Abstract
miR-363-3p has been shown to suppress tumor growth and metastasis in various human cancers. However, the function of miR-363-3p in osteosarcoma (OS) has not been determined. In our study, we found that the expression of miR-363-3p was significantly downregulated in OS tissues compared with adjacent normal tissues. miR-363-3p expression was associated with the poor overall survival rate of OS patients. Moreover, we found that overexpression of miR-363-3p markedly inhibited the proliferation, migration, and invasion of U2OS and MG63 cells. Moreover, we found that SOX4 was a direct target of miR-363-3p in OS cells. Overexpression of miR-363-3p significantly inhibited the expression of SOX4. Expression levels of miR-363-3p and SOX4 were negatively correlated in OS tissues. Finally, we found that restoration of SOX4 attenuated the suppressive effects of miR-363-3p on the proliferation, migration, and invasion of U2OS and MG63 cells. Therefore, our findings demonstrated that miR-363-3p served as a tumor suppressor in OS tissues by targeting SOX4.
Key words: miR-363-3p, Proliferation, Invasion, SOX4, Osteosarcoma (OS)
INTRODUCTION
Osteosarcoma (OS) is one of the most common and malignant bone tumors among children and adolescents and contributes to a large number of cancer-related deaths worldwide1–3. Although OS patients have some benefit from intensive chemotherapy combined with aggressive surgical techniques, the 5-year overall survival rate is still very low due to tumor recurrence and metastasis4,5. In the past few decades, a lot of studies have tried to explore the pathogenesis of OS. However, the underlying molecular mechanism still remains elusive. There is an urgent need to develop novel and effective diagnostic and therapeutic strategies for OS.
MicroRNAs (miRNAs) are a class of noncoding RNAs 18–25 nucleotides in length that can regulate gene expression by targeting the 3′-UTR of their specific target mRNAs6. miRNAs have been reported to serve as pivot regulators in the genesis of various human cancers, such as colorectal cancer, gastric cancer, and OS7,8. Through regulating cellular proliferation, migration, invasion, and apoptosis, miRNAs can act as oncogenes or tumor suppressors. For example, Cai et al. reported that miR-504 enhances the growth and metastasis of OS by targeting tumor protein p53 inducible nuclear protein 1 (TP53INP1)9. In addition, Yuan and colleagues demonstrated that miR-494 inhibits cell proliferation and metastasis via targeting of cyclin-dependent kinase 6 (CDK6) in OS10. This accumulating evidence demonstrates that miRNAs may be promising biomarkers and therapeutic targets for cancer diagnosis and treatment. Therefore, it is of great importance to define the functions of key miRNAs in cancers.
miR-363-3p has been shown to inhibit tumor growth in several types of cancers, including lung adenocarcinoma, papillary thyroid carcinoma, and colorectal cancer11–13. Nevertheless, the biological functions of miR-363-3p and its underlying mechanism in OS remain largely unknown. In our study, we found that the expression of miR-363-3p was upregulated in OS tissues and cell lines. The expression of miR-363-3p is negatively correlated with the prognosis of OS patients. Moreover, we found that overexpression of miR-363-3p significantly inhibited the proliferation, migration, and invasion of OS cells by directly targeting sex-determining region Y box 4 (SOX4). Our findings suggest that miR-363-3p might be a promising therapeutic target for OS treatment.
MATERIALS AND METHODS
Clinical Specimens and Cell Lines
Human OS tumor samples were obtained from 41 patients at the Jingzhou Central Hospital (Hubei Province, P.R. China). The identities of all tumor samples were confirmed by an experienced pathologist. This study was approved by the Research Ethics Committee of Jingzhou Central Hospital. Informed consent was obtained from all subjects participating in the study. Tissue samples were collected at surgery, immediately frozen in liquid nitrogen, and stored until total RNAs were extracted.
Human OS cell lines KHOS, U2OS, and MG-63 and the normal osteoblast cell NHOst were purchased from the Type Culture Collection of the Chinese Academy of Sciences. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL, Carlsbad, CA, USA). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air.
Oligonucleotide and Transfection
miR-363-3p mimic and corresponding negative control mimic (miR-NC) were purchased from GenePharma (Guangzhou, P.R. China). This plasmid was transfected into OS cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Cells were collected 48 h after transfection, and the relative expression levels were evaluated by reverse transcription and quantitative real-time PCR (RT-qPCR).
Cell Proliferation Assay
Cells (3,000 cells per well) were seeded onto 96-well plates and incubated in corresponding medium supplemented with 10% FBS. After the indicated incubation times (24, 48, and 72 h), we added cell counting kit-8 (CCK-8) solution (Dojindo, Japan) into each well, followed by 1–2 h of incubation. The absorbance value at 450 nm was then measured. Experiments were carried out in triplicate.
Colony Formation Assay
For the colony formation ability assay, OS cells were counted at 24 h posttransfection and seeded into 24-well plates at 400 cells per well. Culture medium was replaced every 3 days. After approximately 14 days, cells were cleaned with 1× PBS, stained with common crystal violet dye, and counted using an inverted microscope (IX83; Olympus Corporation, Tokyo, Japan).
In Vitro Migration and Invasion Assays
The migration and invasion abilities of the two types of OS cells were evaluated by a Transwell assay (24-well insert, 8-mm pore size with polycarbonate membrane; Corning Costar, Lowell, MA, USA). Briefly, transfected OS cells (1 × 105 cells/chamber) were suspended in 200 μl of serum-free DMEM and seeded into the upper chamber of Boyden chambers that were coated with (for invasion assay) or without (for migration assay) Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA). DMEM with 20% FBS was added in the lower chamber as a chemoattractant. After 24 h, the cells in the top chambers were removed with a cotton swab, washed with PBS, and the bottom chambers were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet, and counted using a phase-contrast microscope (Olympus) at randomly selected five fields.
Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
RNA was isolated from harvested cells or human tissues with TRIzol reagent (Invitrogen) according to the manufacturer’s instruction. To measure the expression levels of miR-363-3p, a stem-loop-specific primer method was used. Expression of U6 was used as an endogenous control. To determine the mRNA levels of SOX4, total RNAs were reverse transcribed using the RT Reagent Kit (Vazyme, Nanjing, P.R. China). GAPDH was used as an internal control. The cDNAs were amplified by RT-qPCR using SYBR Green Master Mix (Vazyme) on a 7900HT system.
Dual-Luciferase Reporter Assay
TargetScan7.1 (www.targetscan.org) was used to predict the putative target genes of miR-363-3p. Wild-type (Wt) or mutant (Mut) 3′-UTR sequence of SOX4 that contains the miR-363-3p-binding site was cloned into the pGL3 dual-luciferase expression vector (Promega, Madison, WI, USA). The OS cells (1 × 105 cells/well) were seeded into 96-well plates and cotransfected with Wt or Mut SOX4-3′-UTR reporter plasmid and miR-363-3p mimic or miR-NC using Lipofectamine 2000. After 48 h of transfection, cell lysates were harvested, and firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity.
Western Blot
Protein extracts were obtained by lysis buffer, and protein concentrations were measured using a BCA Protein Assay Kit (Beyotime Biotechnology, Haimen, P.R. China). Proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes (Millipore). The membranes were blocked in 5% defatted milk for 1 h and then incubated with primary antibodies overnight at 4°C. Afterward, membranes were probed with horseradish peroxidase-linked secondary antibodies (1:2,000; Abcam) for 1 h. Protein bands were detected using an ECL Plus Western Blotting System (Thermo Fisher Scientific, Waltham, MA, USA). Band intensity was analyzed by Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical Analysis
All experiments were performed three times, and data were analyzed with GraphPad Prism 5 (La Jolla, CA, USA). The correlation between miR-363-3p expression and SOX4 levels in OS tissues was analyzed using Spearman’s rank test. Statistical evaluation for data analysis was determined by t-test or one-way analysis of variance. The differences were considered to be statistically significant at p < 0.05.
RESULTS
miR-363-3p Was Upregulated in OS Tissues and Cell Lines
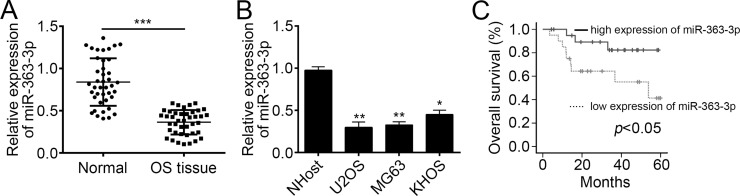
To check the function of miR-363-3p, we first analyzed its expression patterns in 41 pairs of OS tissues and adjacent normal tissues using RT-qPCR. The results indicated that miR-363-3p was significantly downregulated in OS tissues compared with adjacent normal tissues (Fig. 1A). Additionally, we also analyzed the expression of miR-363-3p in OS cell lines (KHOS, U2OS, and MG63 cells) and the normal osteoblast cell NHOst by RT-qPCR. We found that the expression of miR-363-3p was markedly downregulated in OS cell lines compared to normal cells (Fig. 1B). Then we divided these samples into miR-363-3p lowly or highly expressed group based on the median value, followed by Kaplan–Meier analysis. The results indicated that low expression of miR-363-3p in OS patients was associated with poor survival rate (Fig. 1C). Therefore, our results implied that miR-363-3p could serve as a prognostic marker and may play a key role in OS progression.
Figure 1.
MicroRNA-363-3p (miR-363-3p) was upregulated in osteosarcoma (OS) tissues and cell lines. (A) Relative expression of miR-363-3p in OS tissues and adjacent normal tissues . (B) Relative expression of miR-363-3p in OS cell lines (U2OS, MG63, and KHOS) and the osteoblast cell line NHOst. (C) The Kaplan–Meier curves of miR-363-3p expression levels. Patients with low miR-363-3p expression showed a poor overall survival compared to those patients with high miR-363-3p expression (log-rank test; p < 0.05). All data are representative of three independent experiments and expressed as mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001.
Overexpression of miR-363-3p Inhibited Cellular Proliferation of OS Cells
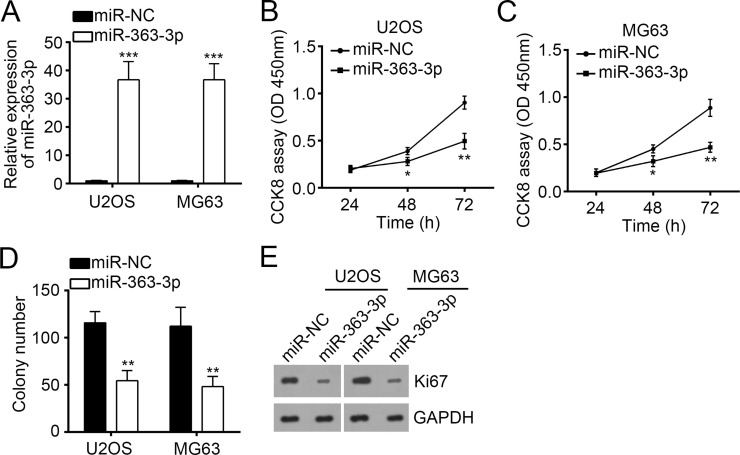
To further explore the role of miR-363-3p in OS, we overexpressed miR-363-3p using a specific mimic in OS cell lines (U2OS and MG63 cells). RT-qPCR data showed that transfection with miR-363-3p mimic effectively increased miR-363-3p expression in U2OS and MG63 cells compared to the cells transfected with miR-NC (Fig. 2A). Then we used these cells to perform CCK-8 and colony formation assays for the evaluation of cellular proliferation ability. The results demonstrated that overexpression of miR-363-3p significantly impaired the cell proliferation ability and colony formation of U2OS and MG63 cells (Fig. 2B–D). Moreover, overexpression of miR-363-3p significantly decreased the expression of Ki-67 in U2OS and MG63 cells (Fig. 2E).
Figure 2.
Overexpression of miR-363-3p inhibited cellular proliferation of OS cells. (A) Reverse transcription and quantitative real-time PCR (RT-qPCR) analysis of miR-363-3p expression in U2OS and MG63 cells transfected with miR-363-3p mimic or miR-NC. (B, C) Cell proliferation curves in U2OS and MG63 cells transfected with miR-363-3p mimic or miR-NC by cell counting kit-8 (CCK-8) assay. (D) Cell proliferation ability was assessed by colony formation assay with U2OS and MG63 cells transfected with miR-363-3p mimic or miR-NC. (E) Western blot analysis of Ki-67 expression in U2OS and MG63 cells transfected with miR-363-3p mimic or miR-NC. GAPDH was used as control. All data are representative of three independent experiments and expressed as mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001.
Overexpression of miR-363-3p Suppressed Cell Migration and Invasion in OS Cells
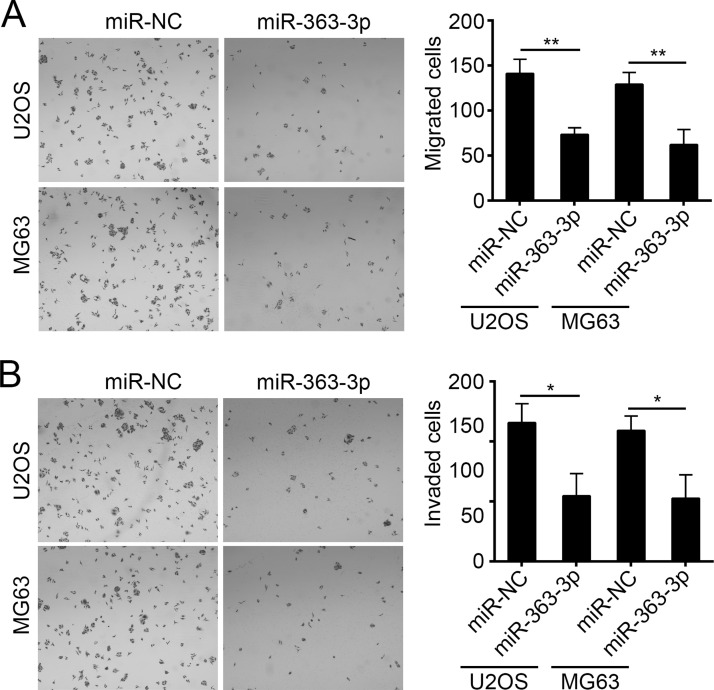
Tumor metastasis is the main cause for poor prognosis of patients. We then investigated the effect of miR-363-3p on cell migration and invasion by Transwell assay. Our results showed that overexpression of miR-363-3p markedly inhibited the number of U2OS and MG63 cells that passed through the chambers (Fig. 3A and B), which suggested that miR-363-3p inhibited OS cell migration and invasion.
Figure 3.
Overexpression of miR-363-3p suppressed cell migration and invasion in OS cells. (A, B) Cell migration and invasion were determined in U2OS and MG63 cells transfected with miR-363-3p mimic or miR-NC by Transwell assay, respectively. All data are representative of three independent experiments and expressed as mean ± SD. *p < 0.05 and **p < 0.01.
SOX4 Was a Target of miR-363-3p in OS Cells
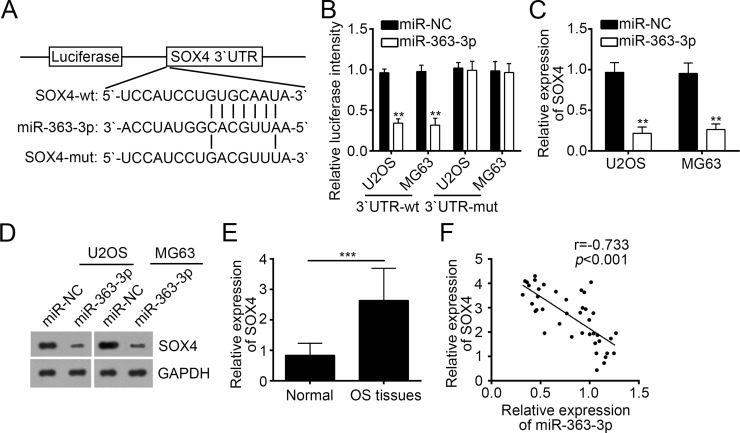
To further explore the mechanism involved in miR-363-3p-regulated OS progression, we analyzed the putative targets of miR-363-3p by a prediction program of TargetScan 7.1. We found that SOX4, a key transcription factor involved in the development of a diversity of tumors, was a potential target of miR-363-3p. There was a potential binding site in the 3′-UTR region of SOX4 mRNA (Fig. 4A). To determine whether the putative binding site has a functional relevance, we performed dual-luciferase reporter assays with U2OS and MG63 cells. Results showed that overexpression of miR-363-3p significantly inhibited the luciferase activity in U2OS and MG63 cells transfected with 3′-UTR-Wt luciferase plasmid (Fig. 4B). However, mutation of this putative binding site abrogated the inhibitory effect by miR-363-3p (Fig. 4B). Moreover, overexpression of miR-363-3p significantly inhibited the mRNA and protein levels of SOX4 in U2OS and MG63 cells (Fig. 4C and D). The above results indicated that SOX4 was a direct target gene of miR-363-3p in OS cells. We then analyzed the expression of SOX4 in OS tissues and found that SOX4 was highly expressed in tumor tissues compared with adjacent normal tissues (Fig. 4E). Notably, we found that there was an inverse correlation between expression levels of miR-363-3p and SOX4 in OS tissues (Fig. 4F).
Figure 4.
Sex-determining region Y box 4 (SOX4) was a target of miR-363-3p in OS cells. (A) Predicted binding site for miR-363-3p in the 3′-UTR of the SOX4 mRNA and the mutation were introduced to this site. (B) Relative luciferase activity was determined in U2OS and MG63 cells cotransfected with SOX4-3′-UTR reporter plasmids (wild type or mutant) and miR-363-3p mimic or miR-NC. (C, D) SOX4 mRNA and protein expression levels in U2OS and MG63 cells transfected with miR-363-3p mimic or miR-NC were detected by RT-qPCR and Western blot, respectively. (E) The expression of SOX4 was negatively associated with the expression of miR-363-3p expression in OS tissues. (F) The SOX4 mRNA expression in the OS tissues and the adjacent nontumor tissues was measured by RT-qPCR. All data are representative of three independent experiments and expressed as mean ± SD. **p < 0.01 and ***p < 0.001.
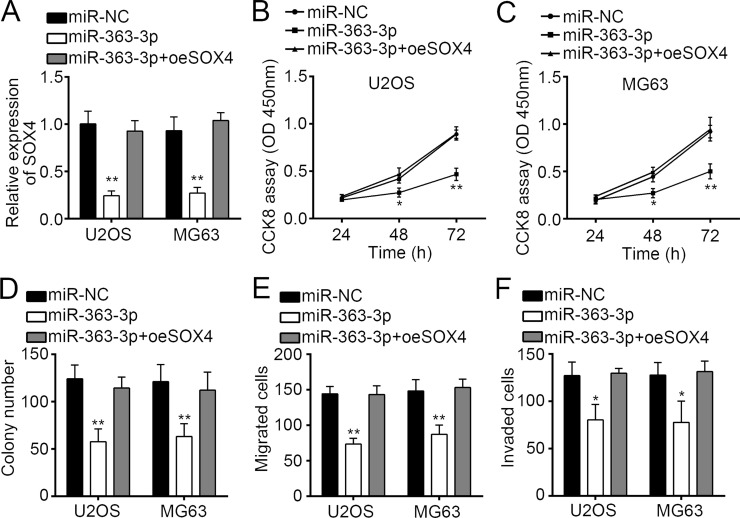
miR-363-3p Suppressed Proliferation, Migration, and Invasion of OS Cells by Targeting SOX4
To determine if SOX4 is responsible for the functional effects of miR-363-3p in OS cells, we restored the expression of SOX4 in miR-363-3p overexpressing U2OS and MG63 cells by transduction with an SOX4-overexpressing plasmid. The results indicated that transfection of the SOX4 plasmid restored its mRNA expression (Fig. 5A). Then we performed CCK-8, colony formation, and Transwell assays. As shown, restoration of SOX4 in miR-363-3p-overexpressing U2OS and MG63 cells reversed the miR-363-3p-mediated inhibitory effects on cellular proliferation, migration, and invasion (Fig. 5B–F). These findings suggested that miR-363-3p exerts a suppressive effect on OS progression, at least in part, by targeting SOX4.
Figure 5.
miR-363-3p suppressed proliferation, migration, and invasion of OS cells by targeting SOX4. (A) SOX4 mRNA levels in U2OS and MG63 cells transfected with MiR-363-3p mimic as well as SOX4 overexpressing plasmids (pCDNA3.1-SOX4) or control. (B–F) Cell proliferation, migration, and invasion activities of U2OS and MG63 cells transfected with miR-363-3p mimic as well as SOX4 overexpressing plasmids were determined by CCK-8, colony formation, and Transwell assays. All data are representative of three independent experiments and expressed as mean ± SD. *p < 0.05 and **p < 0.01.
DISCUSSION
miRNAs have been shown to play crucial functions in the development and progression of human OS via targeting specific genes for expression regulation14,15. In the present study, we explored the biological function of miR-363-3p in OS cells. Increasing evidence showed that miR-363-3p had an important role in various human cancers. The dysregulation of miR-363-3p expression often led to the development or progression of cancers. For example, miR-363-3p overexpression was related to the aggression of gastric cancer16 and glioblastoma17. On the contrary, miR-363-3p played a tumor suppressor role in some other cancers, including ovarian cancer18, hepatocellular carcinoma19, lung adenocarcinoma11, papillary thyroid carcinoma12, colorectal cancer13, and head and neck cancer20. These findings indicate that miR-363-3p exerted different roles in different cancers. Until now, the function of miR-363-3p has not been investigated in OS. In the present study, we found that the expression of miR-363-3p was significantly downregulated in OS tissues and cell lines. Moreover, we found that the expression of miR-363-3p was negatively related with the prognosis of OS patients. Higher expression of miR-363-3p indicated better survival rate of OS patients, which suggests that miR-363-3p may be a promising prognostic biomarker. In addition, we found that overexpression of miR-363-3p significantly suppressed the proliferation, migration, and invasion of OS cells. Therefore, our findings demonstrated that miR-363-3p functions as a tumor suppressor in OS development and progression.
It is widely acknowledged that miRNAs could target the 3′-UTR of their target mRNAs to participate in various biological functions6. To determine the mechanism of miR-363-3p-mediated regulation in OS cells, we screened the target genes by TargetScan 7.1. SOX4 was one of the potential targets of miR-363-3p in OS cells. SOX4 belonging to the SOX [SRY-related high-mobility group (HMG)-box] family of transcription factors has been reported to be involved in many biological processes21. SOX4 was shown to regulate cell proliferation, migration, invasion, apoptosis, and epithelial-to-mesenchymal transition in some cancers22,23. In OS, some researchers have also demonstrated its key functions in the regulation of OS progression. For example, Wu et al. reported that upregulation of miR-25-3p inhibits proliferation, migration, and invasion of OS cells in vitro by directly targeting SOX424. Additionally, Liu and colleagues indicated that miR-132 inhibits cell growth and metastasis in OS cell lines possibly by targeting Sox425. Given these reported roles of SOX4, we chose SOX4 among all possible target genes for further studies. By dual-luciferase activity reporter assay, qRT-PCR, and Western blot, we verified that SOX4 was a target gene of miR-363-3p in OS cells. We also showed that there was an inverse correlation between the expression of miR-363-3p and SOX4 in OS tissues. We found that the expression of SOX4 was upregulated in OS tissues. Furthermore, through functional experiments, we found that restoration of SOX4 abolished the suppressive effects of miR-363-3p overexpression on OS cell proliferation, migration, and invasion. Our findings implied that miR-363-3p played its functions in OS, at least in part, via directly targeting SOX4.
In conclusion, our study suggested that miR-363-3p was upregulated in OS tissues and cell lines, and its expression was correlated with the prognosis of OS patients. Moreover, ectopic expression of miR-363-3p suppressed the proliferation, migration, and invasion of OS cell lines via targeting SOX4. These findings suggest that miR-363-3p might be a promising therapeutic target for OS treatment.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Geller DS, Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8(10):705–18. [PubMed] [Google Scholar]
- 3. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. [DOI] [PubMed] [Google Scholar]
- 4. Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis: Clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol. 2009;39(8):514–22. [DOI] [PubMed] [Google Scholar]
- 5. Liu Y, Zhu ST, Wang X, Deng J, Li WH, Zhang P, Liu BS. MiR-200c regulates tumor growth and chemosensitivity to cisplatin in osteosarcoma by targeting AKT2. Sci Rep. 2017;7(1):13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 7. Han Z, Shi L. Long non-coding RNA LUCAT1 modulates methotrexate resistance in osteosarcoma via miR-200c/ABCB1 axis. Biochem Biophys Res Commun. 2018;495(1):947–53. [DOI] [PubMed] [Google Scholar]
- 8. Zeng B, Shi W, Tan G. MiR-199a/b-3p inhibits gastric cancer cell proliferation via down-regulating PAK4/MEK/ERK signaling pathway. BMC Cancer 2018;18(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai QC, Zeng SX, Dai X, Wu JL, Ma W. miR-504 promotes tumour growth and metastasis in human osteosarcoma by targeting TP53INP1. Oncol Rep. 2017;38(5):2993–3000. [DOI] [PubMed] [Google Scholar]
- 10. Yuan W, Wang D, Liu Y, Tian D, Wang Y, Zhang R, Yin L, Deng Z. miR494 inhibits cell proliferation and metastasis via targeting of CDK6 in osteosarcoma. Mol Med Rep. 2017;16(6):8627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Chen T, Huang H, Jiang Y, Yang L, Lin Z, He H, Liu T, Wu B, Chen J, Kamp DW, Liu G. miR-363-3p inhibits tumor growth by targeting PCNA in lung adenocarcinoma. Oncotarget 2017;8(12):20133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu J, Li Q, Li R, Ren PY, Dong S. MicroRNA-363-3p inhibits papillary thyroid carcinoma progression by targeting PIK3CA. Am J Cancer Res. 2017;7(1):148–58. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Hu FY, Min J, Cao XN, Liu L, Ge ZQ, Hu JB, Li XL. MiR-363-3p inhibits the epithelial-to-mesenchymal transition and suppresses metastasis in colorectal cancer by targeting Sox4. Biochem Biophys Res Commun. 2016;474(1):35–42. [DOI] [PubMed] [Google Scholar]
- 14. Kushlinskii NE, Fridman MV, Braga EA. Molecular mechanisms and microRNAs in osteosarcoma pathogenesis. Biochemistry-Moscow 2016;81(4):315–28. [DOI] [PubMed] [Google Scholar]
- 15. Golbakhsh MR, Boddouhi B, Hatami N, Goudarzi PK, Shakeri M, Yahaghi E, Taheriazam A. Down-regulation of microRNA-182 and microRNA-183 predicts progression of osteosarcoma. Arch Med Sci. 2017;13(6):1352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang PF, Sheng LL, Wang G, Tian M, Zhu LY, Zhang R, Zhang J, Zhu JS. miR-363 promotes proliferation and chemo-resistance of human gastric cancer via targeting of FBW7 ubiquitin ligase expression. Oncotarget 2016;7(23):35284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Floyd DH, Zhang Y, Dey BK, Kefas B, Breit H, Marks K, Dutta A, Herold-Mende C, Synowitz M, Glass R, Abounader R, Purow BW. Novel anti-apoptotic microRNAs 582-5p and 363 promote human glioblastoma stem cell survival via direct inhibition of caspase 3, caspase 9, and Bim. PLoS One 2014;9(5):e96239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin Y, Xu T, Zhou S, Cui M. MicroRNA-363 inhibits ovarian cancer progression by inhibiting NOB1. Oncotarget 2017;8(60):101649–58. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Lu YB, Jiang Q, Yang MY, Zhou JX, Zhang Q. Long noncoding RNA NNT-AS1 promotes hepatocellular carcinoma progression and metastasis through miR-363/CDK6 axis. Oncotarget 2017;8(51):88804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun Q, Zhang JJ, Cao W, Xu W, Xu Q, Ming Y, Wu XB, Chen WT. Dysregulated miR-363 affects head and neck cancer invasion and metastasis by targeting podoplanin. Int J Biochem Cell Biol. 2013;45(3):513–20. [DOI] [PubMed] [Google Scholar]
- 21. Xi J, Feng J, Zeng ST. Long noncoding RNA lncBRM facilitates the proliferation, migration and invasion of ovarian cancer cells via upregulation of Sox4. Am J Cancer Res. 2017;7(11):2180–9. [PMC free article] [PubMed] [Google Scholar]
- 22. Tong ZG, Meng XF, Wang JS, Wang LX. MicroRNA-338-3p targets SOX4 and inhibits cell proliferation and invasion of renal cell carcinoma. Exp Therap Med. 2017;14(5):5200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Gao ZM, Han LG, Xu F, Liu K, Shen Y. Long noncoding RNA CASC2 inhibits metastasis and epithelial to mesenchymal transition of lung adenocarcinoma via suppressing SOX4. Eur Rev Med Pharmacol Sci. 2017;21(20):4584–90. [PubMed] [Google Scholar]
- 24. Wu XL, Zhou HZ, Yue B, Li MH, Liu FX, Qiu CS, Chen BH, Ma XX. Upregulation of microRNA-25-3p inhibits proliferation, migration and invasion of osteosarcoma cells in vitro by directly targeting SOX4. Mol Med Rep. 2017;16(4):4293–300. [DOI] [PubMed] [Google Scholar]
- 25. Liu YL, Li Y, Liu JC, Wu YT, Zhu QS. MicroRNA-132 inhibits cell growth and metastasis in osteosarcoma cell lines possibly by targeting Sox4. Int J Oncol. 2015;47(5):1672–84. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]