Abstract
Astragaloside IV (AS-IV) is an active ingredient in Astragalus membranaceus and is involved in various biological processes, such as regulating the immune system, and counteracting inflammation and malignancy. The aim of this study was to explore the effect of AS-IV on non-small cell lung cancer (NSCLC) cells. Cell counting kit (CCK)-8 assay and flow cytometry were performed to investigate cell survival and cell death, and Western blotting was performed to assess protein expression. We found that AS-IV inhibited the migration and proliferation of NSCLC cells and caused a noticeable increase in cell death. Furthermore, the expression of Bax, a marker of cell death, was increased, whereas the expression of Bcl-2, an antiapoptotic protein, was reduced. AS-IV also promoted cleavage of caspase-3, another indication of apoptosis. Finally, the Akt/GSK-3β/β-catenin axis was suppressed in response to AS-IV. Taken together, these findings provide evidence that AS-IV inhibits NSCLC development via inhibition of the Akt/GSK-3β/β-catenin signaling axis. We therefore propose that AS-IV represents a promising novel agent for the treatment of NSCLC.
Key words: Astragaloside IV (AS-IV), Non-small cell lung cancer (NSCLC), Akt, GSK-3β, β-Catenin
INTRODUCTION
Non-small cell lung cancer (NSCLC) is a major global public health problem and responsible for over 25% of cancer-related deaths annually1,2. Overall, NSCLC has a low 5-year survival rate compared to other malignancies, at 14% for men and 17% for women3,4. Current NSCLC therapies involve a combination of surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy5–7. Although significant advances have been made in the systemic treatment of NSCLC, one of the major limitations has been the development of cellular drug resistance8. Drug resistance can be either preexistent (intrinsic) or acquired after drug exposure. The majority of patients experience well-established toxicities from cytotoxic chemotherapy without a survival advantage.9 Up to 50%–75% of patients who are initially responsive to the small molecule anti-EGFR inhibitor gefitinib become resistant to this therapy in 5–10 months, indicating tumor progression. At present, drug resistance to anti-EGFR inhibitors is the result of secondary EGFR gene mutations, EGFR/Met gene amplification, and/or activation of key signaling pathway10. As a result, it has become necessary to develop innovative and effective strategies to prevent and/or overcome drug resistance11. A number of natural products have been shown to effectively regulate cancer progression and to also enhance the anticancer effects of various anticancer agents. For example, kanglaite is a Chinese herbal medicine that contains extracts from coix seeds. Various studies have shown that kanglaite has anticancer effects, particularly for gastric, lung, and liver cancer. This herbal medicine has undergone clinical trials and is now used in combination with conventional therapy to improve patient quality of life12–14.
Astragaloside IV (AS-IV) is isolated from a Chinese herb, Astragalus mongholicus. It is known to have a variety of immunomodulatory activities and has been widely used to regulate the immune system and provide neuroprotection15–18. It has previously been shown that AS-IV can inhibit the invasion and migration of malignant pulmonary cells via regulation of Treg cells and the PKC-α-ERK1/2-NF-κB axis19,20. The goal of the present study was to investigate the potential biological activities of AS-IV and to better understand the key signaling pathways involved in NSCLC.
MATERIALS AND METHODS
Cell Culture and Reagents
HCC827, A549, and NCI-H1299 human NSCLC cell lines were obtained from ATCC (Manassas, VA, USA). AS-IV was obtained from Sigma-Aldrich (St. Louis, MO, USA). Cells were cultured in DMEM containing 10% FBS, under 5% CO2 conditions at 37°C for 24 h.
Evaluation of Cell Growth and Proliferation
A CCK-8 assay was performed to evaluate cell growth and proliferation. Briefly, the culture media was supplemented with 100 μl of CCK-8 liquid, and cells were incubated for 4 h at 37°C. Treatment was terminated at predetermined time points. A multiwell spectrophotometer (Bio-Rad; Hercules, CA, USA) at 490 nm was used to evaluate absorbance at 450 nm.
Flow Cytometry
Cells were washed with ice-cold PBS and then centrifuged at 1,000 rpm for 5 min. The supernatant was removed, and binding buffer was added to the cell suspension. Cells were stained with FITC-annexin V and propidium iodide (PI) for 10 min at room temperature. A FACS scan flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) was used to analyze the fluorescent signals, and FlowJo software version 7.6 was used to estimate the rate of cell death.
Scratch Test
Cells were plated onto six-well plates (cell number: 4 × 105 cells/well), and trypsin was used to digest the cells. Scratches were made in the cell monolayer using a sterile pipet tip (10 μl). Cells were washed with amicrobic PBS to eliminate detached cells, and the medium was replaced with serum-free medium. Scratch distance was measured and documented immediately and after 24 h.
Western Blot Analysis
Cell lysates were prepared using lysis buffer, and the Bradford assay (Bio-Rad) was performed to quantify the amount of cellular proteins, which were then subjected to SDS-PAGE using 8%–15% polyacrylamide gels (Bio-Rad). Proteins were subsequently transferred to a PVDF membrane (Millipore, Bedford, MA, USA). Membranes were blocked with 5% BSA and then incubated overnight at 4°C with specific primary antibodies diluted in TBST: anti-Bax (5023), anti-phospho-AKT (4060), anti-phospho-GSK (9323), anti-phospho-β-catenin (4176), anti-β-actin (3700), anti-Bcl2 (3498), anti-caspase 3 (9664), anti-AKT (4691), anti-GSK (12456), and anti-β-catenin (8480) (Cell Signaling Technology, Beverly, MA, USA). The membranes were incubated with the appropriate secondary antibodies following washing. Bands were detected using the Pierce detection reagent (Pierce, Rockford, IL, USA) and analyzed by Chemiluminescence Imaging System (Ultra-Lum, Claremont, CA, USA).
Statistical Analysis
Results are presented as mean ± SEM. Two-tailed Student’s t-test or ANOVA before Tukey’s post hoc analysis was performed to determine the differences between groups. Statistical significance was defined as a value of p < 0.05.
RESULTS
AS-IV Inhibits NSCLC Cell Proliferation
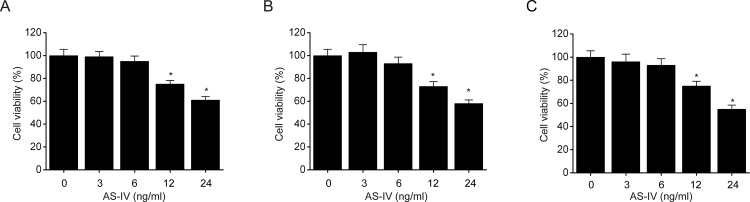
A CCK-8 assay was performed to investigate the cytotoxic effects of AS-IV on NSCLC cells. Three different human NSCLC cell lines, A549, HCC827, and NCI-H1299, were treated with AS-IV. We found that AS-IV (12 and 24 ng/ml) significantly inhibited the proliferation of A549 (Fig. 1A), NCIH1299 (Fig. 1B), and HCC827 (Fig. 1C) cells. These results show that AS-IV is able to prohibit NSCLC cell survival.
Figure 1.
Astragaloside IV (AS-IV) inhibits non-small cell lung cancer (NSCLC) cell proliferation. NSCLC cell lines A549 (A), NCI-H1299 (B), and HCC827 (C) were incubated for 48 h with different amounts of AS-IV (3, 6, 12, and 24 ng/ml). Cell survival was measured using a CCK-8 assay. Results are presented as mean ± SEM of three independent experiments. *p < 0.05, compared to the control group.
AS-IV Enhances NSCLC Cell Death
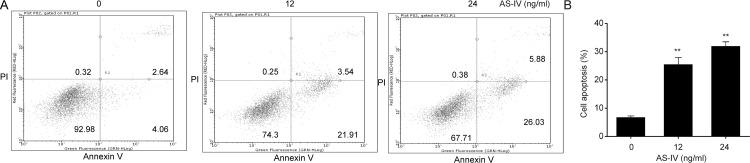
To determine the role of AS-IV in NSCLC cell death, flow cytometry was performed to evaluate annexin V-FITC/PI double staining in the three NSCLC cell lines. We found that AS-IV (12 and 24 ng/ml) significantly induced apoptosis in A549 cell lines (Fig. 2)
Figure 2.
AS-IV enhances NSCLC cell death. A549 cells were incubated with AS-IV (12, and 24 ng/ml) for 48 h. (A, B) Flow cytometric analysis of apoptosis. Results are presented as mean ± SEM of three independent experiments. **p < 0.01, compared to the control group.
AS-IV Inhibits NSCLC Cell Migration
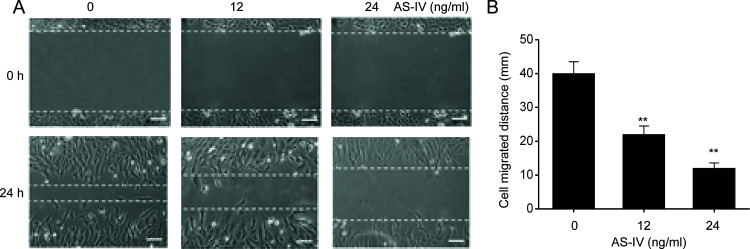
We next determined the effect of AS-IV on NSCLC cell migration using the well-established scratch test. Migration was noticeably reduced in A549 cells supplemented with AS-IV compared to controls (Fig. 3). These findings show that AS-IV suppresses the migration of NSCLC cells.
Figure 3.
AS-IV suppresses migration of NSCLC cells. A549 cells were treated with AS-IV (12 and 4 ng/ml) for 24 h. (A) Scratch test was performed to assess cell migration. (B) Cell migration distance. Results are presented as mean ± SEM of three independent experiments. **p < 0.01, compared to the control group.
AS-IV Regulates the Expression of Bcl-2 and Bax in NSCLC Cells
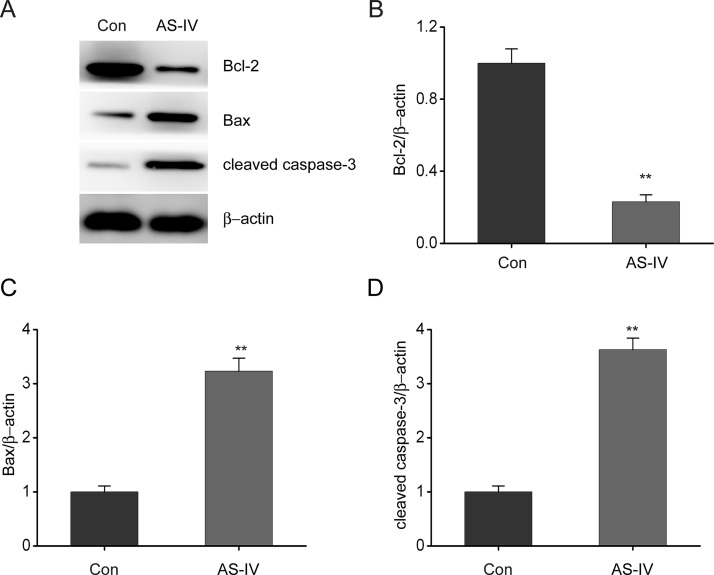
Western blotting was performed to determine the effect of AS-IV on Bcl-2 and Bax expression in NSCLC cells. We found that AS-IV treatment significantly reduced Bcl-2 expression but promoted Bax expression in NSCLC cells, compared to the control group (Fig. 4A–C). Moreover, AS-IV treatment caused a marked increase in the expression of cleaved caspase 3 compared to the control group (Fig. 4D). These results show that AS-IV enhances the expression of proteins that are related to NSCLC cell death.
Figure 4.
AS-IV regulates expression of Bax, Bcl2, and caspase 3 in NSCLC cells. A549 cells were treated with AS-IV (24 ng/ml) for 24 h. (A–D) Representative immunoblots (A), Bcl-2 (B), Bax (C), and cleaved caspase 3 expression (D) in A549 cells. Results are presented as mean ± SEM of three independent experiments. **p < 0.01, compared to the control group.
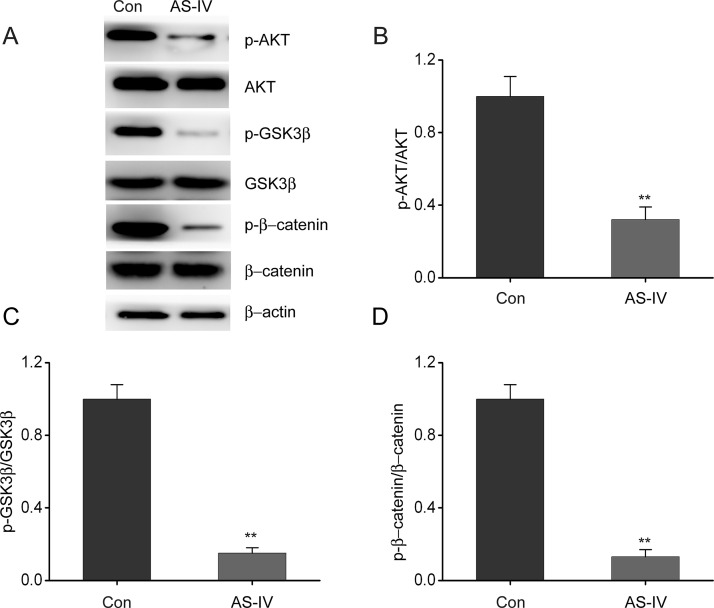
AS-IV Inhibits Stimulation of the Akt/GSK-3β/β-Catenin Axis in NSCLC Cells
Previous studies have shown that the Akt/GSK-3β/β-catenin axis is involved in cell growth and proliferation in a wide range of human cancers21. In order to assess the underlying mechanisms by which AS-IV was exerting its cytotoxic effects, we performed Western blot analysis to examine the expression of components of the Akt/GSK-3β/β-catenin axis. AS-IV treatment resulted in significant inhibition of the phosphorylation of β-catenin, GSK-3β, and Akt (Fig. 5). These findings suggest that AS-IV inhibits NSCLC progression by suppressing stimulation of the Akt/GSK-3β/β-catenin axis.
Figure 5.
AS-IV inhibits stimulation of the Akt/GSK-3β/β-catenin axis in NSCLC cells. A549 cells were treated with AS-IV (24 ng/ml) for 24 h. (A–D) Representative immunoblots (A), p-AKT (B), p-GSK-3β (C), and p-β-catenin (D) expression in A549 cells. Results are presented as mean ± SEM of three independent experiments. **p < 0.01, compared to the control group.
DISCUSSION
The major finding of the present study is that AS-IV inhibits NSCLC growth and proliferation via inhibition of the Akt/GSK-3β/β-catenin axis. AS-IV prevents stimulation of the Akt/GSK-3β/β-catenin axis, which promotes Bax expression and suppresses Bcl2 expression. AS-IV also enhances caspase 3 stimulation, ultimately leading to cell death. Moreover, with increased concentrations, AS-IV shows significant effect on the suppression of cell migration.
Cytotoxic chemotherapy has been the main systemic approach used to treat advanced, metastatic NSCLC. Nevertheless, drug resistance, caused by extensive use, has made chemotherapy difficult and often unsuccessful22–24. Traditional Chinese medicine has been widely utilized due to its promising ability to treat inflammatory disorders25, allergic responses26, and malignancies27. Previous studies have shown that AS-IV is effective at relieving inflammatory reactions, as well as treating cardiovascular illnesses and promoting sensitivity to drugs with respect to malignant cells, via modulation of intracellular signaling pathways16,28. In the present study, we showed that AS-IV inhibits cell growth, proliferation, and migration, and is able to induce NSCLC cell death. Moreover, Bcl-2 proteins participate in the regulation of cell death, which is dependent on mitochondria. Upstream of mitochondria, Bax promotes cell death while Bcl-2 suppresses cell death. The comparative ratio of the two proteins (Bax/Bcl2) is crucial to the stimulation of cell death29. Consequently, we found that AS-IV suppressed Bcl-2 expression while promoting Bax expression, which then results in caspase 3 cleavage leading to activation of cell death.
The class I PI3K/Akt signaling axis mediates several key cellular processes, including cell viability, migration, and cell cycle progression30. PI3K/Akt malfunction enhances the development of malignancies, as well as vascularization of numerous tumors31,32. Previous studies have shown that AKT stimulates GSK3β phosphorylation, which triggers the nuclear aggregation of β-catenin, which is related to TCF4. This then stimulates the expression of various transcription factors linked to EMT, such as Twist1, ZEB1, and SNAI133. GSK-3β also participates in preventing malignancy development by cytoplasmic phosphorylation as well as degeneration of β-catenin via the Akt pathway34. There is increasing evidence to suggest that β-catenin is upregulated in NSCLC tumor cells when compared to normal cells. It has been shown that activation of the β-catenin pathway stimulates proliferation and leads to the generation and development of NSCLC35,36. In the present study, we showed that AS-IV inhibited the Akt phosphorylation and subsequent GSK-3β function, which in turn stimulated the ubiquitination of β-catenin. These findings demonstrate that AS-IV can promote β-catenin phosphorylation modulated via GSK-3β. The phosphorylation of β-catenin causes it to become unstable and consequently reduces its nuclear aggregation. β-Catenin-regulated transcription is crucial for cell proliferation, as well as the progression of NSCLC.
In summary, our research shows that the biological effects of AS-IV in NSCLC are mediated via inhibition of the Akt/GSK-3β/β-catenin signaling pathway. Although further in vivo animal studies are required to confirm its in vitro preclinical cytotoxicity, our findings suggest that AS-IV is a promising novel agent for the treatment of NSCLC.
ACKNOWLEDGMENT
This work was supported by Heilongjiang University of Chinese Medicine PhD Innovation Fund (No. 2015bs05).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Kwon HW, Ham SY, Shin SW. Advanced non-small-cell lung cancer. N Engl J Med. 2017;377(20):1997–8. [DOI] [PubMed] [Google Scholar]
- 2. Sohal SS, Walters EH. Advanced non-small-cell lung cancer. N Engl J Med. 2017;377(20):1998–9. [DOI] [PubMed] [Google Scholar]
- 3. Reck M, Rabe KF. Advanced non-small-cell lung cancer. N Engl J Med. 2017;377(20):1999. [DOI] [PubMed] [Google Scholar]
- 4. Chuang MC, Yang YH, Tsai YH, Hsieh MJ, Lin YC, Lin CK, Chen PC, Yang TM. Survival benefit associated with metformin use in inoperable non-small cell lung cancer patients with diabetes: A population-based retrospective cohort study. PLoS One 2018;13(1):e0191129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang S, Gao A, Liu J, Sun Y. First-line therapy for advanced non-small cell lung cancer with activating EGFR mutation: Is combined EGFR-TKIs and chemotherapy a better choice? Cancer Chemother Pharmacol. 2018;81(3):443–53. [DOI] [PubMed] [Google Scholar]
- 6. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, Felip E, Zeron-Medina J, Garrido P, Brosseau S, Zalcman G, Mazieres J, Caramela C, Lahmar J, Adam J, Chaput N, Soria JC, Besse B. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller ED, Fisher JL, Haglund KE, Grecula JC, Xu-Welliver M, Bertino EM, He K, Shields PG, Carbone DP, Williams TM, Otterson GA, Bazan JG. The addition of chemotherapy to radiation therapy improves survival in elderly patients with stage III non-small cell lung cancer. J Thorac Oncol. 2018;13(3):426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guibert N, Hu Y, Feeney N, Kuang Y, Plagnol V, Jones G, Howarth K, Beeler JF, Paweletz CP, Oxnard GR. Amplicon-based next-generation sequencing of plasma cell-free DNA for detection of driver and resistance mutations in advanced non-small cell lung cancer. Ann Oncol. 2018;29(4):1049–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wangari-Talbot J, Hopper-Borge E. Drug resistance mechanisms in non-small cell lung carcinoma. J Can Res Updates 2013;2(4):265–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu S, Yang H, Ge X, Su L, Zhang A, Liang L. Drug resistance analysis of gefitinib-targeted therapy in non-small cell lung cancer. Oncol Lett. 2016;12(5):3941–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao X, Tang RX, Xie QN, Lin JY, Shi HL, Chen G, Li ZY. The clinical value of miR-193a-3p in non-small cell lung cancer and its potential molecular mechanism explored in silico using RNA-sequencing and microarray data. FEBS Open Biol. 2018;8(1):94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiang M, Li R, Zhang Z, Song X. [Advances in the research of the regulation of Chinese traditional medicine monomer and its derivatives on autophagy in non-small cell lung cancer]. Zhongguo Fei Ai Za Zhi 2017;20(3):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang CY, Ju DT, Chang CF, Muralidhar RP, Velmurugan BK. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. Biomedicine (Taipei) 2017;7(4):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samarakoon SR, Ediriweera MK, Nwokwu CDU, Bandara CJ, Tennekoon KH, Piyathilaka P, Karunaratne DN, Karunaratne V. A study on cytotoxic and apoptotic potential of a triterpenoid saponin (3-O-alpha-L-arabinosyl oleanolic acid) isolated from Schumacheria castaneifolia Vahl in human non-small-cell lung cancer (NCI-H292) cells. Biomed Res Int. 2017;2017:9854083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Wang X, Zheng D, Lin X, Wei Z, Zhang D, Li Z, Zhang Y, Wu M, Liu X. Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy. Biomater Sci. 2018;6(7):1834–45. [DOI] [PubMed] [Google Scholar]
- 16. Tang B, Zhang JG, Tan HY, Wei XQ. Astragaloside IV inhibits ventricular remodeling and improves fatty acid utilization in rats with chronic heart failure. Biosci Rep. 2018;38(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li H, Wang P, Huang F, Jin J, Wu H, Zhang B, Wang Z, Shi H, Wu X. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol Appl Pharmacol. 2018;340:58–66. [DOI] [PubMed] [Google Scholar]
- 18. Xia L, Guo D, Chen B. Neuroprotective effects of astragaloside IV on Parkinson disease models of mice and primary astrocytes. Exp Ther Med. 2017;14(6):5569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang A, Zheng Y, Que Z, Zhang L, Lin S, Le V, Liu J, Tian J. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J Cancer Res Clin Oncol. 2014;140(11):1883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng X, Gu J, Zhang M, Yuan J, Zhao B, Jiang J, Jia X. Astragaloside IV inhibits migration and invasion in human lung cancer A549 cells via regulating PKC-alpha-ERK1/2-NF-kappaB pathway. Int Immunopharmacol. 2014;23(1):304–13. [DOI] [PubMed] [Google Scholar]
- 21. Pei R, Si T, Lu Y, Zhou JX, Jiang L. Salvianolic acid A, a novel PI3K/Akt inhibitor, induces cell apoptosis and suppresses tumor growth in acute myeloid leukemia. Leuk Lymphoma 2017;1–9. [DOI] [PubMed] [Google Scholar]
- 22. Liu Z, Bao Y, Li B, Sun X, Wang L. Does ALK-rearrangement predict favorable response to the therapy of bevacizumab plus pemetrexed in advanced non-small-cell lung cancer? Case report and literature review. Clin Transl Med. 2018;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khozin S, Abernethy AP, Nussbaum NC, Zhi J, Curtis MD, Tucker M, Lee SE, Light DE, Gossai A, Sorg RA, Torres A, Patel P, Blumenthal GM, Pazdur R. Characteristics of real-world metastatic non-small cell lung cancer patients treated with nivolumab and pembrolizumab during the year following approval. Oncologist 2018;23(3):328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito M, Horita N, Nagashima A, Kaneko T. Carboplatin plus pemetrexed for the elderly incurable chemo-naive nonsquamous non-small cell lung cancer: Meta-analysis. Asia Pac J Clin Oncol. 2018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25. Tsai WH, Yang CC, Li PC, Chen WC, Chien CT. Therapeutic potential of traditional Chinese medicine on inflammatory diseases. J Tradit Complement Med. 2013;3(3):142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang RX, You RL, Wang L, Lei L, Wang Z. In vitro experiment of allergic reactions induced by traditional Chinese medicine injections. Zhongguo Zhong Yao Za Zhi 2015;40(13):2503–7. [PubMed] [Google Scholar]
- 27. Lei X, Chen M, Huang M, Li X, Shi C, Zhang D, Luo L, Zhang Y, Ma N, Chen H, Liang H, Ye W, Zhang D. Desacetylvinblastine monohydrazide disrupts tumor vessels by promoting VE-cadherin internalization. Theranostics 2018;8(2):384–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li C, Yang F, Liu F, Li D, Yang T. NRF2/HO-1 activation via ERK pathway involved in the anti-neuroinflammatory effect of astragaloside IV in LPS induced microglial cells. Neurosci Lett. 2018;666:104–10. [DOI] [PubMed] [Google Scholar]
- 29. Fan L, Tan B, Li Y, Zhao Q, Yuan H, Liu Y, Wang D, Zhang Z. Upregulation of miR185 promotes apoptosis of the human gastric cancer cell line MGC803. Mol Med Rep. 2018;17(2):3115–22. [DOI] [PubMed] [Google Scholar]
- 30. Tang SL, Gao YL, Wen-Zhong H. Knockdown of TRIM37 suppresses the proliferation, migration and invasion of glioma cells through the inactivation of PI3K/Akt signaling pathway. Biomed Pharmacother. 2018;99:59–64. [DOI] [PubMed] [Google Scholar]
- 31. Sun K, Wang S, He J, Xie Y, He Y, Wang Z, Qin L. NCOA5 promotes proliferation, migration and invasion of colorectal cancer cells via activation of PI3K/AKT pathway. Oncotarget 2017;8(64):107932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu J, Zhang L, Chen Q, Lin J, Wang S, Liu R, Zhang W, Miao K, Shou T. Knockdown of CPEB4 expression suppresses cell migration and invasion via Akt pathway in non-small cell lung cancer. Cell Biol Int. 2018;42(11):1484–91. [DOI] [PubMed] [Google Scholar]
- 33. Liu L, Zhou XM, Yang FF, Miao Y, Yin Y, Hu XJ, Hou G, Wang QY, Kang J. TRIM22 confers poor prognosis and promotes epithelial-mesenchymal transition through regulation of AKT/GSK3beta/beta-catenin signaling in non-small cell lung cancer. Oncotarget 2017;8(37):62069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leung N, Turbide C, Balachandra B, Marcus V, Beauchemin N. Intestinal tumor progression is promoted by decreased apoptosis and dysregulated Wnt signaling in Ceacam1-/- mice. Oncogene 2008;27(36):4943–53. [DOI] [PubMed] [Google Scholar]
- 35. Chandrasekaran B, Tyagi A, Sharma AK, Cai L, Ankem M, Damodaran C. Molecular insights: Suppression of EGFR and AKT activation by a small molecule in non-small cell lung cancer. Genes Cancer 2017;8(9–10):713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang B, Tang Z, Gong H, Zhu L, Liu X. Wnt5a promotes epithelial-to-mesenchymal transition and metastasis in non-small-cell lung cancer. Biosci Rep. 2017;37(6). [DOI] [PMC free article] [PubMed] [Google Scholar]