Abstract
G-protein-coupled estrogen receptor (GPER) was found to promote non-small cell lung cancer (NSCLC) by estrogen, indicating the potential necessity of inhibiting GPER by a selective antagonist. This study was performed to elucidate the function of GPER-selective inhibitor G15 in NSCLC development. Cytoplasmic GPER (cGPER) and nuclear GPER (nGPER) were detected by immunohistochemical analysis in NSCLC samples. The relation of GPER and estrogen receptor β (ERβ) expression and correlation between GPER, ERβ, and clinical factors were analyzed. The effects of activating GPER and function of G15 were analyzed in the proliferation of A549 and H1793 cell lines and development of urethane-induced adenocarcinoma. Overexpression of cGPER and nGPER was detected in 80.49% (120/150) and 52.00% (78/150) of the NSCLC samples. High expression of GPER was related with higher stages, poorer differentiation, and high expression of ERβ. The protein level of GPER in the A549 and H1793 cell lines was increased by treatment with E2, G1 (GPER agonist), or fulvestrant (Ful; ERβ antagonist) and decreased by G15. Administration with G15 reversed the E2- or G1-induced cell growth by inhibiting GPER. In urethane-induced adenocarcinoma mice, the number of tumor nodules and tumor index increased in the E2 or G1 group and decreased by treatment with G15. These findings demonstrate that using G15 to block GPER signaling may be considered as a new therapeutic target in NSCLC.
Key words: G15, G-protein-coupled estrogen receptor (GPER), Non-small cell lung cancer (NSCLC), G1, E2
INTRODUCTION
By regulating cell growth and differentiation, estrogens, especially endogenous 17β-estradiol (E2), influence normal physiological functions such as menstrual cycle, reproduction, regulation of bone density, functions of brain, etc1–3. Moreover, reported evidence reveals that estrogen is associated with the carcinogenesis of certain types of cancer, such as breast4, endometrial, ovarian5,6, and lung cancers7. Recently, studies revealed that E2 activated estrogen receptor β (ERβ), inducing the proliferation of non-small cell lung cancer (NSCLC) cells in culture7–9, human tumor xenografts8, and animal models of lung cancer10.
It has been demonstrated that antiestrogen treatments exhibited obvious efficiency in the treatment of breast cancer11. Antiestrogen strategies including selective estrogen receptor (ER) modulators such as tamoxifen, pure antagonists (antagonistic in all tissues) such as fulvestrant, and aromatase inhibitors such as anastrozole have been successfully applied for the treatment and prevention of various cancers3,12,13. Increasing evidence indicates that patients with NSCLC will benefit from interfering with estrogen signaling. In more than 6,500 survivors of breast cancer, significantly lower subsequent lung cancer mortality was found in women who received any antiestrogen treatment14. Another study evaluated 2,320 women with or without exposure to antiestrogen treatments and found strong association between decreased lung cancer mortality and antiestrogen treatments15. Importantly, accumulating evidence now focuses on the potential efficiency of inhibition of ER using fulvestrant in the suppression of NSCLC7,12,16. Safety and potential antitumor activity of combined use of gefitinib [an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor] and fulvestrant in postmenopausal women have been shown in a phase I study17. However, a phase II clinical study18 to evaluate whether the addition of fulvestrant enhances the antitumor efficacy of erlotinib (another EGFR tyrosine kinase inhibitor) demonstrated that progression-free survival and response rates were similar between the two treatment arms in unselected patients. It reveals that potential mechanisms limiting efficacy may exist and a new inhibition strategy is needed based on the antiestrogen theory.
G-protein-coupled estrogen receptor (GPER), recently demonstrated to be expressed in lung tumors, acts as a third ER promoting the development of breast, endometrial, and ovarian cancers19–24. Fulvestrant has been found to activate GPER, stimulating the proliferation and migration of breast cancer cells25 and the proliferation of both endometrial and ovarian cancer cells22,24. These phenomena reveal that antiestrogen strategy based on fulvestrant has the potential to activate GPER, resulting in the development of tumors. A GPER-selective antagonist G15 was identified in 200926. Structurally similar to G1, G15 is effective in inhibiting E2- and G1-mediated effects26–28. The core structures of G15 have been used to generate several agents that can be used for the potential treatment of GPER-expressing tumors in vivo29. However, the effects of G15 in the inhibition of GPER in NSCLC are unclear.
In this study, the expression of GPER was detected in the tumor tissues of NSCLC patients, and its relationship with clinical factors was analyzed. Furthermore, the role of GPER in NSCLC in vitro and in vivo was investigated, which provided evidence that the inhibition of GPER by the selective antagonist G15 should be considered.
MATERIALS AND METHODS
Construction of Tissue Microarray and Immunohistochemistry
The procedure is similar to that described previously30. Patients with primary NSCLC (diagnosed between October 2008 and December 2013) who underwent surgery in the Department of Thoracic Surgery (affiliated to the Tongji Hospital of Huazhong University of Science and Technology Tongji Medical College) were enrolled into the study after obtaining appropriate approval from the Institutional Review Board (IRB; ID No. 20141101). Clinical and pathological information from the patients was collected, and the database was tabulated in an anonymous manner. Samples of tissue sections from primary tumors were identified, and 1.5-mm cores of the identified tissues were punched from the donor blocks and inserted into a recipient block. Where available, the edge of the primary tumor and the corresponding normal lung tissue were identified and included into the tissue microarray (TMA). The TMA was cut into 5-μm sections, and immunohistochemistry (IHC) was performed as previously described30. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
IHC staining was performed using a GPER antibody (1:200; Novus Biologicals, Littleton, CO, USA), which was confirmed to be specific for GPER31, and an ERβ antibody (1:200; AB1410; Chemicon International, Temecula, CA, USA). GPER and ERβ staining were evaluated pathologically in a double-blind manner under a light microscope. Cells positive for GPER or ERβ appeared yellow or yellowish brown in the nucleus or cytoplasm, or they contained yellowish brown granules. A pathological examination and semiquantitation based on the staining intensity and proportion of positive cells were performed as previously reported32. Positive cells were scored as follows: 1, ≤20% positive cells; 2, 20%–50% positive cells; 3, 50%–75% positive cells; and 4, ≥75% positive cells. Staining intensity was evaluated as follows: 1, negative; 2, weakly positive; 3, moderately positive; and 4, strongly positive. A score of 1–16 was given by multiplying the staining intensity and proportion of positive cells: (−), ≤4; (+), >4 and ≤8; (++), >8 and ≤12; and (+++), >12 and ≤16.
Cell Lines and Reagents
A549 and H1793 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and grown for 2 weeks (five passages). A549 cells were cultured in 1640 medium supplemented with 10% fetal bovine serum (FBS) at 37°C with 5% CO2. H1793 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium supplemented with 5% FBS, 0.005 mg/ml insulin, 0.01 mg/ml transferrin, 30 nM sodium selenite, 10 nM hydrocortisone, and extra 2 mM l-glutamine at 37°C with 5% CO2. For experiments, cells were plated in phenol red-free medium that was supplemented with either charcoal-stripped serum or no serum for at least 24 h prior to adding the ligand, and 48 h later they were treated with the following agents: E2 (Sigma-Aldrich, St. Louis, MO, USA), (1-(4-(6-bromobenzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl)-ethanone, 1-[(3aS,4R,9bR-rel)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone) (G1, a GPER agonist; Cayman Chemical, Ann Arbor, MI, USA), fulvestrant (an ER antagonist and also a GPER agonist; Cayman Chemical), and (4-(6-bromo-benzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c] quinoline) (G15, a GPER antagonist; Cayman Chemical) at the indicated concentrations. For cell culture experiments, all reagents used were formulated in 100% dimethyl sulfoxide.
Cell Proliferation Assay
Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8) assay. A549 and H1793 cells were seeded in 96-well plates at an initial density of 3 × 103 cells per well and incubated overnight to allow adherence. Subsequent to washing, cells were treated with E2, G1, fulvestrant, and G15 at different concentrations. Cells of each group were incubated for 1–5 days. Nine duplicate wells were used for each group. At the end of the culture period, the viability of cells was measured using the CCK-8 assay according to the manufacturer’s instructions. In brief, 90 μl of fresh serum-free medium and 10 μl of CCK-8 reagent were added into each well after decanting the old medium, and the culture was continued at 37°C for 2 h. The optical density (OD) at 450 nm was measured using a microplate reader (Promega, Madison, WI, USA). The above steps were repeated three times, and the mean was calculated.
Cell proliferation was also quantified by the uptake of 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay, which was performed using the CellLight™ EdU imaging detection kit (RiboBio, Guangzhou, P.R. China) according to the manufacturer’s instructions. The cell proliferation rate was calculated using the formula (EdU add-in cells/Hoechst-stained cells) × 100%. The assay was performed in triplicate and repeated three times in independent experiments.
Urethane-Induced Adenocarcinoma Model and Treatments
Four-week-old female Kunming mice were maintained in an environment with a standardized barrier system (System Barrier Environment No. 00021082) in the Experimental Animal Center of Tongji Hospital of Huazhong University of Science and Technology (Institutional Animal Care and Use Committee No. S365). After mice underwent ovariectomy, lung cancer was induced by urethane (Sigma-Aldrich) as previously described10. Then mice were subcutaneously administered with E2, G1, E2 + fulvestrant (Ful), G1 + Ful, E2 + Ful + G15, Ful, G15, and blank control. E2 (0.09 mg/kg), G1 (0.14 mg/kg), Ful (2.4 mg/kg), and G15 (1.46 mg/kg) were prepared in ethanol. These drugs were injected twice weekly. After 14 weeks, mice were sacrificed and the lungs were collected. The lungs were macroscopically observed, and tumor nodules were counted as previously described10. The total number of lung nodules was counted in each mouse using light microscopy. The lung nodules were divided into two groups. One group was fixed in 10% formalin and embedded in paraffin. The largest lobe was stained with hematoxylin and eosin (H&E) for pathological examinations, and other lobes were sectioned for TMA. The other group was stored in liquid nitrogen at −80°C for further use (Western blot analysis).
Western Blot Analysis
Cells were detached using trypsin, washed three times with phosphate-buffered saline, treated with lysis buffer [25 mM Tris-HCl (pH 7.4), 1% Triton X-100, 150 mM NaCl, 5% ethylenediaminetetraacetic acid, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 10 mg of aprotinin and leupeptin] and incubated for 30 min on ice. Lung cancer tissues were lysed in PMSF, followed by homogenization and determination of the concentration of protein. The lysate was centrifuged for 10 min at 12,000 rpm, and the supernatant was collected. Concentrations of protein were measured using the Bradford method (Bio-Rad, Hercules, CA, USA). A 50-μg aliquot of protein per lane was electrophoresed on 8%–12% sodium dodecyl sulfate-polyacrylamide gel and electroblotted on polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). The transferred membranes were blocked with 5% nonfat dry skim milk in Tris-buffered saline [TBST; 25 mM Tris-HCl (pH 7.4), 125 mM NaCl, 0.05% Tween 20] and incubated at 4°C overnight with the appropriate primary antibodies, which were specific for the protein GPER (1:600) from Novus Biologicals. After being washed with TBST, the membranes were incubated with a horseradish peroxidase-labeled secondary antibody (1:2,000) for 1 h at 37°C before detection using ECL-Plus Western Blotting Detection Reagents (Pierce, Rockford, lL, USA). Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control for protein loading and analysis.
Statistical Analysis
Statistical analysis was performed using IBM SPSS version 19 (SPSS Inc., Chicago, IL, USA). Differences in the expression of GPER between NSCLC and benign pulmonary lesion (BPL) were examined using the chi-square test. Associations between the expression of GPER and clinicopathological determinants were evaluated using the chi-square test and Fisher’s exact test (for nominal variables) as appropriate. Spearman rank correlation was used to analyze the correlation between the expression of GPER and ERβ. Student’s t-test was used to evaluate the statistical significance of the in vitro studies. One-way analysis of variance was used to analyze the influence of E2 and G1 on tumorigenesis. A value of p < 0.05 was considered statistically significant.
RESULTS
Expression of GPER in NSCLC
Tumor samples from 150 patients (106 men and 44 women; mean age, 57 ± 7 years; range, 45–71 years) were available for constructing a TMA. All female patients were in menopause. Additionally, 76 patients with squamous cell carcinoma and 74 patients with adenocarcinoma were found equally distributed among the selected patients. All carcinomas were classified according to the pathological tumor–node–metastasis staging system (UICC, 7th edition, 2009). Of the total patients, 79 presented with stages IA–IIB NSCLC, and 71 presented with stages IIIA–IV NSCLC. Furthermore, poorly differentiated NSCLC was found in 38 patients, and moderate to well-differentiated NSCLC was found in 112 patients. In addition, 100 control subjects (54 men and 46 women; mean age, 55 ± 10 years; range, 45–66 years) with BPL who underwent surgery at our department were enrolled. All female patients were in menopause. Pulmonary tuberculomas, bronchiectasis, and sclerosing hemangiomas were found in 62, 31, and 7 of these patients, respectively.
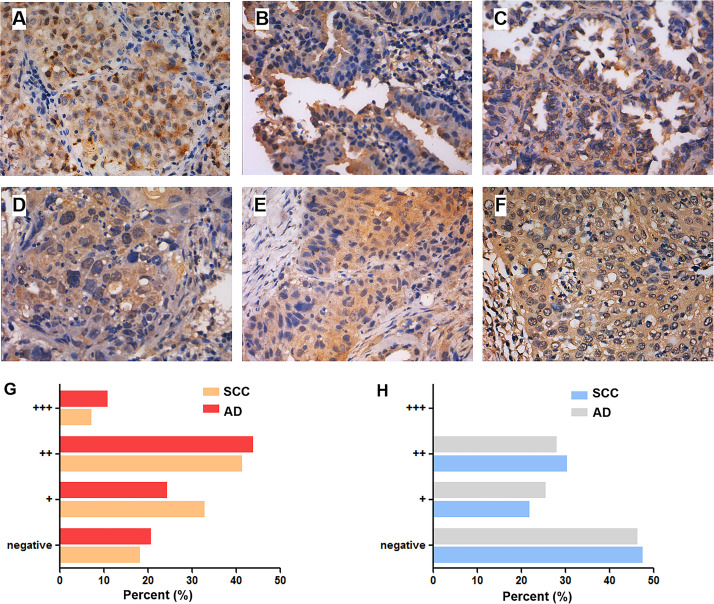
GPER+ granules were mainly found in the cytoplasm and sometimes the nuclei of lung cancer cells (Fig. 1). In BPL, only a few cells were mildly or moderately positive for GPER (+∼++). When accounting for cytoplasmic GPER (cGPER) and nuclear GPER (nGPER) separately in the NSCLC group, 80.49% (120/150) of the samples were positive for cGPER, which was markedly higher than that of BPL (35.00%, 49/140) (p < 0.001). The proportion of samples positive for nGPER was 52.00% (78/150), which was significantly higher than that in benign pulmonary tissues (28.00%, 28/100) (p < 0.001; data not shown). There was no marked correlation between the expression of cGPER and gender, age, smoking index, or histological type. The expression of cGPER in NSCLC at stages IIIA–IV (88.73%) was markedly higher than at stages IA–IIB (72.15%) (p = 0.014). The expression of cGPER in poorly differentiated NSCLC (97.37%) was significantly increased compared to moderate- to well-differentiated NSCLC (74.11%) (p = 0.001). There was no marked correlation between the expression of nGPER and gender, age, smoking index, histological type, lymph node metastasis, TNM stage, or degree of differentiation (Table 1). The expression of cGPER was positively correlated with that of ERβ and especially in female patients with adenocarcinoma (r = 0.242 and 0.457, respectively; p < 0.05) (Table 2). The expression of nGPER did not show any correlation with ERβ (r = 0.019, p < 0.05) (Table 2).
Figure 1.
Expression of G-protein-coupled estrogen receptor (GPER) in lung adenocarcinoma and squamous cell carcinoma. The expression of GPER in lung adenocarcinoma is shown in well (A), moderately (B), and poorly (C) differentiated degrees. The expression of GPER in lung squamous cell carcinoma is shown in well (D), moderately (E), and poorly (F) differentiated degrees. Original magnification: 400×. The bar graphs summarize the percentage of lung adenocarcinoma and squamous cell carcinoma that express various amounts of cytoplasmic GPER (G) and nuclear GPER (H) proteins.
Table 1.
Correlation Between the Expression of Cytoplasmic G-Protein-Coupled Estrogen Receptor (cGPER), Nuclear GPER (nGPER), Estrogen Receptor β (ERβ), and the Clinicopathological Characteristics of Non-Small Cell Lung Cancer (NSCLC)
| cGPER | nGPER | ERβ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| − | + | p | − | + | p | − | + | p | |
| Gender | 1.00 | 0.591 | 0.179 | ||||||
| Male | 21 | 85 | 49 | 57 | 10 | 76 | |||
| Female | 9 | 35 | 23 | 21 | 14 | 50 | |||
| Age | 0.131 | 0.300 | 0.097 | ||||||
| <55 | 6 | 42 | 20 | 28 | 4 | 44 | |||
| ≥55 | 24 | 78 | 52 | 50 | 20 | 82 | |||
| Smoking index | 1.000 | 1.000 | 0.025* | ||||||
| <400 | 15 | 58 | 35 | 38 | 17 | 56 | |||
| ≥400 | 15 | 62 | 37 | 40 | 7 | 70 | |||
| HT | 0.418 | 0.872 | 0.003* | ||||||
| Squamous cell carcinoma | 13 | 63 | 37 | 39 | 19 | 57 | |||
| Adenocarcinoma | 17 | 57 | 35 | 39 | 5 | 69 | |||
| TNM stage | 0.014* | 0.517 | 0.826 | ||||||
| IA–IIB | 22 | 57 | 40 | 39 | 12 | 67 | |||
| IIIA–IV | 8 | 63 | 32 | 39 | 12 | 59 | |||
| Degree of differentiation | 0.001* | 0.575 | 0.442 | ||||||
| Moderate to well | 29 | 83 | 52 | 60 | 20 | 92 | |||
| Poor | 1 | 37 | 20 | 18 | 4 | 34 | |||
p < 0.05.
Table 2.
Correlation Between the Expression of Aromatase, cGPER, nGPER, and ERβ in Female Lung Adenocarcinoma (Spearman’s Test)
| cGPER | nGPER | |||
|---|---|---|---|---|
| r | p | r | p | |
| ERβ | 0.457 | 0.022* | 0.019 | 0.852 |
G15 Inhibited the Response of GPER Stimulated by E2 in NSCLC Cell Lines
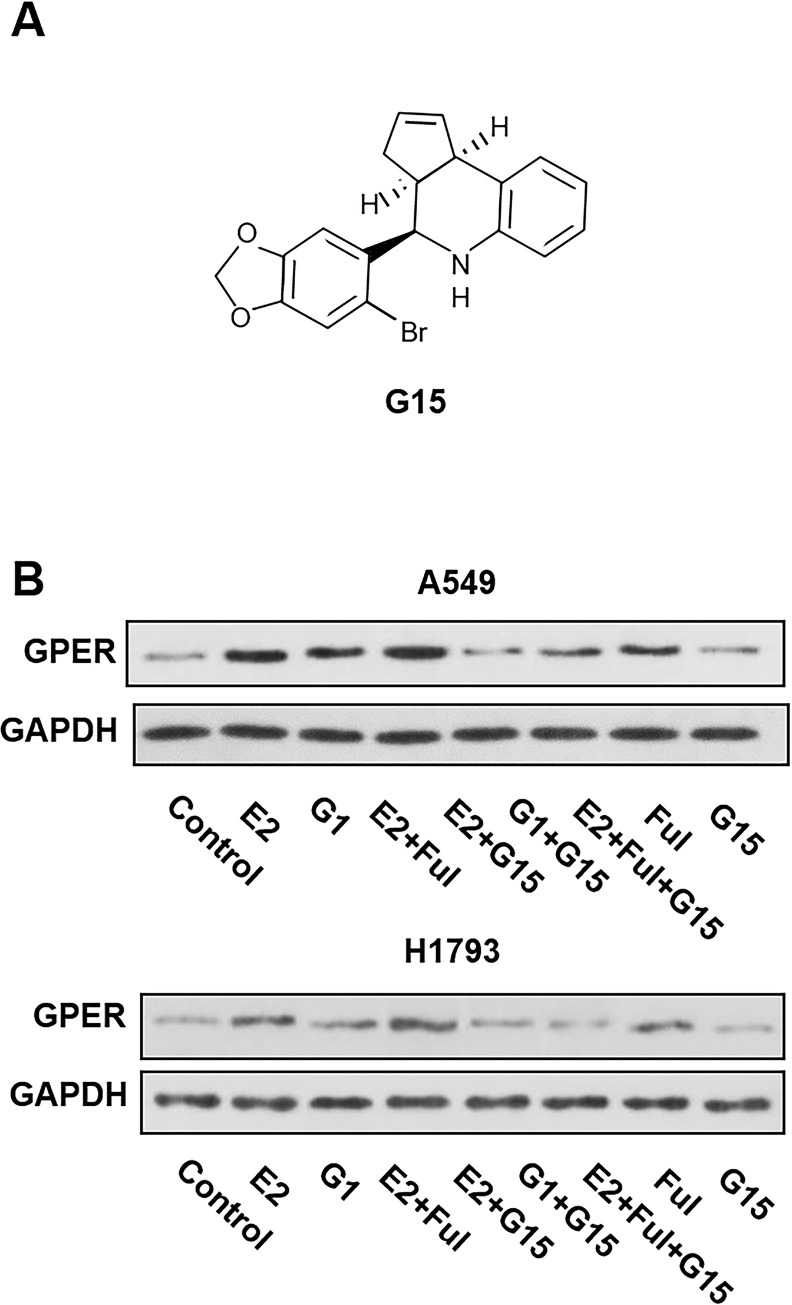
To determine the effects of G15 (chemical structure is shown in Fig. 2A) on the expression of GPER protein in NSCLC cell lines, A549 and H1793 cells were pretreated with E2 (10 nM), G1 (10 nM), E2 + Ful (1 μM), E2 + G15 (1 μM), G1 + G15, E2 + Ful + G15, Ful, and G15 at indicated concentrations. After 48 h, changes in the expression of GPER were examined by Western blot. Treatment with E2 and G1 both upregulated the expression of GPER, suggesting the potential reactivity of GPER in NSCLC cells. In addition, fulvestrant, a selective ER degrader, also upregulated the expression of GPER. The GPER protein level was downregulated under E2 + G15, G1 + G15, and E2 + Ful + G15 treatments. Interestingly, E2 + Ful increased the expression of GPER compared to the control group (Fig. 2B). These results indicated that E2, G1, and Ful stimulated GPER expression in NSCLC cells. Fulvestrant did not inhibit the response of GPER to E2 unless G15 was added synchronously.
Figure 2.
(A) Chemical structure of G15. (B) G15 inhibited the response of GPER stimulated by 17β-estradiol (E2) and G1 in the A549 and H1793 cell lines. The synchronized cells were treated with E2 (10 nM), G1 (10 nM), E2 + fulvestrant (Ful) (1 μM), E2 + G15 (1 μM), G1 + G15, E2 + Ful + G15, Ful, and G15 for 2 days. The protein expression of GPER was determined using Western blot. The data represent means ± SEM from three different experiments.
G15 Inhibited GPER-Mediated Proliferation Stimulated by E2 in NSCLC Cell Lines
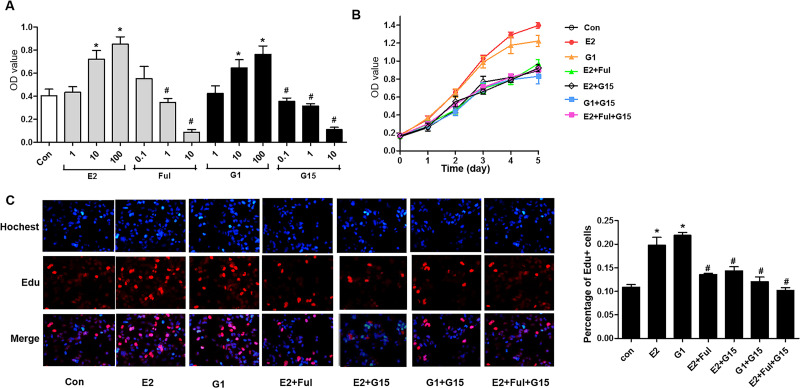
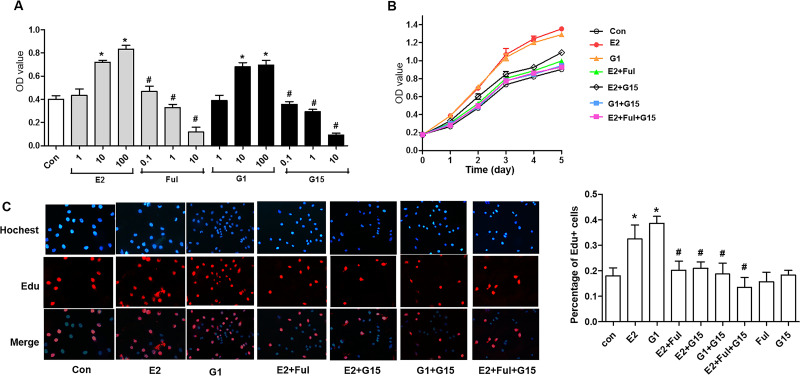
To investigate the effects of G15 on tumor cell proliferation, the growth of A549 and H1793 cells was first examined using the CCK-8 assay after administering 1, 10, and 100 nM selective agonist G1 and 1, 10, and 100 nM E2, and 10 nM E2 with 0.1, 1, and 10 μM Ful and 10 nM G1 with 0.1, 1, and 10 μM G15. The OD values increased in a dose-dependent manner in both the G1 and E2 groups (Figs. 3A and 4A). The A549 and H1793 cells were grouped as E2 (10 nM), G1 (10 nM), E2 + Ful (1 μM), E2 + G15 (1 μM), G1 + G15, and E2 + Ful + G15. Between the first and the fifth days, the OD values increased gradually. The OD values between the different days were significantly different (p < 0.001). E2 and G1 induced both A549 and H1793 cells to grow faster than in the control group (p < 0.001). G15, fulvestrant, or a combination of the above two decreased the proliferation by E2 dramatically (p < 0.001), but no significant differences in the OD value between these three groups were observed (Figs. 3B and 4B). To confirm the biological effects of G15 in S phase cell cycle progression, A549 cells were measured using EdU assay after treating with E2, G1, E2 + Ful, E2 + Ful, G1 + G15, and E2 + Ful + G15 as mentioned. As expected, the percentage of EdU+ cell proliferation increased by nearly onefold by E2 or G1 compared to control group. Administration with G15 reversed the E2- or G1-induced tumor growth (p = 0.0001) (Figs. 3C and 4C). This result indicated that both G1 and E2 enhanced the proliferation of A549 and H1793 cells. G15 and fulvestrant can block the proliferative effects by G1 or E2, respectively. Moreover, G15 could also inhibit the proliferation stimulated by E2/GPER signaling.
Figure 3.
G15 inhibited GPER-mediated proliferation stimulated by E2 and G1 in A549 cell lines. (A) The synchronized A549 cells were treated with E2 or G1 at different concentrations (1, 10, and 100 nM), or Ful (0.1, 1, and 10 μM) and G15 (0.1, 1, and 10 μM) in combination with 10 nM E2 and 10 nM G1 for 2 days. The viability of the cell was analyzed using the Cell Counting Kit-8 assay. (B) The synchronized A549 cells were treated with E2 (10 nM), G1 (10 nM), E2 + Ful (1 μM), E2 + G15 (1 μM), G1 + G15, and E2 + Ful + G15 for 1–5 days. Cell Counting Kit-8 was used to evaluate the viability of cells. The optical density (OD) value proportional to the cell number was measured and plotted on the growth curve. (C) The synchronized A549 cells were treated with E2 (10 nM), G1 (10 nM), E2 + Ful (1 μM), E2 + G15 (1 μM), G1 + G15, and E2 + Ful + G15 for 2 days. 5-Ethynyl-2′-deoxyuridine (EdU) assay of relative Hoechst-stained cells and EdU add-in cells was used to analyze cell proliferation. The data represent mean ± SEM from three different experiments (*p < 0.05 vs. control group, #p < 0.05 vs. E2 group).
Figure 4.
G15 inhibited GPER-mediated proliferation stimulated by E2 and G1 in H1793 cell lines. (A) The synchronized H1793 cells were treated with E2 or G1 at different concentrations (1, 10, and 100 nM), or fulvestrant (0.1, 1, and 10 μM) and G15 (0.1, 1, and 10 μM) in combination with 10 nM E2 and 10 nM G1 for 2 days. The viability of cells was analyzed using the Cell Counting Kit-8 assay. (B) The synchronized H1793 cells were treated with E2 (10 nM), G1 (10 nM), E2 + Ful (1 μM), E2 + G15 (1 μM), G1 + G15, and E2 + Ful + G15 for 1–5 days. The Cell Counting Kit-8 was used to evaluate the viability of cells. The OD value proportional to the cell number was measured and plotted on the growth curve. (C) The synchronized H1793 cells were treated with E2 (10 nM), G1 (10 nM), E2 + Ful (1 μM), E2 + G15 (1 μM), G1 + G15, and E2 + Ful + G15 for 2 days. The EdU assay of relative Hoechst-stained cells and EdU add-in cells was used to analyze cell proliferation. The data represent mean ± SEM from three different experiments (*p < 0.05 vs. control group, #p < 0.05 vs. E2 group).
G15 Inhibited GPER-Mediated Proliferation Stimulated by E2 in Urethane-Induced Adenocarcinoma Mice
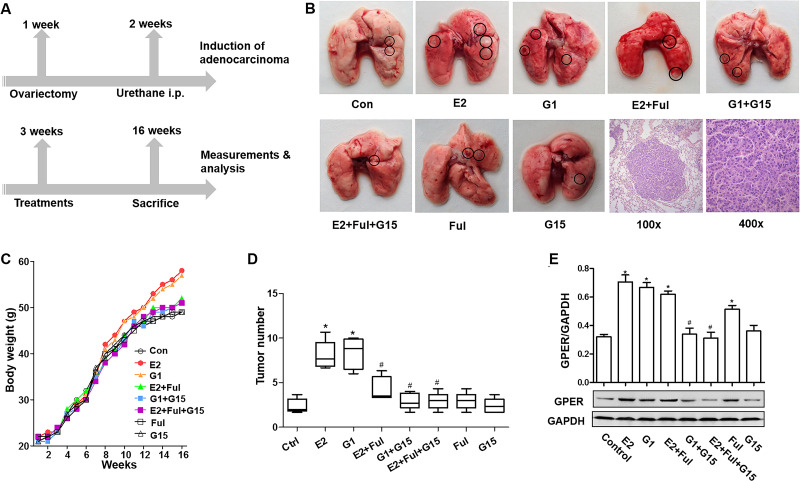
Urethane-induced adenocarcinoma mice model was established to detect the influence of G15 in the generation and progression of lung cancer (Fig. 5A). Visible tumors were observed on the lung surface after 14 weeks of induction (Fig. 5B). The rate of tumor formation was 94.29% (33/35). H&E staining showed that the induction rate of lung adenocarcinoma was 93.94% (31/33) (Fig. 4B). Moreover, parameters including the body weight and number of tumor nodules in the E2 and G1 groups increased compared with the control group. The upregulation of body weight by E2 was reversed by using G15, fulvestrant, or a combination of the two. The parameters such as number of tumor nodules decreased in the E2 + Ful + G15 group but increased in the E2 + Ful group compared to the control group (Fig. 5C and D). The protein expression levels of the GPER from tumor tissues were higher in the E2, G1, and E2 + Ful groups compared with the control group and were lower in the G1 + G15 and E2 + Ful + G15 groups compared with the E2 or G1 group, respectively (Fig. 5E). These findings further indicate a therapeutic effect of G15 on growth and progression of lung tumor in vivo.
Figure 5.
G15 inhibited GPER-mediated proliferation stimulated by E2 in urethane-induced adenocarcinoma mice. (A) Kunming mice were allowed to acclimatize to the environment for 1 week and then underwent an ovariectomy. One week later, animals were intraperitoneally administered with urethane. One week later, mice received 13 weeks of blank control, E2, G1, E2 + Ful, G1 + G15, E2 + Ful + G15, Ful, and G15 subcutaneously twice a week. (B) Gross examination of urethane-induced lung tumors in different groups and representative image of adenocarcinoma [hematoxylin and eosin (H&E) staining]. Original magnification: 100× (left) and 400× (right). (C, D) The body weight and number of nodules in the lungs (number of lung nodules in every mouse of each group) in different groups. (E) GPER protein expression in tumor tissues from different groups was detected using Western blot. The data represent means ± SEM (*p < 0.05 vs. control group, #p < 0.05 vs. E2 or G1 group, respectively).
DISCUSSION
Estrogens play an important role in the development of NSCLC7,13. Previous studies on the use of hormonal replacement therapy and antiestrogen support the idea that estrogen acts as a promoter of lung cancer aggression, which may affect not only the biology but also the outcome of lung cancer7,12. ERβ is widely considered as the major target of endocrine treatment for NSCLC, and a pure ER antagonist, fulvestrant, provides considerable inhibition efficiency for NSCLC in vitro and in vivo7,12,33. Treatment of fulvestrant was well tolerated in a phase II clinical trial18. However, limited efficacy reveals little benefit to patients, raising questions about whether interfering with estrogen signaling only by treating with fulvestrant will benefit NSCLC. Interestingly, another estrogen response receptor, GPER, was found highly expressed in NSCLC tissues and cell lines compared to normal controls31. Thus, the blocking of GPER apart from targeting ERβ should be taken into consideration. In this study, the presence of GPER in NSCLC tissues was exclusively correlated with the expression of ERβ. Additional use of GPER antagonist G15 was found to suppress the development of NSCLC both in vitro and in vivo, revealing that inhibition of GPER by G15 exhibited a new antiestrogen strategy of NSCLC.
Our results demonstrated that GPER-selective agonists G1 and E2 activated GPER and induced proliferation of A549 and H1793 cells. In mice treated with E2 or G1, the body weight and number of tumor nodules in the E2 and G1 groups were markedly higher than those in the control group. These results suggested that E2 and G1 could promote the development of NSCLC independently. Of greater importance, these parameters in the E2 or G1 group were reversed in the fulvestrant, G15, or combination groups. In recent studies, antagonists of ERα and ERβ did not completely block estrogen-mediated nongenomic signaling34, and E2 was found to stimulate proliferation in ER− breast, endometrial, and ovarian cancer cells20–24. These findings led researchers to consider a third ER, GPER, that contributed to these effects. GPER was found to promote the progression of breast, endometrial, ovarian, and thyroid cancers35,36. GPER was also found to be expressed higher in TMAs of human lung and mice lung tumor tissues31. Differential distribution of GPER was also found in breast cancer35, implying that cytoplasmic nuclear expression of GPER conveyed different functions. In our study, the findings indicated that cytoplasmic nuclear expression of GPER correlates with different clinical parameters in NSCLC tissues. We found that GPER was responsive to estrogen stimulation and acted as a promoter of NSCLC, inducing generation and proliferation of tumors. Moreover, inhibition of GPER by selective antagonist G15 revealed tumor-blocking effects in NSCLC cell lines and a urethane-induced NSCLC mice model. Classical ERs are accepted as the predominant nuclear receptors involved in the genomic effects of estrogen. ERs located at the plasma membrane also modulate nongenomic cell signaling pathways via ERs. GPER mainly regulates rapid nongenomic signaling, activates metalloproteinases, and induces the release of heparin-binding epidermal growth factor, which binds and activates EGFR, leading to the downstream activation of signaling molecules such as ERK1 and ERK235.
G15 was identified as a GPER-selective antagonist26. Structurally similar with G1 (Fig. 2A), G15 is effective in inhibiting G1- or E2-mediated proliferation of lung tumor in vitro and in vivo. Similarly, it has been reported that G15 abolished the proliferative effects of GPER in breast cancer37, seminoma38, and cancer-associated fibroblasts39. It is reported here that the inhibition of GPER by G15 led to the inhibition of growth of A549 and H1793 cells and in urethane-induced lung adenocarcinoma mice, as GPER protein level decreased without the influence of expression of ERβ protein. Especially when G15 was added, the upregulation of GPER by E2 and fulvestrant was blocked, revealing a potential protumor mechanism that exists in fulvestrant-based antiestrogen strategies.
Furthermore, it was found that the completely inhibitory effect by G15 on the development of NSCLC was mediated through the inhibition of GPER. Antiestrogen-based therapeutic treatments may be beneficial for the prevention of lung cancer in both men and women. Potential strategies to target the estrogen signaling for lung cancer treatment include7 aromatase inhibitors to block the synthesis of estrogen16, antagonists of ERβ to anti-estrogens, and targeting growth factor pathways such as EGFR33, insulin-like growth factor 1 receptor32, or vascular endothelial growth factor receptor40 that are activated by estrogen signaling. With regard to the blocking of ERβ, fulvestrant, one of the most used antagonists, acts as a pure antagonist but not as a “partial” antagonist such as tamoxifen, downregulating ERβ by increasing its rate of degradation. However, fulvestrant has been found to stimulate, through the activation of GPER, the proliferation and migration in breast cancer cells25 and enhance the proliferation in both endometrial and ovarian cancer cells22,24, which indicates an estrogen-like action of fulvestrant. We hypothesize that fulvestrant blocked the activation of ERβ by E2 but stimulated the activation of GPER. The use of the ERβ inhibitor fulvestrant alone in NSCLC may not block the effects of estrogen completely. However, it should be pointed out that the time of follow-up to patients is limited, and more data are needed to evaluate the relationship between GPER and ERβ signaling. The well-known ER antagonist fulvestrant exerts activation effects on GPER-mediated signaling, confirming the potential opposite functions elicited by estrogenic/anti-estrogenic agents through each type of ER. Based on the findings, the use of G15 to block GPER signaling may be considered as an additional therapeutic target in NSCLC.
In conclusion, these data provide a comprehensive insight into the role of GPER in NSCLC, and targeting GPER by selective inhibitor G15 may be a more potential therapy in patients with NSCLC. However, more investigations, including prospective clinical studies, are warranted in the future. In this regard, the opposite functional activity elicited by antiestrogens through the classical ERs and GPER, as stated earlier, could represent a therapeutic concern toward the pharmacological inhibition of all types of ER.
ACKNOWLEDGMENTS
The authors thank Ensong Guo, Xiao Wei, Hao Lu, and Changlin Zhang from the Key Laboratory of Cancer Invasion and Metastasis (Huazhong University of Science and Technology), Ministry of Education, for their help. This study was funded by the National Natural Science Foundation of China (NSFC) (Grant Nos. 81272590 and 81402163) and Wuhan Municipal Human Resources and Social Security Bureau (Grant No. 2011415).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Koos RD. Minireview: Putting physiology back into estrogens’ mechanism of action. Endocrinology 2011;152(12):4481–8. [DOI] [PubMed] [Google Scholar]
- 2. Shang Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer 2006;6(5):360–8. [DOI] [PubMed] [Google Scholar]
- 3. Shang Y. Hormones and cancer. Cell Res. 2007;17(4):277–9. [DOI] [PubMed] [Google Scholar]
- 4. Huang B, Warner M, Gustafsson JA. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol Cell Endocrinol. 2015;418(Pt 3):240–4. [DOI] [PubMed] [Google Scholar]
- 5. Ito K, Utsunomiya H, Niikura H, Yaegashi N, Sasano H. Inhibition of estrogen actions in human gynecological malignancies: New aspects of endocrine therapy for endometrial cancer and ovarian cancer. Mol Cell Endocrinol. 2011;340(2):161–7. [DOI] [PubMed] [Google Scholar]
- 6. Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: Estrogen pathway in colorectal cancer. Clin Cancer Res. 2013;19(21):5842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siegfried JM, Stabile LP. Estrongenic steroid hormones in lung cancer. Semin Oncol. 2014;41(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62(7):2141–50. [PubMed] [Google Scholar]
- 9. Pietras RJ, Marquez-Garban DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res. 2007;13(16):4672–6. [DOI] [PubMed] [Google Scholar]
- 10. Tang H, Liao Y, Xu L, Zhang C, Liu Z, Deng Y, Jiang Z, Fu S, Chen Z, Zhou S. Estrogen and insulin-like growth factor 1 synergistically promote the development of lung adenocarcinoma in mice. Int J Cancer 2013;133(10):2473–82. [DOI] [PubMed] [Google Scholar]
- 11. Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2016;34(14):1689–701. [DOI] [PubMed] [Google Scholar]
- 12. Miki Y, Abe K, Suzuki S, Suzuki T, Sasano H. Suppression of estrogen actions in human lung cancer. Mol Cell Endocrinol. 2011;340(2):168–74. [DOI] [PubMed] [Google Scholar]
- 13. Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer 2011;11(8):597–608. [DOI] [PubMed] [Google Scholar]
- 14. Bouchardy C, Benhamou S, Schaffar R, Verkooijen HM, Fioretta G, Schubert H, Vinh-Hung V, Soria JC, Vlastos G, Rapiti E. Lung cancer mortality risk among breast cancer patients treated with anti-estrogens. Cancer 2011;117(6):1288–95. [DOI] [PubMed] [Google Scholar]
- 15. Lother SA, Harding GA, Musto G, Navaratnam S, Pitz MW. Antiestrogen use and survival of women with non-small cell lung cancer in Manitoba, Canada. Horm Cancer 2013;4(5):270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, Dubinett SM, Pietras RJ. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65(24):11287–91. [DOI] [PubMed] [Google Scholar]
- 17. Traynor AM, Schiller JH, Stabile LP, Kolesar JM, Eickhoff JC, Dacic S, Hoang T, Dubey S, Marcotte SM, Siegfried JM. Pilot study of gefitinib and fulvestrant in the treatment of post-menopausal women with advanced non-small cell lung cancer. Lung Cancer 2009;64(1):51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garon E, Siegfried JM, Dubinett SM, Elashoff RM, Park DJ, Parikh RJ, Patel R, Hu EH, Reckamp KL, Adams B, Martinez D, Wang HJ, Kabbinavar F, Dacic S, Brennan M, Laux I, Marquez-Garban DC, Stabile LP, Slamon DJ, Pietras RJ. Results of TORI-L-03, a randomized, multicenter phase II clinical trial of erlotinib (E) or E+fulvestrant (F) in previously treated advanced non-small cell lung cancer (NSCLC). In: Proceedings of the 104th Annual Meeting of the American Association for Cancer Research Philadelphia (PA): AACR; 2013; abstract 4664. [Google Scholar]
- 19. Chen Y, Li Z, He Y, Shang D, Pan J, Wang H, Chen H, Zhu Z, Wan L, Wang X. Estrogen and pure antiestrogen fulvestrant (ICI 182 780) augment cell-matrigel adhesion of MCF-7 breast cancer cells through a novel G protein coupled estrogen receptor (GPR30)-to-calpain signaling axis. Toxicol Appl Pharmacol. 2014275(2):176–81. [DOI] [PubMed] [Google Scholar]
- 20. Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14(10):1649–60. [DOI] [PubMed] [Google Scholar]
- 21. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005;307(5715):1625–30. [DOI] [PubMed] [Google Scholar]
- 22. Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Andò S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67(4):1859–66. [DOI] [PubMed] [Google Scholar]
- 23. Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005;146(2):624–32. [DOI] [PubMed] [Google Scholar]
- 24. Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006;20(3):631–46. [DOI] [PubMed] [Google Scholar]
- 25. Pandey DP, Lappano R, Albanito L, Madeo A, Maggiolini M, Picard D. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. EMBO J. 2009;28(5):523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5(6):421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jenei-Lanzl Z, Straub RH, Dienstknecht T, Huber M, Hager M, Grassel S, Kujat R, Angele MK, Nerlich M, Angele P. Estradiol inhibits chondrogenic differentiation of mesenchymal stem cells via nonclassic signaling. Arthritis Rheum. 2010;62(4):1088–96. [DOI] [PubMed] [Google Scholar]
- 28. Gingerich S, Kim GL, Chalmers JA, Koletar MM, Wang X, Wang Y, Belsham DD. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience 2010;170(1):54–66. [DOI] [PubMed] [Google Scholar]
- 29. Ramesh C, Nayak TK, Burai R, Dennis MK, Hathaway HJ, Sklar LA, Prossnitz ER, Arterburn JB. Synthesis and characterization of iodinated tetrahydroquinolines targeting the G protein-coupled estrogen receptor GPR30. J Med Chem. 2010;53(3):1004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Z, Liao Y, Tang H, Chen G. The expression of estrogen receptors beta2, 5 identifies and is associated with prognosis in non-small cell lung cancer. Endocrine 2013;44(2):517–24. [DOI] [PubMed] [Google Scholar]
- 31. Jala VR, Radde BN, Haribabu B, Klinge CM. Enhanced expression of G-protein coupled estrogen receptor (GPER/GPR30) in lung cancer. BMC Cancer 2012;12:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang H, Liao Y, Chen G, Xu L, Zhang C, Ju S, Zhou S. Estrogen upregulates the IGF-1 signaling pathway in lung cancer through estrogen receptor-beta. Med Oncol. 2012;29(4):2640–8. [DOI] [PubMed] [Google Scholar]
- 33. Garon EB, Pietras RJ, Finn RS, Kamranpour N, Pitts S, Marquez-Garban DC, Desai AJ, Dering J, Hosmer W, von Euw EM, Dubinett SM, Slamon DJ. Antiestrogen fulvestrant enhances the antiproliferative effects of epidermal growth factor receptor inhibitors in human non-small-cell lung cancer. J Thorac Oncol. 2013;8(3):270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wehling M. Specific, nongenomic actions of steroid hormones. Annu Rev Physiol. 1997;59:365–93. [DOI] [PubMed] [Google Scholar]
- 35. Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barton M. Position paper: The membrane estrogen receptor GPER—Clues and questions. Steroids 2012;77(10):935–42. [DOI] [PubMed] [Google Scholar]
- 37. Tao S, He H, Chen Q, Yue W. GPER mediated estradiol reduces miR-148a to promote HLA-G expression in breast cancer. Biochem Biophys Res Commun. 2014;451(1):74–8. [DOI] [PubMed] [Google Scholar]
- 38. Chevalier N, Vega A, Bouskine A, Siddeek B, Michiels JF, Chevallier D, Fénichel P. GPR30, the non-classical membrane G protein related estrogen receptor, is overexpressed in human seminoma and promotes seminoma cell proliferation. PLoS One 2012;7(4):e34672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luo H, Yang G, Yu T, Luo S, Wu C, Sun Y, Liu M, Tu G. GPER-mediated proliferation and estradiol production in breast cancer-associated fibroblasts. Endocr Relat Cancer 2014;21(2):355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siegfried JM, Gubish CT, Rothstein ME, Henry C, Stabile LP. Combining the multitargeted tyrosine kinase inhibitor vandetanib with the antiestrogen fulvestrant enhances its antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2012;7(3):485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]