Abstract
Because of the characteristics of high invasiveness, relapse, and poor prognosis, the management of malignant gliomas has always been a great challenge. Nod-like receptor (NLR) family pyrin domain containing 3 (NLRP3) is a crucial component of the NLRP3 inflammasome, a multiprotein complex that can trigger caspase 1/interleukin-1 (IL-1)-mediated inflammatory response once activated and participates in the pathogeny of diverse inflammatory diseases as well as cancers. We examined the function of NLRP3 in the development of glioma. Glioma cells were treated with NLRP3 interference or overexpression vectors, recombinant IL-1β, IL-1β antibody, and NF-κB inhibitor. Cell proliferation and invasion were assessed by CCK-8 and Transwell assays. Gene expression was detected by PCR, Western blot, and ELISA. NLRP3 and NF-κB p65 increased and were positively correlated in glioma tissues. NLRP3 knockdown suppressed glioma cell growth and invasion with the decrease of IL-1β and NF-κB p65. Conversely, forced expression of NLRP3 promoted cell growth. NLRP3 silencing suppressed ectogenous IL-1β-elevated cell proliferation and invasion, whereas IL-1β elimination impaired the proproliferation effect of NLRP3 hyperexpression. Furthermore, NF-κB blockage abrogated IL-1β and NLRP3 hyperexpression increased cell growth and invasion. NLRP3 promoted the growth and invasion of gliomas via the IL-1β/NF-κB p65 signals.
Key words: Glioma, NLRP3, Interleukin-1β (IL-1β), NF-κB
INTRODUCTION
Malignant gliomas are lethal primary brain tumors and have the characteristics of high invasiveness, relapse, and poor prognosis1. Considering the difficulty of complete resection resulting from the diffuse infiltration, adjuvant therapies, such as radiotherapy, cytotoxic chemotherapy, and immunological and targeted therapies, are frequently applied following surgery2,3. Even so, the benefits are still unoptimistic, especially for glioblastoma, only 5.5% of which can survival for 5 years after diagnosis4. Therefore, it is important to explore the underlying mechanisms of glioma metastasis.
Numerous studies have paid attention to the effect of inflammasome, a multiprotein complex that can trigger caspase 1/interleukin-1 (IL-1)/IL-18-mediated inflammatory response once activated by the stimuli of microbes or intracellular noxious substances5,6. According to the difference of a pivotal component, including AIM2, NLRP1, NLRP3, NLRC4, and NLRP6, inflammasomes are divided into different subtypes7. Nod-like receptor family pyrin domain containing 3 (NLRP3) is a member of pattern recognition receptors. It is a pivotal component of the NLRP3 inflammasome and is implicated in the occurrence of many diseases8–10. Moreover, some studies reveal the important role of NLRP3 inflammasome in human cancers. For example, suppression of the elevated NLRP3 inflammasome with a specific inhibitor impaired the survival and invasion capacities of A253 cells11. Excessive NLRP3 inflammasome activation accounted for the development of 5-fluorouracil resistance in oral squamous cell carcinoma12.
However, the function of NLRP3 in gliomas is still elusive. To better understand the pathogenesis of gliomas, we performed this research to investigate the adjustment mechanisms of NLRP3.
MATERIALS AND METHODS
Antibodies and Reagents
The antibodies and reagents used were as follows: NLRP3 (Ab210491; Abcam, Cambridge, MA, USA), nuclear factor κB (NF-κB) p65 [#8242; Cell Signaling Technology (CST), Danvers, MA, USA], IL-1β (Ab156791; Abcam); GAPDH (#5174; CST), anti-IL-1β (AB8320; Abcam), recombinant human IL-1β (201-LB ; RD), PDTC (P8765; Sigma-Aldrich, St. Louis, MO, USA).
Tissue Samples and Cells
Tumor tissues and adjacent normal tissues were collected from glioma patients who underwent surgery in The First Affiliated Hospital of Kunming Medical University. Informed consent from the all patients was obtained in advance. U251 and U87 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from HyClone (SH30243.01; Logan, UT, USA). Fetal bovine serum (FBS) was purchased from Gibco (16000-044; Grand Island, NY, USA). The cells were cultured in DMEM containing 10% FBS and 1% penicillin–streptomycin (PS) in a 37°C incubator with 5% CO2.
Cell Transfection
Interference sequence that targets NLRP3 (GCTGTAACATTCGGAGATT, position 3052) was synthesized and integrated into pLKO.1 plasmid (Addgene, Cambridge, MA, USA). Then the expression vectors were transformed into competent cells of DH5a (TransGen, Beijing, P.R. China). The bacteria solution was coated on LB agar plates and cultured. The transformants that contained pLKO.1-shNLRP3 plasmids were authenticated by polymerase chain reaction (PCR) and gene sequencing. Finally, pLKO.1-shNLRP3, psPAX2, and pMD2G plasmids were isolated and cotransfected into 293T cells for the packaging of recombinant lentiviral vectors. The culture supernatants were collected. For the construction of NLRP3 hyperexpression vectors, the coding sequence of NLRP3 was integrated into pLVX-Puro plasmids. The remaining procedures were similar with those mentioned above. Glioma cells were divided in different groups and transfected with different recombinant lentiviral vectors.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated from samples by TRIzol (1596-026; Invitrogen Life Technologies, Carlsbad, CA, USA), and cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (#K1622; Thermo Fisher Scientific Inc., Rockford, IL, USA). Reaction was performed on the ABI 7300 Real-Time PCR System (Applied Biosystems Life Technologies, Foster City, CA, USA) with the SYBR Green qPCR Master Mixes (#K0223; Thermo Fisher Scientific Inc.). Relative mRNA quantity was calculated by the 2−ΔCt method. The primers used were as follows: NLRP3 (NM_001079821.2), 5′-CTGGAGGATGTGGACTTG-3′ (forward) and 5′-GTCTGCCTTCTCTGTCTG-3′ (reverse); NF-κB p65 (NM_001145138.1), 5′-GAATGGCTCGTCTGTAGTG-3′ (forward) and 5′-TGGTATCTGTGCTCCTCTC-3′ (reverse); GAPDH (NM_001256799.1), 5′-CACCCACTCCTCCACCTTTG-3′ (forward) and 5′-CCACCACCCTGTTGCTGTAG-3′ (reverse).
Western Blotting
Samples were treated with RIPA Lysis Buffer (Solarbio, Beijing, P.R. China) to extract the total protein. The proteins were quantified and stored at −20°C before use, and 10% sodium dodecyl sulfate polyacrylamide gel was prepared to isolate the proteins. After transferring to the nitrocellulose membrane, the bands were blocked with 5% nonfat milk. Then the corresponding primary and secondary antibodies were diluted to appropriate concentrations and added to the protein bands, respectively. Finally, the protein bands were scanned with Tanon 5200 (Tanon, Shanghai, P.R. China). Integrated density value was used to calculate the relative protein quantity.
Enzyme-Linked Immunosorbent Assay (ELISA)
IL-1β concentrations were detected with the human IL-1β ELISA Kit by reference to the manufacturer’s instructions. Briefly, cell culture supernatant was collected for use. Blank and sample wells were set on the ELISA plate. Sample dilution (40 μl) and 10 μl of cell culture supernatant were added to the sample wells. After incubating for 30 min at 37°C, the wells were washed with washing buffer and filled with 50 μl of horseradish peroxidase (HRP)-conjugated reagent. After incubating and washing as above, chromogen solution was added to each well. Finally, stop solution was used to halt the reaction and the absorbance at 450 nm was detected. IL-1β concentrations were calculated according to the standard curve.
Cell Counting Kit-8 (CCK-8) Assay
U87 and U251 cells were seeded into 96-well plates (3 × 103/well) and cultured overnight. Then the cells were divided into different groups and exposed to different treatments. After incubating for 0, 24, 48, and 72 h, CCK-8 solution was added to each well. The absorbance at 450 nm was detected to evaluate cell viability.
Transwell Assay
U87 and U251 cells were seeded into six-well plates and divided into different groups. After treatment for 48 h, cells were digested and added to the chambers coated with Matrigel (9 × 104/well). Forty-eight hours later, the chambers were immersed in 4% paraformaldehyde and 0.5% crystal violet solution consecutively. Cell counting was performed under a microscope.
Statistical Analysis
All the experiments were performed at least three times. Results were presented as mean ± standard deviation (SD) and were analyzed with GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA). Student’s t-test and one-way analysis of variance (ANOVA) were applied to the analysis. The correlation of NLRP3 and NF-κB p65 expression in tissue samples was examined by Bravais–Pearson correlation coefficient. A value of p < 0.05 was regarded as statistically significant.
RESULTS
NLRP3 and NF-κB p65 Were Upregulated and Positively Correlated in Glioma Tumor Tissues
It has been reported that the NF-κB pathway is implicated in inflammatory response and tumorigenesis13,14. To investigate the expression of NLRP3 and NF-κB p65 in gliomas, 40 glioma tumor tissues and 18 adjacent normal tissues from glioma patients were collected for qRT-PCR and Western blot analysis. As shown in Figure 1A and B, NLRP3 and NF-κB p65 transcription increased in glioma tumor tissues compared with normal tissues. Moreover, the mRNA level of NLRP3 was positively correlated with that of NF-κB p65 (r = 0.7333, two-tailed p < 0.0001) (Fig. 1C). Consistent with the elevated transcription, NLRP3 and NF-κB p65 protein levels also increased (Fig. 1D and E). In summary, upregulated NLRP3 might promote glioma formation and progression by activating NF-κB signaling.
Figure 1.
NLRP3 and nuclear factor κB (NF-κB) p65 were upregulated and positively correlated in glioma tumor tissues. The mRNA levels of NLRP3 and NF-κB p65 were detected by quantitative real-time polymerase chain reaction (qRT-PCR) in glioma tumor tissues (n = 40) and adjacent normal tissues (n = 18). The protein levels were detected by Western blot in 12 pairs of tissue specimens. (A) Relative mRNA levels of NLRP3. (B) Relative mRNA levels of NF-κB p65. (C) Correlation analysis of NLRP3 and NF-κB p65 expression in glioma tumor tissues. (D) Western blot results of NLRP3 and NF-κB p65 expression. (E) Quantification of NLRP3 and NF-κB p65 expression. ***p < 0.001.
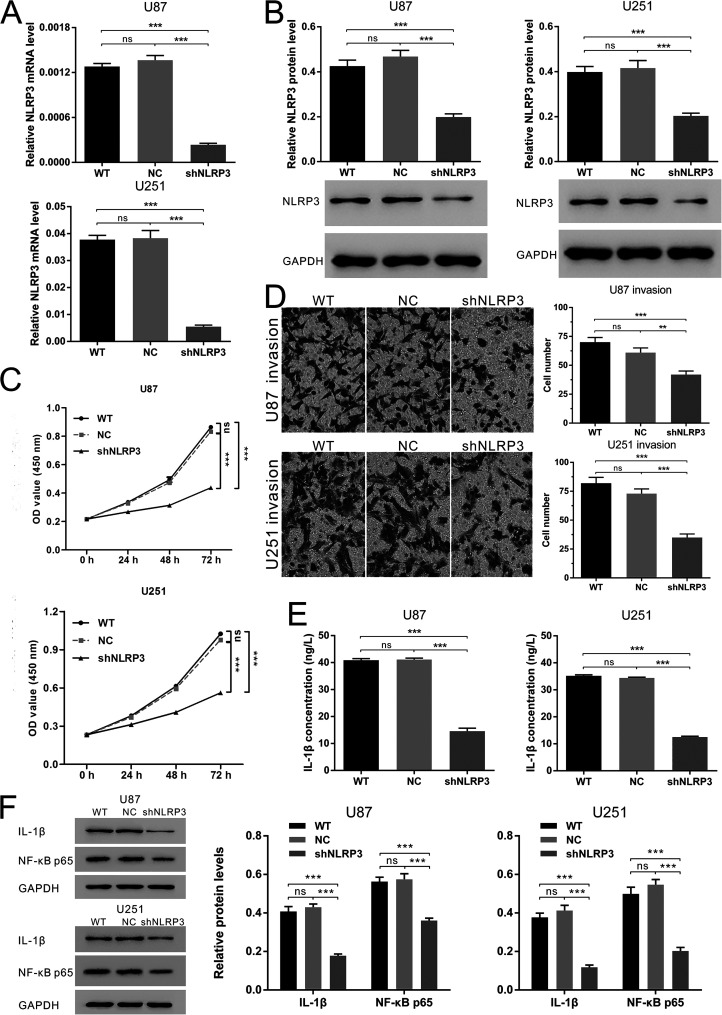
NLRP3 Knockdown Suppressed Glioma Cell Growth and Invasion Through the Decrease of IL-1β and NF-κB p65
Next, we examined the biological function of NLRP3 in shRNA-mediated NLRP3-silenced glioma cell lines. NLRP3-shRNA was transferred into U87 and U251 cells by lentiviral vectors to block the expression of NLRP3. Transfection efficiency was verified by qRT-PCR and Western blot (Fig. 2A and B). As shown in Figure 2C and D, cell proliferation and invasion were significantly suppressed with NLRP3 silencing. Because of the regulation of NLRP3 inflammasome on the generation and secretion of bioactive IL-1β, we performed ELISA to detect the extracellular IL-1β. As expected, NLRP3 knockdown reduced IL-1β concentration in cell culture supernatants (Fig. 2E). Western blot further revealed the downregulation of intracellular IL-1β. These results partly indicated the attenuation of NLRP3 inflammasome activation. In addition, NF-κB p65 reduced with NLRP3 silencing. In conclusion, NLRP3 silencing might suppress glioma cell growth and invasion via the NLRP3 inflammasome pathway-mediated NF-κB p65 decrease.
Figure 2.
NLRP3 knockdown suppressed glioma cell growth and invasion through the decrease of interleukin-1β (IL-1β) and NF-κB p65. NLRP3 expression was transferred with shRNA in U87 and U251 cells to investigate the function of NLRP3 on cellular processes and the signal pathways involved. (A) The results of qRT-PCR to identify the silencing of NLRP3. (B) The results of Western blot to identify the silencing of NLRP3. (C) Cell counting kit-8 (CCK-8) assay for cell proliferation after NLRP3 silencing. (D) Transwell assay for cell invasion after NLRP3 silencing. All the pictures were obtained from the visual fields under a microscope (200×). (E) IL-1β secretion detected by enzyme-linked immunosorbent assay (ELISA). (F) Western blot results of IL-1β and NF-κB p65 expression. WT: normal cultured glioma cells; NC: glioma cells transfected with empty vectors; shNLRP3: glioma cells transfected with NLRP3-shRNA; ns: no significant difference. **p < 0.01. ***p < 0.001.
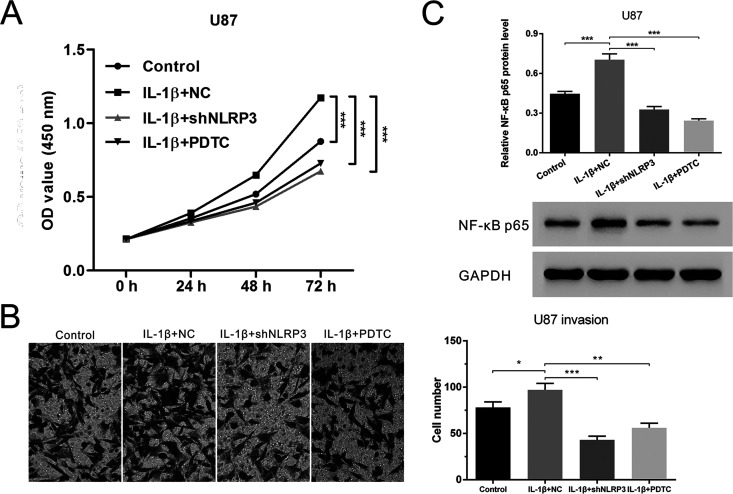
NLRP3 Silencing or NF-κB Blockage Abrogated IL-1β-Increased Cell Proliferation and Invasion
Recent studies reported that IL-1β stimulated cell proliferation and migration15,16. In addition, NLRP3 inflammasome activation could stimulate IL-1β secretion. We added exogenous IL-1β to glioma cells to simulate the excessive activation of NLRP3 inflammasome and further investigated the impact of NLRP3 on glioma cell survival and invasion. U87 cells were pretreated with 5 ng/ml of recombinant human IL-1β before transfection with NLRP3-shRNA or treatment with PDTC. CCK-8 assay demonstrated that IL-1β promoted cell growth. Moreover, the proproliferation effect was significantly inhibited in the presence of NLRP3-shRNA or PDTC (Fig. 3A). Similarly, IL-1β-increased U87 invasion and NF-κB p65 expression were suppressed with NLRP3 knockdown or NF-κB blockage (Fig. 3B and C). Collectively, both NLRP3 silencing and NF-κB blockage could suppress IL-1β-elevated proliferation and invasion capacities of glioma cells.
Figure 3.
NLRP3 silencing or NF-κB blockage abrogated IL-1β-increased cell proliferation and invasion. U87 cells were pretreated with IL-1β and then transfected with NLRP3-shRNA or treated with PDTC. (A) Cell growth detected by CCK-8 assay. (B) Cell invasion detected by Transwell assay. (C) NF-κB p65 expression detected by Western blot. Control: normal cultured U87 cells; IL-1β + NC: U87 cells treated with IL-1β and transfected with empty vectors; IL-1β + shNLRP3: U87 cells treated with IL-1β and transfected with NLRP3-shRNA; IL-1β + PDTC: U87 cells treated with IL-1β and PDTC. *p < 0.05, **p < 0.01, ***p < 0.001.
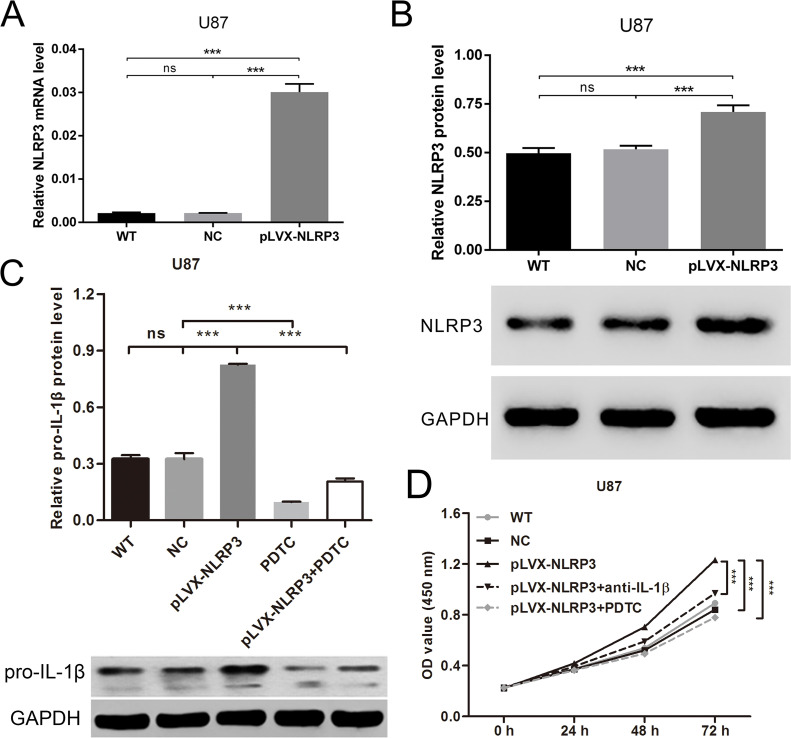
IL-1β Elimination or NF-κB Blockage Abrogated NLRP3 Hyperexpression-Increased Cell Proliferation
NLRP3-hyperexpressed U87 cells were used to examine the effect of NLRP3 from another perspective. Figure 4A and B demonstrates that NLRP3 transcription and translation were enhanced by pLVX-NLRP3, suggesting the hyperexpression of NLRP3. To examine whether the pro-IL-1β amount is enough after NLRP3 overexpression and the effect of NF-κB blocking on pro-IL-1β levels, the pro-IL-1β expression response to NLRP3-pLVX transfection and/or PDTC treatment was measured by Western blot. As shown in Figure 4C, NLRP3 overexpression significantly increased the pro-IL-1β level and PDTC treatment decreased the pro-IL-1β level in U87 cells. Thus, depletion of endogenous IL-1β with neutralizing antibody was performed to simulate the blockage of NLRP3 inflammasome activation. According to the CCK-8 assay (Fig. 4D), forced expression of NLRP3 promoted cell growth, whereas IL-1β depletion could partly attenuate the promotion effect. Analogously, PDTC inhibited NLRP3 hyperexpression-accelerated cell growth. Taken together, NLRP3 could promote glioma cell proliferation via the IL-1β/NF-κB p65 signals.
Figure 4.
IL-1β elimination or NF-κB blockage abrogated NLRP3 hyperexpression and increased cell proliferation. U87 cells were transfected with NLRP3-pLVX and then treated with IL-1β neutralizing antibody or PDTC. (A) Forced expression of NLRP3 detected by qRT-PCR. (B) Forced expression of NLRP3 detected by Western blot. U87 cells were transfected with NLRP3-pLVX and/or treated with PDTC. (C) The expression of pro-IL-1β was measured by Western blot. (D) Cell growth detected by CCK-8 assay. WT: normal cultured U87 cells; NC: U87 cells transfected with empty vectors; pLVX-NLRP3: U87 cells transfected with NLRP3-pLVX; pLVX-NLRP3 + anti-IL-1β: U87 cells transfected with NLRP3-pLVX and treated with IL-1β neutralizing antibody; pLVX-NLRP3 + PDTC: U87 cells transfected with NLRP3-pLVX and treated with PDTC. ***p < 0.001.
DISCUSSION
Inflammation is an essential defense response relying on innate immunity. Transitory inflammatory reaction can protect host cells against harmful factors, such as infection and tissue damage. However, prolonged inflammatory reaction is important for the pathogenesis of numerous diseases, including cancers17–19. On the one hand, chronic inflammation-mediated cytokine secretion constitutes the inflammatory microenvironment, which impacts the biological processes of cells. For example, IL-1 altered the secretome associated with tumor progression in glioma20. On the other hand, tumor microenvironment-recruited immune cells can facilitate tumor metastasis through cytokine-mediated signaling cascades. For instance, B cells promoted bladder cancer metastasis though the regulation of IL-8 on the downstream androgen receptor and MMP signals21. Pattern recognition receptors are needed for inflammation initiation. Consequently, NLRP3 may play a crucial role in tumorigenesis and metastasis.
In the present study, NLRP3 knockdown and hyperexpression were used to identify whether NLRP3 could alert the malignant phenotypes of glioma cell lines. Previous studies demonstrated the regulatory effects of the NF-κB pathway on innate immune and inflammation. Dysregulation of NF-κB is implicated in diverse autoimmune and inflammatory diseases13,22. According to the detection in clinical samples, NLRP3 and NF-κB p65 increased in glioma tissues compared with adjacent normal tissues, and the mRNA levels were positively correlated. NLRP3 silencing remarkably suppressed the growth and invasion of glioma cell lines with the decrease of IL-1β and NF-κB p65 expression. Conversely, forced expression of NLRP3 promoted glioma cell growth. According to a previous study, IL-1β could stimulate glioma cell proliferation, migration, and invasion16. Our research confirmed the facilitation effects with the application of recombinant human IL-1β. Moreover, NLRP3 knockdown could reverse IL-1β-elevated proliferation and invasion. IL-1β neutralizing antibody also weakened the proproliferation effect of NLRP3 hyperexpression. Furthermore, PDTC-induced NF-κB blockage antagonized the impacts of IL-1β and NLRP3 hyperexpression. All of these suggest that NLRP3 affects glioma formation and progression via the IL-1β/NF-κB p65 signals.
Tarassishin et al. investigated the role of IL-1β in malignant gliomas23. Results showed that IL-1β expression and secretion increased in glioma cells compared with human astrocytes and were regulated by annexin A2. The secretome of glioma cells induced by IL-1β could promote angiogenesis. Furthermore, NLRP3 inflammasome was implicated in the processing of IL-1β. Another research study showed that NLRP3 silencing with shRNA suppressed the expression of caspase 1 and IL-1β and decelerated tumor growth in U87 xenograft mouse model24, which revealed the anticancer effect of NLRP3 silencing in vivo. Different from these studies, our present study emphasized the specific effect of NLRP3 on glioma formation and progression through NLRP3 knockdown or overexpression. In addition, we investigated the function of NLRP3 on several cellular processes in glioma cell lines, such as cell proliferation and invasion. Except for the momentous discoveries, there are also some limitations of this study. The expression of other components of NLRP3 inflammasome, such as caspase 1, is not detected after NLRP3 silencing or hyperexpression. In addition, the relevant downstream molecules of the NF-κB pathway are still unknown, which needs further investigation.
Not only NF-κB but also JAK/STAT and MAPK signaling pathways are required for innate immunity25,26. Whether they are correlated with NLRP3 inflammasome in cancers is worthy of exploration. It has been reported that some agents exert pharmacological activities though the inhibition of NLRP3 inflammasome and NF-κB signaling, such as the anti-inflammatory effect of rhein in vitro and in vivo27, suppression of lung injury by silybin in mice28, and the treatment for imiquimod-induced psoriasis by BAY 11-708229. Therefore, the application of targeting NLRP3 in cancer therapy needs to be further validated.
In conclusion, as a pivotal component of the NLRP3 inflammasome, NLRP3 exerts carcinogenesis in gliomas through the proinflammatory IL-1β/NF-κB signaling. Anti-NLRP3 may be a promising therapy option for gliomas.
ACKNOWLEDGMENTS
The study was supported by the Applied Basic Project of Yunnan Province (Joint Grant of Kunming Medical University) (2015FB48) and the Special Grant for High-level Personnel of Yunnan Province (D-201619).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Altieri R, Agnoletti A, Quattrucci F, Garbossa D, Calamo Specchia FM, Bozzaro M, Fornaro R, Mencarani C, Lanotte M, Spaziante R, Ducati A. Molecular biology of gliomas: Present and future challenges. Transl Med UniSa. 2014;10(7):29–37. [PMC free article] [PubMed] [Google Scholar]
- 2. Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E, Balana C, Chinot O, Bendszus M, Reijneveld JC, Dhermain F, French P, Marosi C, Watts C, Oberg I, Pilkington G, Baumert BG, Taphoorn MJB, Hegi M, Westphal M, Reifenberger G, Soffietti R, Wick W. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–29. [DOI] [PubMed] [Google Scholar]
- 3. Ghotme KA, Barreto GE, Echeverria V, Gonzalez J, Bustos RH, Sanchez M, Leszek J, Yarla NS, Gomez RM, Tarasov VV, Ashraf GM, Aliev G. Gliomas: New perspectives in diagnosis, treatment and prognosis. Curr Top Med Chem. 2017;17(12):1438–47. [DOI] [PubMed] [Google Scholar]
- 4. Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18(Suppl 5):v1–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10(2):417–26. [DOI] [PubMed] [Google Scholar]
- 6. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Broz P, Dixit VM. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–20. [DOI] [PubMed] [Google Scholar]
- 8. Wree A, McGeough MD, Inzaugarat ME, Eguchi A, Schuster S, Johnson CD, Pena CA, Geisler LJ, Papouchado BG, Hoffman HM, Feldstein AE. NLRP3 inflammasome driven liver injury and fibrosis: Roles of IL-17 and TNF. Hepatology 2018;67(2):736–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464(7293):1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013;493(7434):674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bae JY, Lee SW, Shin YH, Lee JH, Jahng JW, Park K. P2X7 receptor and NLRP3 inflammasome activation in head and neck cancer. Oncotarget 2017;8(30):48972–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng X, Luo Q, Zhang H, Wang H, Chen W, Meng G, Chen F. The role of NLRP3 inflammasome in 5-fluorouracil resistance of oral squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12(8):715–23. [DOI] [PubMed] [Google Scholar]
- 14. Karin M, Greten FR. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–59. [DOI] [PubMed] [Google Scholar]
- 15. Eun SY, Ko YS, Park SW, Chang KC, Kim HJ. IL-1beta enhances vascular smooth muscle cell proliferation and migration via P2Y2 receptor-mediated RAGE expression and HMGB1 release. Vascul Pharmacol. 2015;72:108–17. [DOI] [PubMed] [Google Scholar]
- 16. Fathima Hurmath K, Ramaswamy P, Nandakumar DN. IL-1beta microenvironment promotes proliferation, migration, and invasion of human glioma cells. Cell Biol Int. 2014;38(12):1415–22. [DOI] [PubMed] [Google Scholar]
- 17. Hussain SP, Harris CC. Inflammation and cancer: An ancient link with novel potentials. Int J Cancer 2007;121(11):2373–80. [DOI] [PubMed] [Google Scholar]
- 18. Luo C, Zhang H. The role of proinflammatory pathways in the pathogenesis of colitis-associated colorectal cancer. Mediators Inflamm. 2017;2017:5126048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng Z, Sze CW, Keng CT, Al-Haddawi M, Liu M, Tan SY, Kwek HL, Her Z, Chan XY, Barnwal B, Loh E, Chang KTE, Tan TC, Tan YJ, Chen Q. Hepatitis C virus mediated chronic inflammation and tumorigenesis in the humanised immune system and liver mouse model. PLoS One 2017;12(9):e0184127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarassishin L, Lim J, Weatherly DB, Angeletti RH, Lee SC. Interleukin-1-induced changes in the glioblastoma secretome suggest its role in tumor progression. J Proteomics 2014;99(1):152–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ou Z, Wang Y, Liu L, Li L, Yeh S, Qi L, Chang C. Tumor microenvironment B cells increase bladder cancer metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs signals. Oncotarget 2015;6(28):26065–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun SC. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17(9):545–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tarassishin L, Casper D, Lee SC. Aberrant expression of interleukin-1beta and inflammasome activation in human malignant gliomas. PLoS One 2014;9(7):e103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li L, Liu Y. Aging-related gene signature regulated by Nlrp3 predicts glioma progression. Am J Cancer Res. 2015;5(1):442–9. [PMC free article] [PubMed] [Google Scholar]
- 25. Yan Z, Gibson SA, Buckley JA, Qin H, Benveniste EN. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin Immunol. 2016;189:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13(9):679–92. [DOI] [PubMed] [Google Scholar]
- 27. Ge H, Tang H, Liang Y, Wu J, Yang Q, Zeng L, Ma Z. Rhein attenuates inflammation through inhibition of NF-kappaB and NALP3 inflammasome in vivo and in vitro. Drug Des Devel Ther. 2017;11:1663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang B, Wang B, Cao S, Wang Y, Wu D. Silybin attenuates LPS-induced lung injury in mice by inhibiting NF-kappaB signaling and NLRP3 activation. Int J Mol Med. 2017;39(5):1111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Irrera N, Vaccaro M, Bitto A, Pallio G, Pizzino G, Lentini M, Arcoraci V, Minutoli L, Scuruchi M, Cutroneo G, Anastasi GP, Ettari R, Squadrito F, Altavilla D. BAY 11-7082 inhibits the NF-kappaB and NLRP3 inflammasome pathways and protects against IMQ-induced psoriasis. Clin Sci. 2017;131(6):487–98. [DOI] [PubMed] [Google Scholar]