Abstract
Although arsenic trioxide (ATO) is a well-known antileukemic drug used for acute promyelocytic leukemia treatment, the development of ATO resistance is still a big challenge. We previously reported that microRNA-204 (miR-204) was involved in the regulation of acute myeloid leukemia (AML) cell apoptosis, but its role in chemoresistance is poorly understood. In the present study, we showed that miR-204 was significantly increased in AML cells after ATO treatment. Interestingly, the increased miR-204 level that was negatively correlated with ATO induced the decrease in cell viability and baculoviral inhibition of apoptosis protein repeat-containing 6 (BIRC6) expression. Overexpression of miR-204 potentiated ATO-induced AML cell growth inhibition and apoptosis. Furthermore, miR-204 directly targets to the 3′-UTR of BIRC6. Upregulation of miR-204 decreased BIRC6 luciferase activity and expression, which subsequently enhanced the expression of p53. Restoration of BIRC6 markedly reversed the effect of miR-204 on the regulation of AML cell sensitivity to ATO. Taken together, our study demonstrates that miR-204 decreases ATO chemoresistance in AML cells at least partially via promoting BIRC6/p53-mediated apoptosis. miR-204 represents a novel target of ATO, and upregulation of miR-204 may be a useful strategy to improve the efficacy of ATO in AML treatment.
Key words: Acute myeloid leukemia (AML), Arsenic trioxide (ATO), miR-204, Apoptosis, BIRC6
INTRODUCTION
Leukemia is a malignant disorder of hematopoietic precursor cells and stem cells1. These cells escape into the blood and accumulate in a large number, leading to the clinical presentations of the disease2. Acute myeloid leukemia (AML) is a highly heterogeneous clonal disorder, characterized by rapid proliferation of leukemia blasts and impaired differentiation of hematopoietic progenitor cells3. Since the advanced healthcare systems, the overall 5-year survival for leukemia patients has reached 70%4. However, in the case of AML, this is only 25%, just in the US, with more than 10,000 estimated deaths in 20164. Although the World Health Organization categorization of AML mentioned that molecular analysis is of great importance to predict the survival rates of AML5, it is still difficult to estimate accurately the prognosis of AML patients due to drug resistance6,7. Thus, it is necessary to develop more effective treatment to increase the sensitivity of AML cells to chemotherapy.
Arsenic trioxide (ATO) is a common antileukemic drug widely used for acute promyelocytic leukemia (APL) treatment8. It effectively induces APL cell differentiation and apoptosis9,10. However, a clinical study has shown that AML patients treated with ATO frequently acquire drug resistance11,12. Although several factors have been reported to contribute to ATO resistance, such as PI3K/AKT pathway activation and specific chromosomal translocations13,14, the mechanisms of ATO resistance are still not yet fully understood.
MicroRNAs (miRNAs) are a class of endogenous, small, and noncoding RNAs with around 22 nucleotides in length, which posttranscriptionally regulate the target genes expression by binding to the 3′-untranslated region (3′-UTR)15,16. Increasing lines of evidence have reported that several miRNAs are important regulators in chemoresistance15,17. miR-204 plays a key role in the development of several malignant tumors, including prostate cancer18, ovarian cancer19, gastric cancer20, and colorectal cancer21. Our previous study reported that miR-204 level was decreased in clinical AML samples and regulated AML cell apoptosis16. However, the effect of miR-204 on chemoresistance of AML cells remains unknown. In this study, we found that miR-204 increases the sensitivity of AML cells to ATO treatment by targeting baculoviral inhibition of apoptosis protein repeat-containing 6 (BIRC6), a gene known to be involved in regulating cell apoptosis, suggesting that miR-204 is a promising target for chemoresistance treatment.
MATERIALS AND METHODS
Materials and Reagents
RPMI-1640 medium, fetal bovine serum (FBS), gentamicin, TRIzol reagent, and Lipofectamine 3000 were obtained from Invitrogen (Carlsbad, CA, USA). Antibodies against BIRC6, p53, and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). miR-204 mimics, miRNA mimics negative control, and miR-204 and U6 primers were from Rio Biotechnology (Guangzhou, P.R. China). Stock solution of ATO prepared in 1 mol/L NaOH was obtained from Sigma-Aldrich. (St. Louis, MO, USA) and diluted in phosphate-buffered saline (PBS) to a concentration of 10 mmol/L.
Patients and Specimen Preparation
The primary AML samples were collected from patients with AML before and after ATO treatment between 2010 and 2015 in the Department of Hematology of the First Affiliated Hospital of Xi’an University. The samples from 48 cases of AML were selected based on the follow-up visits: 1) subjects with a good response to ATO (10 mg daily until remission then 10 mg for 2 weeks every 2 months), who were given as a prolonged outpatient maintenance course over 2 years; 2) subjects received no other chemotherapies. The research protocols were approved by the ethics committee of Xi’an University and performed in accordance with the Declaration of Helsinki and Good Clinical Practice. Informed consent was obtained from all individual participants included in the study.
Cell Culture
Human AML cell lines, AML-5 and HL-60, were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI-1640 medium containing 10% FBS and 50 μg/ml gentamicin in a humidified incubator with 5% CO2 at 37°C.
Cell Viability Assay
The viability of AML-5 or HL-60 cells was measured by Cell Counting Kit-8 (CCK-8; Yiyuan Biotechnology, Guangzhou, P.R. China) according to the manufacturer’s instructions. Briefly, the cells were seeded in 96-well plates (2 × 103 cells/well) and rendered quiescent by replacing the medium with 0.2% FBS for 24 h. After indicated treatment according to the experimental design, the cells were incubated with fresh medium containing 10 μl of CCK-8 reagent for 4 h at 37°C, 5% CO2. The absorbance was read at 450–540 nm using a microplate reader (Bio-Tek, Winooski, VT, USA).
Quantitative Real-Time PCR
Total RNA from AML cells was isolated using TRIzol reagent according to manufacturer’s instructions and was reversed transcribed using the miRNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) or SuperScript III First-Strand Synthesis system (Qiagen, Valencia, CA, USA). For the determination of miR-204 level, cDNA was mixed with TaqMan® Micro Assay kit (Thermo Fisher Scientific Inc., Rockford, IL, USA) and amplified using 7500 Fast Real-Time PCR Systems (Applied Biosystems). To examine the mRNA expression of BIRC6, PCR reaction was performed using Fast SYBR® Green Master Mix kit (Applied Biosystems). The specific primer sequences of BIRC6 and GAPDH were synthesized and provided by the Shanghai Biological Engineering Technology Services Co. Ltd. (Shanghai, P.R. China): BIRC6 sense 5′-CCTGACTTCACTTCCGGCTA-3′ and antisense 5′-GAGCTGCTGTGCCTCTGTAA-3′; GAPDH sense 5′-GCCATCGTCACCAACTGGGAC-3′ and antisense 5′-CGATTTCCCGCTCGGCCGTGG-3′. The mRNA level of target genes was normalized by U6 or GAPDH, and calculated using the 2−ΔΔCT method.
Cell Transfection
Human BIRC6 cDNA was purchased from OpenBioSystems (Huntsville, AL, USA) and cloned into pSMCV expression vector (OpenBioSystems), which was confirmed by sequencing. AML-5 or HL-60 cells were transfected with miR-204 mimics (20 nmol/L), miRNA mimics negative control (20 nmol/L), BIRC6 plasmid or empty vector for 48 h using Lipofectamine 3000 according to the manufacturer’s instruction.
Apoptosis Detection Assay
The apoptosis of AML cells was measured with the FITC–Annexin-V Apoptosis Detection kit (Beyotime Institute of Biotechnology, Shanghai, P.R. China) using FACS Caliber flow cytometry (Becton Dickinson, San Diego, CA, USA) according to the manufacturer’s instruction. In brief, the cells were washed with PBS, centrifuged at 1,000 × g for 10 min, and then the cell pellets were resuspended and incubated with annexin V–FITC and propidium iodide (PI) for 20 min at room temperature. The stained cells were counted with flow cytometry and analyzed using WinMDI software (The Scripps Research Institute, La Jolla, CA, USA).
Cell Cycle Analysis
Distribution of cell cycle in AML cells was evaluated by flow cytometry. The cells were harvested by centrifugation at 1,000 × g for 10 min at 4°C. The pellets were washed with cold PBS twice, and then fixed with 70% ethanol for 30 min on ice. Before flow cytometry analysis, the samples were incubated with 50 μg/ml of PI dissolved in PBS for 30 min at 37°C. Cellular DNA contents were analyzed using a Becton Dickinson FACScan flow cytometer. The subG1, G1 and S/G2/M populations were quantified with the ModFit software program (Verity Software House, Topsham, ME, USA).
Western Blotting Analysis
Cells were washed with PBS and lysed in a lysis buffer containing 50 mM HEPES (Promocell, Heidlberg, Germany), 1% Triton X-100, and protease and phosphatase inhibitors (Pierce Biotechnology, Rockford, IL, USA). Cellular protein concentration from total cell lysates (20 μg) was quantified using a bicinchoninic acid kit (Bio-Rad, Hercules, CA, USA). Samples containing equal amounts of protein were electrophoresed on 6%–8% SDS-PAGE gels and transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% nonfat milk in TBST (in mmol/L, 10 Tris-HCl, 150 NaCl, 0.05% Tween-20, pH 7.6) and probed with the indicated primary antibodies (1:1,000) at 4°C overnight. Then the membranes were washed with TBST three times and incubated with HRP-conjugated secondary antibodies (Cell Signaling Technology, Billerica, MA, USA) for 1 h. The membranes were exposed to enhanced chemiluminescence kit according the manufacturer’s instructions (Beyotime Institute of Biotechnology). Image quantification was performed by ImageJ software (Version 1.41; NIH, Maryland, MD, USA).
Luciferase Assay
The 3′-UTR of BIRC6 (GenBank ID: NM_016252), which is predicted to contain the binding site for miR-204, was cloned into the pmirGLO dual-luciferase miRNA target expression vector (Promega, Madison, WI, USA). The mutant BIRC6 3′-UTR was constructed by substitution of 4 bp from the seed region of miR-204. The cells (1 × 105) were cotransfected with BIRC6 3′-UTR or mutant BIRC6 3′-UTR and miR-204 mimics or miRNA mimics negative control. Forty-eight hours later, the cells were harvested, and the luciferase activity was assessed using a dual-luciferase reporter system (Promega) according to the manufacturer’s protocols.
Statistical Analysis
All data were given as mean value ± SEM; n value represents the number of independent experiments. The regression analysis between miR-204 level and cell viability or BIRC6 expression was determined by the Pearson correlation test. The statistical significance was analyzed by two-tailed Student t-test or one-way ANOVA, followed by the Bonferroni multiple comparison test using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
miR-204 Level Was Negatively Correlated With AML Cell Viability and BIRC6 Expression After ATO Treatment
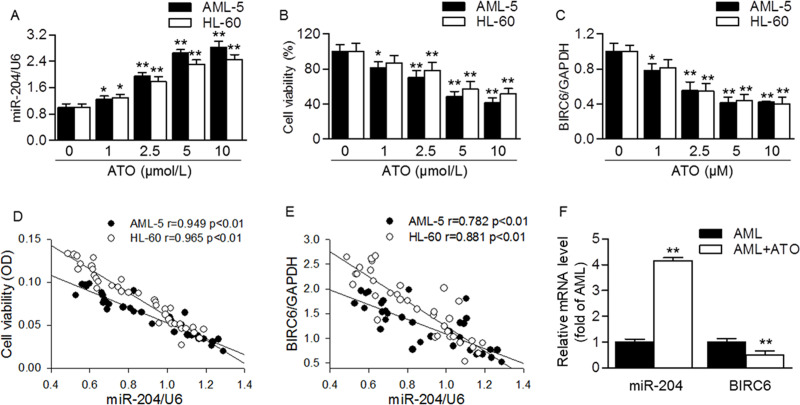
To investigate the involvement of miR-204 in ATO sensitivity, we first examined the effect of ATO on miR-204 level. The result showed that ATO induced the level of miR-204 in a dose-dependent manner in AML-5 and HL-60 cells, respectively (Fig. 1A). On the contrary, the viability of AML-5 and HL-60 cells was gradually inhibited by ATO treatment (Fig. 1B). Moreover, ATO also decreased the mRNA expression of BIRC6 (Fig. 1C). Importantly, the increased miR-204 level was negatively correlated with cell viability and BIRC6 expression (Fig. 1D and E). Similar tendencies were also observed in primary AML samples from AML patients. After ATO treatment, miR-204 level was dramatically increased, but BIRC6 expression was inhibited (Fig. 1F). These results suggest that the increased miR-204 level may be involved in ATO-mediated inhibition of AML cell viability and BIRC6 expression.
Figure 1.
MicroRNA-204 (miR-204) level was negatively correlated with arsenic trioxide (ATO)-induced decrease in cell viability and baculoviral inhibition of apoptosis protein repeat-containing 6 (BIRC6) expression in acute myeloid leukemia (AML) cells. (A) AML-5 or HL-60 cells were treated with different concentrations of ATO (0, 1, 2.5, 5, or 10 μmol/L) for 48 h. The miR-204 level was determined by quantitative real-time PCR. (B) Cell viability was assessed by cell counting kit (CCK-8) assay. (C) The mRNA expression of BIRC6 was examined by quantitative real-time PCR. *p < 0.05; **p < 0.01 versus 0 μmol/L, n = 6–8. (D, E) The cell viability (D) and BIRC6 expression (E) were negatively correlated with miR-204 level, respectively. (F) Forty-eight cases of AML patients with a good response to ATO were selected after 2-year outpatient maintenance, and then the RNA of AML samples was harvested. Quantitative real-time PCR analysis of miR-204 and BIRC6 mRNA levels in primary AML samples before and after ATO treatment. **p < 0.01 versus AML (before ATO treatment).
miR-204 Upregulation Promoted ATO-Induced AML Cell Apoptosis
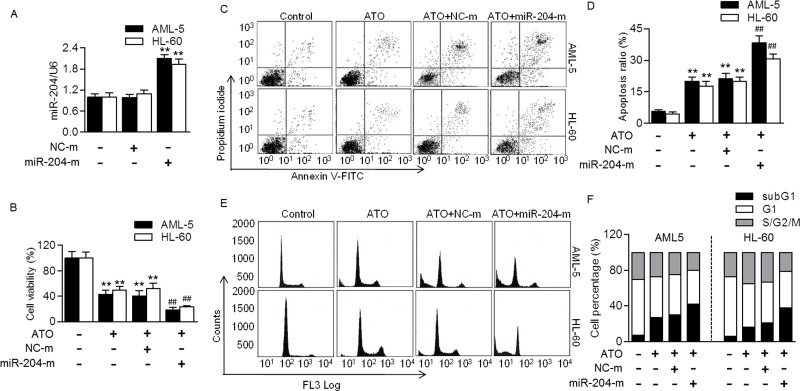
To further explore the functional role of miR-204 in regulating chemosensitivity of AML cells, we next tested the effects of miR-204 overexpression on cellular functions. Quantitative real-time PCR confirmed that miR-204 was successfully overexpressed after transfection with miR-204 mimics (Fig. 2A). miR-204 upregulation significantly enhanced ATO-induced decrease in AML-5 and HL-60 cell viability. Compared with the ATO group, the cell viability after miR-204 overexpression was decreased from 42.5% to 18.7% in AML-5 cells and from 49.2% to 23.3% in HL-60 cells, respectively (Fig. 2B). The Annexin V/PI double staining by flow cytometry showed that overexpression of miR-204 markedly potentiated ATO-induced apoptosis in AML-5 and HL-60 cells compared with the ATO group with an increase in the percentage of apoptotic cells from 19.9% to 38.3% and 17.8% to 30.8%, respectively (Fig. 2C and D). Furthermore, the effect of miR-204 overexpression on cell cycle distribution was also examined. The results revealed that the subG1 population induced by ATO was further augmented in cells treated with miR-204 mimics, indicating the increase in apoptotic cells. In AML-5 and HL-60 cells, the subG1 percentages in ATO-treated cells before or after miR-204 mimic transfection were 27.0% versus 40.5%, and 16.8% versus 38.2%, respectively (Fig. 2E and F). The data indicate that miR-204 increases ATO chemosensitivity via promoting cell apoptosis rather than inhibiting cell proliferation.
Figure 2.
Upregulation of miR-204 promoted ATO-induced AML cell apoptosis. (A) Human AML cell lines, AML-5 and HL-60, were transfected with miR-204 mimics (miR-204-m; 20 nmol/L) or mimic negative control (NC-m) for 48 h. Quantitative real-time PCR was performed to test the level of miR-204. **p < 0.01 versus control, n = 4. (B) The cells were transfected with miR-204 mimics or mimic negative control for 48 h, and then treated with ATO (5 μmol/L) for another 48 h. Cell viability was examined. **p < 0.01 versus control; ##p < 0.01 versus ATO, n = 6. (C) Cell apoptosis was examined by annexin V/propidium iodide (PI) staining using flow cytometry. (D) The percentage of apoptosis ratio was quantified. (E) Cell cycle was quantified by flow cytometry. (F) Graphs correspond to the distribution of cell population in different phases. n = 6.
miR-204 Targeted to BIRC6 and Enhanced p53 Expression
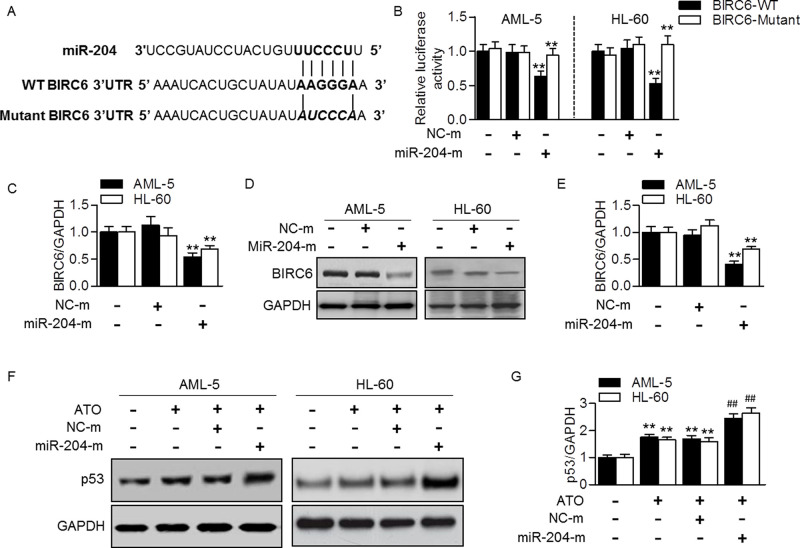
Computational mRNA target analysis by bioinformatic software Targetscan shows that miR-204 contains a potential binding site in BIRC6. A 6-bp fragment of BIRC6 3′-UTR is complementary to the miR-204 seed sequence (Fig. 3A). To confirm whether miR-204 directly binds to BIRC6 and inhibits its translation, we upregulated miR-204 level and measured its effect on BIRC6 3′-UTR luciferase activity. By cotransfection with miR-204 mimics and BIRC6 3′-UTR luciferase reporter into AML-5 or HL-60 cells, the luciferase assay showed that miR-204 overexpression markedly decreased the luciferase activity of BIRC6 3′-UTR (Fig. 3B). In addition, the effect of miR-204 mimics on BIRC6 expression was determined. Quantitative real-time PCR showed that BIRC6 mRNA expression was reduced in cells treated with miR-204 mimics (Fig. 3C). Consistently, the protein expression of BIRC6 after miR-204 mimic transfection was also decreased (Fig. 3D and E). Since p53 is an important anticarcinogenic factor that can be regulated by BIRC622,23, we investigated the effect of miR-204 on p53 expression in the presence of ATO. The results showed that ATO-induced increase in p53 expression was significantly enhanced after miR-204 overexpression (Fig. 3F and G). These data suggest that miR-204 increases the sensitivity of AML cells to ATO at least partially via BIRC6/p53-dependent apoptotic pathway.
Figure 3.
miR-204 targeted at BIRC6 and increased p53 expression. (A) The human miR-204 with BIRC6 3′-untranslated region (3′-UTR) with seed sequence. (B) Luciferase activity of BIRC6 3′-UTR was examined in AML-5 or HL-60 cells treated with miR-204 mimics (miR-204-m, 20 nmol/L) or mimic negative control (NC-m) for 48 h. **p < 0.01 versus control, n = 6. (C) BIRC6 mRNA expression was determined by quantitative real-time PCR. **p < 0.01 versus control, n = 5. (D) Western blotting analysis of BIRC6. Representative images were shown. (E) Densitometric analysis of BIRC6 was performed. **p < 0.01 versus control, n = 6. (F) The cells were treated with miR-204 mimics for 48 h before ATO incubation (5 μmol/L). p53 expression was tested. (G) Densitometric analysis of BIRC6 was performed. **p < 0.01 versus control; ##p < 0.01 versus ATO, n = 5.
Rescued BIRC6 Expression Abolished the Effect of miR-204 on AML Cell Apoptosis Induced by ATO
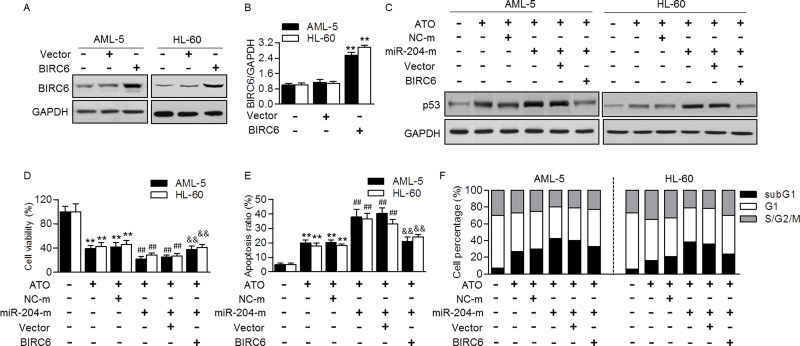
To confirm the critical role of BIRC6 in miR-204-mediated ATO sensitivity, BIRC6 expression was restored in AML cells, and its effect on p53-mediated apoptosis was measured. Expectedly, transfection with BIRC6 plasmid upregulated BIRC6 expression in AML-5 and HL-60 cells (Fig. 4A and B). Importantly, miR-204 mimics that mediated the enhanced effect on p53 expression were markedly reversed after restoration of BIRC6 (Fig. 4C). CCK-8 assay also showed that the effect of miR-204 mimics on ATO-mediated cell growth and apoptosis was significantly attenuated in cells transfected with BIRC6 plasmid (Fig. 4D and E). Similarly, the enhanced population of subG1 phase induced by miR-204 overexpression was inhibited after BIRC6 upregulation (Fig. 4F), further supporting that downregulation of BIRC6 contributes to the effect of miR-204 on ATO sensitivity in AML cells.
Figure 4.
Restoration of BIRC6 reversed the effect of miR-204 on AML cell apoptosis induced by ATO. (A) Western blotting analysis of BIRC6 expression in AML-5 or HL-60 cells transfected with BIRC6 plasmid for 48 h. Representative images are shown. (B) Densitometric analysis of BIRC6 was performed. **p < 0.01 versus control, n = 4. (C) The cells were cotransfected with miR-204 mimics (miR-204-m, 20 nmol/L) and BIRC6 plasmid for 48 h and then treated with ATO (5 μmol/L) for 48 h. p53 protein expression was determined by Western blotting analysis. (D, E) After the treatment mentioned in (C), cell viability (D) and apoptosis (E) were examined. **p < 0.01 versus control; ##p < 0.01 versus ATO; &&p < 0.01 versus ATO + miR-204-m, n = 6. (F) Cell cycle was quantified by flow cytometry, and the graphs correspond to the distribution of cell population in different phases. n = 6.
DISCUSSION
In the past decade, miRNA regulation has been suggested as an important mechanism that is involved in a wide range of cancer development1,17. Because they are more stable than many mRNA moieties and even some proteins, many investigators have thought that this stability can be exploited to function as a novel gene therapeutic approach in some diseases, including leukemia, such as AML and chronic myeloid leukemia3,24. The alteration of miRNAs may indicate important clinicopathological significance of AML25–27. For example, miR-192 level was reduced in the specimens of AML patients, and this miRNA caused G0/G1 cell cycle arrest and inhibited cell proliferation by targeting CCNT225. In addition, a study of miR-22 found that it was downregulated in primary AML samples, suggesting that miR-22 may play an antitumor role with therapeutic potential in AML26. Our recent work also reported that miR-204 was downregulated in blood samples of AML patients and different AML cell lines. Overexpression of miR-204 inhibited the growth of AML cells16. In this study, we used two different APL cell lines: AML-5 and HL-60 cells. The results showed that ATO, a well-recognized antileukemic drug, significantly increased miR-204 level, suggesting that the antileukemic effects of ATO may be associated with the changes in miR-204 level. Indeed, enforcing miR-204 markedly potentiated ATO-induced apoptosis of AML cells. Moreover, we selected AML patients with a good response to ATO that represented normal recovery marrow after ATO treatment. The data clearly showed that the levels of miR-204 and BIRC6 were dramatically altered after ATO treatment. Although the sample quantity was low, it could still indicate that higher miR-204 and lower BIRC6 may be associated with favorable prognosis of AML.
Inhibitor of apoptosis proteins (IAPs) family plays a critical role in regulating cell survival and death, which is closely associated with apoptosis resistance in a variety of cancer cells28,29. BIRC6 is the largest member of IAP family with a unique ubiquitin-conjugating domain23,30. The role of BIRC6 in AML has been well documented. For example, upregulation of BIRC6 was associated with unfavorable response to therapy in childhood AML, indicating BIRC6 may contribute to carcinogenic effects in AML31,32. p53, a well-known anticarcinogenic regulator, has been demonstrated to act as a substrate of BIRC6 E3 ubiquitin ligase23. BIRC6 can interact with p53 and facilities its degradation22. Therefore, the feedback loop between BIRC6 and p53 is significant for the regulation of cancer cell apoptosis and drug resistance.
Previous studies have reported that BIRC6 is negatively regulated by several miRNAs by binding to specific sequence in the 3′-UTR region, such as miR-181a, miR-342, and miR-446h23,33,34. In this study, we evidenced that miR-204 level was negatively correlated with BIRC6 expression during ATO treatment. Luciferase assay further showed that BIRC6 was also a potential target of miR-204. Upregulation of miR-204 suppressed BIRC6 expression and luciferase activity and subsequently increased p53 expression. This suggests that miR-204 inhibits BIRC6 expression and in turn activates p53-dependent apoptotic pathway. Given that miR-204 enhances ATO sensitivity and targets to BIRC6, we speculated that the increased expression of BIRC6 may contribute to the drug resistance of AML cells. Indeed, higher BIRC6 expression was detected in childhood AML31,32. Similar to the previous observations that BIRC6 upregulation was also resistant to various anticancer drug in different cancer cells31,35–37, the effect of miR-204 on apoptosis through BIRC6 was further confirmed in our study using BIRC6 plasmid in AML cells. However, a previous study reported that reduced BIRC6 expression was associated with an immature myeloid phenotype of AML samples rather than immature myeloid cells38. These apparent discrepancies may be related to different cell subtypes in distinct leukemic entities and must be reconciled by additional studies.
In conclusion, this study is the first to our knowledge to demonstrate the important role of miR-204 in regulating ATO sensitivity in AML cells, suggesting that miR-204 could be a potential target for overcoming chemoresistance in AML.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Kayser S, Levis MJ. Advances in targeted therapy for acute myeloid leukaemia. Br J Haematol. 2018;180(4):484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Itzykson R, Duchmann M, Lucas N, Solary E. CMML: Clinical and molecular aspects. Int J Hematol. 2017;105(6):711–9. [DOI] [PubMed] [Google Scholar]
- 3. Foran JM. New prognostic markers in acute myeloid leukemia: Perspective from the clinic. Hematology Am Soc Hematol Educ Program 2010;2010:47–55. [DOI] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 5. Godley LA, Cunningham J, Dolan ME, Huang RS, Gurbuxani S, McNerney ME, Larson RA, Leong H, Lussier Y, Onel K, Odenike O, Stock W, White KP, Le Beau MM. An integrated genomic approach to the assessment and treatment of acute myeloid leukemia. Semin Oncol. 2011;38(2):215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albano F, Chiurazzi F, Mimmi S, Vecchio E, Pastore A, Cimmino C, Frieri C, Iaccino E, Pisano A, Golino G, Fiume G, Mallardo M, Scala G, Quinto I. The expression of inhibitor of Bruton’s tyrosine kinase gene is progressively up regulated in the clinical course of chronic lymphocytic leukaemia conferring resistance to apoptosis. Cell Death Dis. 2018;9(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brinkmann K, Kashkar H. Targeting the mitochondrial apoptotic pathway: A preferred approach in hematologic malignancies? Cell Death Dis. 2014;5:e1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norsworthy KJ, Altman JK. Optimal treatment strategies for high-risk acute promyelocytic leukemia. Curr Opin Hematol. 2016;23(2):127–36. [DOI] [PubMed] [Google Scholar]
- 9. Mi JQ, Chen SJ, Zhou GB, Yan XJ, Chen Z. Synergistic targeted therapy for acute promyelocytic leukaemia: A model of translational research in human cancer. J Intern Med. 2015;278(6):627–42. [DOI] [PubMed] [Google Scholar]
- 10. Wang S, Zhou M, Ouyang J, Geng Z, Wang Z. Tetraarsenictetrasulfide and arsenic trioxide exert synergistic effects on induction of apoptosis and differentiation in acute promyelocytic leukemia cells. PLoS One 2015;10(6):e0130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomita A, Kiyoi H, Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O 3) in acute promyelocytic leukemia. Int J Hematol. 2013;97(6):717–25. [DOI] [PubMed] [Google Scholar]
- 12. Kim J, Aftab BT, Tang JY, Kim D, Lee AH, Rezaee M, Kim J, Chen B, King EM, Borodovsky A, Riggins GJ, Epstein EH Jr, Beachy PA, Rudin CM. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell 2013;23(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roy NK, Bordoloi D, Monisha J, Padmavathi G, Kotoky J, Golla R, Kunnumakkara AB. Specific targeting of Akt kinase isoforms: Taking the precise path for prevention and treatment of cancer. Curr Drug Targets 2017;18(4):421–35. [DOI] [PubMed] [Google Scholar]
- 14. Tenen DG. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer 2003;3(2):89–101. [DOI] [PubMed] [Google Scholar]
- 15. Seca H, Almeida GM, Guimaraes JE, Vasconcelos MH. miR signatures and the role of miRs in acute myeloid leukaemia. Eur J Cancer 2010;46(9):1520–7. [DOI] [PubMed] [Google Scholar]
- 16. Wang Z, Luo H, Fang Z, Fan Y, Liu X, Zhang Y, Rui S, Chen Y, Hong L, Gao J, Zhang M. MiR-204 acts as a potential therapeutic target in acute myeloid leukemia by increasing BIRC6-mediated apoptosis. BMB Rep. 2018;51(9):444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gabra MM, Salmena L. microRNAs and acute myeloid leukemia chemoresistance: A mechanistic overview. Front Oncol. 2017;7:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song CJ, Chen H, Chen LZ, Ru GM, Guo JJ, Ding QN. The potential of microRNAs as human prostate cancer biomarkers: A meta-analysis of related studies. J Cell Biochem. 2018;119(3):2763–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu X, Shen H, Yin X, Long L, Chen X, Feng F, Liu Y, Zhao P, Xu Y, Li M, Xu W, Li Y. IL-6R/STAT3/miR-204 feedback loop contributes to cisplatin resistance of epithelial ovarian cancer cells. Oncotarget 2017;8(24):39154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shrestha S, Yang CD, Hong HC, Chou CH, Tai CS, Chiew MY, Chen WL, Weng SL, Chen CC, Chang YA, Lee ML, Huang WY, Hsu SD, Chen YC, Huang HD. Integrated microRNA-mRNA analysis reveals miR-204 inhibits cell proliferation in gastric cancer by targeting CKS1B, CXCL1 and GPRC5A. Int J Mol Sci. 2017;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin Y, Zhang B, Wang W, Fei B, Quan C, Zhang J, Song M, Bian Z, Wang Q, Ni S, Hu Y, Mao Y, Zhou L, Wang Y, Yu J, Du X, Hua D, Huang Z. miR-204-5p inhibits proliferation and invasion and enhances chemotherapeutic sensitivity of colorectal cancer cells by downregulating RAB22A. Clin Cancer Res. 2014;20(23):6187–99. [DOI] [PubMed] [Google Scholar]
- 22. Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268(10):2764–72. [DOI] [PubMed] [Google Scholar]
- 23. Liu XY, Zhang FR, Shang JY, Liu YY, Lv XF, Yuan JN, Zhang TT, Li K, Lin XC, Liu X, Lei Q, Fu XD, Zhou JG, Liang SJ. Renal inhibition of miR-181a ameliorates 5-fluorouracil-induced mesangial cell apoptosis and nephrotoxicity. Cell Death Dis. 2018;9(6):610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lim EL, Trinh DL, Ries RE, Wang J, Gerbing RB, Ma Y, Topham J, Hughes M, Pleasance E, Mungall AJ, Moore R, Zhao Y, Aplenc R, Sung L, Kolb EA, Gamis A, Smith M, Gerhard DS, Alonzo TA, Meshinchi S, Marra MA. MicroRNA expression-based model indicates event-free survival in pediatric acute myeloid leukemia. J Clin Oncol. 2017;35(35):3964–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ke S, Li RC, Lu J, Meng FK, Feng YK, Fang MH. MicroRNA-192 regulates cell proliferation and cell cycle transition in acute myeloid leukemia via interaction with CCNT2. Int J Hematol. 2017;106(2):258–65. [DOI] [PubMed] [Google Scholar]
- 26. Jiang X, Hu C, Arnovitz S, Bugno J, Yu M, Zuo Z, Chen P, Huang H, Ulrich B, Gurbuxani S, Weng H, Strong J, Wang Y, Li Y, Salat J, Li S, Elkahloun AG, Yang Y, Neilly MB, Larson RA, Le Beau MM, Herold T9, Bohlander SK, Liu PP, Zhang J, Li Z, He C, Jin J, Hong S, Chen J. miR-22 has a potent anti-tumour role with therapeutic potential in acute myeloid leukaemia. Nat Commun. 2016;7:11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun YP, Lu F, Han XY, Ji M, Zhou Y, Zhang AM, Wang HC, Ma DX, Ji CY. MiR-424 and miR-27a increase TRAIL sensitivity of acute myeloid leukemia by targeting PLAG1. Oncotarget 2016;7(18):25276–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Srinivasula SM, Ashwell JD. IAPs: What’s in a name? Mol Cell 2008;30(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fulda S. Inhibitor of apoptosis (IAP) proteins in hematological malignancies: Molecular mechanisms and therapeutic opportunities. Leukemia 2014;28(7):1414–22. [DOI] [PubMed] [Google Scholar]
- 30. Martin SJ. An Apollon vista of death and destruction. Nat Cell Biol. 2004;6(9):804–6. [DOI] [PubMed] [Google Scholar]
- 31. Ismail EA, Mahmoud HM, Tawfik LM, Habashy DM, Adly AA, El-Sherif NH, Abdelwahab MA. BIRC6/Apollon gene expression in childhood acute leukemia: Impact on therapeutic response and prognosis. Eur J Haematol. 2012;88(2):118–27. [DOI] [PubMed] [Google Scholar]
- 32. Sung KW, Choi J, Hwang YK, Lee SJ, Kim HJ, Lee SH, Yoo KH, Jung HL, Koo HH. Overexpression of Apollon, an antiapoptotic protein, is associated with poor prognosis in childhood de novo acute myeloid leukemia. Clin Cancer Res. 2007;13(17):5109–14. [DOI] [PubMed] [Google Scholar]
- 33. Crippa E, Folini M, Pennati M, Zaffaroni N, Pierotti MA, Gariboldi M. miR-342 overexpression results in a synthetic lethal phenotype in BRCA1-mutant HCC1937 breast cancer cells. Oncotarget 2016;7(14):18594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Druz A, Chu C, Majors B, Santuary R, Betenbaugh M, Shiloach J. A novel microRNA mmu-miR-466h affects apoptosis regulation in mammalian cells. Biotechnol Bioeng. 2011;108(7):1651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Houdt WJ, Emmink BL, Pham TV, Piersma SR, Verheem A, Vries RG, Fratantoni SA, Pronk A, Clevers H, Borel Rinkes IH, Jimenez CR, Kranenburg O. Comparative proteomics of colon cancer stem cells and differentiated tumor cells identifies BIRC6 as a potential therapeutic target. Mol Cell Proteomics 2011;10(12):M111011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen Z, Naito M, Hori S, Mashima T, Yamori T, Tsuruo T. A human IAP-family gene, apollon, expressed in human brain cancer cells. Biochem Biophys Res Commun. 1999;264(3):847–54. [DOI] [PubMed] [Google Scholar]
- 37. Okumu DO, East MP, Levine M, Herring LE, Zhang R, Gilbert TSK, Litchfield DW, Zhang Y, Graves LM. BIRC6 mediates imatinib resistance independently of Mcl-1. PLoS One 2017;12(5):e0177871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schlafli AM, Torbett BE, Fey MF, Tschan MP. BIRC6 (APOLLON) is down-regulated in acute myeloid leukemia and its knockdown attenuates neutrophil differentiation. Exp Hematol Oncol. 2012;1(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]