt/NF-κB/MGMT signaling pathway. Oncol Lett. 2017;14(6):6597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang SH, Li S, Lu G, Xue H, Kim DH, Zhu JJ, Liu Y. Metformin treatment reduces temozolomide resistance of glioblastoma cells. Oncotarget 2016;7(48):78787–803. [DOI] [PMC free article] [PubMed] [Google Scholar]