Abstract
Nasopharyngeal cancer (NPC) is a malignant epithelial carcinoma of the head and neck. Cancer therapy targeting programmed cell death protein-1 (PD-1) or programmed death ligand-1 (PD-L1) is revolutionary. However, the tumorigenic mechanism of PD-L1 is not yet clear in NPC. Here we demonstrated an oncogenic role of PD-L1 via activating PI3K/AKT in NPC cells. PD-L1 overexpression was frequently detected in NPC biopsies and cell lines by qRT-PCR. PD-L1 overexpression and knockdown demonstrated that PD-L1 promoted NPC cell invasion and metastasis in vitro and in vivo. Mechanistically, PD-L1 prominently activated the epithelial–mesenchymal transition (EMT) process in a PI3K/AKT-dependent manner. Taken together, we found that PD-L1 overexpression confers NPC cell malignancy and aggressiveness via activating the downstream PI3K/AKT signaling. Thus, these results provide a basis for diagnosis and treatment of NPC.
Key words: PD-L1, Epithelial–mesenchymal transition (EMT), Nasopharyngeal carcinoma (NPC), PI3K/AKT
INTRODUCTION
Nasopharyngeal cancer (NPC) is a malignant epithelial carcinoma of the head and neck. Currently, it is believed that the incidence of NPC is also characterized by ethnic susceptibility, regional concentration, and familial aggregation1. The nasopharynx is more secluded, and the early symptoms are not obvious. Many NPC patients have been diagnosed in the middle and late stages, and the average 5-year survival rate of patients with middle and advanced stage is around 70%2. Therefore, exploring the molecular mechanism of progression and metastasis of NPC and new intervention targets have important clinical significance for improving the cure rate and survival rate of NPC, to prolong patients’ life.
Epithelial–mesenchymal transition (EMT) is important for tumor cell invasion and metastasis3. EMT is a particular phenomenon of the epithelial cells transformed into mesenchymal cells in physiological and pathological conditions4. The concept of EMT was developed by Greenberg and Hay in 1982. They found that the epithelial cells formed into mesenchymal cell samples in collagen gel by forming pseudopodia and loss of polarity, obtained stromal cells markers such as N-cadherin, vimentin, and ultimately obtained migration ability5. Invasion and metastasis are significant aggressive phenotypes of human cancers and the most lethal attributes of cancer death. Prevention of tumor invasion and metastasis has attracted great attention in clinical research6–10. Therefore, studying the molecular mechanism of EMT is of great significance to understand the invasion and metastasis of tumors and to develop novel interventional therapy for tumors. In this study, we investigated whether programmed death ligand 1 (PD-L1) was involved in EMT, invasion, and metastasis of NPC, and the underlying mechanisms. Our results showed, for the first time, that PD-L1 triggered EMT-like cellular marker alterations and promoted invasion and metastasis of NPC by activating the PI3K/AKT pathway.
Numerous studies have found that PD-L1 is overexpressed in a variety of tumor tissues. Highly expressed PD-L1 interacts with its receptor programmed cell death protein-1 (PD-1), can cause apoptosis and immune disability of tumor antigen-specific T cells, can make tumor cells escape immune surveillance, and can promote tumor occurrence and development11. Antibodies targeting PD-1 or PD-L1 can effectively block the recognition process between PD-1 and PD-L1, and clinical research results have suggested that they have good therapeutic effects on melanoma12, lung cancer13, and kidney cancer14. However, the tumorigenic mechanism of PD-L1 is not yet clear. We found that PD-L1 could promote tumor invasion and metastasis in NPC in our research.
MATERIALS AND METHODS
Tissue Samples
The tissues samples were obtained from patients that did not receive chemoradiotherapy. All participants signed the consent forms before carrying out this research and approval was received from the Human Ethics Committee of Gannan Medical College.
Cell Culture
NPC cell lines (5-8F, 6-10B, CNE1, CNE2, SUNE1, HONE1, C666-1) and normal nasopharyngeal epithelial cells (NP69) were obtained from the Cancer Institute of Southern Medical University (Guangzhou, P.R. China). The authenticity of cell lines in our study was verified with the DNA fingerprinting method. All cell lines used in this study were maintained in RPMI-1640 medium (Invitrogen, Shanghai, P.R. China) with 10% FBS (HyClone, Logan, UT, USA), 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen), and were incubated at 5% CO2 at 37°C.
Migration and Invasion Assays
We determined the effect of PD-L1 on NPC cell migration and invasion in vitro by using a traditional Transwell assay/modified Boyden chamber. For Transwell migration assay, 1 × 105 cells were seeded into the upper chamber (BD Biosciences, San Jose, CA, USA) with serum-free DMEM. Boyden invasion assay was performed using Matrigel (BD Biosciences) in the upper chamber. DMEM with 10% FBS was put into the lower compartment as chemoattractant. Cells were allowed to migrate for 12 h. The remaining cells in the upper chamber were scraped out by cotton swap. The cells that had migrated to the lower surface of the membrane were fixed with 100% methanol and stained with hematoxylin solution (Sigma-Aldrich, St. Louis, MO, USA), followed by counting in five random optical fields. The detection procedures were as described in our previous publication10.
RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR
The TRIzol reagent, iScript cDNA synthesis kit, and SYBR PrimeScript miRNA RT-PCR Kit were purchased from TaKaRa Bio (Dalian, P.R. China). The procedures of RNA isolation, reverse transcription, and qRT-PCR were carried out according to the manufacturer’s protocol and our previous publication15. The primers used to amplify PD-L1 (Invitrogen) were 5′-TGGCATTTGCTGAACGCATTT-3′ (forward) and 5′-TGCAGCCAGGTCTAATTGTTTT-3′ (reverse). The primers used for amplifying E-cadherin (Invitrogen) were 5′-TGCCCAGAAAATGAAAAAGG-3′ (forward) and 5′-GTGTATGTGGCAATGCGTTC-3′ (reverse). The primers used for amplifying vimentin (Invitrogen) were 5′-GAGAACTTTGCCGTTGAAGC-3′ (forward) and 5′-GCTTCCTGTAGGTGGCAATC-3′ (reverse). The primers used for amplifying β-catenin (Invitrogen) were 5′-AGGTCTGAGGAGCAGCTTCA-3′ (forward) and 5′-ATTGTCCACGCTGGATTTTC-3′ (reverse). The primers used for amplifying N-cadherin (Invitrogen) were 5′-ACAGTGGCCACCTACAAAGG-3′ (forward) and 5′-CCGAGATGGGGTTGATAATG-3′ (reverse). The primers used for amplifying GAPDH (Invitrogen) were 5′-ACCCAGAAGACTGTGGATGG-3′ (forward) and 5′-TCTAGACGGCAGGTCAGGTC-3′ (reverse).
Western Blotting
For each sample, 25 μg of total protein extracts was separated on SDS-PAGE gels and transferred to PVDF membranes, which were blocked with 5% BSA for 1 h and incubated with primary antibodies against PD-L1, E-cadherin, vimentin, PI3K, pPI3K, AKT, and pAKT (Cell Signaling Technology, Danvers, MA, USA) and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). All the primary antibodies were incubated overnight at 4°C, followed by incubation with corresponding secondary antibody and detection.
Immunohistochemistry
The staining procedure was performed according to manufacturer recommendation protocol and the detailed steps have been described previously15.
In Vivo Metastasis Analysis in Nude Mice
Female BALB/c nude mice (4–5 weeks) were from Wenzhou Medical University and were maintained in microisolator cages under aseptic conditions. For in vivo metastasis assays, the vector- or PD-L1-expressing CNE2 cells (1.0 × 106) were subcapsularly transplanted into the liver of nude mice (eight mice/per group), respectively. All animals were sacrificed on the 30th day. This study was carried out in accordance with the Guide for the Care and Use of Laboratory Animals of Wenzhou Medical University. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Wenzhou Medical University. Subcapsular transplantation of NPC cells was performed under sodium pentobarbital anesthesia to minimize suffering.
Statistical Analysis
SPSS 16.0 software was used for statistical analysis. Data are presented as means ± SD of at least three independent experiments. The results were analyzed using a two-tailed Student’s t-test. A value of p < 0.05 was considered statistically significant.
RESULTS
PD-L1 Overexpression Was Frequently Detected in NPC Biopsies and Cell Lines
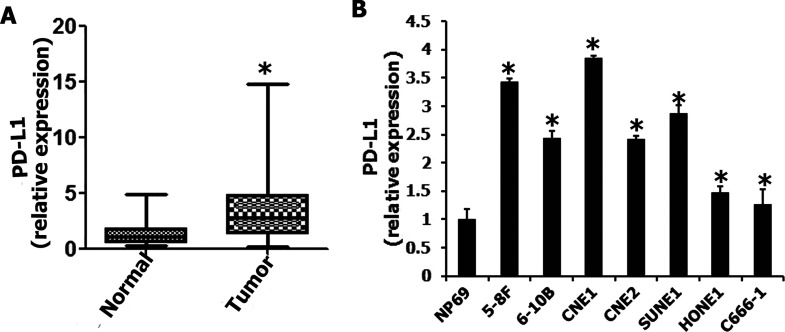
We evaluated the expression of PD-L1 mRNA in 81 NPC biopsies and 20 noncancerous nasopharyngeal epithelial biopsies by qRT-PCR. High expression of PD-L1 was detected in NPC specimens (Fig. 1A). In addition, the overexpression of PD-L1 was found in NPC cell line (5-8F, 6-10B, CNE1, CNE2, SUNE1, HONE1, and C666-1) cells (Fig. 1B). Therefore, PD-L1 overexpression was more frequently detected in NPC than in noncancerous nasopharyngeal epithelial biopsies and NP69 cell line.
Figure 1.
The expression of programmed death ligand-1 (PD-L1) in nasopharyngeal carcinoma tissues and cells. (A) PD-L1 was upregulated in nasopharyngeal carcinoma tissues. (B) PD-L1 was upregulated in nasopharyngeal carcinoma cells. *p < 0.05 compared to control.
Ectopic Expression of PD-L1 in NPC Cells Induced EMT-Like Molecular Changes and Enhanced Cell Migration and Invasion by Activating the PI3K/AKT Pathway
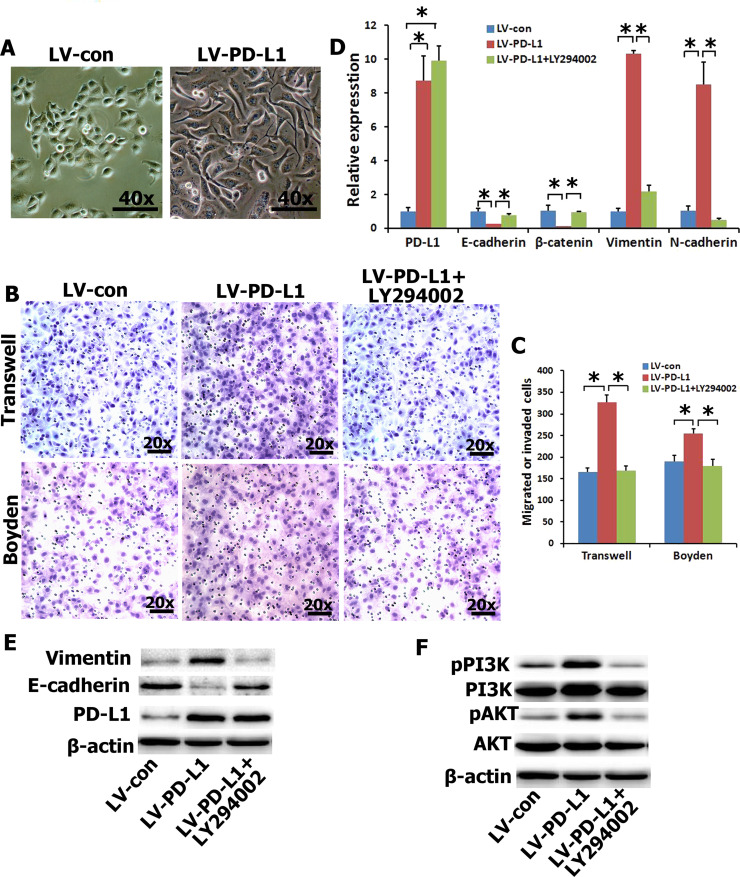
EMT is a central mechanism contributing to invasion and metastasis of various cancers7,16,17. To understand whether PD-L1 overexpression directly induces EMT, and migration and invasion of NPC cells, we examined the surface markers and phenotypic changes of CNE2 cells with ectopic expression of PD-L1. The PD-L1 transgene was successfully overexpressed in CNE2 cells (Fig. 2D and E). PD-L1-expressing CNE2 cells exhibited typical EMT-like phenotypes (Fig. 2A). Therefore, we also examined the effects of PD-L1 on the migration and invasion of NPC cells based on Transwell migration and Boyden invasion assays. As shown in Figure 2B and C, PD-L1-expressing CNE2 cells displayed significantly enhanced migration and invasion abilities in comparison with vector-expressing cells. The qRT-PCR and Western blotting results demonstrated that ectopic expression of PD-L1 significantly reduced the expression of epithelial markers (i.e., E-cadherin and/or β-catenin) and significantly increased the expression of mesenchymal markers (vimentin and/or N-cadherin) in CNE2 cells (Fig. 2D and E). In mechanism studies, we found that PI3K/AKT signaling contributed most significantly to the upregulation of PD-L1 in NPC cells (Fig. 2F). In order to further determine whether the PI3K/AKT pathway affects alteration of EMT gene in PD-L1-expressing CNE2 cells, a PI3K/AKT inhibitor (LY294002) was used to treat PD-L1-expressing CNE2 cells. The inhibition was specific for PI3K at concentrations up to 20 μM. The results showed that treatment with LY294002 significantly abolished the effect of PD-L1 on migration and invasion (Fig. 2B and C), and EMT gene (Fig. 2D and E). Taken together, these results suggested that PD-L1 overexpression enhanced the migration and invasion of NPC cells in vitro by inducing EMT-like cellular marker alterations via activating the PI3K/AKT signaling pathway.
Figure 2.
Enhanced cell migration and invasion in PD-L1-expressing CNE2 cells via the PI3K/AKT pathway. (A) CNE2 cells coupled with an alteration of cellular morphology. (B, C) The migration and invasion were analyzed with an in vitro migration assay using a Transwell chamber and an in vitro invasion assay using a Matrigel-coated Boyden chamber, respectively. PI3K inhibitor (LY294002) abolished the migration and invasion capability induced by PD-L1. Representative photomicrograph is presented. The migrated cells were plotted as the average number of cells per field of view from five different experiments, as described in Materials and Methods. (D, E) The mRNA and protein expressions of EMT-related genes were detected by quantitative real-time (qRT)-PCR and Western blot with PD-L1-overexpression in CNE2 cells and pretreated with LY294002 (20 μM). (F) The protein expressions of PI3K/AKT signal pathway were detected by Western blot. *p < 0.05 compared to control.
Silence of Endogenous PD-L1 Reversed EMT Gene Change and Reduced the Migration and Invasion Abilities of NPC Cells by Inhibition of the PI3K/AKT Pathway
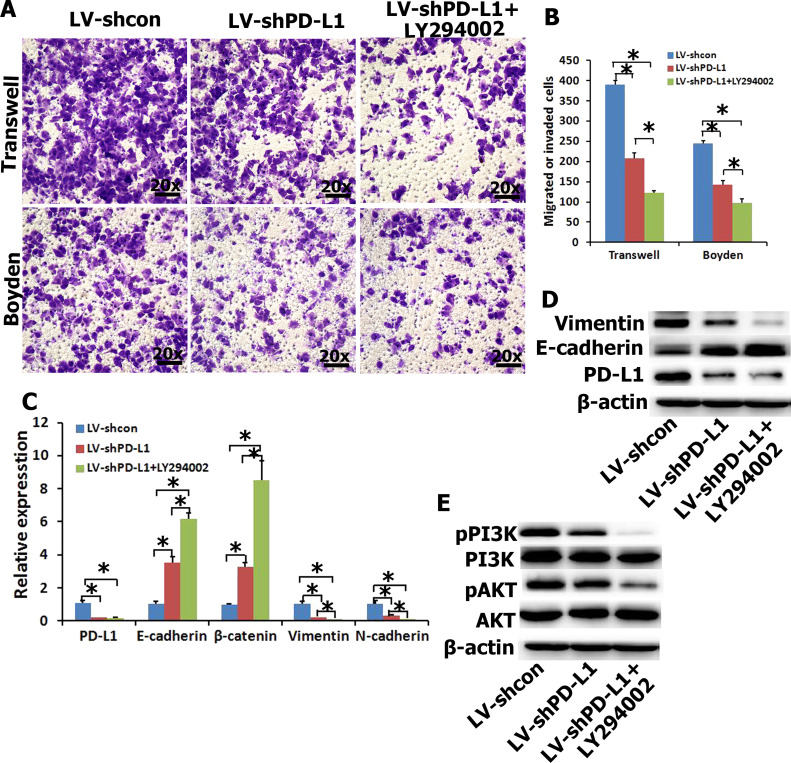
To further examine the effects of PD-L1 on EMT, and migration and invasion of NPC cells, endogenous PD-L1 in SUNE1 cells was silenced using specific shRNA. The shRNAPD-L1 specifically knocked down endogenous PD-L1 mRNA (Fig. 3C) and protein (Fig. 3D) expression in SUNE1 cells. Transwell migration and Boyden invasion assays also demonstrated that knockdown of endogenous PD-L1 by shRNA markedly inhibited the migration and invasion of SUNE1 cells (Fig. 3A and B). As indicated in Figure 3C and D, silencing endogenous PD-L1 in SUNE1 cells increased the expression of epithelial markers (i.e., E-cadherin and/or β-catenin) and concomitantly reduced the expression of mesenchymal markers (i.e., vimentin and/or N-cadherin) at both mRNA and protein levels. In addition, PI3K/AKT signal transduction was suppressed by PD-L1 inhibition (Fig. 3E). In order to further determine whether the PI3K/AKT pathway affects alteration of EMT gene in shRNAPD-L1 SUNE1 cells, LY294002 was used to treat shRNAPD-L1 SUNE1 cells. Relative migration and invasion (Fig. 3A and B) as well as expression levels of mesenchymal markers (i.e., vimentin and/or N-cadherin) were decreased by LY294002 relative to the shRNAPD-L1group. Moreover, the effects of pretreatment with LY294002 on shRNAPD-L1 SUNE1 cells increased the expression of epithelial markers (i.e., E-cadherin and/or β-catenin) (Fig. 3C and D). Taken together, suppression of endogenous PD-L1 expression in NPC cells reversed the EMT-like molecular changes and reduced the migration and invasion of NPC cells by inhibiting the PI3K/AKT signal pathway.
Figure 3.
Underexpression of PD-L1 decreases cell migration and invasiveness in SUNE1 cells via the PI3K/AKT pathway. (A, B) The migration and invasion were analyzed with an in vitro migration assay using a Transwell chamber and an in vitro invasion assay using a Matrigel-coated Boyden chamber, respectively. The migrated cells were plotted as the average number of cells per field of view from five different experiments, as described in Materials and Methods. (C, D) The mRNA and protein expressions of EMT-related genes were detected by qRT-PCR and Western blot when interfering with PD-L1 expression in SUNE1 cells and pretreated with LY294002 (20 μM). (E) The protein expressions of PI3K/AKT signal pathway were detected by Western blot. *p < 0.05 compared to control.
PD-L1 Positively Modulates the Metastasis of NPC Cells In Vivo
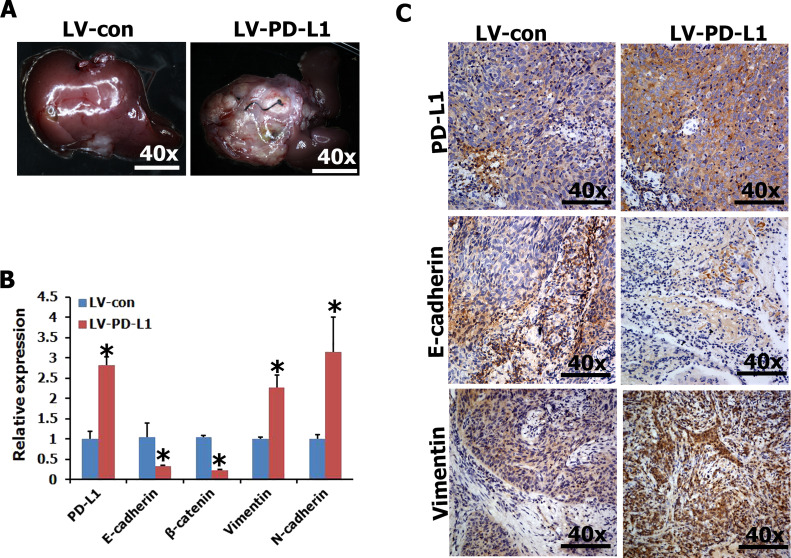
Local invasion and distant metastasis are common clinical features of NPC. To determine whether PD-L1 improves the migration and invasion of NPC cells in vivo, the vector- or PD-L1-expressing CNE2 cells were subcapsularly transplanted into the liver of nude mice and formed tumors in the liver. We found that the size of tumors on the liver surface derived from PD-L1-expressing CNE2 cells was significantly larger than that derived from vector-expressing CNE2 cells (Fig. 4A). Moreover, qRT-PCR and IHC analysis revealed that PD-L1 expression of primary tumors derived from PD-L1-expressing CNE2 cells was significantly higher than that of primary tumors derived from vector-expressing CNE2 cell (Fig. 4B and C). In addition, we detected the expression of epithelial markers (i.e., E-cadherin and/or β-catenin) and mesenchymal markers (vimentin and/or N-cadherin) by qRT-PCR and IHC in primary tumors. The results revealed that ectopic expression of PD-L1 CNE2 cells exhibited typical EMT-like phenotypes, including downregulation of epithelial marker E-cadherin and upregulation of mesenchymal marker vimentin (Fig. 4B and C). Thus, PD-L1 positively regulates the metastasis of NPC cells in vivo.
Figure 4.
Enforced expression of PD-L1 in CNE2 cells promoted metastasis in vivo. (A) Representative livers from nude mice 30 days after subcapsular liver transplantation of vector-expressing (LV-con) or PD-L1-expressing (LV-PD-L1) CNE2 cells (eight mice/group). (B, C) The expression of PD-L1 and EMT-related genes was detected in tumor tissues by qRT-PCR and immunohistochemistry. *p < 0.05 compared to control.
DISCUSSION
Current studies have shown that PD-L1 expression is closely related to tumor prognosis. In a previous PD-L1 study, Shi et al.18 found that high PD-L1 expression was associated with TNM stage and prognosis in tumor cells of colorectal cancer patients. Karim et al.19 found that PD-L1 expression in cervical cancer tissues was expressed in a few samples and affected the prognosis of patients. Nomi et al.20 found that PD-L1+ patients with pancreatic cancer had worse prognosis than negative patients. Muenst et al.21 found that the expression of PD-L1 in breast cancer tissues was related to tumor size, pathological classification, and lymph node metastasis, and the total survival time of positive patients was significantly shortened. Frigola et al.22 and Thompson et al.23 found that PD-L1 expression was associated with tumor staging and prognosis in renal cancer patients. Azuma et al.24 found that high expression of PD-L1 in tumor cells of non-small cell lung cancer was associated with EGFR mutation and had a poor prognosis. In addition, relevant studies also showed that PD-L1 expression was high in the cancer tissues of patients with ovarian cancer25, malignant melanoma26, and esophageal cancer27, and the prognosis was poor. Therefore, PD-L1 has been proven to be a poor prognostic factor.
We, for the first time, have proved that PD-L1+ expression is closely related to EMT in NPC. PD-L1 may have important significance in the malignant progress of NPC, and blocking PD-L1 and its receptor can improve the clinical outcome of cancer treatment. In the immune response mechanism of the tumor microenvironment, PD-L1 is a negative regulatory molecule, though it can combine with PD-1 by inhibiting T-cell proliferation and promoting cell secretion immunosuppressive factor12. In patients with renal cell carcinoma, those with high expression of PD-L1 are four times more likely to metastasize and die than those with negative PD-L123. These studies and this current study suggest that PD-L1 has a general promotion effect on the occurrence and development of different types of tumors. Although studies have shown that PD-L1 has poor prognosis in NPC28, few studies have revealed the molecular mechanism of PD-L1 expression in NPC cells.
ACKNOWLEDGMENT
This work was supported by Wenzhou Science and Technology Project (Y20170015, 2017Y0359) and the Natural Science Foundation of Zhejiang Province (CN) (LQ18H070005).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet 2005;365(9476):2041–54. [DOI] [PubMed] [Google Scholar]
- 2. Wu X, Huang PY, Peng PJ, Lu LX, Han F, Wu SX, Hou X, Zhao HY, Huang Y, Fang WF, Zhao YY, Xue C, Hu ZH, Zhang J, Zhang JW, Ma YX, Liang WH, Zhao C, Zhang L. Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2013;24(8):2131–6. [DOI] [PubMed] [Google Scholar]
- 3. Bonnomet A, Brysse A, Tachsidis A, Waltham M, Thompson EW, Polette M, Gilles C. Epithelial-to-mesenchymal transitions and circulating tumor cells. J Mammary Gland Biol Neoplasia 2010;15(2):261–73. [DOI] [PubMed] [Google Scholar]
- 4. Vincent-Salomon A, Thiery JP. Host microenvironment in breast cancer development: Epithelial-mesenchymal transition in breast cancer development. Breast Cancer Res. 2003;5(2):101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. [DOI] [PubMed] [Google Scholar]
- 8. Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. [DOI] [PubMed] [Google Scholar]
- 9. Li J, Yang S, Yan W, Yang J, Qin YJ, Lin XL, Xie RY, Wang SC, Jin W, Gao F, Shi JW, Zhao WT, Jia JS, Shen HF, Ke JR, Liu B, Zhao YQ, Huang WH, Yao KT, Li DJ, Xiao D. MicroRNA-19 triggers epithelial-mesenchymal transition of lung cancer cells accompanied by growth inhibition. Lab Invest. 2015;95:1056–70. [DOI] [PubMed] [Google Scholar]
- 10. Wang SC, Lin XL, Li J, Zhang TT, Wang HY, Shi JW, Yang S, Zhao WT, Xie RY, Wei F, Qin YJ, Chen L, Yang J, Yao KT, Xiao D. MicroRNA-122 triggers mesenchymal-epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoA. PLoS One 2014;9:e101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keir ME, Butte MJ, Freeman GJ. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. [DOI] [PubMed] [Google Scholar]
- 13. Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94(1):107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19(5):1021–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie R, Lin X, Du T, Xu K, Shen H, Wei F, Hao W, Lin T, Lin X, Qin Y, Wang H, Chen L, Yang S, Yang J, Rong X, Yao K, Xiao D, Jia J, Sun Y. Targeted disruption of miR-17-92 impairs mouse spermatogenesis by activating mTOR signaling pathway. Medicine (Baltimore) 2016;95(7):e2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. [DOI] [PubMed] [Google Scholar]
- 17. Christofori G. New signals from the invasive front. Nature 2006;441:444–50. [DOI] [PubMed] [Google Scholar]
- 18. Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, Yang AG, Wen WH. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One 2013;8:e76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15:6341–7. [DOI] [PubMed] [Google Scholar]
- 20. Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–7. [DOI] [PubMed] [Google Scholar]
- 21. Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, Weber WP, Soysal SD. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H, Kwon ED. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. [DOI] [PubMed] [Google Scholar]
- 24. Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, Hoshino T, Nakanishi Y, Okamoto I. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected non-small cell lung cancer. Ann Oncol. 2014;25:1935–40. [DOI] [PubMed] [Google Scholar]
- 25. Wang Q, Lou W, Di W, Wu X. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 2007;104:3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010;116:1757–66. [DOI] [PubMed] [Google Scholar]
- 27. Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2015;11:2947–53. [DOI] [PubMed] [Google Scholar]
- 28. Zhang J, Fang W, Qin T, Yang Y, Hong S, Liang W, Ma Y, Zhao H, Huang Y, Xue C, Huang P, Hu Z, Zhao Y, Zhang L. Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med Oncol. 2015;32(3):86. [DOI] [PubMed] [Google Scholar]