Abstract
Transforming growth factor-β1 (TGF-β1)-induced epithelial–mesenchymal transition (EMT) of non-small cell lung cancer (NSCLC) may contribute to tumor metastasis. TGF-β1-induced EMT in H1975 cells (a human NSCLC cell line) resulted in the adoption of mesenchymal responses that were predominantly mediated via the TGF-β1–integrin signaling pathway. Ursolic acid has been previously reported to inhibit tumor growth and metastasis in several cancers. However, whether ursolic acid can attenuate TGF-β1-induced EMT in H1975 cells and its underlying mechanisms remain unknown. In this study, ursolic acid significantly attenuated the TGF-β1-induced decrease in E-cadherin level and elevated the level of N-cadherin. Furthermore, ursolic acid inhibited the mesenchymal-like responses in H1975 cells, including cell migration, invasion, and activity of matrix metallopeptidase (MMP)-2 and -9. Finally, our new findings provided evidence that ursolic acid could inhibit EMT in NSCLC through TGF-β1 signaling pathway-mediated integrin αVβ5 expression, and this might be the potential mechanism of resveratrol on the inhibition of invasion and metastases in NSCLC. We conclude that ursolic acid attenuated TGF-β1-induced EMT in H1975 cells and thus might be a promising therapeutic agent for treating NSCLC.
Key words: Non-small cell lung cancer (NSCLC), Epithelial–mesenchymal transition (EMT), Ursolic acid, Metastasis
INTRODUCTION
The epithelial-to-mesenchymal transition (EMT) is a major phenotype of cancer metastasis and invasion that occurs in epithelial tumors and accounts for 90% of human tumors1. EMT is characterized by the loss of epithelial characteristics and the acquisition of mesenchymal characteristics; loss of epithelial markers such as E-cadherin and the induction of mesenchymal markers including N-cadherin and vimentin are hallmarks of early and late stage events of EMT, respectively2. Morphologically, cancer cells change from a polarized, epithelial shape to a spindle-shaped phenotype. Epithelial tumor cells become more motile and invasive after undergoing EMT. Various growth and differentiation factors can induce or regulate the process of EMT in cancers. Tumor growth factor (TGF)-β1 has received much attention as a characterized inducer of EMT during cancer progression and metastasis3. TGF-β1 triggers the signal for EMT through a heteromeric complex of two type I and two type II transmembrane serine/threonine kinase receptors. TGF-β1-induced activation of the receptor complex leads to the activation of Smad2 and Smad3 through phosphorylation of the type I receptors. Next, trimers consisting of phosphorylated Smad2/3 and Smad4 translocate to the nucleus, where they cooperate with transcription factors such as Snail and Twist to repress the expression of epithelial markers and activate the expression of mesenchymal markers at the mRNA level. This signaling is referred to as TGF-β1-activated Smad signaling in EMT4.
Tumor migration and invasion by TGF-β1-induced crosstalk between signaling pathways, including Smad, non-Smad, and Wnt signaling pathways, accompany the increased expression and activity of matrix metalloproteinases (MMPs), which have been recognized as major contributors to the proteolytic degradation of the extracellular matrix that is required for tumor cell migration and invasion5. Additionally, focal adhesion kinase (FAK), Src, and paxillin are functionally interdependent molecules related to EMT-mediated tumor cell migration and invasion. As mentioned above, the processes of EMT-mediated tumor cell migration and invasion are regulated in a complex manner by several molecules and signals. To control both tumor metastasis and tumor growth, the upstream signaling molecules involved in this process (e.g., integrin) have been considered as potentially druggable target molecules6. Integrin αVβ5, a transmembrane protein, plays a pivotal role in many cellular events, including cell cycle, differentiation, and proliferation, by regulating the crosstalk between multiple signaling pathways (e.g., PI3K/Akt, Wnt, and NF-κB). A recent study has reported that integrin αVβ5 modulates cell proliferation and invasion by regulating EMT-related genes7. These results suggest that the inhibition of integrin may be a promising therapeutic strategy for treating EMT-related disorders including cancer metastasis.
Ursolic acid (Fig. 1) is a pentacyclic triterpenoid compound that is derived from a range of medicinal herbs, including Rosemarinus officinalis, Eribotrya japonica, Calluna vulgaris, and Oldenlandia diffusa 8. Ursolic acid has been demonstrated to exert a number of anticancer activities, including the inhibition of tumorigenesis, tumor promotion, and angiogenesis, as well as the induction of apoptosis in various cancer cell lines, including breast, gastric, cervical cancer, hepatocellular carcinoma, and human non-small cell lung cancer (NSCLC)9–12. In addition, ursolic acid has been reported to inhibit in vivo tumor growth in various animal models, and to suppress invasion and migration in human breast and ovarian cancer cells.13,14 However, to the best of our knowledge, no promising data has been reported to demonstrate the potential for ursolic acid to inhibit the process of EMT and metastasis in NSCLC cells. Therefore, the present study aimed to investigate the inhibitory effect of ursolic acid on the growth and invasive phenotype of H1975 human NSCLC cells. This study is the first to report that ursolic acid blocks the TGF-β1-induced EMT in H1975 human lung adenocarcinoma cells by regulating the integrin αVβ5 signaling pathway.
Figure 1.
The structure of ursolic acid.
MATERIALS AND METHODS
Cell Culture and Drug Treatment
Human NSCLC cell line H1975 was obtained from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (CBTCCCAS, Shanghai, P.R. China). H1975 cells were maintained in RPMI-1640 medium (Gibco-BRL/Invitrogen, Carlsbad, CA, USA) containing 10% (v/v) fetal bovine serum (FBS; Wisent, Quebec, Canada), 100k U/L penicillin, and 100 mg/L streptomycin (Gibco-BRL/Invitrogen) at 37°C in a humidified 5% CO2 atmosphere. Ursolic acid was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) prior to incubation with or without TGF-β1 (Roche, Mannheim, Germany) at a concentration of 5 ng/ml for 24 h. To investigate the effects of TGF-β1 or ursolic acid on cell morphology, an integrin αVβ5 inhibitor SB273005 (Selleck, Houston, TX, USA) was used at 100 nM with or without TGF-β1. The concentration of DMSO in the medium never exceeded 0.1% to avoid toxicity in the H1975 cells. Each experiment was independently performed at least three times.
Cell Proliferation Assay
The cell viability was determined by CellTiter 96® AQueous One Solution cell proliferation assay (Promega, Madison, WI, USA). Briefly, cells were seeded in 96-well cell culture plates and treated with indicated agents. After incubation for the indicated time period, 20 μl of One Solution reagent was added to each well, and incubation was continued for an additional 4 h. The absorbance was measured at 490 nm using Synergy™ HT Multi-Mode Microplate Reader (Bio-Tek, Winooski, VT, USA). The effect of the indicated agents on cell viability was assessed as the percent of cell viability compared with vehicle-treated control cells, which were arbitrarily assigned 100% viability. The concentration of ursolic acid resulting in 50% inhibition of control growth (IC50) was calculated by Graphpad Prism 5 statistics software.
Western Blotting Analyses
Cellular proteins were extracted using RIPA buffer [50 mM Tris/HCl; pH 7.4; 150 mM NaCl; 1% (v/v) NP-40; 0.1% (w/v) SDS; SolarBio, Beijing, P.R. China] containing 1% (v/v) PMSF (SolarBio), 0.3% (v/v) protease inhibitor (Sigma-Aldrich), and 0.1% (v/v) phosphorylated proteinase inhibitor (Sigma-Aldrich). Lysates were centrifuged at 15,000 rpm at 4°C for 15 min, and the supernatant was collected for total protein. A BCA protein assay kit (Biotime Biotech, Haimen, China) was used to determine the protein concentration. Equal amounts of protein (15 μg) were separated on an SDS-PAGE gel [10% (v/v) polyacrylamide] and transferred onto a PVDF membrane. Nonspecific binding was blocked using 8% (w/v) milk in TBS-T for 2 h at room temperature (RT). The membranes were then incubated with primary antibodies against GAPDH (Abmart, Shanghai, P.R. China), E-cadherin (Cell Signaling Technology, Danvers, MA, USA), and N-cadherin (Cell Signaling Technology) overnight at 4°C. After several washes with TBS-T, the membranes were incubated in HRP-conjugated goat anti-rabbit and anti-mouse IgG or HRP-conjugated mouse anti-goat IgG (all at a 1:5,000 dilution; Abmart) for 2 h at RT and then washed. The target proteins were visualized using enhanced chemiluminescence (Millipore, Braunschweig, Germany) according to the manufacturer’s recommendations, and quantified using density analysis normalized against GAPDH and expressed as the fold change compared to control.
Immunofluorescence
Cells grown on chamber slides were washed with PBS for 15 min (total), fixed in 4% paraformaldehyde for 30 min at RT, and permeabilized with 0.1% Triton X-100 at RT for 5 min. After several washes with PBS for 15 min (total), nonspecific binding was blocked with 3% bovine serum albumin (BSA) in PBS for 1 h at RT. Next, the cells were incubated with the following primary antibody: human integrin, which was diluted at 1:100 in PBS with 1% BSA. After the cells were incubated with the primary antibody for 2 h at RT, the cells were washed with PBS and incubated with Alexa Fluor 488-conjugated anti-rabbit IgG or TRITC-conjugated anti-mouse IgG (Zhongshan Biotechnology, Beijing, P.R. China) at 1:50 in PBS with 1% BSA for 1 h at RT. After several washes for 15 min (total) with PBS, the cell nuclei were visualized with Hoechst 33258 staining at a concentration of 10 μg/ml for 10 min at RT. The slides were then washed again, dried, mounted, and examined using a fluorescence microscope.
Cell Migration Assay
Cells were grown as a confluent monolayer in six-well plates and were pretreated with DMSO or ursolic acid for 24 h. To initiate migration, the cell layer was scratched using a pipette tip. Next, the cells were incubated with or without 5 ng/ml of TGF-β1.
Invasion Assay
Cells (2.5 × 104) were suspended in serum-free medium and seeded into the inserts of a 24-well BD BioCoat Matrigel Invasion Chamber (BD, Beijing, P.R. China) in triplicate. Inserts were cocultured in a well of FBS-containing media as an attractant. Chambers along with inserts were incubated at 37°C and 5% CO2 atmosphere for 6 h. Inserts were then removed, and the upper surface of the membrane was scrubbed to remove nonmigrating cells. Inserts were then fixed with methanol and stained with 1% crystal violet. Multiple 10× magnification images per well were acquired, and the average counts were calculated15.
MMP-2/9 Activity Assay
The activity of MMP-2/9 was determined by QuickZyme human MMP activity assay (QucikZyme BioSciences, Netherlands) according to the manufacturer’s instructions. Briefly, after treatment with ursolic acid for 24 h, cells were washed with fresh medium and replaced with serum-free medium. After 24 h, the medium was collected and centrifuged at 10,000 × g for 10 min. Respective supernatant was added to the 96-well strip coated with MMP-2/9 antibody and incubated at 4°C overnight. After washing with wash buffer four times, 50 μl of assay buffer was added into the well, followed by adding 50 μl of detection reagent. After incubation at 37°C for 1 h, OD405 was measured with a Tecan plate reader (Männedorf, Switzerland)16.
Quantitative Real-Time PCR
Total RNA was isolated using the TRIzol reagent (Invitrogen) according to the manufacturer’s recommendations. The cDNA was then synthesized with 1 μg of total RNA using a PrimeScript RT reagent kit (Takara Bio, Tokyo, Japan) according to the protocol. RT-qPCR was performed using IQTM SYBR Green supermix and the iQ5 real-time detection system (Bio-Rad, Hercules, CA, USA). The comparative cycle threshold (Ct) method was applied to quantify the expression levels through calculating the two (−ΔΔCt) method. The primers used for PCR were as follows: GAPDH: 5′-TGTGGGCATCAATGGATTTGG-3′ (forward) and 5′-ACACCATGTATTCCGGGTCAAT-3′ (reverse); E-cadherin: 5′-CGAGAGCTACACGTTCACGG-3′ (forward) and 5′-GGGTGTCGAGGGAAAAATAGG-3′ (reverse). N-cadherin: 5′-TTTGATGGAGGTCTCCTAACACC-3′ (forward) and 5′-ACGTTTAACACGTTGGAAATGTG-3′ (reverse). The GAPDH RNA expression was used to normalize the target gene levels.
Statistical Analysis
The data are expressed as the mean ± SD. The number of independent experiments is represented by “n.” Multiple comparisons were performed using one-way ANOVA followed by Tukey’s multiple comparison test, where a value of p < 0.05 was considered significant.
RESULTS
TGF-β1 Induced EMT in H1975 cells
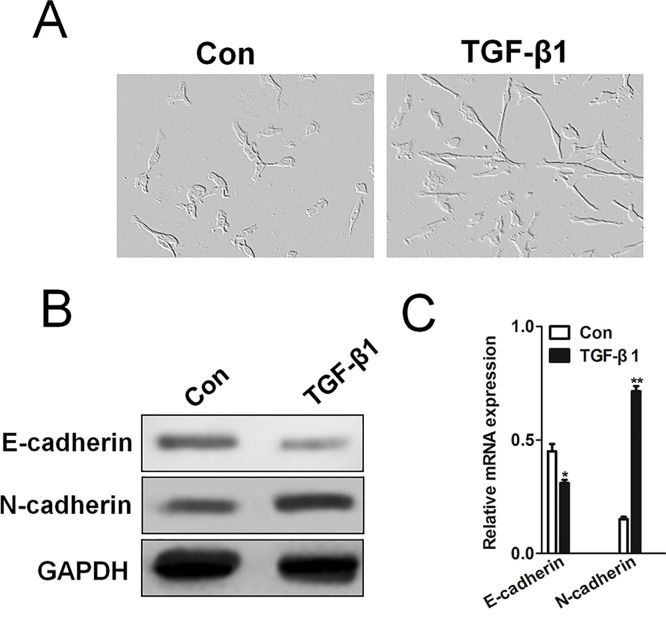
It has previously been reported that TGF-β1 remarkably induces EMT in several tumor cell lines, including breast cancer and hepatocellular carcinoma cells17,18. Based on our intention to mechanistically dissect the role of EMT in experimental NSCLC cells, we first characterized TGF-β1 in human NSCLC cells H1975. H1975 cells cultured in basal media exhibited classic cobblestone morphology. After stimulation with TGF-β1 at 5 ng/ml for 24 h, the cells adopted a more fibroblast-like, elongated, and narrower, spindle-shaped morphology (Fig. 2A). We stimulated NSCLC H1975 cells with TGF-β1 at 5 ng/ml for 24 h and examined the expression of EMT markers in H1975 cells, such as E-cadherin and N-cadherin by Western blotting assay (Fig. 2B). Consistent with the immunological finding, qRT-PCR analyses (Fig. 2C) showed that TGF-β1 stimulation significantly inhibited the mRNA of the epithelial marker E-cadherin and increased the expression of the mesenchymal marker N-cadherin, compared to control cells. Together, these findings show that TGF-β1 directly induces EMT in NSCLC cells.
Figure 2.
Transforming growth factor (TGF)-β1-induced epithelial–mesenchymal transition (EMT) in H1975 cells. (A) H1975 cells were exposed to 5 ng/ml TGF-β1 for 24 h. Compared to the basal condition, TGF-β1-treated H1975 cells lost their cobblestone shape and adopted a fibroblast-like, spindle-shaped morphology. (B) Western blotting revealed that TGF-β1 significantly downregulated the expression of the epithelial marker E-cadherin and upregulated the expression of the mesenchymal marker N-cadherin. (C). Quantitative real-time (qRT)-PCR analysis of E-cadherin and N-cadherin mRNA levels in H1975 cells. PCR values were normalized to the levels of GAPDH. Data are presented as the mean ± SD from three independent measurements. *p < 0.05, **p < 0.01 versus control.
Ursolic Acid Attenuates Changes in the Expression of EMT Markers Induced by TGF-β1
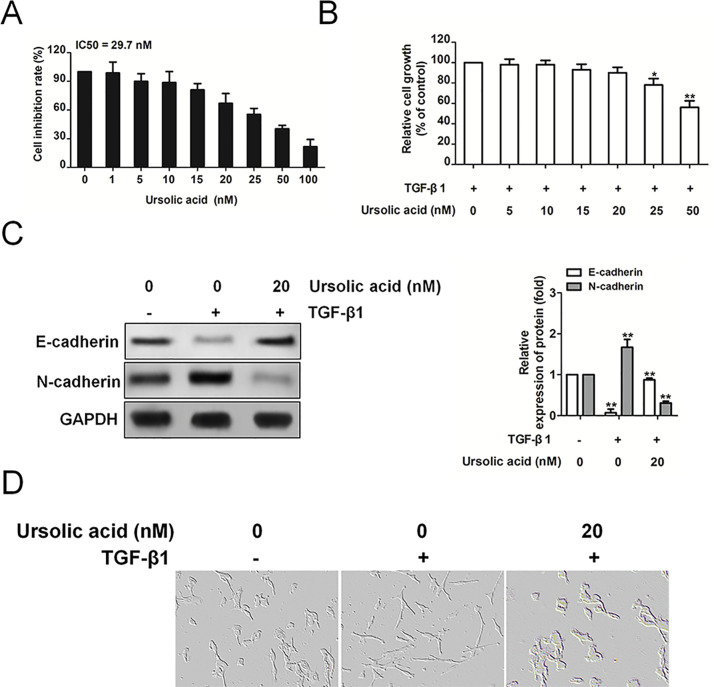
To investigate the effect of ursolic acid on the growth of H1975 cancer cells in vitro, a CellTiter 96® AQueous One Solution cell proliferation assay was performed on cells exposed to various concentrations of ursolic acid. As demonstrated in Figure 3A, administration of the H1975 cells with ursolic acid for 24 h inhibited proliferation in a dose-dependent manner, with a half maximal inhibitory concentration of 29.7 nM. Before assessing the effect of ursolic acid on TGF-β1-induced EMT in H1975 cells, we performed another cell viability assay to determine the concentration of ursolic acid to use in this EMT progress study. No significant differences in H1975 cell viability were observed at ursolic acid concentrations up to 25 nM in the presence of TGF-β1 (Fig. 3B), so we used up to 20 nM ursolic acid in the following experiments. The effect of ursolic acid on TGF-β1-induced EMT was first evaluated by checking the cadherin switch (Fig. 3C). Consistently, in response to TGF-β1, E-cadherin was decreased, whereas N-cadherin was induced. Nevertheless, ursolic acid inhibited the TGF-β1-induced cadherin switch. Finally, we observed that ursolic acid attenuated TGF-β1-induced morphological changes from cuboidal epithelial cells to fibroblast-like spindle-shaped cells (Fig. 3D). In summary, our findings reveal that ursolic acid inhibits changes in the expression of EMT markers that are induced by TGF-β1.
Figure 3.
Ursolic acid inhibits the proliferation of H1975 cells and its EMT markers. (A) H1975 cells were treated with various concentrations of ursolic acid for 48 h and then subjected to CellTiter 96® AQueous One Solution cell proliferation assay. The results represent the mean ± SD for triplicates. The values are expressed as percentage of viable cells normalized to percentage of viable cells in 0.5% DMSO-treated cells. The concentration of ursolic acid resulting in 50% inhibition of control growth (IC50) was calculated by SPSS statistics software using Probit model. (B). The effect of ursolic acid on the viability of H1975 cells in the presence of TGF-β1. Briefly, cells were treated with TGF-β1 (5 ng/ml) alone or in combination with ursolic acid for 24 h, and then cell viability was measured by CellTiter 96® AQueous One Solution cell proliferation assay analysis. The results represent the mean ± standard error of the mean for triplicates. *p < 0.05 and **p < 0.01 versus control. (C) H1975 cells were treated with TGF-β1 (5 ng/ml) alone or in combination with ursolic acid for 24 h. Immunoblot analysis of the effect of ursolic acid on the TGF-β1-induced expression of epithelial and mesenchymal markers. (D) H1975 cells were exposed to TGF-β1 alone or in combination with ursolic acid. Compared to the control, TGF-β1-treated H1975 cells lost their cobblestone shape and adopted a fibroblast-like, spindle-shaped morphology, whereas ursolic acid-treated H1975 cells lost their fibroblast-like, spindle-shaped morphology and adopted a cobblestone shape.
Ursolic Acid Inhibits TGF-β1-Induced H1975 Cell Migration and Invasion
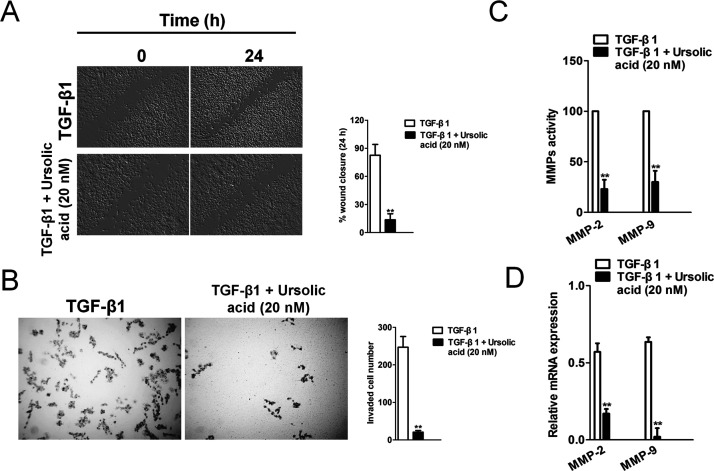
Next, we examined the effect of ursolic acid on the TGF-β1-induced migration and invasion of H1975 cells. The wound healing assay revealed that ursolic acid inhibited the TGF-β1-induced migration of H1975 cells (Fig. 4A). In the Transwell chamber assay, ursolic acid significantly inhibited the TGF-β1-induced invasion of H1975 cells across the Matrigel-coated membrane (Fig. 4B). In addition, we examined whether ursolic acid inhibits the activation of gelatinases such as MMP-2 and MMP-9 and the expression of their mRNAs. As expected, TGF-β1 strongly induced the activation of MMP-2 and MMP-9; however, ursolic acid dramatically inhibited the induction of those factors (Fig. 4C). Ursolic acid also significantly inhibited the TGF-β1-induced expression of both MMP-2 and MMP-9 mRNAs (Fig. 4D). These results demonstrate that ursolic acid has the potential to inhibit TGF-β1-induced EMT in H1975 cells.
Figure 4.
Ursolic acid inhibits TGF-β1-induced H1975 cell migration and invasion. (A) A cell migration assay was performed by scratching the cell layer prior to drug treatment, and time-lapse images were obtained from 0 to 24 h. TGF-β1 increased the migration activity, and ursolic acid decreased TGF-β1-induced cell migration. (B) H1975 cells were subjected to Transwell invasion assay in the absence or presence of ursolic acid. (C) H1975 cells were untreated or treated with TGF-β1 and the indicated concentrations of ursolic acid. The effect of ursolic acid on the TGF-β1-induced activation of MMP-2/9 was evaluated by MMP activity assay. (D) H1975 cells were treated with the indicated concentrations of ursolic acid in the absence or presence of TGF-β1, and MMP-2/9 transcription was evaluated using qPCR. PCR values were normalized to the levels of GAPDH. Data are presented as the mean ± SD from three independent measurements. **p < 0.01 versus control.
Ursolic Acid Inhibits TGF-β1-Induced Activation of Integrin αVβ5 Signaling
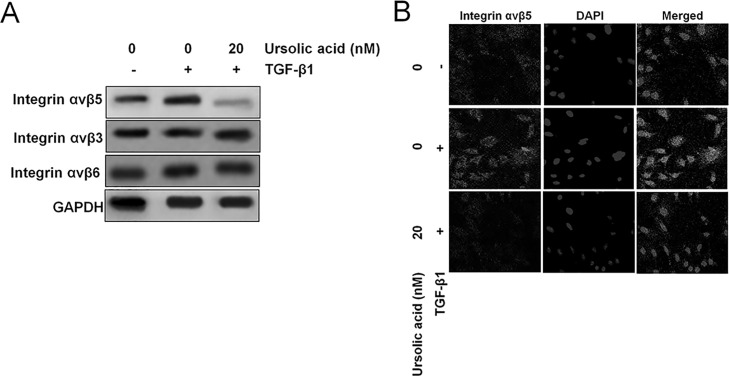
Integrin signaling is known to feed downstream of TGF-β1, so we tested the effect of ursolic acid on TGF-β1-induced activation of integrin αVβ5 signaling19. In this study, the cells were divided into three groups: a control group (DMSO), a TGF-β1 group (DMSO + TGF-β1), and a TGF-β1 + ursolic acid group. Both Western blotting (Fig. 5A) and immunofluorescence (Fig. 5B) demonstrated that treatment of H1975 cells with TGF-β1 significantly increased the expression of integrin αVβ5. Compared to the treatment with TGF-β1 alone, H1975 cells treated with both TGF-β1 and ursolic acid showed a remarkable downregulation of integrin αVβ5. Taken together, these results suggest that ursolic acid might inhibit TGF-β1-induced EMT by blocking the TGF-β1-induced integrin αVβ5 signaling in H1975 cells.
Figure 5.
Ursolic acid inhibits TGF-β1-induced integrin αVβ5 signaling. (A) The effect of ursolic acid on TGF-β1-induced expression of integrin αVβ5 was evaluated using Western blot analysis. Briefly, H1975 cells were treated with TGF-β1 alone or in combination with ursolic acid for 24 h. Western blotting analysis of integrin αVβ5 expression by H1975 cells. (B) Immunofluorescence revealed that H1975 cells exposed to TGF-β1 significantly increased their expression of integrin αVβ5, while ursolic acid inhibited integrin αVβ5 levels induced by TGF-β1.
Integrin αVβ5 Pharmacological Inhibitor Blocks the Inhibitory Effect of Ursolic Acid on TGF-β1-Induced EMT of H1975 Cells
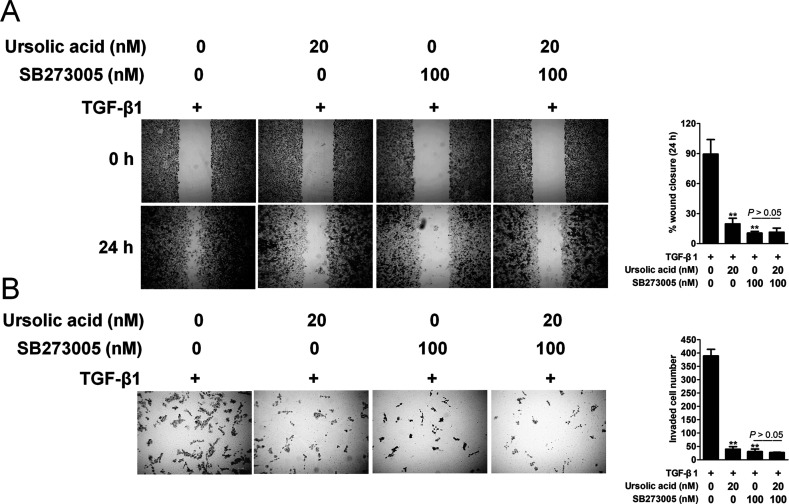
To elucidate whether the inhibition of integrin αVβ5 signaling is necessary for the effect of ursolic acid on TGF-β1-induced EMT of H1975 cells, we pretreated H1975 cells with SB273005, a pharmacologcal inhibitor of integrin. In order to exclude the influence of ursolic acid or inhibitor on NSCLC cell metastasis, we designed a control experiment with or without TGF-β1. As shown in Figure 6A, in the presence of TGF-β1, ursolic acid, SB273005, and ursolic acid combined with SB273005 inhibited H1975 migration. However, there was no significant difference between the SB273005 alone treatment and the combination treatment group. Similarly, in the presence of TGF-β1, ursolic acid, SB273005, and ursolic acid combined with SB273005 have marked effects on H1975 cell invasion. However, there was no significant difference between SB273005 alone treatment and the combination treatment group (Fig. 6B). These data indicate that ursolic acid-inhibited EMT and cell metastasis are dependent on integrin signaling in NSCLC.
Figure 6.
Integrin αVβ5 inhibitor SB273005 blocked the effects of ursolic acid on TGF-β1-induced EMT in H1975 cells. (A) Effects of SB273005 on the migration of H1975 cells with TGF-β1. A cell migration assay was performed by scratching the cell layer prior to drug treatment, and time-lapse images were obtained from 0 to 24 h. H1975 cells were treated with SB273005 (100 nM) alone or ursolic acid combined with SB273005 for 24 h in the presence of TGF-β1. (B) H1975 cells were subjected to Transwell invasion assay in the presence of SB273005 (100 nM) alone or ursolic acid combined with SB273005. Data are presented as the mean ± SD from three independent measurements. **p < 0.01 versus control.
DISCUSSION
EMT is thought to play an important role in cellular transdifferentiation during cancer metastasis. TGF-β1-induced EMT in tumor cells is a classic pathological model employed in studies on cancer cell metastasis progress20,21. It has been reported that ursolic acid could suppress TGF-β1-induced EMT in human proximal tubular epithelial cells22. However, whether ursolic acid can inhibit EMT in NSCLC cells induced by TGF-β1 was unknown. In this study, we used H1975 cells as a model of human NSCLC cells and explored the inhibitory effects of ursolic acid on EMT. H1975 cells demonstrated many features of NSCLC cells, and most studies investigating EMT have used H1975 cells as a model of NSCLC. In addition, a few studies have used both H1975 cells and primary NSCLC to study EMT and showed that H1975 cells presented similar features as the primary model during the EMT process. Thus, H1975 cells are a suitable human model to study EMT23,24.
Our results showed that TGF-β1 induced H1975 cells to lose their classic cobblestone-like morphology and to adopt a mesenchymal spindle-like appearance. Moreover, E-cadherin expression was decreased, and the expression of mesenchymal marker N-cadherin was increased. E-cadherin is an adherent junction protein that is specifically expressed in epithelial cells. Loss of E-cadherin is a universal feature of EMT, and E-cadherin can be used as an epithelial marker25. However, the acquisition of a mesenchymal phenotype is more difficult to define due to the lack of specificity in many of the available phenotypic markers. Thus, we succeeded in establishing an EMT model in NSCLC to study the therapeutic effect of ursolic acid on this pathological process. In this study, we used Western blotting and qPCR to detect the expression of two EMT markers, and these two assays showed that ursolic acid alleviated changes in the expression of these markers induced by TGF-β1, indicating that ursolic acid could attenuate TGF-β1-induced EMT in NSCLC, which supports our hypothesis. We also evaluated whether ursolic acid could reverse TGF-β1-induced morphological changes. Our results showed that the ursolic acid completely prevented cells from undergoing EMT induced by TGF-β1.
By undergoing the EMT process in metastasis, NSCLC cells also adopted a mesenchymal and fibroblast-like response, which directly promoted cell migration and invasion. In this study, we investigated the effects of ursolic acid on the pathological responses induced by TGF-β1, including cell migration ability and activity of MMP-2 and -9. Our study revealed that ursolic acid could inhibit TGF-β1-induced cell migration. MMP-2 and -9 contributed to the activation of TGF-β1 and the disruption of basement membranes in NSCLC. This study showed that ursolic acid significantly decreased MMP-2 and -9 secretion induced by TGF-β1.
TGF-β1 induced EMT in NSCLC via two specific pathways, a Smad-dependent pathway and a Smad-independent pathway26. The former, which is known as the TGF-β-Smad2/3 pathway, plays a predominant role in TGF-β1-induced EMT in H1975 cells27. The latter, the Smad-independent pathway, includes several pathways, such as the integrin, Akt, JNK, Rho, p38 mitogen-activated protein kinase (MAPK), and NF-κB pathways, etc28. Of further interest, we examined the possible pathway of how ursolic acid participates in the cell metastasis of H1975 cells and found decreased integrin αVβ5 in TGF-β1-upregulated NSCLC cells.
Taken together, our study provided the first line of evidence that ursolic acid could attenuate the EMT process induced by TGF-β1 in human NSCLC H1975 cells associated with the inhibition of the TGF-β1-integrin signaling pathway in the absence of regulatory effects on cell morphology. More importantly, ursolic acid also inhibited H1975 cells from adopting the pathological mesenchymal and fibroblast-like behaviors induced by TGF-β1, including cell migration, invasion, integrin expression, and MMP-2 and -9 activity. Thus, ursolic acid may be a promising therapeutic agent for the treatment of NSCLC metastasis.
ACKNOWLEDGMENT
National Natural Science Foundation of China (No. 81273647); Natural Science Foundation of Fujian Province (2013J01365, 2016J01509); Young and middle-aged talent training project in Fujian provincial health system (2015-ZQN-ZD-2); Fujian Province health education joint research project (WKJ-FJ-19).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13(2):97–110. [DOI] [PubMed] [Google Scholar]
- 2. Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR, Goel A. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 2013;62(9):1315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu X, Guo H, Chen X, Xiao J, Zou Y, Wang W, Chen Q. Effect of RhoC on the epithelial-mesenchymal transition process induced by TGF-beta1 in lung adenocarcinoma cells. Oncol Rep. 2016;36(6):3105–12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Zhang X, Zhang P, Shao M, Zang X, Zhang J, Mao F, Qian H, Xu W. SALL4 activates TGF-beta/SMAD signaling pathway to induce EMT and promote gastric cancer metastasis. Cancer Manag Res. 2018;10:4459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237(3):273–81. [DOI] [PubMed] [Google Scholar]
- 6. Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25(4):234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart RL, O’Connor KL. Integrin beta4 is a controversial target for non-small cell lung cancer-reply. Hum Pathol. 2016;61:222–3. [DOI] [PubMed] [Google Scholar]
- 8. Wu S, Zhang T, Du J. Ursolic acid sensitizes cisplatin-resistant HepG2/DDP cells to cisplatin via inhibiting Nrf2/ARE pathway. Drug Des Devel Ther. 2016;10:3471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin R, Li T, Tian JX, Xi P, Liu RH. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit Rev Food Sci Nutr. 2018;58(4):568–74. [DOI] [PubMed] [Google Scholar]
- 10. Wang S, Meng X, Dong Y. Ursolic acid nanoparticles inhibit cervical cancer growth in vitro and in vivo via apoptosis induction. Int J Oncol. 2017;50(4):1330–40. [DOI] [PubMed] [Google Scholar]
- 11. Liu T, Ma H, Shi W, Duan J, Wang Y, Zhang C, Li C, Lin J, Li S, Lv J, Lin L. Inhibition of STAT3 signaling pathway by ursolic acid suppresses growth of hepatocellular carcinoma. Int J Oncol. 2017;51(2):555–62. [DOI] [PubMed] [Google Scholar]
- 12. Lai MY, Leung HW, Yang WH, Chen WH, Lee HZ. Up-regulation of matrix metalloproteinase family gene involvement in ursolic acid-induced human lung non-small carcinoma cell apoptosis. Anticancer Res. 2007;27(1A):145–53. [PubMed] [Google Scholar]
- 13. Jin H, Pi J, Yang F, Jiang J, Wang X, Bai H, Shao M, Huang L, Zhu H, Yang P, Li L, Li T, Cai J, Chen ZW. Folate-chitosan nanoparticles loaded with ursolic acid confer anti-breast cancer activities in vitro and in vivo. Sci Rep. 2016;6:30782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang J, Wang W, Qian L, Zhang Q, Lai D, Qi C. Ursolic acid inhibits the proliferation of human ovarian cancer stem-like cells through epithelial-mesenchymal transition. Oncol Rep. 2015;34(5):2375–84. [DOI] [PubMed] [Google Scholar]
- 15. Wang MJ, Zhang H, Li J, Zhao HD. microRNA-98 inhibits the proliferation, invasion, migration and promotes apoptosis of breast cancer cells by binding to HMGA2. Biosci Rep. 2018;38(5):BSR20180571. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Wang L, Shi Y, Ju P, Liu R, Yeo SP, Xia Y, Owlanj H, Feng Z. Silencing of diphthamide synthesis 3 (Dph3) reduces metastasis of murine melanoma. PLoS One 2012;7(11):e49988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma CQ, Yang Y, Wang JM, Du GS, Shen Q, Liu Y, Zhang J, Hu JL, Zhu P, Qi WP, Qian YW, Fu Y. The aPKCiota blocking agent ATM negatively regulates EMT and invasion of hepatocellular carcinoma. Cell Death Dis. 2014;5:e1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zarzynska JM. Two faces of TGF-beta1 in breast cancer. Mediators Inflamm. 2014;2014:141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parvani JG, Galliher-Beckley AJ, Schiemann BJ, Schiemann WP. Targeted inactivation of beta1 integrin induces beta3 integrin switching, which drives breast cancer metastasis by TGF-beta. Mol Biol Cell 2013;24(21):3449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lv X, Li L, Lv L, Qu X, Jin S, Li K, Deng X, Cheng L, He H, Dong L. HOXD9 promotes epithelial-mesenchymal transition and cancer metastasis by ZEB1 regulation in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:133. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Kaowinn S, Kim J, Lee J, Shin DH, Kang CD, Kim DK, Lee S, Kang MK, Koh SS, Kim SJ, Chung YH. Cancer upregulated gene 2 induces epithelial-mesenchymal transition of human lung cancer cells via TGF-beta signaling. Oncotarget 2017;8(3):5092–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sohn EJ, Won G, Lee J, Yoon SW, Lee I, Kim HJ, Kim SH. Blockage of epithelial to mesenchymal transition and upregulation of let 7b are critically involved in ursolic acid induced apoptosis in malignant mesothelioma cell. Int J Biol Sci. 2016;12(11):1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okayama H, Kohno T, Ishii Y, Shimada Y, Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S, Watanabe S, Sakamoto H, Kumamoto K, Takenoshita S, Gotoh N, Mizuno H, Sarai A, Kawano S, Yamaguchi R, Miyano S, Yokota J. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012;72(1):100–11. [DOI] [PubMed] [Google Scholar]
- 24. Wu Z, Li Y, Zhang G. Downregulation of microRNA-301a inhibited proliferation, migration and invasion of non-small cell lung cancer by directly targeting DLC1. Oncol Lett. 2017;14(5):6017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bendris N, Arsic N, Lemmers B, Blanchard JM. Cyclin A2, Rho GTPases and EMT. Small GTPases 2012;3(4):225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li H, Da LJ, Fan WD, Long XH, Zhang XQ. Transcription factor glioma-associated oncogene homolog 1 is required for transforming growth factor-beta1-induced epithelial-mesenchymal transition of non-small cell lung cancer cells. Mol Med Rep. 2015;11(5):3259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li N, Xu H, Fan K, Liu X, Qi J, Zhao C, Yin P, Wang L, Li Z, Zha X. Altered beta1,6-GlcNAc branched N-glycans impair TGF-beta-mediated epithelial-to-mesenchymal transition through Smad signalling pathway in human lung cancer. J Cell Mol Med. 2014;18(10):1975–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo K. Signaling cross talk between TGF-beta/Smad and other signaling pathways. Cold Spring Harb Perspect Biol. 2017;9(1):a022137. [DOI] [PMC free article] [PubMed] [Google Scholar]