Abstract
The long noncoding RNA (lncRNA) H19 has been described to participate in the metastasis of various tumors. Nevertheless, whether H19 promotes or impedes tongue squamous cell carcinoma (TSCC) cell migration and invasion remains controversial. Here we found that the expression of H19 was elevated in TSCC tissues compared with adjacent normal tissues. Moreover, we demonstrated that the expression of H19 was higher in metastasized tumors compared with unmetastasized tumors. Consistently, TSCC cells express higher levels of H19 than human squamous cells. Subsequently, depletion of H19 impaired the migration and invasion abilities of TSCC cells. Mechanistically, we demonstrated that H19 functions as a competing endogenous RNA (ceRNA) to sponge miRNA let-7a, leading to an increase in a let-7a target, the key regulator of tumor metastasis HMGA2, which is enriched in TSCC tissues and cell lines. Intriguingly, inhibition of let-7a significantly rescued the short hairpin H19 (shH19)-induced decrease in TSCC migration and invasion. These findings revealed that the H19/let-7a/HMGA2/EMT axis plays a critical role in the regulation of TSCC migration and invasion, which may provide a new therapeutic target for TSCC.
Key words: H19, HMGA2, Epithelial–mesenchymal transition (EMT), Migration, Invasion, Tongue squamous cell carcinoma (TSCC)
INTRODUCTION
Tongue squamous cell carcinoma (TSCC) is one of the most common and devastating oral cancers1–3. Despite tremendous improvements in multimodal diagnosis and treatment techniques in the past few decades, the prognosis for patients with TSCC has remained relatively unchanged for the past three decades because of its proclivity for local invasion and distant metastasis4. Therefore, increased understanding of the complex migration and invasion mechanisms of TSCC cells is imperative to the development of effective and mechanism-based therapeutic modalities for this malignancy.
Long noncoding RNAs (lncRNAs) are defined as a class of non-protein-coding transcripts (>200 nucleotides long)5. An increasing number of lncRNAs have been identified to be involved in some important biological processes, such as imprinting control, cell differentiation, and tumorigenesis6–8. Recently, increasing evidence suggests that lncRNA H19 plays an important role in the tumorigenesis and metastasis of diverse cancers9–11. For example, H19-derived miR-675 promotes tumorigenesis and metastasis via downregulating c-Cbl and Cbl-b in breast cancer12. Moreover, H19 sponged let-7 to release LIN28, subsequently promoting breast cancer stem cell maintenance13. H19 also serves as a competing endogenous RNA (ceRNA) to regulate the bioavailability of tumor suppressor let-7 during the process of endometrial cancer metastasis11. In pancreatic carcinoma, H19 facilitates tumor metastasis via H19–let-7–high-mobility group AT-hook 2 (HMGA2)-mediated epithelial–mesenchymal transition (EMT)14. Although these findings have indicated that H19 has an important function in regulating tumorigenicity and metastasis of various tumors, little is known about the role of H19 in the metastasis of TSSC and the underlying mechanisms.
HMGA2, a DNA-binding protein, acts as an architectural transcription regulator that can assemble and maintain enhanceosomes15,16. HMGA2 is aberrantly expressed in many types of cancer and regulates the level of both oncogenes and tumor suppressors17. A high level of HMGA2 in cancer is associated with increased invasiveness, stemness, and poor prognosis18–21. A recent study demonstrated that overexpression of HMGA2 promotes tongue cancer metastasis through the EMT pathway22.
Our study demonstrates that H19 is highly expressed in TSCC tissues and cell lines. Depletion of H19 attenuates the migration and invasion capabilities of TSCC cells. Moreover, H19-mediated TSCC cell migration and invasion were through HMGA2-associated EMT in a let-7-dependent manner. Inhibition of let-7 significantly rescued the short hairpin H19 (shH19)-mediated decrease in TSCC cell migration and invasion. In conclusion, our study revealed the role of H19 in TSCC cell migration and invasion and shed light on the mechanism of the H19/let-7/HMGA2 pathway.
MATERIALS AND METHODS
Clinical Samples and Cell Lines
All TSCC samples were obtained from 16 diagnosed patients with prior patient consent and the approval of the Institutional Clinical Ethics Review Board in the Dalian Stomatological Hospital (Table 1). Samples were frozen in liquid nitrogen for RNA extraction. The human TSCC cell lines CAL27, SCC9, SCC15, and SCC25, and FaDu, a human squamous cell line derived from the hypopharynx, were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). CAL27 cells were cultured in DMEM (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), and the other cells were cultured in RPMI-1640 (Thermo Scientific, Waltham, MA, USA) medium supplemented with 10% FBS. For all cell lines, 100 IU/ml penicillin and 100 μg/ml streptomycin were added to the culture medium, and all of the cells were incubated at 37°C in a humidified atmosphere of 95% air 5% CO2.
Table 1.
Demographic Information of the Tongue Squamous Cell Carcinoma (TSCC) Tissue Donors
| Patients | Sex | Age (Years) | Stage |
|---|---|---|---|
| 1 | Female | 48 | III |
| 2 | Female | 31 | III |
| 3 | Male | 55 | II |
| 4 | Male | 49 | I |
| 5 | Female | 45 | IV |
| 6 | Male | 51 | II |
| 7 | Female | 54 | II |
| 8 | Male | 40 | IV |
| 9 | Female | 51 | IV |
| 10 | Male | 36 | II |
| 11 | Male | 38 | I |
| 12 | Female | 58 | III |
| 13 | Male | 43 | IV |
| 14 | Female | 38 | II |
| 15 | Female | 40 | IV |
| 16 | Female | 48 | III |
Plasmid Construction
Wild-type human H19 (WT H19) and mutant human H19 (Mut H19) were constructed as previously described23, and psiCHECK2-let-7 4× was built according to the previous study24. The bioinformatics tool Miranda (http://www.microrna.org) was used to validate the let-7a binding sites within the full-length transcripts of HMGA2. The putative let-7a-binding site within HMGA2 3′-UTR fragments was inserted into the luciferase reporter vector psiCHECK2 (Promega, Madison, WI, USA) between the Xho1 and Not1 sites.
MicroRNA Mimic and MicroRNA Inhibitor Transfection
Transient transfection was performed using Lipofectamine 2000 (Invitrogen Life Techhinologies) following the manufacturer’s protocol. miRNA let-7 mimic, pre-miR negative control (NC), miRNA let-7 inhibitor, and anti-miR NC were purchased from Qiagen (let-7a; Cat. No. MS00006482; Valencia, CA, USA). All primer sequences are listed in Table 2.
Table 2.
MicroRNA (miRNA) Mimic and miRNA Inhibitor Sequences, shRNA Sequence, and Primers
| Sequence 5′>3′ | Antisequence 5′>3′ | |
|---|---|---|
| mlet-7 | UUGUACUACACAAAAGUACUG | CUAUACAACCUACUACCUCAUU |
| ilet-7 | AACUAUACAACCUACUACCUCA | |
| shH19-1 | GAGTTAGCAAAGGTGACATCT | |
| shH19-2 | GACCTCATCAGCCCAACATCA | |
| shNC | TTCTCCGAACGTGTCACGTTTC | |
| β-actin | ATCAAGATCATTGCTCCTCCTGAG | CTGCTTGCTGATCCACATCTG |
| H19 | GCACCTTGGACATCTGGAGT | TTCTTTCCAGCCCTAGCTCA |
| HMGA2 | AAGTTGTTCAGAAGAAGCCTGCTCA | TGGAAAGACCATGGCAATACAGAAT |
| Vimentin | ATTCAAGCATTCCAGCTCTCA | TTGACTGACCCTTGAAGTGGCGAT |
| E-cadherin | CCTAGACTTGTGCCATGATGC | ACCTAGCAAGTCCCGTCAACCA |
| N-cadherin | TGCCAACTGAAGTCGTGGATT | GGCACACCTGGTACCACTTTACA |
| Twist1 | TGACCTAACGTGGTACCGTAA | CGGATCCGATTTCCCGCGTTT |
| Zeb1 | ACGGTACTTAGGCAACCTGAC | TTAGCCTAGCCCGTAATGGCTT |
| Snail1 | TTACGGATCGGTAAACCGTTA | TTACGATCGAAGTACGTAATGC |
mlet-7, let-7 mimic; ilet-7, let-7 inhibitor; shH19, short hairpin H19; shNC, short hairpin negative control; HMGA2, high-mobility group AT-hook 2.
Stable Cell Lines
To establish stable CAL27-shH19 cell lines, shRNA lentiviruses (GenePharma, Shanghai, P.R. China) were added into six-well plates for 48 h. Cells were then selected in the presence of 1 mg/ml puromycin (Sigma-Aldrich, St. Louis, MO, USA) for at least 72 h. All primer sequences are listed in Table 2.
Dual-Luciferase Reporter Assay
CAL27 cells (1 × 104) were seeded into each well of 48-well plates, and the following steps were carried out as previously described25. Human CAL27 cells were cotransfected with 10 ng of the indicated luciferase reporter and 48 nM let-7a mimic using Lipofectamie 2000 (Invitrogen Life Technologies). After 48 h, the luciferase activities were eveluated by Dual-Luciferase Reporter Assay Kit (Promega); the relative luciferase activity was normalized to Renilla luciferase activity. Results represent the average of triplicate samples from three independent experiments.
Western Blot
Total proteins were extracted using RIPA buffer supplemented with PMSF, and protein concentrations were quantified using the BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Jiangsu, P.R. China) according to the manufacturer’s instructions. Equal amounts of proteins were separated by 10% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were then transferred to the nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). After blocking with TBST/5% nonfat milk for 1 h at room temperature, the membrane was incubated with antibodies against HMGA2 (1:2,000; 8179; Cell Signaling Technology, Inc., Danvers, MA, USA), vimentin (1:1,000; 5741; Cell Signaling Technology, Inc.), E-cadherin (1:1,000; ab1416; Abcam, Cambridge, UK), N-cadherin (1:1,000; ab18203; Abcam), and GAPDH (1:10,000; ab8245; Abcam) followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody (1:5,000; A0208 or A0216; Beyotime Institute of Biotechnology). Antibody binding was detected with an enhanced chemiluminescence kit (Amersham, Chiltern, UK) using Bio-Rad ChemiDoc XRS+ Imaging System following the manufacturer’s instructions.
RNA Extraction and RT-qPCR
Total RNA was extracted from CAL27 using the TRIzol reagent (Invitrogen Life Technologies) following the manufacturer’s instructions. The cDNA was generated with an oligo-dT primer using SuperScript III RT System (Invitrogen Life Technologies) in 20 μl of reaction mixture containing 5 μg of total RNA. RT-qPCR was performed using a ViiA7 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and the QuantiNova™ SYBR-Green PCR Kit (Qiagen) according to the manufacturer’s instructions. All primer sequences are listed in Table 2. Relative mRNA expression levels for the genes of interest were analyzed using the 2−ΔΔCt method as previously described26.
Transwell Migration Assay
The migration assay was carried out in 24-well chambers with polycarbonate 8-μm pore membrane filters (Transwell; Corning Life Sciences, Corning, NY, USA). CAL27 cells were resuspended in serum-free cell medium at 1 × 105 cells/ml. Cells (200 μl) were placed in the upper Transwell chambers, and 500 μl of growth medium supplemented with 10% FBS was used as an attractant in the lower chambers. After incubation at 37°C for 24 h, the migrated cells were fixed with 4% paraformaldehyde (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 20 min and stained with 0.5% crystal violet (Shanghai Sengon Company, P.R. China) for 5 min. After washing with distilled water, the images were obtained using an inverted microscope (Olympus, Tokyo, Japan), and five random fields at 10× magnification were counted.
Microfluidic Chip Fabrication
The tumor invasion microfluidic device adopted in the present study consisted of a PDMS (poly-dimethyl-siloxane; Silgard 184; Dow Chemical, Midland, MI, USA) microchannel layer and a bottom coverslip. It contains one central chamber (5,000 μm long, 1,000 μm wide, and 80 μm high) and two side channels (6,000 μm long, 500 μm wide, and 80 μm high). Matrigel (BD Biosciences, San Jose, CA, USA) mixed with the same volume of cell culture medium was injected into the central chamber. The microfluidic device was then incubated at 37°C for 20 min to allow complete solidification of the Matrigel. One of the side channels contained CAL27 cell suspension (1% FBS in DMEM) at a concentration of 6 × 106/ml, and another channel contained whole-cell culture medium (10% FBS in DMEM). After the tumor cells were injected into the chip, we incubated it at 37°C in an upright position for 2 h to settle the tumor cells on the hydrogel surface. Then the chip was returned to its original position.
Statistical Analysis
Data were expressed as mean ± SD of three independent experiments with GraphPad Prism software. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) software (version 16.0). The significant differences between the two groups were determined using the two-tailed Student’s t-test for unpaired data. A value of p < 0.05 was considered statistically significant.
RESULTS
H19 Was Upregulated in TSCC Tissues and Cell Lines
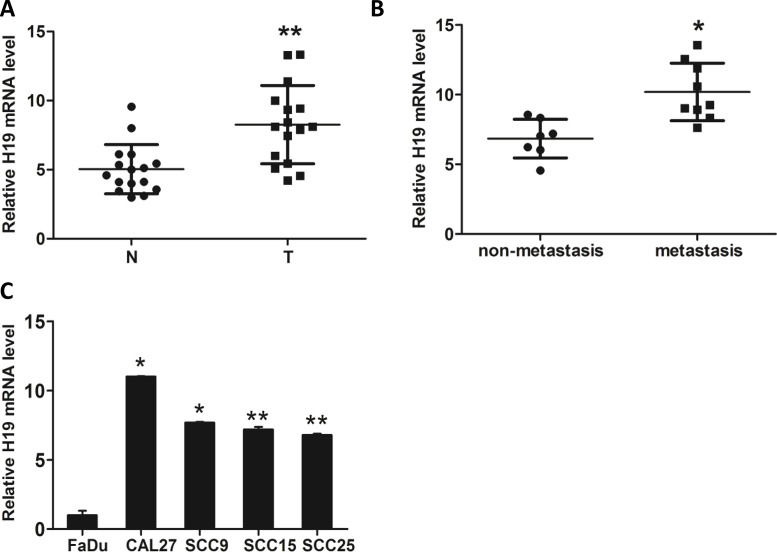
To investigate the role of lncRNA H19 in TSCC cell migration and invasion, we examined the expression of H19 in TSCC tissues and corresponding adjacent nontumorous tissues. We found that H19 was significantly highly expressed in TSCC tissues (Fig. 1A), especially in tissues that will metastasize (clinical II, III, and IV stages) (Fig. 1B). Moreover, the H19 level in four TSCC cell lines and one human squamous cell line was examined using RT-qPCR. H19 was significantly highly expressed in the TSCC cell lines CAL27, SCC9, SCC15, and SCC25 compared with the normal squamous cell line FaDu. The level of H19 in CAL27, which we selected to further study the molecular mechanisms, was the highest among all of the cell lines (Fig. 1C).
Figure 1.
Long noncoding RNA (lncRNA) H19 expression is assessed in tongue squamous cell carcinoma (TSCC) tissues and cell lines. (A) The expression of H19 in TSCC tissue and cell lines was detected by RT-qPCR. (B) RT-qPCR demonstrated that the expression of H19 in the metastasizing group (n = 9) was significantly higher than that in the nonmetastasis group (n = 7). (C) H19 levels were evaluated by RT-qPCR in four TSCC cell lines, and normal human epithelial squamous cells were used as controls. The relative H19 mRNA level was normalized to β-actin. The statistical differences were analyzed using the paired t-test (n = 3, *p < 0.05 , **p < 0.01).
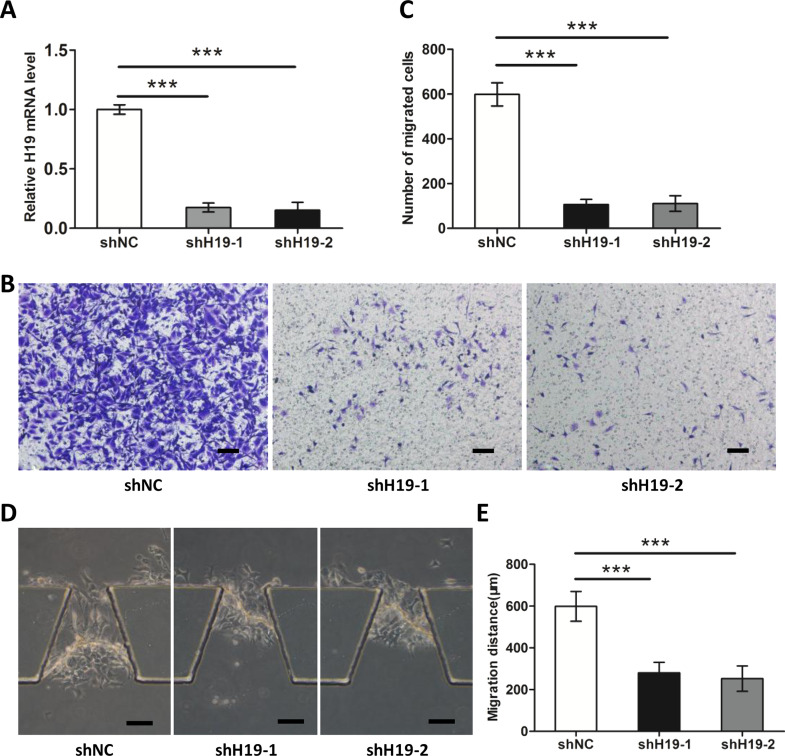
H19 Is Essential for TSCC Cell Migration and Invasion
To investigate the impact of H19 on TSCC cell migration and invasion, we established stable H19 knockdown CAL27 cells. Figure 2A showed the efficiency of shRNA-mediated downregulation of H19. The impact of H19 on the migration ability of TSCC cells was evaluated using Transwell assays. Figure 2B and C shows that downregulation of H19 significantly impaired the migration ability of CAL27 cells compared with shRNA control. Subsequently, we examined the invasion capacity of CAL27 with an invasion microfluidic device. Figure 2D and E shows that downregulation of H19 attenuated the migration distance of CAL27, representing an impaired invasion ability.
Figure 2.
H19 modulated migration and invasion of TSCC cells. (A) The interfering efficiency of the lentivirus encoding H19-targeting shRNAs (shH19) was confirmed by RT-qPCR, compared with negative control lentivirus [short hairpin negative control (shNC)]. Knockdown of H19 reduced TSCC cell migration (B, C) and invasion abilities (D, E). Scale bar: 100 μm. Numbers are mean ± SD (n = 3). ***p < 0.001.
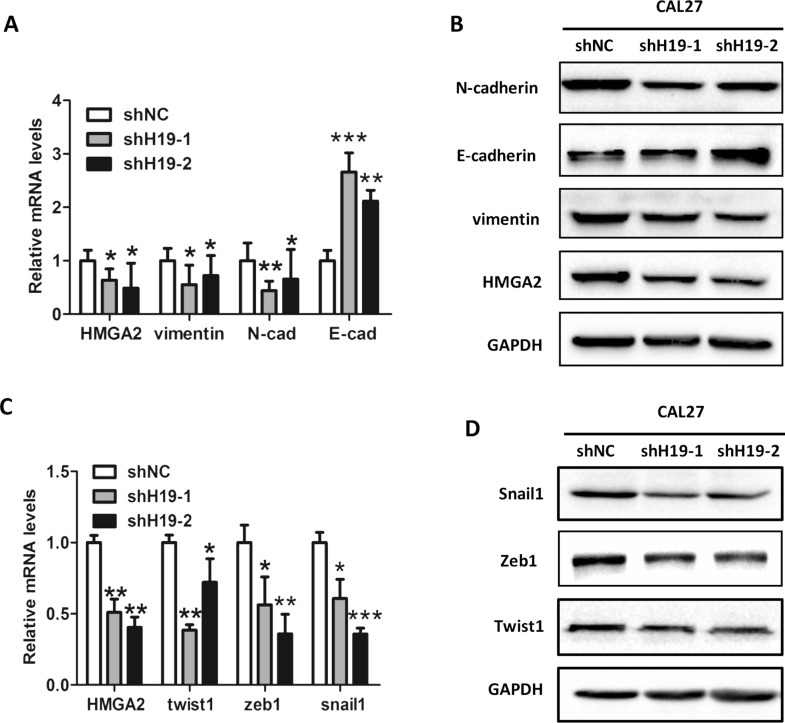
Knockdown of H19 Attenuates HMGA2-Mediated EMT
A previous study demonstrated that overexpression of HMGA2 promotes tongue cancer metastasis through the EMT pathway22, which prompted us to investigate if H19-mediated TSCC cell migration and invasion are associated with HMGA2-associated EMT. Our results showed that knockdown of H19 led to decreases in both the mRNA (Fig. 3A) and protein levels (Fig. 3B) of HMGA2, as well as HMGA2 downstream EMT markers vimentin and N-cadherin, but an increase in E-cadherin. In concordance, depletion of H19 results in decreases in mRNA (Fig. 3C) and protein levels (Fig. 3D) of EMT-related transcription factors, such as twist, zeb1, and snail1.
Figure 3.
Knockdown of H19 attenuates high-mobility group AT-hook 2 (HMGA2)-mediated epithelial–mesenchymal transition (EMT). The mRNA (A) and protein levels (B) of HMGA2, vimentin, N-cadherin, and E-cadherin were confirmed by RT-qPCR and Western blot in H19-depleted CAL27 cells. GAPDH was probed as the loading control. The mRNA (C) and protein levels (D) of EMT-related transcription factors twist, zeb1, and snail1 were confirmed by RT-qPCR and Western blot. GAPDH was probed as loading control. Numbers are mean ± SD (n = 3, *p < 0.05 , **p < 0.01, and ***p < 0.001).
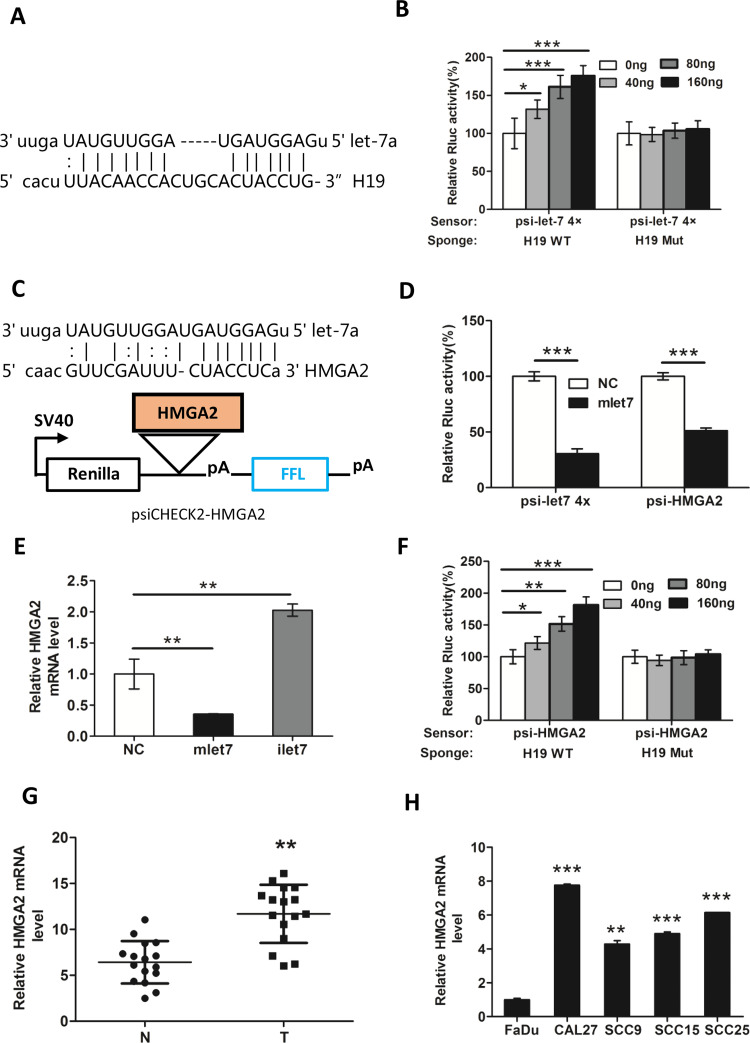
H19 Knockdown Decreased HMGA2 Expression in a let-7a-Dependent Manner
H19 was reported as a sponge to antagonize let-711. As shown in Figure 4A, the bioinformatics tool Miranda (http://www.microrna.org) was used to validate the let-7a binding sites within the full-length lncRNA H19 (both putative binding sites work). PsiCHECK2-let-7 4× (sensor) was transfected into CAL27 cells together with increasing amounts of WT H19 (WT, sponge) or H19 containing a mutated let-7 binding site (Mut H19). The Rluc activity increased in response to WT H19 but not Mut H19 in a dose-dependent manner (Fig. 4B), suggesting that H19 specifically sequestered endogenous let-7, thereby preventing it from inhibiting Rluc expression. Next, we cloned the 3′-UTR putative fragment of HMGA2 into the psiCHECK2 plasmid (psiCHECK-HMGA2) (Fig. 4C). PsiCHECK-HMGA2 and psiCHECK-let-7 4× were transfected into CAL27 cells together with let-7 mimic (mlet-7) in parallel with NCs. The results showed that mlet-7 significantly repressed the relative luciferase activity of reporter psiCHECK2-HMGA2 (Fig. 4D). In addition, HMGA2 mRNA level decreased in the presence of mlet-7 and increased in the presence of let-7a inhibitors (ilet-7) (Fig. 4E). Moreover, when psiCHECK-HMGA2 (sensor) and increasing amounts of WT H19 (sponge) were cotransfected into the CAL27 cells, the relative luciferase activity was elevated in response to WT H19, but not to Mut H19, in a dose-dependent manner (Fig. 4F). These results revealed that H19 can block the bioactivity of let-7a to release its target HMGA2, which was involved in TSCC cell migration and invasion. In addition, Figure 4G and H demonstrated that the mRNA levels of HMGA2 were significantly higher in TSCC tissues and cell lines than in normal tissues. Collectively, we found that the H19/let-7a/HMGA2 axis played a critical role in TSCC cell migration and invasion.
Figure 4.
H19 knockdown decreases the expression of HMGA2 in a let-7-dependent manner. (A) The let-7a binding sites within the full-length transcripts of H19 were validated by the bioinformatics analysis Miranda. (B) let-7 sensor (psiCHECK2-let-7 4×) was transfected into CAL27 cells, together with 0, 20, 40, or 80 ng of sponge plasmid wild type (WT) H19 or mutant (Mut) H19. (C) The diagram represents the psiCHECK2-HMGA2 sensor plasmid. The let-7 binding sites of HMGA2 were inserted to the cloning site. (D) Using psiCHECK2-HMGA2, interaction of HMGA2 and miRNA let-7a was measured by luciferase assay in CAL27. (E) CAL27 cells were transfected with 48 nM control miRNA (NC), let-7 mimic (mlet-7), or let-7 inhibitor (ilet-7). RNAs were extracted 48 h later, and RT-qPCR analysis was performed. (F) let-7 sensor (psiCHECK-HMGA2) was transfected into CAL27 cells, together with 0, 20, 40, or 80 ng of sponge plasmid WT H19 or Mut H19. The expression of HMGA2 in (G) TSCC tissues and (H) cell lines was detected by RT-qPCR. GAPDH was probed as the loading control. Numbers are mean ± SD (n = 3, *p < 0.05, **p < 0.01, and ***p < 0.001).
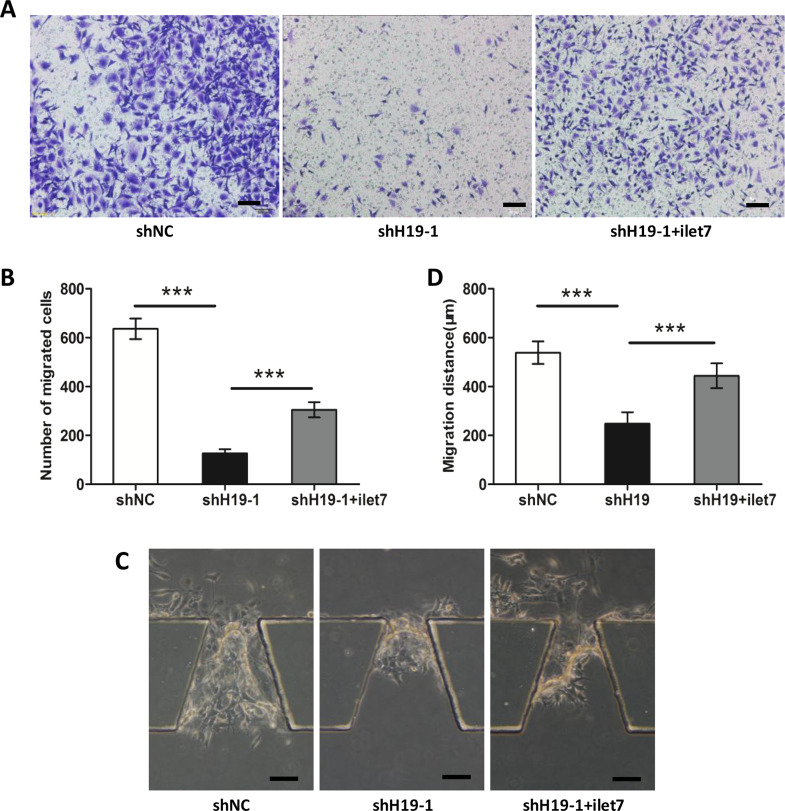
ilet-7 Restores shH19-Induced Inhibition of TSCC Cell Migration and Invasion
To illuminate the crucial role of let-7a in H19-mediated TSCC cell migration and invasion, H19-shRNA together with let-7 inhibitor (ilet-7) was transfected into CAL27 cells. ilet-7 significantly rescued the attenuation of migration via H19-shRNA in CAL27 cells (Fig. 5A and B). In addition, Figure 5C and D shows that ilet-7 also rescued the inhibition of invasion in CAL27 cells by H19-shRNA. Collectively, these results indicated that H19 modulates the TSCC cell migration and invasion partly through let-7a.
Figure 5.
ilet-7 restores shH19-induced inhibition of TSCC metastasis. (A, B) shH19 was transfected together with ilet-7 in CAL27 cells. Transwell assays showed that the migration of CAL27 cells was rescued compared with CAL27 cells only transfected with shH19. (C, D) Invasion microfluidic model demonstrated that the invasion ability of CAL27 cells was restored after we transfected shH19 together with ilet-7 relative to cells only transfected with shH19. Scale bar: 100 μm. Numbers are mean ± SD (n = 3, ***p < 0.001).
DISCUSSION
In the present study, we revealed a previously unappreciated role of H19 in the regulation of TSCC cell migration and invasion. H19 was highly expressed in TSCC tissues compared with corresponding adjacent normal tissues. Consistently, H19 expression was detected at higher levels in TSCC cells than in normal squamous cells (Fig. 1). H19 function was investigated in shRNA-mediated knockdown studies, and we demonstrated that H19 enhanced TSCC cell migration and invasion abilities (Fig. 2). let-7a was validated as a tumor suppressor, whose function could be attenuated by H19. HMGA2 is known to be targeted by let-7 and is dysregulated in many types of cancer, including TSCC. Moreover, HMGA2 is a key regulator of tumor metastasis. We demonstrated that ilet-7 could significantly rescue the impaired migration and invasion abilities induced by shH19, which indicates that let-7 plays a critical role in the H19-induced TSCC cell migration and invasion. Collectively, our studies suggested that the H19/let-7/HMGA2 axis may contribute to the migration and invasion of TSCC cells.
lncRNA H19 is highly enriched during embryogenesis and downregulated after birth, except in adult skeletal muscle and heart. H19 was characterized as a tumor oncogenic factor in several types of cancer such as breast cancer, hepatocellular carcinoma27, ovarian carcinoma28, and lung cancer29. However, other studies demonstrated that H19 may act as a tumor suppressor. For example, loss of IGF-II/H19 imprinting in the developing kidney could contribute to sporadic Wilms tumor30, and the extinction of H19 expression accelerated rhabdomyosarcoma development31. Here we explored the oncogenic role of H19 in TSCC. Our study revealed a high expression level of H19 in TSCC cells and TSCC tissues, which induced migration and invasion via upregulating the oncogene HMGA2. Thus, our data suggested that H19 could act as an oncogene in the progression of TSCC.
Although numerous studies demonstrated that lncRNAs have critical functions in human malignancy, the molecular mechanisms by which lncRNAs modulate tumor metastasis remain elusive32. Recent studies showed that lncRNAs mostly function as endogenous “sponges” for miRNAs and act as posttranscriptional regulators33. For example, lncRNA CASC2 sponges miR-18a, acting as a ceRNA in colorectal cancer34. lncRNA linc00673 regulated non-small cell lung cancer proliferation, migration, and invasion through sponging miR-150-5p35. Therefore, we hypothesized that H19 also targeted miRNAs in TSCC.
In our study, bioinformatics analysis showed that let-7a had a putative binding site in the 3′-UTR region of HMGA2. The dual-luciferase reporter assay revealed that mlet-7 could remarkably reduce the luciferase activity of psi-HMGA2, and H19 could directly bind let-7a. Moreover, our data revealed that ilet-7 could reverse the function of H19 depletion on TSCC cell migration and invasion. Thus, these results suggested that H19 could restrain the function of let-7a via sponging it. A previous study showed that let-7a could suppress pancreatic cancer metastasis by targeting HMGA214. Our data revealed that H19 depletion suppressed the mRNA and protein expression level of HMGA2. Furthermore, we found that mlet-7 downregulated the level of HMGA2 and ilet-7 upregulated HMGA2 expression. Collectively, these data indicated that the regulation of HMGA2 in TSCC by H19 required the activity of let-7a.
HMGA2 is highly expressed in undifferentiated cells during embryogenesis but is silenced after birth36,37. Therefore, HMGA2 is rarely detected in normal adult tissues. However, HMGA2 is usually reactivated in many types of malignant tumors. A high level of HMGA2 has been associated with cancer proliferation, metastasis, and poor prognosis in multiple types of cancer38. Additionally, HMGA2 has been demonstrated to be a key factor involved in EMT, which is vital for tumor metastasis39–43. The expression of HMGA2 and EMT markers was detected after depleting H19 in CAL27 cells. The results showed that H19 sponged let-7, leading to derepression of HMGA2, which mediated EMT. Thus, we demonstrated that H19 might promote TSCC progression via the H19/let-7a/HMGA2 signaling pathway axis.
Collectively, this study revealed that the lncRNA/miRNA/mRNA axis contributes to the migration and invasion of TSCC cells. H19 functions as a ceRNA to sponge miRNA let-7 and subsequently promotes TSCC cell migration and invasion. Targeting this newly identified regulatory axis provides therapeutic opportunities for TSCC patients.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52(4):195–215. [DOI] [PubMed] [Google Scholar]
- 4. Patel RS, Clark JR, Dirven R, Wyten R, Gao K, O’Brien CJ. Prognostic factors in the surgical treatment of patients with oral carcinoma. ANZ J Surg. 2009;79(1–2):19–22. [DOI] [PubMed] [Google Scholar]
- 5. Shore AN, Herschkowitz JI, Rosen JM. Noncoding RNAs involved in mammary gland development and tumorigenesis: There’s a long way to go. J Mammary Gland Biol Neoplasia 2012;17(1):43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu X, Zhou C, Li R, Deng Y, Zhao L, Zhai W. Long noncoding RNA AFAP1-AS1 promoted tumor growth and invasion in cholangiocarcinoma. Cell Physiol Biochem. 2017;42(1):222–30. [DOI] [PubMed] [Google Scholar]
- 7. Lin C, Wang Y, Zhang S, Yu L, Guo C, Xu H. Transcriptional and posttranscriptional regulation of HOXA13 by lncRNA HOTTIP facilitates tumorigenesis and metastasis in esophageal squamous carcinoma cells. Oncogene 2017;133(38):5392–406. [DOI] [PubMed] [Google Scholar]
- 8. Wu F, Li J, Du X, Zhang W, Lei P, Zhang Q. Long noncoding RNA AB019562 promotes cell proliferation and metastasis in human hepatocellular carcinoma. Mol Med Rep. 2017;16(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matouk I, Raveh E, Ohana P, Lail RA, Gershtain E, Gilon M, De Groot N, Czerniak A, Hochberg A. The increasing complexity of the oncofetal h19 gene locus: Functional dissection and therapeutic intervention. Int J Mol Sci. 2013;14(2):298–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333(2):213–21. [DOI] [PubMed] [Google Scholar]
- 11. Yan L, Zhou J, Gao Y, Ghazal S, Lu L, Bellone S, Yang Y, Liu N, Zhao X, Santin AD, Taylor H, Huang Y. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene 2015;34(23):3076–84. [DOI] [PubMed] [Google Scholar]
- 12. Vennin SN, Dahmani F, Julien S, Bertucci F, Finetti P, Chassat T, Bourette RP, Le Bourhis X, Adriaenssens E. H19 non coding RNA-derived miR-675 enhances tumorigenesis. Oncotarget 2015;4976:29209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng F, Li TT, Wang KL, Xiao GQ, Wang JH, Zhao HD, Kang ZJ, Fan WJ, Zhu LL, Li M, Cui B, Zheng FM, Wang HJ, Lam EW, Wang B, Xu J, Liu Q. H19/let-7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance. Cell Death Dis. 2017;8(1):e2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma C, Nong K, Zhu H, Wang W, Huang X, Yuan Z, Ai K. H19 promotes pancreatic cancer metastasis by derepressing let-7’s suppression on its target HMGA2-mediated EMT. Tumour Biol. 2014;35(9):9163–9. [DOI] [PubMed] [Google Scholar]
- 15. Fedele M, Fusco A. HMGA and cancer. Biochim Biophys Acta 2010;1799(1–2):48–54. [DOI] [PubMed] [Google Scholar]
- 16. Ozturk N, Singh I, Mehta A, Braun T, Barreto G. HMGA proteins as modulators of chromatin structure during transcriptional activation. Front Cell Dev Biol. 2014;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Narita M, Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA, Myers MP, Lowe SW. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell 2006;126(3):503–14. [DOI] [PubMed] [Google Scholar]
- 18. Cai J, Shen G, Liu S, Meng Q. Downregulation of HMGA2 inhibits cellular proliferation and invasion, improves cellular apoptosis in prostate cancer. Tumour Biol. 2016;37(1):699–707. [DOI] [PubMed] [Google Scholar]
- 19. Motoyama K, Inoue H, Nakamura Y, Uetake H, Sugihara K, Mori M. Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family. Clin Cancer Res. 2008;14(8):2334–40. [DOI] [PubMed] [Google Scholar]
- 20. Lee CT, Wu TT, Lohse CM, Zhang L. High-mobility group AT-hook 2: An independent marker of poor prognosis in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45(11):2334–40. [DOI] [PubMed] [Google Scholar]
- 21. Jiang W, Finniss S, Cazacu S, Xiang C, Brodie Z, Mikkelsen T, Poisson L, Shackelford DB, Brodie C. Repurposing phenformin for the targeting of glioma stem cells. Oncotarget 2016;7:56456–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao XP, Zhang H, Jiao JY, Tang DX, Wu YL, Pan CB. Overexpression of HMGA2 promotes tongue cancer metastasis through EMT pathway. J Transl Med. 2016;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kallen AN, Zhou X-B, Xu J, Qiao C, Ma J, Yan L, Lu L, Liu C, Yi J-S, Zhang H, Min W, Bennett AM, Gregory RI, Ding Y, Huang Y. The imprinted H19 lncRNA antagonizes Let-7 microRNAs. Mol Cell 2013;52(1):101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iwasaki S, Kawamata T, Tomari Y. Drosophila argonaute1 and argonaute2 employ distinct mechanisms for translational repression. Mol Cell 2009;34(1):58–67. [DOI] [PubMed] [Google Scholar]
- 25. Qiu C, Ma Y, Wang J, Peng S, Huang Y. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res. 2010;38(4):1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 27. Ariel I, Miao HQ, Ji XR, Schneider T, Roll D, de Groot N, Hochberg A, Ayesh S. Imprinted H19 oncofetal RNA is a candidate tumour marker for hepatocellular carcinoma. Mol Pathol. 1998;51:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy SK, Huang Z, Wen Y, Spillman MA, Whitaker RS, Simel LR, Nichols TD, Marks JR, Berchuck A. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol Cancer Res. 2006;4(4):283–92. [DOI] [PubMed] [Google Scholar]
- 29. Kondo M SH, Ueda R, Osada H, Takagi K, Takahashi T, Takahashi T. Frequent loss of imprinting of the H19 gene is often associated with its overexpression in human lung cancers. Oncogene 1995;10:1193–8. [PubMed] [Google Scholar]
- 30. Okamoto K, Morison IM, Taniguchi T, Reeve AE. Epigenetic changes at the insulin-like growth factor II/H19 locus in developing kidney is an early event in Wilms tumorigenesis. Proc Natl Acad Sci USA 1997;94:5367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Casola S, Pedone PV, Cavazzana AO, Basso G, Luksch R, d’Amore ES, Carli M, Bruni CB, Riccio A. Expression and parental imprinting of the H19 gene in human rhabdomyosarcoma. Oncogene 1997;14:1503–10. [DOI] [PubMed] [Google Scholar]
- 32. Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer 2013;108(12):2419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanchez-Mejias A, Tay Y. Competing endogenous RNA networks: Tying the essential knots for cancer biology and therapeutics. J Hematol Oncol. 2015;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang G, Wu X, Li S, Xu X, Zhu H, Chen X. The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep. 2016;6:26524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H, Kong J, Ding K, Shen HM, Wu H, Xia D, Wu Y. Long non-coding RNA linc00673 regulated non-small cell lung cancer proliferation, migration, invasion and epithelial mesenchymal transition by sponging miR-150-5p. Mol Cancer 2017;16(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu J, Liu Z, Shao C, Gong Y, Hernando E, Lee P, Narita M, Muller W, Liu J, Wei JJ. HMGA2 overexpression-induced ovarian surface epithelial transformation is mediated through regulation of EMT genes. Cancer Res. 2011;71(2):349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nishino J, Kim I, Chada K, Morrison SJ. HMGA2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell 2008;135(2):227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fusco A, Fedele M. Roles of HMGA proteins in cancer. Nat Rev Cancer 2007;7(12):899–910. [DOI] [PubMed] [Google Scholar]
- 39. Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174(2):175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watanabe S, Ueda Y, Akaboshi S, Hino Y, Sekita Y, Nakao M. HMGA2 maintains oncogenic RAS-induced epithelial-mesenchymal transition in human pancreatic cancer cells. Am J Pathol. 2009;174(3):854–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Q, Liu T, Zheng S, Gao X, Lu M, Sheyhidin I, Lu X. HMGA2 is down-regulated by microRNA let-7 and associated with epithelial-mesenchymal transition in oesophageal squamous cell carcinomas of Kazakhs. Histopathology 2014;65(3):408–17. [DOI] [PubMed] [Google Scholar]
- 42. Morishita A, Zaidi MR, Mitoro A, Sankarasharma D, Szabolcs M, Okada Y, D’Armiento J, Chada K. HMGA2 is a driver of tumor metastasis. Cancer Res. 2013;73(14):4289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zha L, Zhang J, Tang W, Zhang N, He M, Guo Y, Wang Z. HMGA2 elicits EMT by activating the Wnt/beta-catenin pathway in gastric cancer. Dig Dis Sci. 2013;58(3):724–33. [DOI] [PubMed] [Google Scholar]