Abstract
Many studies have shown that downregulated miR-203 level is in a variety of cancers including gastric cancer (GC). However, the precise molecule mechanisms of miR-203 in GC have not been well clarified. In the current study, we investigated the biological functions and molecular mechanisms of miR-203 in GC cell lines. We found that miR-203 is downregulated in GC tissues and cell lines. Moreover, the low level of miR-203 was associated with increased expression of annexin A4 in GC tissues and cell lines. The invasion and EMT of GC cells were suppressed by overexpression of miR-203. However, downregulation of miR-203 promoted invasion and EMT of GC cells. Bioinformatics analysis predicted that annexin A4 was a potential target gene of miR-203. Next, luciferase reporter assay confirmed that miR-203 could directly target annexin A4. Consistent with the effect of miR-203, downregulation of annexin A4 by siRNA inhibited the invasion and EMT of GC cells. Introduction of annexin A4 in GC cells partially blocked the effects of miR-203 mimic. Introduction of miR-203 directly targeted annexin A4 to inhibit the invasion and EMT of GC cells. Overall, reactivation of the miR-203/annexin A4 axis may represent a new strategy for overcoming metastasis of GC.
Key words: Gastric cancer (GC), miR-203, Annexin A4, Invasion, Epithelial–mesenchymal transition (EMT)
INTRODUCTION
Gastric cancer (GC) is the fourth most common cancer worldwide and the second leading cause of cancer-related deaths in China1,2. The major therapeutic option for GC is surgical resection. However, patients with advanced GC often show poor survival over time due to tumor recurrence and metastasis3,4. Therefore, it is essential to explore novel therapeutic strategies to improve GC treatment.
Annexins are a structurally homologous family of calcium-dependent phospholipid-binding proteins5. The 12 human annexins play different roles in regulating the activity of phospholipase A2, membrane trafficking, cell division, differentiation, apoptosis, and blood coagulation5–8. In addition, annexins play critical roles in carcinogenesis9. Recent reports show that upregulation and downregulation of annexin members in multiple cancers are closely associated with progression of cancers9. However, the expressions of annexins such as A1, A2, A3, A5, and A7 in GC are still unclear.
MicroRNAs (miRNAs) are some endogenous small noncoding RNA molecules that could bind to the target messenger RNAs (mRNAs) in the 3′-untranslated regions (3′-UTRs) and regulated their expression10. miRNAs play crucial roles in numerous biological processes including cell differentiation, cell proliferation, cell apoptosis, and tumor progression, including GC11. For example, microRNA-219-5p suppresses the proliferation, migration, and invasion of GC cells by modulating the LRH-1/Wnt/β-catenin signaling pathway12. miR-326 inhibits GC cell growth via downregulation of NOB1 expression13. MicroRNA-509-3p inhibits the proliferation and migration through increasing XIAP expression in GC cells14. Thus, these evidences showed that miRNAs play an important role in GC.
In this study, the level of annexin A4 had been demonstrated to be highest among annexins in GC tissues in comparison to the adjacent tissues. Furthermore, we found that miR-203 could directly target annexin A4 in GC cells using the online database, TargetScan 6.2. Currently, miR-203 has attracted much attention because several studies have reported that miR-203 is frequently decreased and functions as a tumor suppressor in hepatocellular carcinoma, cervical cancer, oral carcinoma, gastric cancer and non-small cell lung cancer15–19. However, until now, the precise mechanism of miR-203 in GC remains unclear. In this study, we also found that the level of miR-203 was frequently downregulated in GC tissues and cell lines. Moreover, our findings showed that the annexin A4 expression was significantly upregulated in GC tissues and cell lines, which was closely associated with the decreased miR-203 level. Introduction of miR-203 suppressed the invasion and epithelial–mesenchymal transition (EMT) of GC cells. Overexpression of annexin A4 reversed the inhibitory effects of miR-203 mimic on GC cells. Hence, our results showed important roles for miR-203 in the pathogenesis of GC and suggested its potential application in its treatment.
MATERIALS AND METHODS
Cell Culture and Human Tissues
Human GC cell lines AGS, BGC-823, MKN45, SGC7901, and a normal human gastric epithelial cell line GES-1 were purchased from the Chinese Academy of Sciences (Shanghai, P.R. China). The cell lines were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin (Gibco) in a humidified chamber with an atmosphere of 5% CO2 at 37°C. Ten pairs of human GC tissues and their adjacent normal tissues were collected from Cangzhou Central Hospital. Patients recruited in this study were newly diagnosed with GC and had not received any treatment. The tumor and adjacent normal tissues were obtained after surgical resection and immediately frozen in liquid nitrogen and then stored at −80°C for analysis. Prior informed consent was obtained, and the study protocol was approved by the ethics committee of Cangzhou Central Hospital.
miRNA Transfection
To upregulate or downregulate the level of miR-203 in SGC7901 cells, the cells were transfected with miR-203 mimic and miR-negative control (miR-NC) or miR-203 inhibitor and miR-negative control of inhibitor (inhibitor-NC) that were synthesized by Gene-Pharma (Shanghai, P.R. China) using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. The pcDNA3.1 vector, pcDNA-annexin A4, siRNA for annexin A4 (si-annexin A4), and siRNA-negative control (si-NC) were synthesized and purified by Gene-Pharma.
Reverse Transcription Polymerase Chain Reaction
Total RNA of SGC7901 was extracted using Trizol reagent (Qiagen, Hilden, Germany) for analyzing miRNA and mRNA levels according to the manufacturer’s protocols. For quantification of miR-203, the TaqMan MicroRNA Reverse Transcription Kit and TaqMan miRNA assay (Applied Biosystems, Carlsbad, CA, USA) were used to perform reverse transcription and PCR according to the manufacturer’s instructions. U6 was used as the internal control. The gene expressions of annexin A4, E-cadherin, N-cadherin, vimentin, Twist1, Slug, and ZEB1 were detected using the SYBR Green PCR kits (Qiagen). GAPDH served as an internal control. U6 snRNA and GAPDH mRNA were used to normalize. Primers used are listed in Table 1.
Table 1.
Sequence of Primers for qRT-PCR
| Gene | Primer Sequence |
|---|---|
| Annexin A1 | F: 5′-GCGGTGAGCCCCTATCCTA-3′ |
| R: 5′-TGATGGTTGCTTCATCCACAC-3′ | |
| Annexin A2 | F: 5′-TCTACTGTTCACGAAATCCTGTG-3′ |
| R: 5′-AGTATAGGCTTTGACAGACCCAT-3′ | |
| Annexin A3 | F: 5′-TTAGCCCATCAGTGGATGCTG-3′ |
| R: 5′-CTGTGCATTTGACCTCTCAGT-3′ | |
| Annexin A4 | F: 5′-GGAGGTACTGTCAAAGCTGCT-3′ |
| R: 5′-GGCAAGGACGCTAATAATGGC-3′ | |
| Annexin A5 | F: 5′-AGCGGGCTGATGCAGAAAC-3′ |
| R: 5′-ACTTCGGGATGTCAACAGAGT-3′ | |
| Annexin A7 | F: 5′-CCTAGTGGCTTTCCTCCAATG-3′ |
| R: 5′-ACCAGGATAGCCTCCAGGG-3′ | |
| E-cadherin | F: 5′-TACACTGCCCAGGAGCCAGA-3′ |
| R: 5′-TGGCACCAGTGTCCGGATTA-3′ | |
| N-cadherin | F: 5′-TCAGGCGTCTGTAGAGGCTT-3′ |
| R: 5′-ATGCACATCCTTCGATAAGACTG-3′ | |
| Vimentin | F: 5′-GACGCCATCAACACCGAGTT-3′ |
| R: 5′-CTTTGTCGTTGGTTAGCTGGT-3′ | |
| Twist1 | F: 5′-GTCCGCAGTCTTACGAGGAG-3′ |
| R: 5′-GCTTGAGGGTCTGAATCTTGCT-3′ | |
| Slug | F: 5′-CGAACTGGACACACATACAGTG-3′ |
| R: 5′-CTGAGGATCTCTGGTTGTGGT-3′ | |
| ZEB1 | F: 5′-GATGATGAATGCGAGTCAGATGC-3′ |
| F: 5′-ACAGCAGTGTCTTGTTGTTGT-3′ | |
| U6 | F: 5′-CTCGCTTCGGCAGCACA-3′ |
| F: 5′-AACGCTTCACGAATTTGCGT-3′ | |
| GAPDH | F: 5′-GAGTCAACGGATTTGGTCGTATTG-3′ |
| R: 5′-CCTGGAAGATGGTGATGGGATT-3′ |
Transwell Invasion Assay
Transwell Matrigel invasion assay using Transwell chambers (8-μm pore size; Millipore, Billerica, MA, USA) precoated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) that contained extracellular matrix proteins was used to determined cell invasion. In brief, 1 × 105 cells in 100 μl of RPMI-1640 containing 1% FBS were seeded in the upper chamber, and 600 μl of RPMI-1640 containing 10% FBS was added to the lower chamber. After 24 h of incubation at 37°C in a 5% CO2 atmosphere, cells that remained in the upper chamber were removed by cotton swabs, and penetrating cells were fixed in methanol and then stained with 0.1% crystal violet. The membranes were then rinsed with 30% glacial acetic acid. Eventually, the washing solution was detected at 540 nm for cell counting measurement.
Measurement of Matrix Metalloproteinase (MMP)-2, MMP-9, and Inhibitors of Metalloproteinases (TIMP)-1 Levels
Enzyme-linked immunosorbent assay (ELISA) kit (USCN, USCN Life Science, Wuhan, P.R. China) was used to determine the levels of MMP-2, MMP-9, and TIMP-1 in the culture supernatants based on the manufacturer’s instructions.
Western Blot Analysis
SGC7901 cells were washed twice in cold PBS and then lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology Jiangsu, P.R. China) containing protease inhibitor cocktail (Millipore). The protein concentration of cell lysates was quantified by BCA kit (Beyotime Institute of Biotechnology), and 50 ng of each of the proteins was separated by SDS-PAGE on 10% gels and then transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore). The membranes were blocked in 5% nonfat milk diluted with TBST at room temperature for 1 h and incubated overnight at 4°C with primary antibodies such as anti-annexin A4, anti-MMP-2, anti-MMP-9, anti-E-cadherin, anti-N-cadherin, anti-vimentin, anti-Twist1, anti-Slug and anti-ZEB1 (1:500; Abcam, Cambridge, UK) and anti-TIMP-1 (1:1,000; Cell Signaling Technology, Danvers, MA, USA). The membranes were then incubated with a goat anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase secondary antibody (1:1,000; Cell Signaling Technology) for 2 h. The proteins were visualized using ECL-plus reagents (Amersham Biosciences Corp., Piscataway, NJ, USA). The density of the bands was measured using the ImageJ software (NIH, Bethesda, MD, USA), and values were normalized to the densitometric values of β-actin (1:5,000; Sigma-Aldrich, St. Louis, MO, USA) in each sample.
Luciferase Reporter Assay
SGC7901 cells were seeded in 24-well plates and incubated for 24 h before transfection. The pMIR-annexin A4-3′-UTR wild type or mutant plasmid was cotransfected with miR-203 mimic or miR-NC, and pRL-TK plasmid (Promega, Madison, WI, USA) into SGC7901 cells. After transfection for 48 h, luciferase reporter gene assay was implemented using the dual-luciferase reporter system (Promega) following the manufacturer’s protocols. All experiments were performed at least three times.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Data from each group were expressed as mean ± standard error of the mean (SEM). Student’s t-test was used to analyze differences between two groups. A one-way ANOVA was performed to detect statistical differences among multiple groups. Differences within the ANOVA were determined using a Tukey’s post hoc test. Differences were considered statistically significant at a value of p < 0.05.
RESULTS
The Expression of Annexin A4 Was Upregulated and the Level of miR-203 Was Downregulated in GC Tissues and Cell Lines
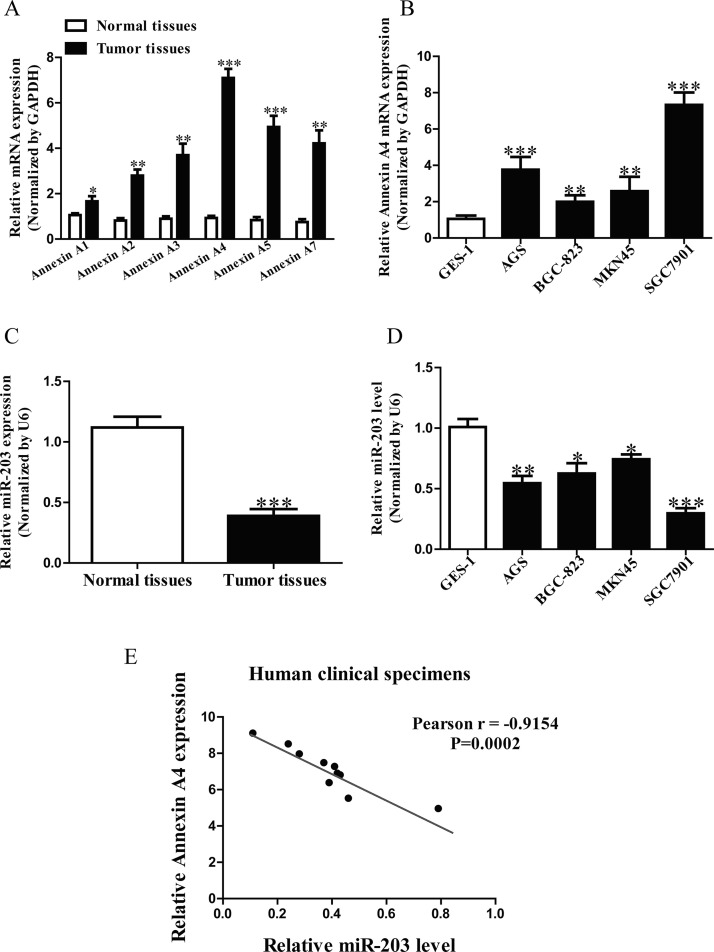
It has been reported that annexins such as annexin A1, A2 A3, A4, A5, and A7 were closely associated with cancers20. In this study, we detected these six annexin genes in GC tissues. The results showed that the mRNA level of annexin A4 was highest in GC tissues among these six annexin genes compared with the adjacent tissues (Fig. 1A). Subsequently, we also determined the mRNA level of annexin A4 in four GC cell lines such as AGS, BGC-823, MKN45, SGC7901 and a normal human gastric epithelial cell line GES-1. Compared with GES-1, the level of annexin A4 in SGC7901 cells was higher than that in other three GC cell lines (Fig. 1B). For further study, we found that miR-203 may directly target annexin A4 using the online database, TargetScan 6.2. Next, our results showed that the level of miR-203 in the GC tissues was significantly lower in comparison to the adjacent tissues (Fig. 1C). We also demonstrated that miR-203 expression was markedly downregulated in SGC7901 cells compared to that in GES-1, as shown in Figure 1D. So SGC7901 cells were used in the following experiments. To determine whether the annexin A4 expression was closely related to miR-203 level in GC tissues, the Pearson’s correlation analysis revealed a significant negative correlation between annexin A4 and miR-203 (Fig. 1E).
Figure 1.
The levels of annexin A4 and miR-203 in gastric cancer (GC) tissues and cell lines. (A) The mRNA levels of annexin A1, A2, A3, A4, A5, and A7 in GC tissues and their corresponding adjacent normal tissues. (B) The mRNA expression of annexin A4 analyzed by qRT-PCR in four GC cell lines (AGS, BGC-823, MKN45, SGC7901) and a normal human gastric epithelial cell line GES-1. (C) Relative miR-203 level in GC tissues and their corresponding adjacent normal tissues. (D) Relative miR-203 level analyzed by qRT-PCR in four GC cell lines (AGS, BGC-823, MKN45, SGC7901) and GES-1. (E) Pearson’s correlation analysis of the relative miR-203 levels of and the relative annexin A4 mRNA levels in GC tissues. All data are presented as mean ± SEM, n = 6. *p < 0.05, **p < 0.01, ***p < 0.001 versus Normal, GC tissues or GES-1.
Effect of miR-203 on the Invasion and Related Molecules of GC Cells
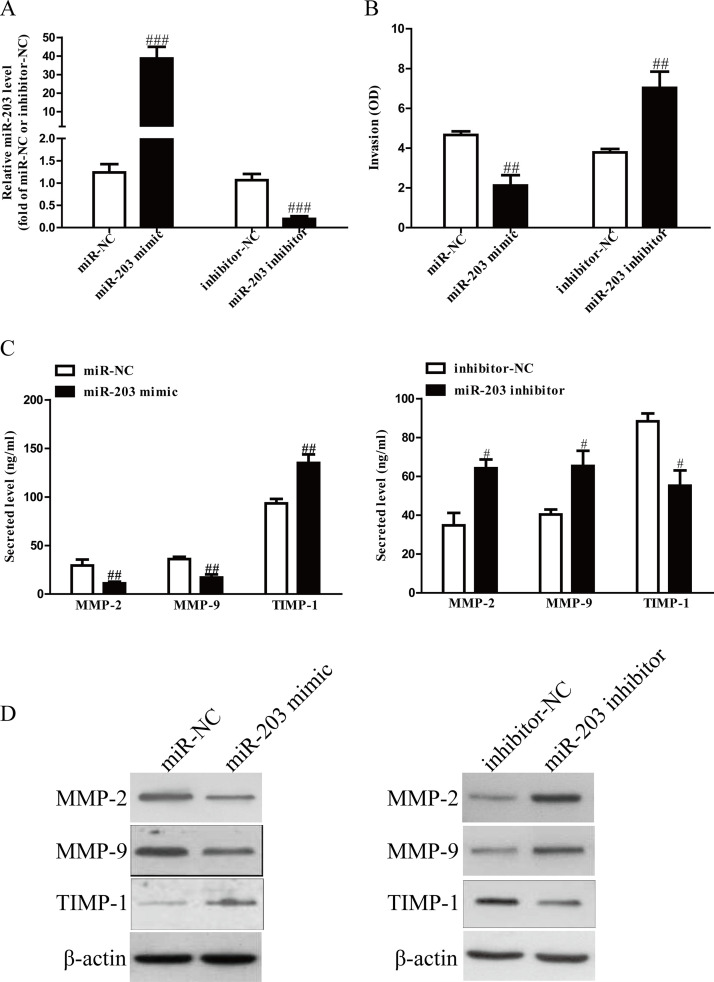
After transfection with miR-203 mimic and inhibitor, the qRT-PCR analysis showed that the level of miR-203 was significantly upregulated and downregulated, respectively (Fig. 2A). These data demonstrated that we efficiently enhanced and reduced miR-203 expression in SGC7901 cells. To study the role of miR-203 in invasion of GC cells, we evaluated the invasive capacities of SGC7901 cells transfected with miR-203 mimic or inhibitor by Transwell invasion assays. Transwell assays illustrated that the invasion of SGC7901 cells was remarkably suppressed in the miR-203 mimic group compared to the miR-NC group, but was significantly promoted in the miR-203 inhibitor group compared to the anti-miR-NC group (Fig. 2B). These findings showed that miR-203 might play a critical role in inhibition of GC cell invasion. The balance between MMPs and TIMP-1 is demonstrated to play an important role of invasion by stimulating degradation of the ECM in GC cells and is associated with enhanced tumor metastatic potential. Our ELISA and Western blot assays indicated that total secretion of MMP-2 and MMP-9 in the culture supernatants and expressions of MMP-2 and MMP-9 were evidently decreased by overexpression of miR-203 in SGC7901 cells, and total secretion of TMIP-1 and protein expression of TMIP-1 were significantly increased (Fig. 2C and D). However, knockdown of miR-203 could enhance the secretion and protein expressions of MMP-2 and MMP-9 and reduce the secretion and protein expressions of TMIP-1 (Fig. 2C and D). Taken together, our findings suggested that downregulated MMP-2 and MMP-9 expressions and upregulated TMIP-1 expression might be the possible mechanisms that contributed to the inhibitory effect of miR-203 mimic on the invasion of SGC7901 cells.
Figure 2.
The effects of miR-203 on invasion and related molecule expressions in SGC7901 cells. SGC7901 cells were transfected with miR-203 mimic or inhibitor. (A) The level of miR-203 analyzed by qRT-PCR. (B) The invasion of SGC7901 cells was assessed by Transwell assay. (C) Levels of total MMP-2, MMP-9, and TIMP-1 were detected in the culture supernatants of cultured SGC7901 cells by ELISA assay. (D) The protein expressions of MMP-2, MMP-9, and TIMP-1 were examined by Western blot. All data are presented as mean ± SEM, n = 4. #p < 0.05, ##p < 0.01, ###p < 0.001 versus miR-NC or inhibitor-NC.
Effects of miR-203 on EMT of GC Cells
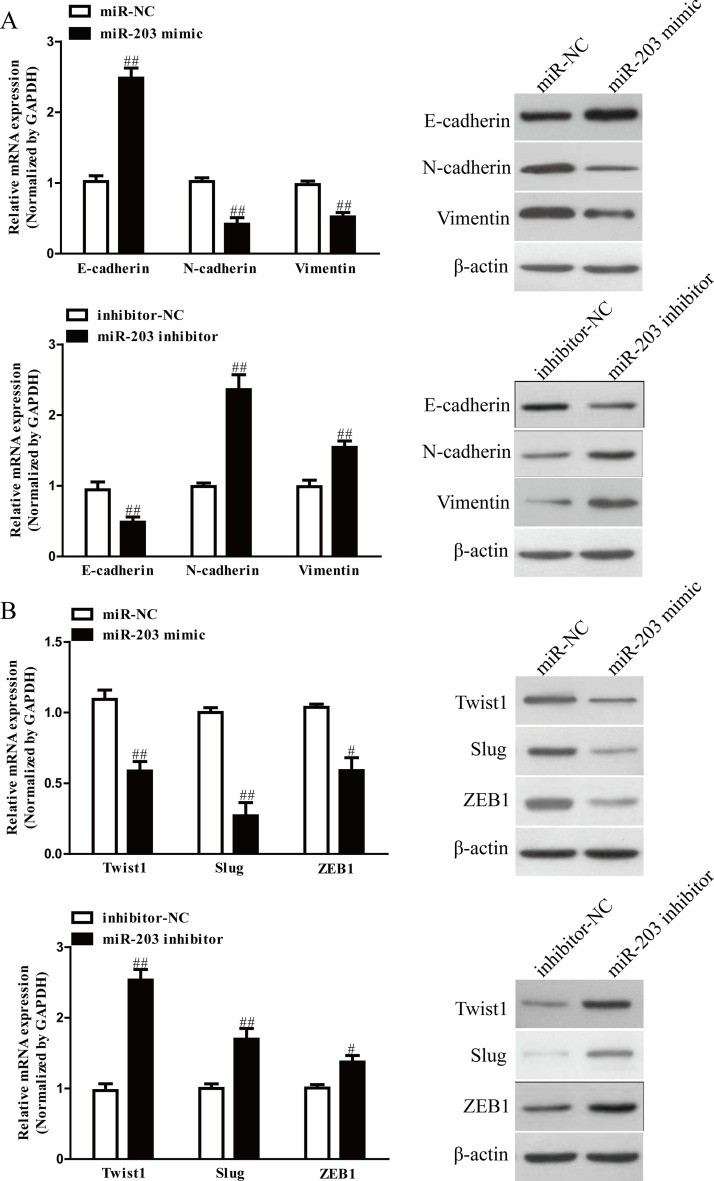
We next explored the effects of miR-203 on the expressions of EMT markers in SGC7901 cells using qRT-PCR and Western blot. Introduction of miR-203 in SGC7901 cells led to increasing the expression of epithelial marker E-cadherin, and decreasing the expressions of mesenchymal markers N-cadherin and vimentin at mRNA and protein levels (Fig. 3A). Moreover, we also determined the expressions of EMT-related transcription factors in SGC7901 cells after transfection with miR-203 mimic. Overexpression of miR-203 significantly reduced the mRNA and protein expressions of Twist1, Slug, and ZEB1 in both cells (Fig. 3B). However, knockdown of miR-203 had opposite effects of miR-203 upregulation on EMT of GC cells (Fig. 3A and B). Altogether, our findings revealed that overexpression of miR-203 could inhibit EMT of GC cells.
Figure 3.
The effects of miR-203 on expressions of EMT-related molecules in SGC7901 cells. (A) The mRNA and protein levels of E-cadherin, N-cadherin, and vimentin were determined by qRT-PCR and Western blot in SGC7901 cells transfected with miR-203 mimic or inhibitor, respectively. (B) The mRNA and protein levels of Twist1, Slug, and ZEB1 were determined by qRT-PCR and Western blot in SGC7901 cells transfected with miR-203 mimic or inhibitor, respectively. All data are presented as mean ± SEM, n = 4. #p < 0.05, ##p < 0.01 versus miR-NC or inhibitor-NC.
Knockdown of Annexin A4 Mimicked the Effects of Overexpression of miR-203
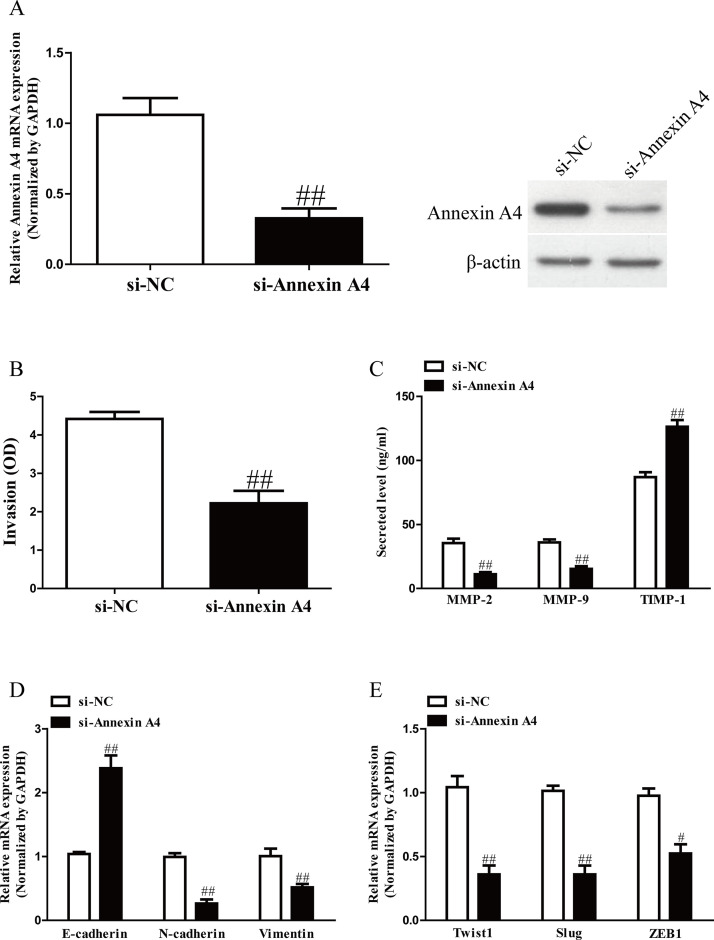
To explore the role of annexin A4 in GC cells, SGC7901 cells were transfected with si-NC or si-annexin A4. The qRT-PCR and Western blot analysis showed that expression of annexin A4 was significantly decreased at mRNA and protein level in SGC7901 cells transfected with si-annexin A4 (Fig. 4A). Transwell assay revealed that downregulation of annexin A4 suppressed invasion of GC cells (Fig. 4B). Moreover, decreased annexin A4 expression could significantly reduce total expressions of MMP-2 and MMP-9 and enhance expression of TMIP-1 in SGC7901 cells (Fig. 4C). In addition, silencing of annexin A4 contributed to upregulation of E-cadherin and downregulation of N-cadherin and vimentin (Fig. 4D). Next, the mRNA levels of Twist1, Slug, and ZEB1 in SGC7901 cells were also significantly reduced by decreasing miR-203 level (Fig. 4E). Hence, knockdown of annexin A4 induced a very similar phenotype to miR-203 overexpression in GC cells.
Figure 4.
Annexin A4 silencing also could inhibit the invasion and EMT in GC cells. SGC7901 cells were transfected with si-annexin A4 or si-NC. (A) The mRNA and protein levels of annexin A4 were determined by qRT-PCR and Western blot, respectively. (B) The invasion of SGC7901 cells was assessed by Transwell assay. (C) Levels of total MMP-2, MMP-9, and TIMP-1 were detected in the culture supernatants of cultured SGC7901 cells by ELISA assay. (D) The expressions of E-cadherin, N-cadherin, and vimentin were determined by qRT-PCR in SGC7901 cells. (E) The expressions of Twist1, Slug, and ZEB1 were determined by qRT-PCR in SGC7901 cells. All data are presented as mean ± SEM, n = 4. #p < 0.05, ##p < 0.01 versus si-NC.
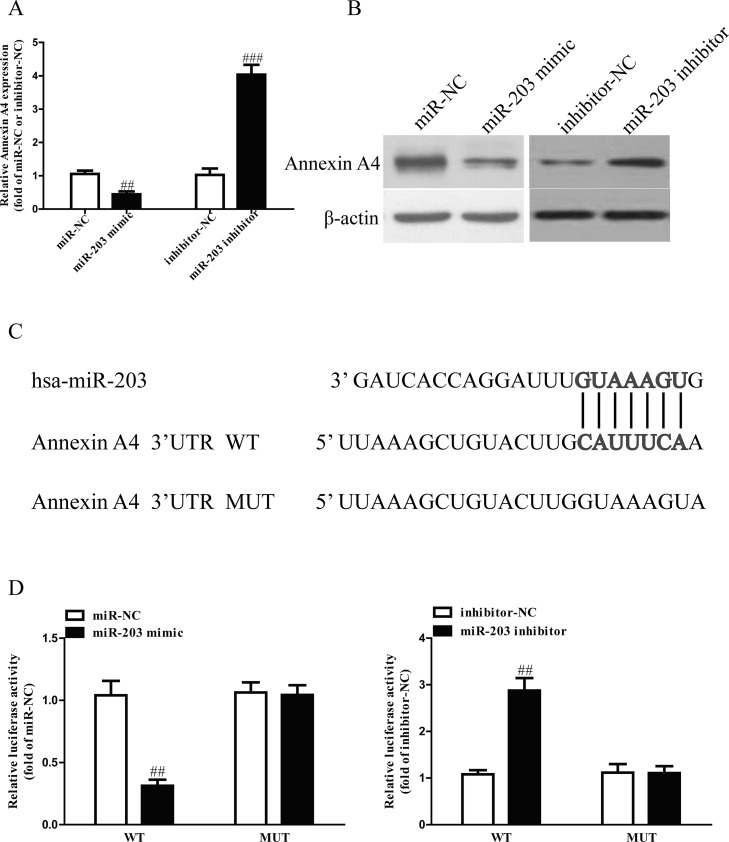
miR-203 Directly Targets Annexin A4 in GC Cells
Based on TargetScan 6.2 database, we predicted that annexin A4 was a binding target of miR-203. Next, we performed qRT-PCR and Western blotting to determine the expression of annexin A4 at mRNA and protein levels in SGC7901 cells transfected with miR-203 mimic or inhibitor. Our findings showed that mRNA and protein levels of annexin A4 were significantly decreased after transfection with miR-203 mimic and were dramatically increased after transfection with miR-203 inhibitor (Fig. 5A and B). Moreover, luciferase reporter assay also confirmed that miR-203 directly targeted annexin A4. Annexin A4 3′-UTR was cloned into a luciferase reporter vector, and the putative miR-203 binding site in the annexin A4 3′-UTR was mutated (Fig. 5C). Our data showed that upregulation of miR-203 markedly inhibited the luciferase activity of pMIR-annexin A4 3′-UTR WT (Fig. 5D). Mutation of the miR-203 binding site in the annexin A4 3′-UTR abolished the effect of miR-203, which indicated that miR-203 directly and negatively regulated the expression of annexin A4.
Figure 5.
Annexin A4 was a direct target of miR-203. (A) The mRNA level of annexin A4 was determined by qRT-PCR in SGC7901 cells transfected with miR-203 mimic or inhibitor. (B) The protein expression of annexin A4 was determined by Western blot in SGC7901 transfected with miR-203 mimic or inhibitor. (C) Schematic representation of annexin A4 3′-UTRs showing putative miRNA target site. (D) The analysis of the relative luciferase activities of annexin A4-WT and annexin A4-MUT in GC cells. All data are presented as mean ± SEM, n = 4. ##p < 0.01, ###p < 0.001 versus miR-NC or inhibitor-NC.
miR-203 Suppressed the Invasion and EMT of GC Cells Through Inhibition of Annexin A4
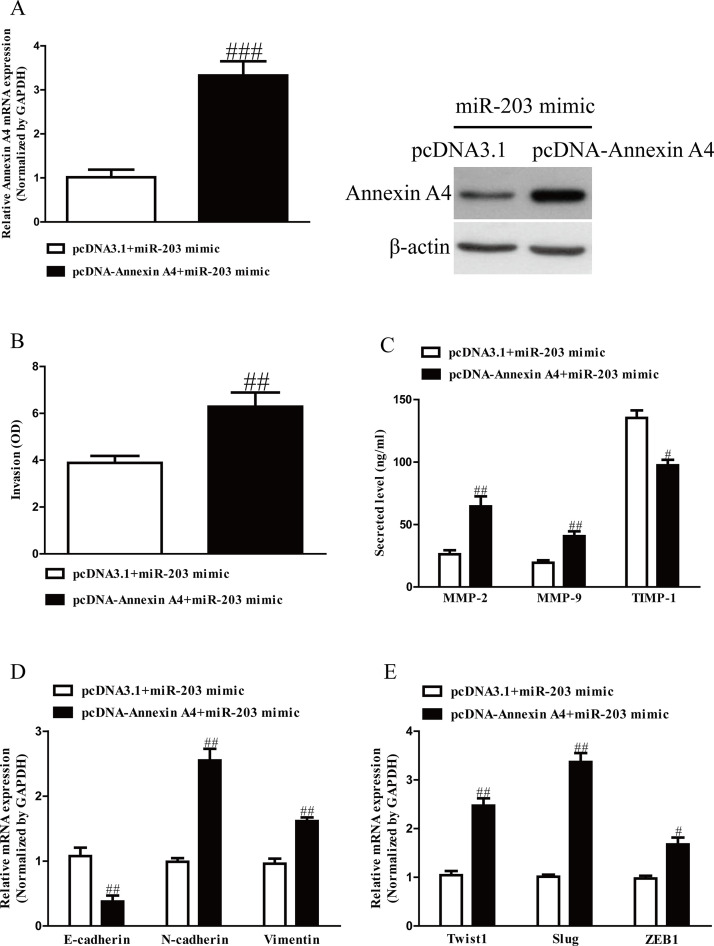
To confirm whether miR-203 inhibited the invasion and EMT of GC cells through annexin A4-dependent mechanism, we cotransfected SGC7901 cells with miR-203 mimic and pcDNA3.1–annexin A4 vector. Our data showed that the expressions of annexin A4 at mRNA and protein levels were dramatically increased after transfection with miR-203 mimic and pcDNA–annexin A4 compared with miR-203 mimic and pcDNA vector in SGC7901 cells (Fig. 6A). Data from Transwell assay indicated that upregulation of annexin A4 could reverse the inhibitory effect of miR-203 overexpression on invasion of GC cells (Fig. 6C). Moreover, introduction of annexin A4 decreased the expression of E-cadherin and increased expressions of N-cadherin and vimentin in SGC7901 cells after transfection with miR-203 mimic (Fig. 6D). Next, upregulation of annexin A4 could also reverse the effects of miR-203 mimic on the expressions of Twist1, Slug, and ZEB1 (Fig. 6E). Therefore, our data clearly confirmed that upregulation of miR-203 inhibited the invasion and EMT of GC cells by directly targeting annexin A4, and knockdown of annexin A4 was essential for the miR-203 mimic-induced inhibition of the invasion and EMT in GC cells.
Figure 6.
Overexpression of annexin A4 partially rescued miR-203-inhibited cell proliferation, invasion, and EMT in GC cells. SGC7901 cells were transfected with either miR-203 mimic with or without pcDNA–annexin A4 vector. (A) The mRNA and protein levels of annexin A4 were determined by qRT-PCR and Western blot, respectively. (B) The invasion of SGC7901 cells was assessed by Transwell assay. (C) Levels of total MMP-2, MMP-9, and TIMP-1 were detected in the culture supernatants of cultured SGC7901 cells by ELISA assay. (D) The expressions of E-cadherin, N-cadherin, and vimentin were determined by qRT-PCR in SGC7901 cells. (E) The expressions of Twist1, Slug, and ZEB1 were determined by qRT-PCR in SGC7901 cells. All data are presented as mean ± SEM, n = 4. #p < 0.05, ##p < 0.01, ###p < 0.001 versus miR-203 mimic + pcDNA3.1.
DISCUSSION
The major cause of death of GC is metastasis, which greatly hinders treatment success21. Therefore, understanding the precise molecular mechanisms underlying GC metastasis is critical to develop novel and effective therapeutic strategies for patients with advanced GC. Despite structural similarities among the members of this family, annexin proteins play different roles in anti-inflammation action, antifibrinolytic activity, vesicle trafficking, cell proliferation, apoptosis, differentiation, and angiogenesis5,6,8,22. A recent study demonstrated that several forms of annexins are closely associated with the development and progression of cancer, and expression patterns of individual annexins are tumor-type specific9. Several of the annexin proteins, including annexin A1, A2, A3, A4, A5, A6, A7, and A11, are involved in the oncogenic process. In this study, we for the first time demonstrated that annexin A4 was the highest expression in GC tissues among these annexin proteins. Then we found that the expression of annexin A4 in GC tissues was higher than that in the adjacent tissues. Our findings showed that miR-203 was frequently downregulated in GC cell lines and tissues. As expected, introduction of miR-203 inhibited the invasion and EMT of SGC7901 cells. Our findings suggested that miR-203 played crucial roles in regulating the invasion and EMT of GC and might be a possible diagnostic and predictive biomarker.
However, the precise mechanism of miR-203 in GC remained unclear. Therefore, in this study, we aimed to elucidate the biological functions and its mechanism of miR-203 in GC. Afterward, qRT-PCR, Western blotting, and luciferase reporter assay confirmed that miR-203 directly targeted annexin A4. Importantly, we also demonstrated that overexpression of annexin A4 partly blocked the inhibitory effects of miR-203 mimic on the invasion and EMT of GC cells. Therefore, we made a conclusion that miR-203 played critical roles in inhibition of the invasion and EMT of GC cells by regulation of annexin A4.
One of the key molecular steps in the process of invasion is degradation of extracellular matrix (ECM) components by proteolytic enzymes23. A previous study has shown that the imbalance between MMP activity and the specific inhibitor TIMPs in GC tissue might be a significant factor in the process of tumor invasion and metastasis24. It has been demonstrated that the expression of MMP-9 in GC tissues was significantly higher, but TIMP-1 was significantly lower than that in normal tissues24. In this article, Transwell assay showed that overexpression or knockdown of miR-203 dramatically suppressed or promoted the invasion of SGC7901 cells compared with miR-NC or anti-miR-NC group, respectively. Moreover, expressions of MMP-2 and MMP-9 were significantly decreased, and expression of TIMP-1 was dramatically increased in SGC7901 after transfection with miR-203 mimic. In addition, one key molecular step in the process of distant metastasis is characterized by EMT. Activation of EMT is usually observed in many types of malignant tumors including GC25,26. EMT capacitates the cancer cells to obtain invasive properties and metastatic growth characteristics. In EMT, genes such as E-cadherin, N-cadherin, β-catenin, fibronectin, and vimentin have been established as the markers of EMT27. We determined the changes of EMT markers in SGC7901 cells transfected with miR-203 mimic and inhibitor. Our data demonstrated that upregulation of miR-203 could dramatically increase the epithelial marker E-cadherin and decrease the mesenchymal markers N-cadherin and vimentin, which suggested that miR-203 might reverse the EMT process to suppress cell invasion and metastasis. Moreover, many transcription factors, such as Snail, Slug, Twist, ZEB1, and ZEB2, are considered as the crucial inducers of EMT28–30. Further study showed that introduction of miR-203 led to downregulating the expressions of Twist1, Slug, and ZEB1. Collectively, our results indicated that overexpressing miR-203 inhibited EMT through downregulation of transcription factors, thereby suppressing cell invasion and metastasis of GC.
Annexin A4 is a member of the annexin family and is important for the diagnosis and prognosis of cancers. Several studies have reported malignancies associated with high expression of annexin A4 include lung, gastric, colorectal, pancreatic, gallbladder, breast, renal, ovarian, laryngeal, and prostate cancers31,32, indicating that annexin A4 may be a critical oncogene contributing to metastasis of cancers. In the present study, annexin A4 was also found to be upregulated in GC cells and tissues. Next, our findings confirmed that annexin A4 was a direct target of miR-203 in GC cells. Furthermore, we found that knockdown of annexin A4 also significantly inhibited the invasion and EMT of GC cells, mimicking the effects of miR-203 overexpression. Besides, restoration of annexin A4 partially blocked the inhibitory effects of miR-203 mimic on the invasion and EMT of GC cells, suggesting that annexin A4 might play an important role in metastasis of GC.
In conclusion, we have demonstrated that relative miR-203 expression was dramatically reduced in GC tissues and cells, whereas the expression of annexin A4 was significantly enhanced in GC tissues and cells. Upregulation of miR-203 inhibited the invasion and EMT of GC cells by direct downregulation of annexin A4. This novel miR-203/annexin A4 axis might provide new insights into the molecular mechanisms underlying metastasis of GC, and overexpression of miR-203 might be a possible therapeutic strategy for the therapy of GC in the future.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Bickenbach K, Strong VE. Comparisons of gastric cancer treatments: East vs. west. J Gastric Cancer 2012;12:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gigek CO, Chen ES, Calcagno DQ, Wisnieski F, Burbano RR, Smith MA. Epigenetic mechanisms in gastric cancer. Epigenomics 2012;4:279–94. [DOI] [PubMed] [Google Scholar]
- 3. Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. [DOI] [PubMed] [Google Scholar]
- 4. Leja M, You W, Camargo MC, Saito H. Implementation of gastric cancer screening—The global experience. Best Pract Res Clin Gastroenterol. 2014;28:1093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerke V, Moss SE. Annexins: From structure to function. Physiol Rev. 2002;82:331–71. [DOI] [PubMed] [Google Scholar]
- 6. Hayes MJ, Moss SE. Annexins and disease. Biochem Biophys Res Commun. 2004;322:1166–70. [DOI] [PubMed] [Google Scholar]
- 7. Lim LH, Pervaiz S. Annexin 1: The new face of an old molecule. FASEB J. 2007;21:968–75. [DOI] [PubMed] [Google Scholar]
- 8. Rand JH. The annexinopathies: A new category of diseases. Biochim Biophys Acta 2000;1498:169–73. [DOI] [PubMed] [Google Scholar]
- 9. Mussunoor S, Murray GI. The role of annexins in tumour development and progression. J Pathol. 2008;216:131–40. [DOI] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 11. Zhang B, Pan X, Cobb GP, Anderson TA. MicroRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. [DOI] [PubMed] [Google Scholar]
- 12. Li C, Dong J, Han Z, Zhang K. microRNA-219-5p represses the proliferation, migration, and invasion of gastric cancer cells by targeting the LRH-1/Wnt/β-Catenin signaling pathway. Oncol Res. 2017;25:617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ji S, Zhang B, Kong Y, Ma F, Hua Y. miR-326 inhibits gastric cancer cell growth through downregulating NOB1. Oncol Res. 2017;25:853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun J, Li J, Zhang W, Zhang J, Sun S, Li G, Song H, Wan D. MicroRNA-509-3p inhibits cancer cell proliferation and migration via upregulation of XIAP in gastric cancer cells. Oncol Res. 2017;25:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei W, Wanjun L, Hui S, Dongyue C, Xinjun Y, Jisheng Z. miR-203 inhibits proliferation of HCC cells by targeting survivin. Cell Biochem Funct. 2013;31:82–5. [DOI] [PubMed] [Google Scholar]
- 16. Zhu X, Er K, Mao C, Yan Q, Xu H, Zhang Y, Zhu J, Cui F, Zhao W, Shi H. miR-203 suppresses tumor growth and angiogenesis by targeting VEGFA in cervical cancer. Cell Physiol Biochem. 2013;32:64–73. [DOI] [PubMed] [Google Scholar]
- 17. Lim HS, Kim CS, Kim JS, Yu SK, Go DS, Lee SA, Moon SM, Chun HS, Kim SG, Kim DK. Suppression of oral carcinoma oncogenic activity by microRNA-203 via downregulation of SEMA6A. Anticancer Res. 2017;37:5425–33. [DOI] [PubMed] [Google Scholar]
- 18. Gao P, Wang S, Jing F, Zhan J, Wang Y. MicroRNA-203 suppresses invasion of gastric cancer cells by targeting ERK1/2/Slug/ E-cadherin signaling. Cancer Biomark. 2017;19:11–20. [DOI] [PubMed] [Google Scholar]
- 19. Cheng R, Lu C, Zhang G, Zhang G, Zhao G. Overexpression of miR-203 increases the sensitivity of NSCLC A549/H460 cell lines to cisplatin by targeting Dickkopf-1. Oncol Rep. 2017;37:2129–36. [DOI] [PubMed] [Google Scholar]
- 20. Jung EJ, Moon HG, Park ST, Cho BI, Lee SM, Jeong CY, Ju YT, Jeong SH, Lee YJ, Choi SK, Ha WS, Lee JS, Kang KR, Hong SC. Decreased annexin A4 expression correlates with tumor progression in papillary thyroid cancer. Proteomics Clin Appl. 2010;4:528–37. [DOI] [PubMed] [Google Scholar]
- 21. Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell 2006;127:679–95. [DOI] [PubMed] [Google Scholar]
- 22. Park JE, Lee DH, Lee JA, Park SG, Kim NS, Park BC, Cho S. Annexin A4 is a potential angiogenic mediator. Biochem Biophys Res Commun. 2005;337:1283–7. [DOI] [PubMed] [Google Scholar]
- 23. Simpson-Haidaris PJ, Rybarczyk B. The role of fibrinogen as an extracellular matrix protein. Ann NY Acad Sci. 2001;936:406–25. [PubMed] [Google Scholar]
- 24. Li Y, Tan BB, Zhao Q, Fan LQ, Wang D, Liu Y. ZNF139 promotes tumor metastasis by increasing migration and invasion in human gastric cancer cells. Neoplasma 2014;61:291–8. [DOI] [PubMed] [Google Scholar]
- 25. Yue H, Tang B, Zhao Y, Niu Y, Yin P, Yang W, Zhang Z, Yu P. MIR-519d suppresses the gastric cancer epithelial-mesenchymal transition via Twist1 and inhibits Wnt/β-catenin signaling pathway. Am J Transl Res. 2017;9:3654–64. [PMC free article] [PubMed] [Google Scholar]
- 26. Wang H, Wang C, Tian W, Yao Y. The crucial role of SRPK1 in IGF-1-induced EMT of human gastric cancer. Oncotarget 2017;8:72157–66. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan MA, Chen HC, Zhang D, Fu J. Twist: A molecular target in cancer therapeutics. Tumor Biol. 2013;34:2497–506. [DOI] [PubMed] [Google Scholar]
- 29. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer 2007;7:415–28. [DOI] [PubMed] [Google Scholar]
- 30. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13:97–110. [DOI] [PubMed] [Google Scholar]
- 31. Wei B, Guo C, Liu S, Sun MZ. Annexin A4 and cancer. Clin Chim Acta 2015;447:72–8. [DOI] [PubMed] [Google Scholar]
- 32. Yao H, Sun C, Hu Z, Wang W. The role of annexin A4 in cancer. Front Biosci. (Landmark Ed) 2016;21:949–57. [DOI] [PubMed] [Google Scholar]