Abstract
Osteosarcomas, especially those with metastatic or unresectable disease, have limited treatment options. The antitumor effects of pharmacologic inhibitors of angiogenesis in osteosarcomas are hampered in patients by the rapid development of tumor resistance, notably through increased invasiveness and accelerated metastasis. Here we demonstrated that thrombospondin 1 (TSP-1) is a potent inhibitor of the growth and metastasis of the osteosarcoma cell line MG-63. Moreover, we demonstrate that upregulation of TSP-1 facilitated expression of vasculostatin in MG-63 cells. In angiogenesis assays, overexpression of TSP-1 inhibited MG-63 cells and induced tube formation of human umbilical vein endothelial cells (HUVECs) in a CD36-dependent fashion. Finally, in xenografted tumors, we observed that TSP-1 overexpression inhibited angiogenesis and tumor growth. These results provided strong evidence for an important role of the TSP-1/CD36/vasculostatin signaling axis in mediating the antiangiogenic activity of osteosarcoma.
Key words: TSP-1, Osteosarcoma, Metastasis, Angiogenesis, CD36
INTRODUCTION
Osteosarcoma is one of the most malignant tumors of mesenchymal origin and primarily occurs in adolescents and children1. There is a large amount of evidence that the pleiomorphic tumor of the bone depends on new blood vessel development, also known as tumor angiogenesis, for osteosarcoma cell growth and metastasis2,3. Vascular endothelial growth factor (VEGF) functions as an indicator for evaluating the prognostic importance of angiogenesis in osteosarcoma4. Monotherapy with VEGF receptor tyrosine kinase inhibitors (TKIs) in sarcoma has become an area of active research5. Nevertheless, recent data have provided strong evidence that tumors may escape or become resistant to the antiangiogenic effects of blocking the VEGF signaling pathway. Therefore, additional antiangiogenic approaches need to be evaluated.
Two members of the thrombospondin (TSP) family, TSP-1 and TSP-2, are important naturally occurring angiogenesis inhibitors6. Both proteins were shown to have potent antiangiogenic activity by inhibiting endothelial cell proliferation, migration, and tube formation in response to multiple angiogenic stimuli and by inhibiting angiogenic responses in a number of in vivo models. The antiangiogenic activity of TSP-1 was localized to the properdin-like type I repeats, also called thrombospondin structural homology repeats (TSRs), in the N-terminal domain of TSP-17. TSP-1 encoded by the THBS1 gene is a major negative regulator of angiogenesis compromising endothelial cell survival, migration, and responses to VEGF8,9. TSP-1 parlays its antiangiogenic activity into inhibition of tumor growth and metastases in many different tumor types, including breast carcinoma and prostate cancer.
CD36 is a 471-amino acid, 88-kDa, heavily glycosylated, and multifunctional membrane protein present on many cell types including microvascular endothelial cells (MVECs)10. Recognition of a specific protein domain known as TSR present in antiangiogenic proteins like TSP-1, TSP-2, and brain angiogenesis inhibitor 1 (BAI-1) by CD36 on MVECs mediates antiangiogenic signaling11. Interaction of TSR with CD36 induces proapoptotic signaling and also downregulates vascular endothelial growth factor receptor-2 (VEGFR2)-mediated pro-angiogenic signaling to inhibit angiogenesis via both direct and indirect MVEC pathways12. Many in vivo and human studies have shown the importance of the anti-angiogenic role of CD36 in pathological processes like tumor growth and wound healing13. Vasculostatin is the extracellular fragment of BAI-1 and has been shown to be a potent antiangiogenic and antitumorigenic factor14. Vasculostatin contains five TSP type 1 repeats (amino acids 264–315, 357–407, 412–462, 470–520, and 525–575) and an integrin-antagonizing RGD motif (amino acids 231–233)15. All five TSP-1 domains of vasculostatin have also been shown to be antiangiogenic in vitro and in vivo. However, the molecular mechanism of the antiangiogenic activity of TSP-1 and vasculostatin has not been studied as well in osteosarcoma.
Thus, in this study, we first demonstrated that TSP-1 overexpression inhibited the viability and colony formation of osteosarcoma MG-63 cells. Our data demonstrated that overexpression of TSP-1 decreased the metastatic phenotype of MG-63 cells and was mechanistically associated with regulation of vasculostatin. Furthermore, we report that ectopic expression of TSP-1 in MG-63 cells induced apoptosis and inhibited tube formation of endothelial cells via the CD36 receptor. In contrast to the experimental antiangiogenic studies that focused on the effects on endothelial cells, with much less attention on MG-63 cell metastasis, we provide strong evidence that TSP-1 significantly inhibited tumor growth of xenotransplanted MG-63 osteosarcoma cells and lung metastasis of MG-63 tumor cells in nude mice. Our findings provided new evidence that TSP-1/CD36/vasculostatin may be a novel anticancer agent for osteosarcoma therapy.
MATERIALS AND METHODS
Cell Lines and Culture
Human osteosarcoma MG-63 cells were obtained from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (CBTCCCAS; Shanghai, P.R. China). SJSA-1, U-2 OS, SW1353, and Saos-2 cells were obtained from Cobioer Biotechnology Co., Ltd. (Nanjing, P.R. China). Cells were maintained in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) or DMEM (Invitrogen) containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Human umbilical vein endothelial cells (HUVECs; CHI Scientific, Iangyin, P.R. China) were cultured in ECGM-MV medium (PromoCell, Heidelberg, Germany) containing fetal calf serum (0.05 ml/ml), endothelial cell growth supplement (0.004 ml/ml), recombinant human epidermal growth factor (10 ng/ml), heparin (22.5 μg/ml), hydrocortisone (1 μg/ml), and supplemented with 50 U/ml penicillin and 50 μg/ml streptomycin.
Lentivirus Infection
Stable overexpression of TSP-1 or stable overexpression of CD63 was carried out using the lentiviral expression system (GeneChem, Shanghai, P.R. China) according to the manufacturer’s instructions. The ORF of TSP-1 or CD36 was cloned into pReciever-LV105 (GeneCopoeia, Rockville, MD, USA). MG-63 cells transfected with vector were used as negative control.
Cell Viability Assay
MG-63 cells (2 × 104 per well) were seeded with 200 μl of growth medium in a 96-well plate. After 48 h, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT; 0.5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well. The cells were then cultured at 37°C for 4 h, and 200 μl of DMSO was added into each well and mixed by shaking at room temperature for 15 min. After that, the absorption rate was measured at a wavelength of 490 nm using a spectrophotometer.
Colony Formation Assay
Two hundred MG-63 cells were seeded into 6-cm plates and incubated for 14 days. Cells were then fixed with 4% paraformaldehyde and dyed with crystal violet. Colonies were counted and photographed16. The experiment was performed independently in triplicate.
Tube Formation Assay
Twenty-four-well plates were coated with 200 μl of Matrigel (BD Pharmingen, San Jose, CA, USA) and incubated for 1 h at 37°C. HUVECs (1 × 105 cells) in ECGM-MV containing indicated culture medium (CM) with or without concurrent addition of 10 μg/ml of blocking CD36 antibody (Abcam, Cambridge, UK) were seeded into duplicate and observed at 12 h17. Images were captured and computer-assisted analyses of the tube length were performed using the Photoshop CS3 software.
Apoptosis Assay
HUVECs were incubated in CM with, or without, concurrent addition of 10 μg/ml of blocking CD36 antibody (Abcam) for 48 h. HUVECs were harvested by trypsinization without EDTA and washed twice in PBS. After staining with annexin V/fluorescein isothiocyanate (FITC) and propidium iodide (PI), the cells were immediately analyzed by flow cytometer FACSCalibur (Becton Dickinson Biosciences, San Jose, CA, USA)18.
Wound Healing Assay
Confluent MG-63 cells were wounded with a 100-μl pipet tip, and detached cells were removed by PBS washes. The cells were treated with FBS-free medium. Initial wound width was measured, and the cells were allowed to migrate for 24 h and were then fixed with 1% glutaraldehyde and photographed19.
Transwell Invasion Assay
The 8-μm pore inserts were coated with Matrigel (Becton and Dickinson Company, NJ, USA). MG-63 cells (5 × 104) were resuspended in 200 μl of serum-free medium and plated into the upper chamber of a Millicell invasion chamber (Millipore, Billerica, MA, USA), and the bottom chamber was filled with normal CM. After incubation for 24 h, the noninvading cells on the upper surface were removed with a cotton-tipped swab, and the invading cells on the bottom surface of the filter were fixed in methanol, stained with 0.1% crystal violet, and counted under a microscope using five randomly selected fields20.
Western Blot Analysis
Cell lysates from MG-63 cells were separated on SDS-PAGE under reducing conditions and transferred to a PVDF membrane and then immunoblotted with a rabbit anti-BAI1 (vasculostatin; sc-100680; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The specifically bound antibody was detected using the enhanced chemiluminescence kit (Millipore).
Quantitative Real-Time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen). First-strand cDNA was synthesized with 1 μg of total RNA using a PrimeScript RT reagent kit (Takara Bio, Tokyo, Japan). Quantitative real-time PCR (qRT-PCR) was performed using IQTM SYBR Green Supermix and the iQ5 real-time detection system (Bio-Rad Laboratories, Hercules, CA, USA). The comparative cycle threshold (Ct) method was applied to quantify the expression levels through calculating the 2(−ΔΔCt) method. The primers used for PCR were as follows (sense and antisense, respectively): GAPDH, CTCACCGGATGCACCAATGTT and CGCGTTGCTCACAATGTTCAT; TSP-1, AGACTCCGCATCGCAAAGG and TCACCACGTTGTTGTCAAGGG; BAI1 (vasculostatin), ACAACCTGGTTCTCAGCATCC and GGACGGTCGTGTTCCTCTG. cDNA amplification and relative expression values were obtained from three independent experiments.
Tumorigenesis Assay
Parental MG-63 cells (3 × 106) or TSP-1-overexpressing MG-63 cells were subcutaneously implanted into athymic, 6-week-old BALB/c nu/nu mice (n = 6 for each group). The tumor volumes were calculated using the following formula: volume = 0.5 × larger diameter × smaller diameter2. At the end of the experiments, mice were euthanized, and then the tumors were isolated21. For histological analyses, tumors were fixed with 4% paraformaldehyde and embedded in paraffin. Immunohistochemical staining for Ki-67 and CD31 was carried out. All animal experiments were approved by Guizhou Medical University Institutional Animal Care and Use Committee.
Lung Metastasis Assay
To evaluate the metastatic potential of MG-63 cells, 2 × 106 parental or TSP-1-overexpressing MG-63 cells in 100 μl of PBS were injected into the tail veins of 6-week-old nude mice (n = 6 for each group). After 12 weeks, the mice were sacrificed, and quantitation of metastatic colonies was performed on representative hematoxylin and eosin (H&E)-stained sections of formalin-fixed and paraffin-embedded lungs.
Statistical Analysis
All experimental data were presented as a means ± SD and analyzed by Student’s t-test or one-way ANOVA using SPSS software. A value of p < 0.05 was considered statistically significant.
RESULTS
Stable Overexpression of TSP-1 Expression in Osteosarcoma Cells
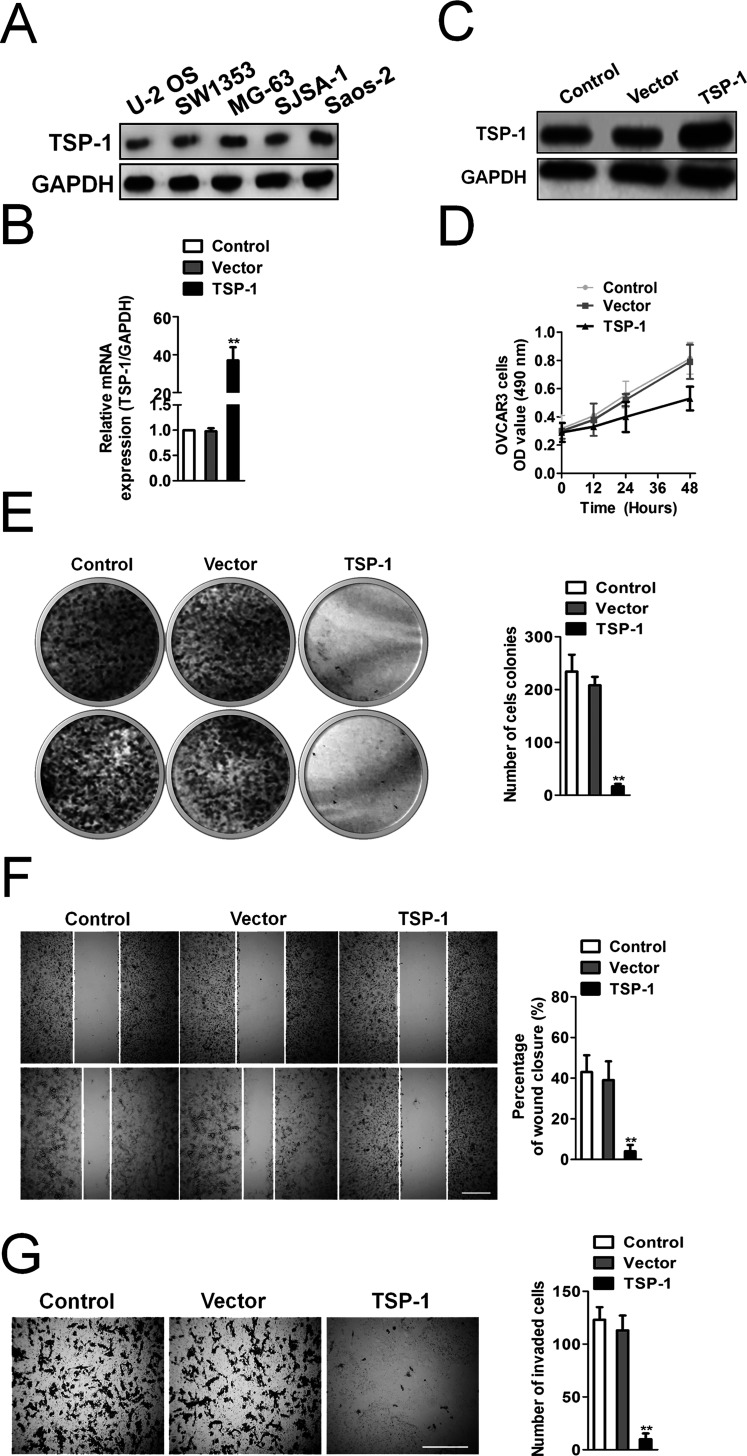
First, we assessed TSP-1 expression across various osteosarcoma cell lines and found that the MG-63 cell line has the highest TSP-1 expression followed by Saos-2, SJSA-1, SW1353, and U-2 OS (Fig. 1A). To explore the role of TSP-1 on osteosarcoma MG-63 cells, we utilized a lentivirus carrying TSP-1 to infect MG-63 for overexpression of TSP-1. qRT-PCR analysis showed that the mRNA level of TSP-1 was remarkably increased in MG-63 cells transfected with TSP-1 compared to control cells (Fig. 1B). Similarly, the levels of TSP-1 protein were also upregulated significantly in cells transfected with TSP-1 compared with control cells (Fig. 1C). Stable TSP-1-overexpressing MG-63 cells were subjected to cell viability analysis. Our data showed that TSP-1-overexpressing cells grew much slower than control cells (Fig. 1D). Consistently, TSP-1-overexpressing cells exhibited decreased colony formation ability (Fig. 1E). Moreover, we further analyzed whether TSP-1 overexpression inhibited the malignancy of MG-63 cells. Wound healing and Transwell assays suggested that TSP-1 overexpression reduced the mobility (Fig. 1F) and invasion (Fig. 1G) of MG-63 cells in vitro.
Figure 1.
Thrombospondin 1 (TSP-1) overexpression inhibits the malignancy of osteosarcoma MG-63 cells. (A) Relative expression of TSP-1 in a panel of osteosarcoma cell lines. (B) Quantitative real-time PCR (qRT-PCR) analysis of TSP-1 expression after infection of TSP-1 lentivirus vectors. The data are presented as means ± SD. For indicated comparisons, **p < 0.01. (C) Western blotting analysis of TSP-1 protein expression. GAPDH was used as internal control. (D) 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) assay. (E) TSP-1-overexpressing MG-63 cells showed decreased efficiency of cell colony formation compared to control cells. Cell colonies were counted in five random fields of vision. Scale bar: 200 μm. The data are presented as means ± SD. For indicated comparisons, **p < 0.01. (F) Effects of TSP-1 overexpression on MG-63 cell migration were determined by wound healing assay. The percentage of wound healing was calculated as the width at indicated time. Scale bar: 200 μm. The data are presented as means ± SD. For indicated comparisons, **p < 0.01. (G) Invasion assay. MG-63 cells were seeded on the upper chamber precoated with Matrigel for 24 h. Cells on the underside of the membrane were fixed and stained with crystal violet. Scale bar: 200 μm. The data are presented as means ± SD. For indicated comparisons, **p < 0.01.
Overexpression of TSP-1 Inhibits Tube Formation of CD36+ Endothelial Cells
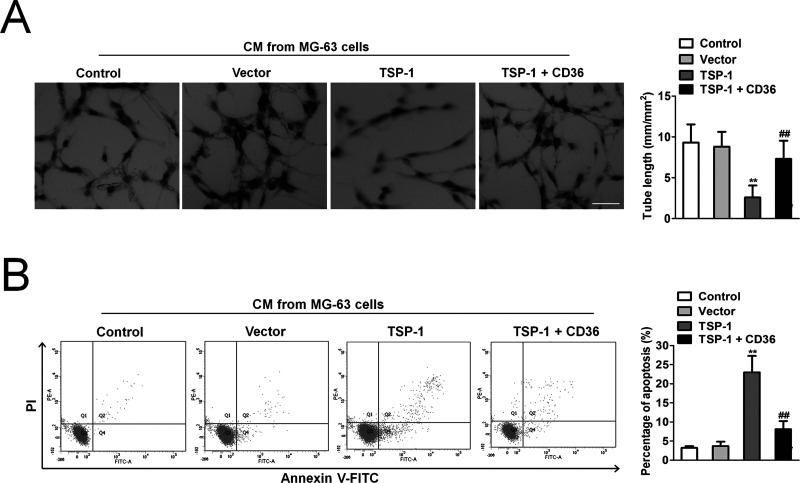
The antiangiogenic efficacy was analyzed by investigating the effect of the CM from MG-63 cells to induce tube formation of HUVECs cultured on Matrigel. After 12 h, formation of networks of tube-like structures was detected in HUVECs cultured with CM from the parental MG-63 cells. In contrast, only sparse tube-like structures were detected when HUVECs were treated with CM from MG-63 cells overexpressing TSP-1, resulting in a significant reduction of the average tube length by more than 50% (Fig. 2A). After incubation of HUVECs with blocking CD36 antibody the addition of CM from MG-63 cells resulted in tube formation that was comparable to untreated control (Fig. 2A), demonstrating that activation of the CD36 receptor was involved in the pronounced inhibition of network formation by TSP-1. Treatment of HUVECs with IgG-Fc did not show any significant effect on CM-induced network formation, providing evidence that the Fc fragment is not involved in the antiangiogenic activity of TSP-1. Flow cytometry showed that treatment of HUVECs with CM from MG-63 cells overexpressing TSP-1 for 72 h resulted in a significant increase in apoptotic endothelial cells compared to HUVECs cultured with CM from control MG-63 cells (Fig. 2B). Parallel incubation with blocking CD36 antibody attenuated MG-63 cells overexpressing TSP-1-induced apoptosis in HUVECs to close to the basal level, indicating that CD36 is required for the apoptotic activity of TSP-1.
Figure 2.
TSP-1-overexpressing MG-63 cells inhibit human umbilical vein endothelial cell (HUVEC) tube formation and induce apoptosis. (A) HUVECs were seeded on Matrigel. Cultures were treated with either PBS, or culture medium (CM) from control MG-63 cells, or CM from MG-63 cells overexpressing TSP-1. HUVECs were incubated with both CM from MG-63 cells overexpressing TSP-1 and blocking CD36 antibody. Incubation of HUVECs with a blocking CD36 antibody abolished the inhibition of HUVEC tube formation induced by TSP-1 overexpression. Scale bar: 50 μm. The data are presented as means ± SD. **p < 0.01 compared to control, ##p < 0.01 compared to cells transfected with TSP-1. (B) Flow cytometric apoptosis assay. Cell apoptosis was assessed by flow cytometry. The data are presented as means ± SD. **p < 0.01 compared to control, ##p < 0.01 compared to cells transfected with TSP-1.
Stable Overexpression of TSP-1 Expression Inhibits MG-63 Tumor Growth and Angiogenesis
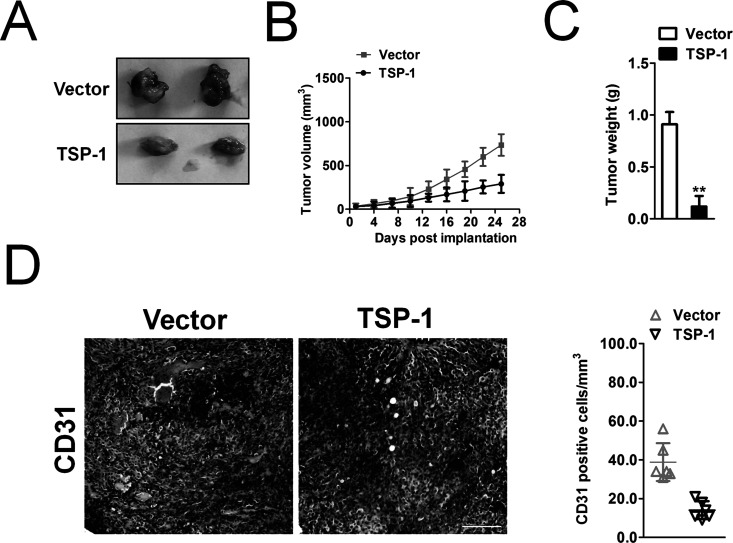
Furthermore, we performed a nude mouse xenograft assay to assess the role of TSP-1 overexpression in vivo. Forty-five days after tumor cell injection, TSP-1-overexpressing tumor xenografts showed a reduction in tumor growth compared to control tumor xenografts (Fig. 3A). The average volumes of the tumor mass derived from TSP-1-overexpressing MG-63 cells were much smaller than those of tumor xenografts derived from control cells (Fig. 3B). The tumor weight also showed that overexpression of TSP-1 inhibited the growth of osteosarcoma cells in nude mice (Fig. 3C). To analyze tumor-associated vascularization, frozen sections of MG-63 tumors were stained for the endothelial cell molecule CD31. Computer-assisted morphometric image analysis revealed that the average vessel size (Fig. 3D) was significantly decreased in tumors derived from TSP-1-overexpressing MG-63 cells, compared with control tumors. These findings suggested that TSP-1 overexpression inhibited growth and angiogenesis of tumor xenografts in vivo.
Figure 3.
MG-63 expressing TSP-1 inhibits tumorigenicity in mice models. (A) Representative picture of tumor mass. (B) Tumor growth kinetics (mean ± SD) of vector cells versus TSP-1-overexpressing MG-63 cells in nude mice. (C) Mean weight of tumor spheres. The data are presented as means ± SD. **p < 0.01. (D). Percentages of CD31 staining in tumor mass from vector cells versus TSP-1-overexpressing MG-63 cells. Scale bar: 200 μm.
Overexpression of TSP-1 or CD36 Increases Vasculostatin In Vitro and In Vivo
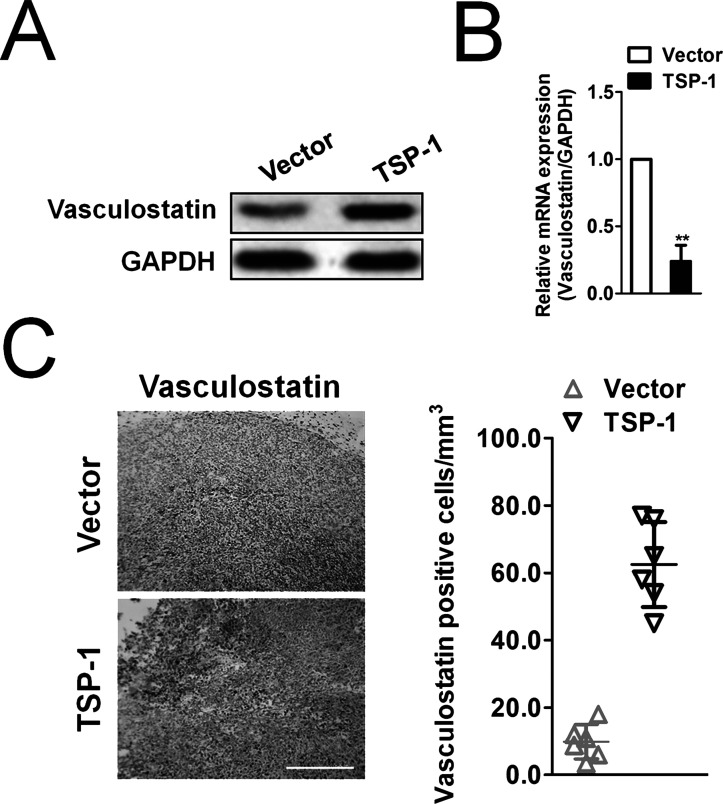
To disclose the possible convergent points and to clarify the potential mechanism by which TSP-1 regulates growth and angiogenesis of osteosarcoma cells, we detected the expression of vasculostatin in MG-63 cancer cells. We found that the levels of vasculostatin were increased in TSP-1-overexpressing MG-63 cells compared with control cells (Fig. 4A and B). Consistently, the expression of vasculostatin was also increased in CD36-overexpressing MG-63 cells compared to control cells (Fig. 4A and B). Moreover, we discovered that the mRNA and protein expression of vasculostatin were increased in the tumor tissues from xenografts of TSP-1-overexpressing MG-63 cells (Fig. 4C). Our data showed that TSP-1 upregulation affected expression of vasculostatin protein, suggesting a positive relationship between TSP-1 and vasculostatin.
Figure 4.
Effects of TSP-1/CD36 on regulation of vasculostatin gene expression. (A) Western blotting analysis of the expression of vasculostatin in TSP-1-overexpressing cells or CD36-overexpressing MG-63 cells. (B) The expression of vasculostatin in TSP-1-overexpressing or CD36-overexpressing MG-63 cells was determined by qRT-PCR. GAPDH was used as an internal control. The data are presented as means ± SD. **p < 0.01 compared to vector. (C) Representative immunohistochemistry staining for expression of vasculostatin in MG-63 osteosarcoma cell grafts grown in nude mice. Scale bar: 200 μm.
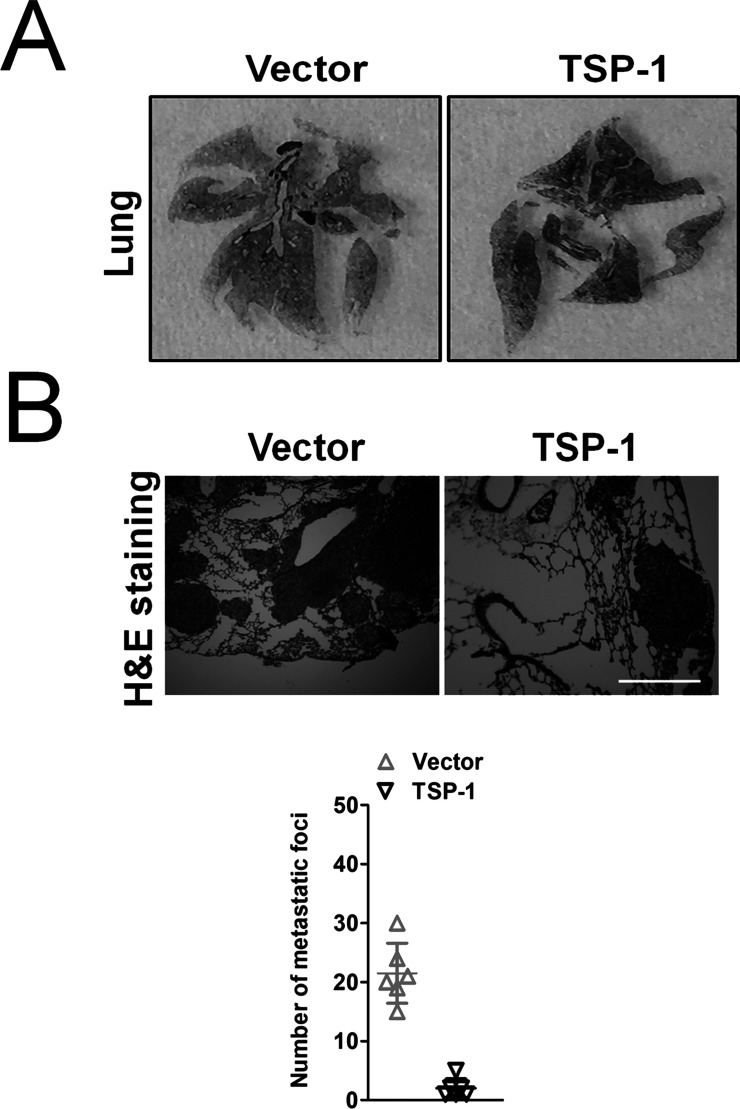
Overregulation of TSP-1 Suppresses Pulmonary Metastases of MG-63 Cells
To determine the role of TSP-1 in pulmonary metastasis in osteosarcoma, TSP-1-overexpressing MG-63 cells were injected into nude mice via the vein tail. Lungs were excised after 21 days (Fig. 5A). The rate of pulmonary metastasis and the number of visible metastases in the TSP-1-overexpressing cell groups were dramatically reduced. Diagnoses of metastatic nodules were confirmed by H&E stains (Fig. 5B).
Figure 5.
Overexpression of TSP-1 inhibits metastases of MG-63 cells in vivo. (A) Photographs of lungs from each group. (B) Representative lungs after hematoxylin and eosin (H&E) staining from each group. Visible lung metastasis number of each group was counted. Scale bar: 200 μm.
DISCUSSION
Approximately 20% of osteosarcoma patients exhibit tumor angiogenesis and lung metastasis when diagnosed, which correlates with poor 5-year survival rates22–25. Despite intense efforts to characterize the genomic patterns of osteosarcoma, effective therapeutic targets and diagnostic markers are still in urgent need. The purpose of this study was to find a possible therapeutic target for osteosarcoma angiogenesis and metastasis. TSP-1 was identified as an angiogenesis inhibitor more than 20 years ago26. The mechanisms underlying this activity were initially demonstrated to involve a specific endothelial cell receptor, CD36, which generates antiangiogenic signals that lead to apoptosis in the presence of angiogenic growth factors8,27. However, the molecular mechanism of the antiangiogenic activity of TSP-1 and vasculostatin has not been studied as well in osteosarcoma.
In the current study, we investigated the effects of TSP-1 overexpression on the regulation of osteosarcoma MG-63 cell viability in vitro and growth in nude mouse xenografts. We found that TSP-1 overexpression reduced MG-63 cell viability and colony formation in vitro. In addition, TSP-1 overexpression suppressed tumor growth in nude mice, consistent with several previous studies documenting that TSP-1 was characterized as a biomarker for metastasis of cancer cells28–30. Indeed, our current in vitro data confirmed that overexpression of TSP-1 expression inhibited the migration and invasion abilities of MG-63 cells compared with the parental cells. Moreover, the expression of vasculostatin was increased in TSP-1-overexpressing MG-63 cells. Finally, we investigated the efficacy of TSP-1 overexpression on MG-63 tumor metastasis in vivo. MG-63 cells were injected into nude mice via the tail vein, and MG-63 cells that metastasized to the lungs were determined. As expected, overexpression of TSP-1 could significantly decrease the lung metastasis of MG-63 cells. Altogether, these data suggest that TSP-1 could be further evaluated as a target for osteosarcoma growth and metastasis.
Angiogenesis is a physiological process through which new blood vessels are grown from preexisting vessels. It is controlled by the complicated and coordinated actions of proangiogenic and antiangiogenic factors24. Under pathological conditions, angiogenesis is finely regulated by many upregulated angiogenic factors, including ligands and receptors. TSP-1 is a potent endogenous inhibitor of tumor growth and angiogenesis. We found that TSP-1 overexpression significantly inhibited endothelial cell tube formation and induced endothelial cell apoptosis. Moreover, blockade of the CD36 receptor almost completely abrogated the reported in vitro effects of TSP-1 overexpression. These data suggested that the antiangiogenic activity of TSP-1 overexpression is mediated by CD36-dependent induction of endothelial cell apoptosis and tube formation. In all, our current study clarifies the function and role of TSP-1 in osteosarcoma metastasis and angiogenesis. Further studies are needed to verify TSP-1 as a biomarker for the prediction of osteosarcoma progression and survival of patients.
ACKNOWLEDGMENT
Overseas student’s scientific and technological innovation projects of Guizhou province (201502).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Rogozhin DV, Bulycheva IV, Konovalov DM, Talalaev AG, Roshchin VY, Ektova AP, Bogoroditsky YS, Strykov VA, Kazakova AN, Olshanskaya YV, Kachanov DY, Tereshchenko GV. [Classical osteosarcoma in children and adolescent]. Arkh Patol. 2015;77(5):68–74. [DOI] [PubMed] [Google Scholar]
- 2. Zhang T, Kastrenopoulou A, Larrouture Q, Athanasou NA, Knowles HJ. Angiopoietin-like 4 promotes osteosarcoma cell proliferation and migration and stimulates osteoclastogenesis. BMC Cancer 2018;18(1):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao GS, Gao ZR, Zhang Q, Tang XF, Lv YF, Zhang ZS, Zhang Y, Tan QL, Peng DB, Jiang DM, Guo QN. TSSC3 promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR pathway to suppress tumorigenesis and metastasis in osteosarcoma, and predicts a favorable prognosis. J Exp Clin Cancer Res. 2018;37(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohba T, Cates JM, Cole HA, Slosky DA, Haro H, Ando T, Schwartz HS, Schoenecker JG. Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells associates with aggressive osteosarcoma. Mol Cancer Res. 2014;12(8):1100–11. [DOI] [PubMed] [Google Scholar]
- 5. Mantovani FB, Morrison JA, Mutsaers AJ. Effects of epidermal growth factor receptor kinase inhibition on radiation response in canine osteosarcoma cells. BMC Vet Res. 2016;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu LY, Ramakrishnan DP, Silverstein RL. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 2013;122(10):1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Firlej V, Mathieu JR, Gilbert C, Lemonnier L, Nakhle J, Gallou-Kabani C, Guarmit B, Morin A, Prevarskaya N, Delongchamps NB, Cabon F. Thrombospondin-1 triggers cell migration and development of advanced prostate tumors. Cancer Res. 2011;71(24):7649–58. [DOI] [PubMed] [Google Scholar]
- 8. Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2(5):a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang X, Kazerounian S, Duquette M, Perruzzi C, Nagy JA, Dvorak HF, Parangi S, Lawler J. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J. 2009;23(10):3368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koch M, Hussein F, Woeste A, Grundker C, Frontzek K, Emons G, Hawighorst T. CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast Cancer Res Treat. 2011;128(2):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Primo L, Ferrandi C, Roca C, Marchio S, di Blasio L, Alessio M, Bussolino F. Identification of CD36 molecular features required for its in vitro angiostatic activity. FASEB J. 2005;19(12):1713–5. [DOI] [PubMed] [Google Scholar]
- 12. Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138(3):707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu J, He Z, Gao X, Wu F, Ding R, Ren Y, Jiang Q, Fan M, Liang C, Wu Z. Oxidized high-density lipoprotein impairs endothelial progenitor cells’ function by activation of CD36-MAPK-TSP-1 pathways. Antioxid Redox Signal. 2015;22(4):308–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cork SM, Kaur B, Devi NS, Cooper L, Saltz JH, Sandberg EM, Kaluz S, Van Meir EG. A proprotein convertase/MMP-14 proteolytic cascade releases a novel 40 kDa vasculostatin from tumor suppressor BAI1. Oncogene 2012;31(50):5144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klenotic PA, Huang P, Palomo J, Kaur B, Van Meir EG, Vogelbaum MA, Febbraio M, Gladson CL, Silverstein RL. Histidine-rich glycoprotein modulates the anti-angiogenic effects of vasculostatin. Am J Pathol. 2010;176(4):2039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji M, Feng Q, He G, Yang L, Tang W, Lao X, Zhu D, Lin Q, Xu P, Wei Y, Xu J. Silencing homeobox C6 inhibits colorectal cancer cell proliferation. Oncotarget 2016;7(20):29216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su X, Wang J, Chen W, Li Z, Fu X, Yang A. Overexpression of TRIM14 promotes tongue squamous cell carcinoma aggressiveness by activating the NF-kappaB signaling pathway. Oncotarget 2016;7(9):9939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ju XN, Mu WN, Liu YT, Wang MH, Kong F, Sun C, Zhou QB. Baicalin protects against thrombin induced cell injury in SH-SY5Y cells. Int J Clin Exp Pathol. 2015;8(11):14021–7. [PMC free article] [PubMed] [Google Scholar]
- 19. Al-Haidari AA, Syk I, Thorlacius H. MiR-155-5p positively regulates CCL17-induced colon cancer cell migration by targeting RhoA. Oncotarget 2017;8(9):14887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alderman C, Sehlaoui A, Xiao Z, Yang Y. MicroRNA-15a inhibits the growth and invasiveness of malignant melanoma and directly targets on CDCA4 gene. Tumour Biol. 2016;37(10):13941–50. [DOI] [PubMed] [Google Scholar]
- 21. Liu G, Wang L, Han H, Li Y, Lu S, Li T, Cheng C. LncRNA ZFAS1 promotes growth and metastasis by regulating BMI1 and ZEB2 in osteosarcoma. Am J Cancer Res. 2017;7(7):1450–62. [PMC free article] [PubMed] [Google Scholar]
- 22. Tsunemi T, Nagoya S, Kaya M, Kawaguchi S, Wada T, Yamashita T, Ishii S. Postoperative progression of pulmonary metastasis in osteosarcoma. Clin Orthop Relat Res. 2003(407):159–66. [DOI] [PubMed] [Google Scholar]
- 23. Ek ET, Dass CR, Contreras KG, Choong PF. Pigment epithelium-derived factor overexpression inhibits orthotopic osteosarcoma growth, angiogenesis and metastasis. Cancer Gene Ther. 2007;14(7):616–26. [DOI] [PubMed] [Google Scholar]
- 24. Xie L, Ji T, Guo W. Anti-angiogenesis target therapy for advanced osteosarcoma. Oncol Rep. 2017;38(2):625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meazza C, Scanagatta P. Metastatic osteosarcoma: A challenging multidisciplinary treatment. Expert Rev Anticancer Ther. 2016;16(5):543–56. [DOI] [PubMed] [Google Scholar]
- 26. Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19(7):597–614. [DOI] [PubMed] [Google Scholar]
- 28. Lin XD, Chen SQ, Qi YL, Zhu JW, Tang Y, Lin JY. Overexpression of thrombospondin-1 in stromal myofibroblasts is associated with tumor growth and nodal metastasis in gastric carcinoma. J Surg Oncol. 2012;106(1):94–100. [DOI] [PubMed] [Google Scholar]
- 29. Rouanne M, Adam J, Goubar A, Robin A, Ohana C, Louvet E, Cormier J, Mercier O, Dorfmuller P, Fattal S, de Montpreville VT, Lebret T, Dartevelle P, Fadel E, Besse B, Olaussen KA, Auclair C, Soria JC. Osteopontin and thrombospondin-1 play opposite roles in promoting tumor aggressiveness of primary resected non-small cell lung cancer. BMC Cancer 2016;16:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yee KO, Connolly CM, Duquette M, Kazerounian S, Washington R, Lawler J. The effect of thrombospondin-1 on breast cancer metastasis. Breast Cancer Res Treat. 2009;114(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]