Abstract
Cluster of differentiation 47 (CD47) overexpression is common in various malignancies. This study investigated whether CD47 promotes human glioblastoma invasion and, if so, the underlying mechanisms involved. CD47 expression was found to be stronger in tissues of patients with glioblastoma and in various cancer cell lines than in normal controls. CD47 downregulation via siRNA suppressed invasion in vitro, whereas CD47 overexpression through plasmid transfection exerted the opposite effect. However, overexpression or knocking down of CD47 had no effect on cell proliferation. Moreover, CD47 expression was related to Akt phosphorylation at the cellular molecular level. Suppression of Akt with a specific inhibitor impaired the invasion ability of CD47-overexpressing cells, indicating that stimulation of the PI3K/Akt pathway served as the downstream regulator of CD47-triggered invasion. These results suggest that CD47 might be a useful predictor of poor prognosis and metastasis and a potential target for treating glioblastomas.
Key words: CD47, Glioblastoma, Invasion, Akt
INTRODUCTION
Gliomas are one of the most prevalent malignancies in the central nervous system (CNS), making up 80% of the primary tumors of the adult brain1. The clinical outcome of patients suffering from terminal glioma and glioblastoma is usually poor, with the median survival being 8–10 months2. Similar to various other malignancies, glioblastoma is characterized by a noticeably high proliferation index. Nevertheless, its noticeable invasion makes it catastrophic and stubborn in terms of treatment, hindering surgical excision and leading to remarkable neurological morbidity and mortality3.
As a widely expressed transmembrane glycoprotein in normal tissues, cluster of differentiation 47 (CD47) regulates “self/don’t-eat-me” signals in normal cells by suppressing phagocytosis through the interaction of signal regulatory protein α (SIRPα) with macrophages4. It has been previously discovered that CD47 expression is increased in diverse cancers, including hematological cancers such as leukemias5 and lymphomas6, as well as solid cancers such as breast cancer7, colon carcinomas8, and hepatocellular carcinoma9. Previous studies have also shown that CD47 expression promotes cancer cell invasion10, indicating that this transmembrane glycoprotein could serve as a target to treat diverse cancers11. Nevertheless, the function and expression of CD47 in glioblastomas are still insufficiently understood.
In this study, CD47 expression in various glioblastoma cell lines and samples was explored in vitro in order to determine its role in glioblastoma cell invasion and proliferation. The results revealed that involvement of the PI3K/Akt pathway was necessary for the invasion ability, which was in agreement with the current understanding about CD47 stimulation of this process. The findings of this study throw light upon the influence of CD47 in enhancing glioblastoma invasion.
MATERIALS AND METHODS
Patients and Samples
Normal and glioblastoma brain tissues were acquired from participants who had received excision surgery at Linyi Third People’s Hospital (Linyi, P.R. China). The specimens were stored immediately at 80°C. The histological patterns of glioblastoma were determined according to World Health Organization classifications, where every sample was identified with the help of two pathologists. No radiotherapy, chemotherapy, or adjunctive therapy had been conducted on any participant prior to the surgery. Informed consent was acquired from each participant. The research was approved by the Ethics Committee of Linyi Third People’s Hospital.
Cell Culture
The glioblastoma cell lines U251, T98G, and U87, as well as the normal astrocyte cell line HEB were purchased from the Institute of Biophysics, Chinese Academy of Sciences (Beijing, P.R. China). Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Shanghai, P.R. China), supplemented with fetal bovine serum (FBS; 10%) and streptomycin and penicillin (100 U/ml; Gibco, Carlsbad, CA USA), was applied for the cell cultures, which were carried out on 10-cm dishes at 37°C under 5% CO2.
Transfection
Lipofectamine 2000 (Invitrogen) was utilized to transfect with pcDNA vectors expressing CD47 (pcDNA3.1-3xFlag-CD47) and siRNA against CD47. Cells were allowed to grow for 24 h. Phosphate-buffered saline (PBS) was used to wash the cells prior to transfection according to the manufacturer’s protocol.
Real-Time PCR
TRIzol reagent (Life Technologies, Carlsbad, CA, USA) was used to extract total RNA from the cells. cDNA was then generated using the TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen Biotech, Beijing, P.R. China). To quantify the CD57 transcripts, real-time quantitative PCR (qPCR) was carried out using the TransStart Top Green qPCR SuperMix (Transgen Biotech). The oligonucleotide primers for GAPDH (internal reference) and CD47 were as follows: GAPDH, 5′-GCA CCG TCA AGG CTG AGA AC-3′ (sense) and 5′-TGG TGA AGA CGC CAG TGGA-3′ (antisense); and CD47, 5′-AGA TCC GGT GGT ATG GAT GAGA-3′ (sense) and 5′-GTC ACA ATT AAA CCA AGG CCA GTAG-3′ (antisense). Every procedure was carried out in triplicate. The cycle threshold (Ct) value for the CD47 cDNA was normalized to that of GAPDH according to the comparative CT (ΔΔCt) method12.
Western Blot Analysis
The cells were cultivated in serum-free DMEM in six-well plates until they reached 80% confluency. The medium was removed, and the cells were washed twice with PBS. A lysis buffer (M-PER® Mammalian Protein Extraction Reagent; Pierce Biotechnology, Rockford, IL, USA) was then added to the cells, following which they were scraped from the plate and centrifuged at 14,000 × g at 4°C for 10 min. The supernatant was then heated for 5 min in Laemmli sample buffer containing dithiothreitol (50 mM). Equivalent quantities of the lysed and heated proteins (30 μg/well) were electrophoresed on polyacrylamide gels (10%; Bio-Rad, Shanghai, P.R. China), following which the isolated proteins were transferred to a polyvinylidene difluoride membrane (GE Healthcare, Pittsburgh, PA, USA). The membrane was blocked with skimmed milk (5%; Bio-Rad) for 1 h at room temperature, and then incubated overnight with the primary antibody at 4°C. Determination of concentrations was conducted as instructed. The membrane was then incubated with the horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature, and the proteins were finally examined using enhanced chemiluminescence kits (GE Healthcare).
Cell Proliferation Assay
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Life Technologies) was applied to evaluate cell proliferation. In brief, cells were seeded in 96-well plates at a density of 4,000 cells per well in 100 μl of DMEM and cultured for 4 days. Each well was supplemented with MTT solution (10 μl) for 1 h daily. The OD values were then determined at 490 nm.
Cell Invasion Assay
Transwell chambers (24-well inserts; 8-μm pore size; Millipore, Billerica, MA, USA) containing Matrigel (200 μg/ml; BD Biosciences, Franklin Lakes, NJ, USA) were used to evaluate cell invasion. In brief, after 24 h of transfection, the cells were treated with mitomycin C (10 μg/ml) for 2 h. Following trypsin treatment, the cells were seeded in the upper chambers at a density of 1 × 105 cells per chamber in serum-free DMEM. The lower chambers containing DMEM-F12 with FBS (15%) served as the chemoattractants. Incubation was carried out for 48 h. Subsequently, cells located at the upper surfaces of the chambers were gently removed. The invading cells located in the lower chambers were fixed in methanol and stained with crystal violet. Light microscopy was then carried out to evaluate the number of cells in five random visual fields.
Statistical Analysis
GraphPad InStat software (GraphPad, San Diego, CA, USA) was applied to analyze all the data. Results are presented as the mean ± standard deviation. The Student’s t-test was used to evaluate differences between groups, where a value of p < 0.05 was regarded as being statistically significant.
RESULTS
Expression of CD47 Is Increased in Glioblastoma Cells and Tissues
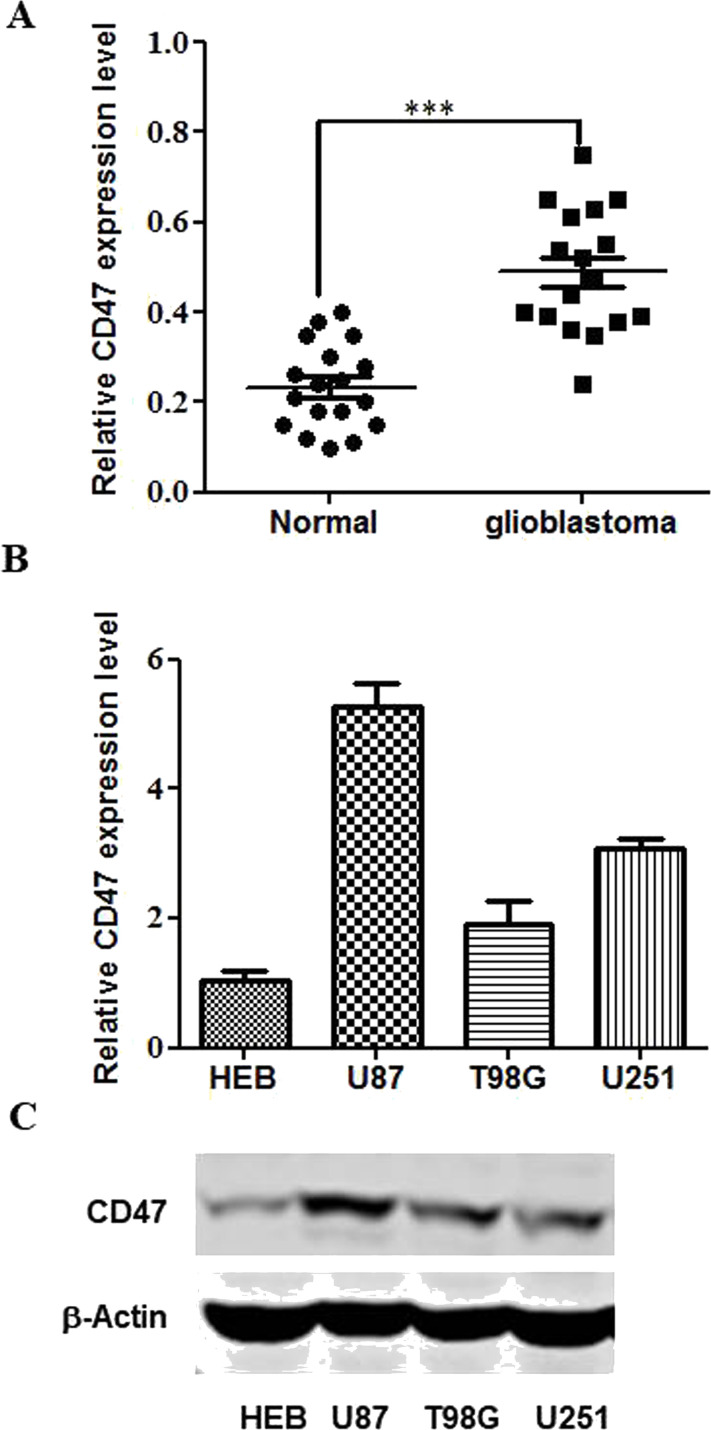
The influence of CD47 on glioblastomas was explored by qPCR using 23 glioblastoma tissue specimens and 10 normal counterparts. CD47 upregulation was evident in most of the glioblastoma tissues (Fig. 1A). Both CD47 transcription and translation were increased in the U251, T98G, and U87 cells in comparison with that in normal HEB astrocytes (Fig. 1B and C). Because U87 had the most noticeable CD47 expression among the glioblastoma cell lines, it was used for the cell invasion and proliferation assays.
Figure 1.
Cluster of differentiation 47 (CD47) is expressed in glioblastomas. (A) Quantitative PCR (qPCR) was applied to evaluate CD47 expression in 23 glioblastoma specimens and 10 normal counterparts. (B) qPCR was applied to evaluate CD47 expression in U251, T98G, and U87 cells in comparison with normal HEB astrocytes. (C) Western blotting was applied to assess CD47 expression in U251, T98G, and U87 cells in comparison with normal HEB astrocytes. ***p < 0.001.
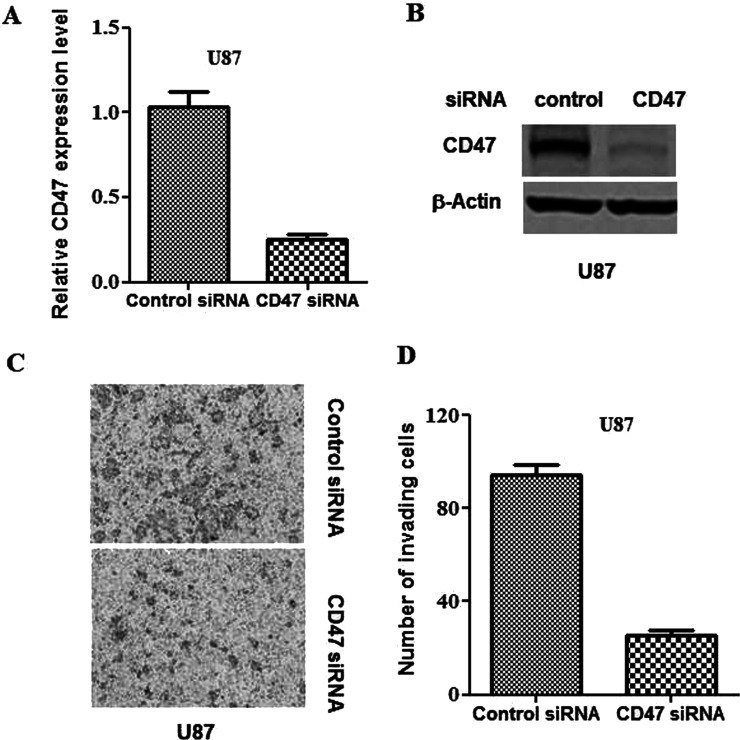
Knockdown of CD47 Impairs Glioblastoma Cell Invasion
To characterize the importance of CD47 activity in glioblastomas, knockdown of the glycoprotein was carried out in U87 cells by transfection with CD47-siRNA. qPCR verified that the CD47-siRNA had decreased the cellular transcription and translation of CD47 (Fig. 2A and B). It was found that the knockdown of CD47 remarkably inhibited the invasion of U87 cells compared with that of control cells (Fig. 2C and D).
Figure 2.
Knockdown of CD47 impairs glioblastoma invasion. CD47 siRNA and control siRNA were transfected, respectively, into U87 cells. (A) qPCR was applied to evaluate CD47 transcription at 24 h subsequent to transfection. (B) Western blotting was applied to evaluate CD47 translation at 24 h subsequent to transfection. (C) Representative images from the in vitro Matrigel invasion assay. (D) Quantification of the invasion assay depicted in (C).
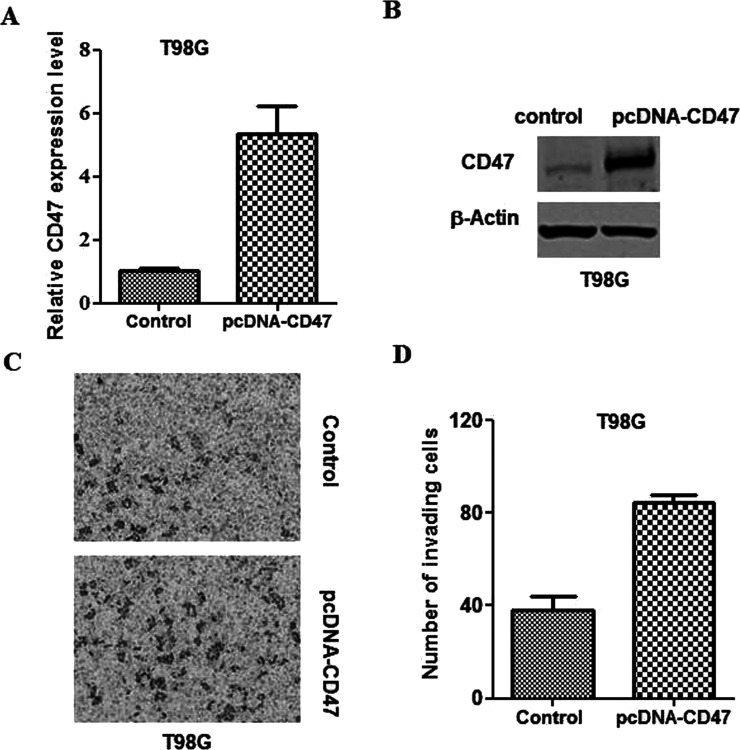
Overexpression of CD47 Promotes Glioblastoma Cell Invasion
To further investigate the influence of CD47 on the glioblastoma invasion ability, T98G cells were transfected with pcDNA3.1-3xFlag-CD47 plasmids. Western blotting and qPCR assays were then applied to confirm the overexpression of CD47 in T98G cells (Fig. 3A and B). It was found that CD47 overexpression noticeably increased T98G cell invasion relative to that of the control cells (Fig. 3C and D).
Figure 3.
CD47 overexpression promotes glioblastoma invasion. Control and pcDNA3.1-3xFlag-CD47 plasmids were transfected into T98G cells. (A) qPCR was applied to evaluate CD47 transcription at 24 h subsequent to transfection. (B) Western blotting was applied to evaluate CD47 translation at 24 h subsequent to transfection. (C) Representative images from the in vitro Matrigel invasion assay. (D) Quantification of the invasion assay depicted in (C).
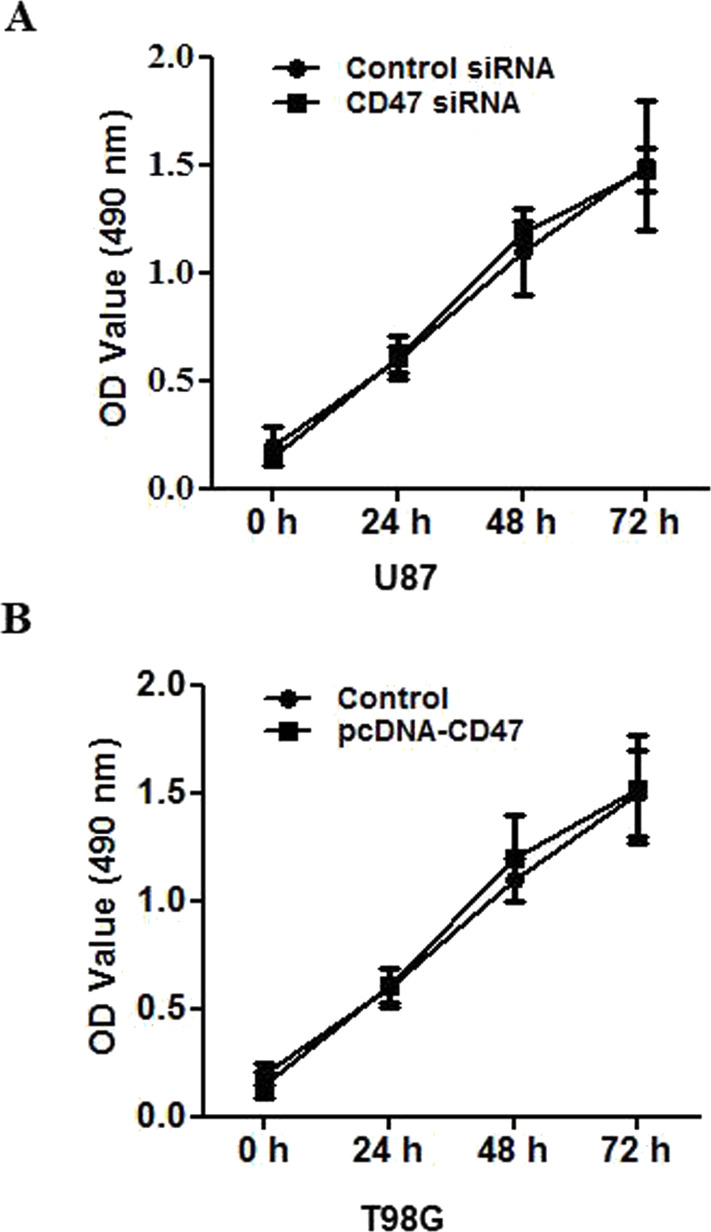
CD47 Is Unnecessary for Glioblastoma Cell Proliferation
To investigate the influence of CD47 on glioblastoma cell proliferation, the MTT assay was carried out using U87 cells transfected with CD47-siRNA or control siRNA. The number of cells in the CD47-siRNA group was similar to that in the control group (Fig. 4A). Relative to the control group, CD47 overexpression had no obvious effect on U87 proliferation over the three time points tested (Fig. 4B).
Figure 4.
CD47 is unnecessary for glioblastoma proliferation. (A) CD47 siRNA and control siRNA were transfected into U87 cells. The cells were subjected to MTT assay at 24 h subsequent to transfection in order to evaluate cell proliferation; five replicates were tested at every time point. (B) Control and pcDNA3.1-3xFlag-CD47 plasmids were transfected into T98G cells. The cells were subjected to MTT assay at 24 h subsequent to transfection in order to evaluate cell proliferation; five replicates were tested at every time point.
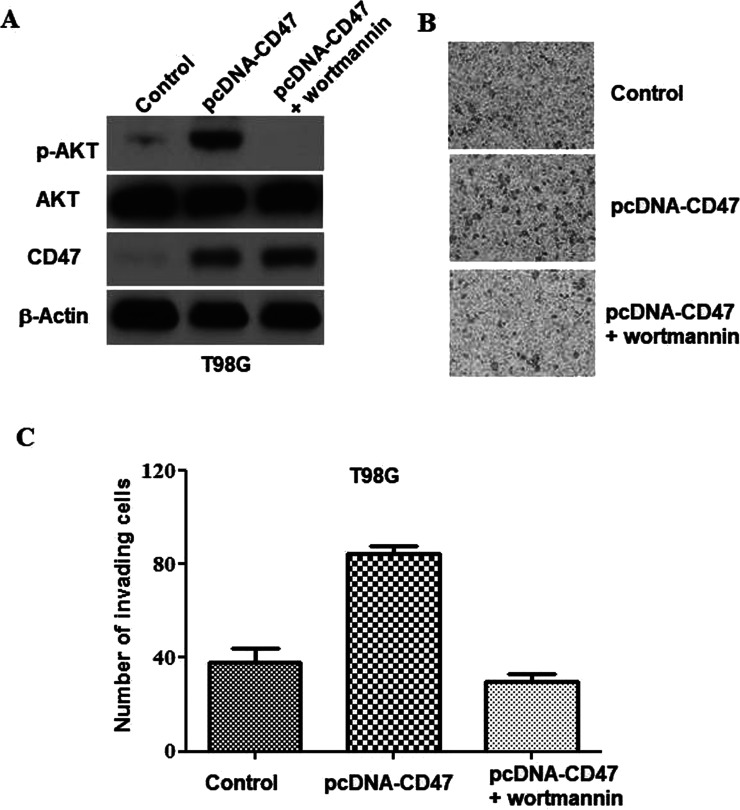
The PI3K/Akt Signaling Pathway Is Necessary for CD47-Triggered Glioblastoma Invasion
Previous research had indicated that receptors of CD47 stimulated the PI3K/Akt pathway13. To throw light on how CD47 triggers glioblastoma invasion on the molecular level, we examined the level of Akt phosphorylation in U87 cells prior and subsequent to CD47 overexpression. Akt phosphorylation was largely increased after CD47 overexpression (Fig. 5A). To determine whether the PI3K/Akt pathway is necessary for the glioblastoma invasion triggered by CD47, Wortmannin (a PI3K suppressor) was used to suppress Akt phosphorylation (Fig. 5A). The invasion assay showed that Wortmannin treatment had eliminated the CD47-triggered invasion of T98G cells (Fig. 5B and C). These data indicated that the PI3K/Akt signaling pathway was important for the glioblastoma invasion activity triggered by CD47.
Figure 5.
The PI3K/Akt signaling pathway is necessary for CD47-triggered glioblastoma invasion. Control and pcDNA3.1-3xFlag-CD47 plasmids were transfected into T98G cells with or without subsequent Wortmannin treatment. (A) Western blotting was applied to evaluate the expression of specific proteins at 24 h subsequent to transfection. (B) Representative images from the in vitro Matrigel invasion assay. (C) Quantification of the invasion assay depicted in (B).
DISCUSSION
In this study, we first proved that CD47 is overexpressed in glioblastoma cells and tissues in comparison with its expression in normal brain specimens and HEB astrocytes. CD47 regulated glioblastoma invasion on the cellular level, which was modulated by the PI3K/Akt pathway. Our findings indicate that CD47 might serve as an essential predictor and treatment target of glioblastomas.
Previous research had suggested that CD47 could serve as an innovative predictor of various cancers6,14–17. Baccelli and colleagues demonstrated that the presence of ductal breast cancer metastasis-initiating cells with the EPCAM+CD44+CD47+MET+/− phenotype was associated with a shorter overall survival and more metastatic lesions4. Additionally, CD47-expressing cancer cells in the circulation led to recurrence and metastasis in patients with breast cancer18. A constitutive increase in CD47 expression was necessary for both metastasis and immune tolerance in non-Hodgkin’s lymphoma14. In the case of colorectal carcinoma, CD47 upregulation was linked with distant metastasis, probably via immunological escape8. In agreement with these research studies, siRNA against CD47 significantly suppressed the growth and pulmonary metastasis of melanomas19. Additionally, blockade of the CD47 signal suppressed cancer development and metastasis, demonstrating its therapeutic merits for various malignancies20,21. In our present study, we provided proof of CD47 overexpression and its biological importance and functional effect in glioblastoma using several cancer cell lines and tissue specimens.
The influence of CD47 on cell proliferation is open for debate. In fact, it has been demonstrated that CD47 is able to enhance cell proliferation or trigger cell death. In the CNS, CD47 overexpression in cultivated nerve cells improved synapse generation and branching. SIRPα binding to CD47 enhanced the generation of the spine as well as filopodia in cultivated nerve cells22,23. Additionally, it was shown that ligation of CD47 to the 4N1 peptide or thrombospondin-1 (TSP-1) modulated the migration and growth of smooth muscle cells in the blood vessels24. Furthermore, CD47 stimulation triggered the dissemination and adhesion of melanoma cells25 and the epithelium of the intestines26, as well as the growth of breast cancer cells27. CD47 stimulation via 4N1 or TSP-1 ligation defended normal and malignant thyroid cells against cell death triggered by ceramide, doxorubicin, and camptothecin28. On the contrary, it was discovered that CD47 triggered the death of chronic B and T lymphatic leukemia cells via reactions independent of caspases29. In our present study, both loss- and gain-of-function methods were adopted, whereupon it was revealed that glioblastoma proliferation was independent of the overexpression or knockdown of CD47. In brief, our results indicated that the influence of CD47 on cell proliferation varied from lineage to lineage. Furthermore, CD47 has been found to be the main receptor related to cell adhesion via its interaction with integrins30. Consequently, the influence of CD47 could vary depending on the adhesion or circulation status of cells.
Our present study suggests that an innovative CD47 pathway participates in the stimulation of PI3K/Akt in glioblastoma. It was widely accepted that PI3K/Akt is a cancer development pathway affecting cell viability, growth, movement, and proliferation in various malignancies, such as astrocytomas, melanomas, and endometrial, breast, renal, ovarian, pulmonary, lymphoid, and thyroid cancers31. With regard to astrocytomas, the PI3K/Akt pathway brings about glycolysis by regulating localization of the glucose transporter Glut1 to the surface of cells and by activating hexokinase, which serves as an enzyme in the glycolytic reaction. Furthermore, the phosphorylation of inositol 1,4,5-triphosphate receptors on Akt suppresses staurosporine-induced cell death in U87 cells32. Akt also inhibits mammalian target of rapamycin in gliomas, which regulates various cellular reactions, such as cytoskeletal architecture, transcription, and autophagy, to enhance cell viability33. Increased Akt phosphorylation subsequent to CD47 overexpression in glioblastoma cells was also evident in our research. Additionally, a PI3K suppressor (Wortmannin) eliminated the CD47-triggered invasion ability of T98G cells.
In summary, the findings of this study suggest that the stimulation of CD47 receptors promotes glioblastoma invasion but not proliferation. It is hypothesized that PI3K/Akt stimulation participates in the pathway related to CD47 expression. Consequently, our research offers a potential target for the development of innovative therapeutic strategies against glioblastomas.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
REFERENCES
- 1. Munshi A. Central nervous system tumors: Spotlight on India. South Asian J Cancer 2016;5:146–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu Y, Yuan J, Zhang Z, Lin L, Xu S. Syndecan-1 expression in human glioma is correlated with advanced tumor progression and poor prognosis. Mol Biol Rep. 2012;39:8979–85. [DOI] [PubMed] [Google Scholar]
- 3. Miller TE, Liau BB, Wallace LC, Morton AR, Xie Q, Dixit D, Factor DC, Kim LJY, Morrow JJ, Wu Q, Mack SC, Hubert CG, Gillespie SM, Flavahan WA, Hoffmann T, Thummalapalli R, Hemann MT, Paddison PJ, Horbinski CM, Zuber J, Scacheri PC, Bernstein BE, Tesar PJ, Rich JN. Transcription elongation factors represent in vivo cancer dependencies in glioblastoma. Nature 2017;547:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baccelli I, Stenzinger A, Vogel V, Pfitzner BM, Klein C, Wallwiener M, Scharpff M, Saini M, Holland-Letz T, Sinn HP, Schneeweiss A, Denkert C, Weichert W, Trumpp A. Co-expression of MET and CD47 is a novel prognosticator for survival of luminal breast cancer patients. Oncotarget 2014;5:8147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr., van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009;138:286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, Park CY, Weissman IL, Majeti R. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, Gilkes DM, He J, Semenza GL. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci USA 2015;112:E6215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steinert G, Scholch S, Niemietz T, Iwata N, Garcia SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, Rahbari NN, Buchler MW, Stoecklein NH, Weitz J, Koch M. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 2014;74:1694–704. [DOI] [PubMed] [Google Scholar]
- 9. Lee TK, Cheung VC, Lu P, Lau EY, Ma S, Tang KH, Tong M, Lo J, Ng IO. Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology 2014;60:179–91. [DOI] [PubMed] [Google Scholar]
- 10. Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013;339:971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA 2012;109:6662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 13. Sick E, Boukhari A, Deramaudt T, Ronde P, Bucher B, Andre P, Gies JP, Takeda K. Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway. Glia 2011;59:308–19. [DOI] [PubMed] [Google Scholar]
- 14. Chao MP, Tang C, Pachynski RK, Chin R, Majeti R, Weissman IL. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood 2011;118:4890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, Otten MA, Finetti P, Van Egmond M, Matozaki T, Kraal G, Birnbaum D, van Elsas A, Kuijpers TW, Bertucci F, van den Berg TK. CD47-signal regulatory protein-alpha (SIRPalpha) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci USA 2011;108:18342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cioffi M, Trabulo S, Hidalgo M, Costello E, Greenhalf W, Erkan M, Kleeff J, Sainz B Jr, Heeschen C. Inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin Cancer Res. 2015;21:2325–37. [DOI] [PubMed] [Google Scholar]
- 17. Xiao Z, Chung H, Banan B, Manning PT, Ott KC, Lin S, Capoccia BJ, Subramanian V, Hiebsch RR, Upadhya GA, Mohanakumar T, Frazier WA, Lin Y, Chapman WC. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett. 2015;360:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bauerle T, Wallwiener M, Holland-Letz T, Hofner T, Sprick M, Scharpff M, Marme F, Sinn HP, Pantel K, Weichert W, Trumpp A. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–44. [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Xu Z, Guo S, Zhang L, Sharma A, Robertson GP, Huang L. Intravenous delivery of siRNA targeting CD47 effectively inhibits melanoma tumor growth and lung metastasis. Mol Ther. 2013;21:1919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chao MP, Weissman IL, Majeti R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu JF, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, Xia B, Bi Q, Wang YP. CD47 blockade inhibits tumor progression human osteosarcoma in xenograft models. Oncotarget 2015;6:23662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyashita M, Ohnishi H, Okazawa H, Tomonaga H, Hayashi A, Fujimoto TT, Furuya N, Matozaki T. Promotion of neurite and filopodium formation by CD47: Roles of integrins, Rac, and Cdc42. Mol Biol Cell 2004;15:3950–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murata T, Ohnishi H, Okazawa H, Murata Y, Kusakari S, Hayashi Y, Miyashita M, Itoh H, Oldenborg PA, Furuya N, Matozaki T. CD47 promotes neuronal development through Src- and FRG/Vav2-mediated activation of Rac and Cdc42. J Neurosci. 2006;26:12397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lymn JS, Patel MK, Clunn GF, Rao SJ, Gallagher KL, Hughes AD. Thrombospondin-1 differentially induces chemotaxis and DNA synthesis of human venous smooth muscle cells at the receptor-binding level. J Cell Sci. 2002;115:4353–60. [DOI] [PubMed] [Google Scholar]
- 25. Barazi HO, Li Z, Cashel JA, Krutzsch HC, Annis DS, Mosher DF, Roberts DD. Regulation of integrin function by CD47 ligands. Differential effects on alpha vbeta 3 and alpha 4beta1 integrin-mediated adhesion. J Biol Chem. 2002;277:42859–66. [DOI] [PubMed] [Google Scholar]
- 26. Broom OJ, Zhang Y, Oldenborg PA, Massoumi R, Sjolander A. CD47 regulates collagen I-induced cyclooxygenase-2 expression and intestinal epithelial cell migration. PLoS One 2009;4:e6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Congote LF, Temmel N. The C-terminal 26-residue peptide of serpin A1 stimulates proliferation of breast and liver cancer cells: Role of protein kinase C and CD47. FEBS Lett. 2004;576:343–7. [DOI] [PubMed] [Google Scholar]
- 28. Rath GM, Schneider C, Dedieu S, Rothhut B, Soula-Rothhut M, Ghoneim C, Sid B, Morjani H, El Btaouri H, Martiny L. The C-terminal CD47/IAP-binding domain of thrombospondin-1 prevents camptothecin- and doxorubicin-induced apoptosis in human thyroid carcinoma cells. Biochim Biophys Acta 2006;1763:1125–34. [DOI] [PubMed] [Google Scholar]
- 29. Manna PP, Frazier WA. The mechanism of CD47-dependent killing of T cells: Heterotrimeric Gi-dependent inhibition of protein kinase A. J Immunol. 2003;170:3544–53. [DOI] [PubMed] [Google Scholar]
- 30. Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–5. [DOI] [PubMed] [Google Scholar]
- 31. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2002;2:489–501. [DOI] [PubMed] [Google Scholar]
- 32. Wojcikiewicz RJ, Luo SG. Phosphorylation of inositol 1,4,5-trisphosphate receptors by cAMP-dependent protein kinase. Type I, II, and III receptors are differentially susceptible to phosphorylation and are phosphorylated in intact cells. J Biol Chem. 1998;273:5670–7. [DOI] [PubMed] [Google Scholar]
- 33. Zeng X, Kinsella TJ. Mammalian target of rapamycin and S6 kinase 1 positively regulate 6-thioguanine-induced autophagy. Cancer Res. 2008;68:2384–90. [DOI] [PubMed] [Google Scholar]