Abstract
A large number of microRNAs (miRNAs) have been previously demonstrated to be dysregulated in breast cancer (BC), and alterations in miRNA expression may affect the initiation and progression of BC. This study showed that miR-664 expression was obviously reduced in BC tissues and cell lines. Resumption of the expression of miR-664 attenuated the proliferation and invasion of BC cells. The molecular mechanisms underlying the inhibitory effects of BC cell proliferation and invasion by miR-664 were also studied. Insulin receptor substrate 1 (IRS1) was identified as a novel and direct target of miR-664. In addition, siRNA-mediated silencing of IRS1 expression mimicked the suppressive effects of miR-664 overexpression in BC cells. Rescue experiments demonstrated that recovered IRS1 expression partially antagonized the inhibition of proliferation and invasion of BC cells caused by miR-664 overexpression. Thus, miR-664 may serve as a tumor suppressor in BC by directly targeting IRS1. Moreover, miR-664 downregulation in BC may contribute to the occurrence and development of BC, suggesting that miR-664 may be a novel therapeutic target for patients with BC.
Key words: miR-664, Breast cancer (BC), Insulin receptor substrate 1 (IRS1), Proliferation, Invasion
INTRODUCTION
Breast cancer (BC) is the most commonly diagnosed cancer and the second leading cause of cancer-related mortality in women globally1. It is characterized by aggressive local invasion, early metastasis, and limited sensitivity to chemotherapy2. Genetic and epigenetic alterations as well as environmental factors have been demonstrated to be involved in BC occurrence and development3,4; however, the exact mechanisms associated with the pathogenesis of BC remain unclear. Currently, the primary treatments for patients with BC are surgical operation followed by hormonotherapy, chemotherapy, radiotherapy, and/or biological therapy5. In the past decade, remarkable improvements in diagnosis and therapy have significantly reduced the death rate of BC patients by over 30%6,7. Nevertheless, the prognosis of BC patients diagnosed at advanced stage remains unsatisfactory. Local recurrence and distant metastasis, even after combined treatments, are two primary reasons behind the poor prognosis of patients with BC8. Hence, an in-depth study of the mechanisms underlying the formation and progression of BC and the search for novel therapeutic methods for patients with this malignancy are necessary.
MicroRNAs (miRNAs) are a multifunctional group of highly conserved 18–23 nucleotide single-stranded and noncoding RNA molecules that are generated by two RNAse III enzymes: DROSHA and DICER9. miRNAs can negatively mediate gene expression through interactions at the 3′-untranslated regions (3′-UTRs) of their target gene in a base pairing manner, and therefore result in either mRNA degradation or translation suppression10. One specific miRNA may regulate the expression of numerous target genes because each seed sequence may match multiple mRNAs11. Dysregulation of miRNAs, which is mainly caused by deletions, amplifications, epigenetic silencing, or mutations in miRNA loci12, has been previously observed in almost all types of human malignancy. Aberrantly expressed miRNAs may play either a tumor suppressive or an oncogenic role in tumorigenesis and tumor development via the regulation of a wide range of pathological processes, such as cell proliferation, cycle, survival, migration, invasion, metastasis, and angiogenesis13,14. Therefore, miRNAs may have the potential to be developed as promising therapeutic targets for the treatment of human cancers.
miR-664 is aberrantly expressed in several types of human cancers, including cervical cancer15, malignant melanoma16, osteosarcoma17,18, lung cancer19, and T-cell acute lymphoblastic leukemia20. However, no specific studies have been performed to detect the expression of miR-664 in BC and investigate the biological roles of miR-664 in BC. The present study aimed to initially determine the expression of miR-664 in BC tissues and cell lines. In addition, the effects of miR-664 on cell proliferation and invasion were studied. Furthermore, the mechanisms underlying the regulatory roles of miR-664 in BC cells were examined.
MATERIALS AND METHODS
Clinical Specimens
A total of 34 pairs of human BC tissues and adjacent relatively normal tissues were obtained from Linyi Central Hospital between June 2015 and February 2017. The patients did not undergo hormonotherapy, chemotherapy, radiotherapy, or biological therapy before surgery. All tissue samples were immediately immersed in liquid nitrogen and stored at −80°C until further RNA extraction. This study was approved by the Ethics Committee of Linyi Central Hospital, and written informed consent was provided by all participants.
Cell Lines and Culture
Four human BC cell lines (MCF-7, MDA-MB-231, BT-474, and SKBR3) and a normal mammary epithelial cell line (MCF-10A) were acquired from the American Type Culture Collection (Manassas, VA, USA). All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin solution (all from Gibco, Grand Island, NY, USA). Cells were grown in a humidified 5% CO2 cell incubator at 37°C.
miRNA Mimic, Small Interfering RNA (siRNA), and Plasmid Transfection
miR-664 mimic and miRNA mimic negative control (miR-NC) were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, P.R. China). siRNA against the expression of insulin receptor substrate 1 (IRS1 siRNA) and NC siRNA were produced by GeneCopoeia (Guangzhou, P.R. China). Empty pcDNA3.1 plasmid and IRS1 overexpression plasmid pcDNA3.1-IRS1 lacking the respective 3′-UTR were chemically synthesized by the Chinese Academy of Sciences (Changchun, P.R. China). During cell transfection, cells were inoculated into six-well plates one night prior to transfection. Cell transfection was carried out using a Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) following the manufacturer’s protocols. The transfection mixture was replaced with fresh DMEM supplemented with 10% FBS at 6–8 h posttransfection.
RNA Extraction and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
A TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was applied to isolate total RNA from tissue specimens or cells. To quantify miRNA expression, total RNA was converted into complementary DNA (cDNA) using a TaqMan microRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Subsequently, a TaqMan MicroRNA Assay Kit (Applied Biosystems) was used to carry out qPCR on an Applied Biosystems® 7500 Real-Time PCR System (Applied Biosystems). U6 small nuclear RNA (snRNA) served as an internal reference for miR-664 expression. For the detection of mRNA expression, cDNA was prepared using PrimeScript 1st Strand cDNA Synthesis Kit (Takara Bio Inc., Tokyo, Japan). Quantification of IRS1 mRNA was performed using a SYBR Premix Ex Taq Kit (Takara Bio Inc.) with GAPDH as an internal control. Relative gene expression was analyzed by the 2−ΔΔCq method21.
Cell Counting Kit-8 (CCK-8) Assay
Transfected cells were trypsinized 24 h after transfection and plated into 96-well plates at a density of 3,000 cells per well. Cell proliferation was detected using the CCK-8 assay at 0, 24, 48, and 72 h after seeding. In brief, 10 μl of CCK-8 reagent (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added into each well and incubated at 37°C in 5% CO2 for another 2 h. The absorbance was determined at a wavelength of 450 nm using an automatic multiwell spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each experiment was performed in triplicate and repeated three times.
Transwell Invasion Assay
Transwell invasion assay was used to measure cell invasion capacity using Matrigel-coated Transwell invasion chambers (BD Biosciences, Franklin Lakes, NJ, USA). Transfected cells were trypsinized at 48 h posttransfection and suspended in FBS-free DMEM. A cell suspension containing 1 × 105 cells was plated in the upper Transwell chambers, while the lower chambers were filled with 500 μl of DMEM containing 20% FBS. The chambers were then incubated for 24 h at 37°C with 5% CO2. The noninvasive cells were gently removed by scrubbing with a cotton swab, while the invasive cells were fixed in 4% formaldehyde, stained with 0.5% crystal violet, washed, and photographed under an inverted microscope (Olympus Corp., Tokyo, Japan). Finally, the number of invasive cells was counted in five randomly selected fields.
Luciferase Reporter Assay
The wild-type (Wt) or mutant (Mut) 3′-UTRs of the IRS1 gene were designed and synthesized by GenePharma Co., Ltd., inserted downstream of the pMIR-GLOTM Luciferase vector (Promega Corp., Madison, WI, USA), and named as pMIR-Wt-IRS1-3′-UTR and pMIR-Mut-IRS1-3′-UTR, respectively . Cells were inoculated into 24-well plates and cotransfected with miR-664 mimic or miR-NC, and pMIR-Wt-IRS1-3′-UTR or pMIR-Mut-IRS1-3′-UTR, using a Lipofectamine® 2000 reagent in accordance with the manufacturer’s instructions. After transfection for 48 h, the luciferase activities were analyzed using a Dual-Luciferase Reporter Assay System (Promega Corp.) according to the manufacturer’s instruction. Renilla luciferase activity was used as an internal control.
Protein Extraction and Western Blot Analysis
Tissues or cells were solubilized in cold radioimmunoprecipitation lysis buffer, and protein concentrations were detected using a BCA Protein Assay Kit (both from Beyotime Institute of Biotechnology, Haimen, P.R. China). Equal quantities of protein were subjected to electrophoresis through 10% SDS-PAGE, transferred onto PVDF membranes (EMD Millipore, Billerica, MA, USA), blocked in 5% nonfat milk dissolved in TBS containing 0.1% Tween 20 (TBST), and then incubated overnight at 4°C with the following primary antibodies: a mouse anti-human monoclonal IRS1 antibody (dilution, 1:1,000; Cat. No. ab201644) or a mouse anti-human monoclonal GADPH antibody (dilution, 1:1,000; Cat. No. ab9484; both Abcam, Cambridge, UK). After washing with TBST three times, the membrane was further incubated with goat anti-rabbit secondary antibody (dilution, 1:5,000; Cat. No. ab205719; Abcam) at room temperature for 2 h. An enhanced chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.) was utilized to visualize the protein signals. GAPDH was used as a loading control for protein level normalization.
Statistical Analysis
Results were expressed as means ± standard deviation (SD) and analyzed with SPSS 19.0 software (IBM Corp., New York, USA). Differences between groups were evaluated using Student’s t-tests or one-way analysis of variance followed by SNK multiple comparison test. A value of p < 0.05 was considered statistically significant.
RESULTS
miR-664 Is Downregulated in BC Tissues and Cell Lines
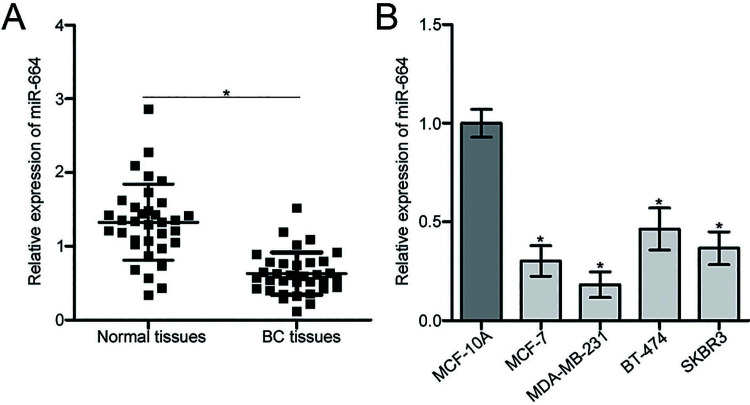
To analyze the expression pattern of miR-664 in BC, we first measured miR-664 expression in 34 pairs of human BC tissues and adjacent relatively normal tissues using RT-qPCR. We found that miR-664 expression was reduced in BC tissues relative to that in adjacent relatively normal tissues (p < 0.05) (Fig. 1A). The expression level of miR-664 was then examined in four human BC cell lines (MCF-7, MDA-MB-231, BT-474, and SKBR3) and a normal mammary epithelial cell line (MCF-10A). The results of the RT-qPCR analysis revealed that the expression level of miR-664 was lower in all four BC cell lines compared with that in MCF-10A (p < 0.05) (Fig. 1B). MCF-7 and MDA-MB-231 cells exhibited relatively lower miR-664 expression compared with the other BC cell lines and were therefore selected for subsequent experiments.
Figure 1.
Expression level of microRNA-664 (miR-664) in breast cancer (BC) tissues and cell lines. (A) Relative expression of miR-664 was determined by reverse transcription quantitative polymerase chain reaction (RT-qPCR) in 34 pairs of human BC tissues and adjacent relatively normal tissues. *p < 0.05 compared with normal tissues. (B) RT-qPCR analysis was performed to detect relative miR-664 expression in four human BC cell lines (MCF-7, MDA-MB-231, BT-474, and SKBR3) and a normal mammary epithelial cell line (MCF-10A). *p < 0.05 compared with MCF-10A.
miR-664 Overexpression Inhibits the Proliferation and Invasion of BC Cells
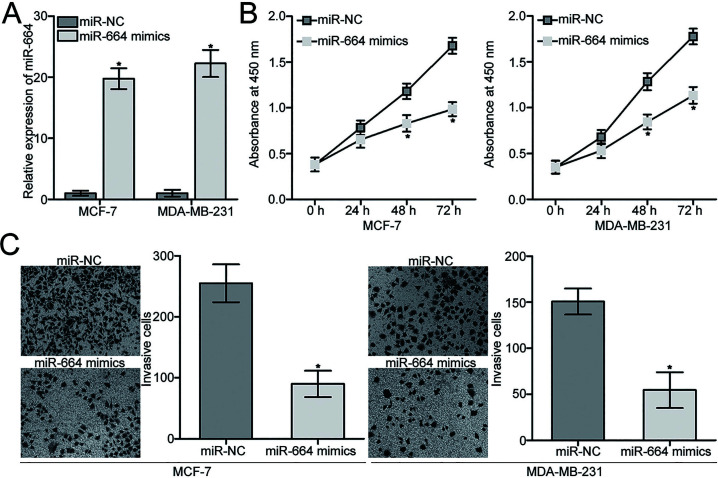
Given the downregulation of miR-664 in BC, we hypothesized that miR-664 may play tumor-suppressing roles in BC progression. To confirm this hypothesis, miR-664 mimics were transfected into MCF-7 and MDA-MB-231 cells to increase endogenous miR-664 expression. Following transfection, the expression of miR-664 was markedly overexpressed in miR-664 mimic-transfected MCF-7 and MDA-MB-231 cells compared with that in the miR-NC group (p < 0.05) (Fig. 2A). CCK-8 and Transwell invasion assays were then performed to determine whether miR-664 may affect cell proliferation and invasion in BC. The results showed that ectopic expression of miR-664 significantly suppressed proliferation (p < 0.05) (Fig. 2B) and invasion (p < 0.05) (Fig. 2C) of MCF-7 and MDA-MB-231 cells. These results suggest that miR-664 may inhibit the progression of BC.
Figure 2.
miR-664 attenuates proliferation and invasion of MCF-7 and MDA-MB-231 cells. (A) MCF-7 and MDA-MB-231 cells were transfected with miR-664 mimic or miRNA mimic negative control (miR-NC). At 48 h after transfection, the transfection efficiency was evaluated by RT-qPCR. *p < 0.05 compared with miR-NC. (B) Cell counting kit-8 (CCK-8) assays were utilized to detect proliferation of MCF-7 and MDA-MB-231 cells transfected with miR-664 mimic or miR-NC. *p < 0.05 compared with miR-NC. (C) Cell invasion capacities of MCF-7 and MDA-MB-231 cells transfected with miR-664 mimic or miR-NC were analyzed by Transwell invasion assays. *p < 0.05 compared with miR-NC.
IRS1 Is a Novel Direct Target Gene of miR-664 in BC
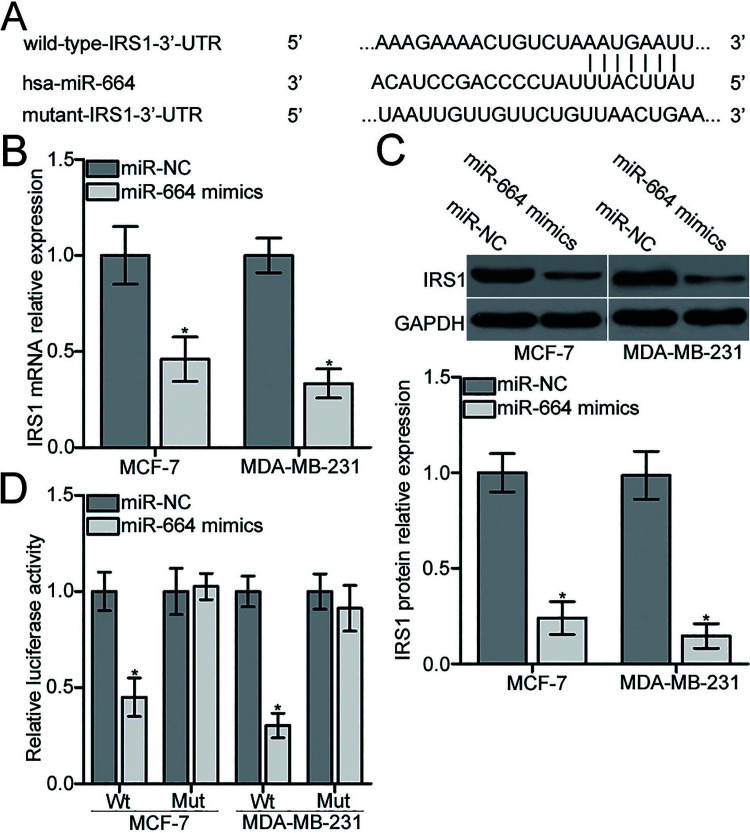
To find the molecular mechanisms underlying the suppressive effects of miR-664 in BC, we conducted a bioinformatics analysis to predict the potential target of miR-664 using miRanda (www.microrna.org/microrna/home.do) and TargetScan (www.targetscan.org/). Among the candidates, IRS1 was selected for further confirmation because it plays important roles in BC progression22–25. As shown in Figure 3A, the 3′-UTR of IRS1 contained a putative miR-664 binding site. To determine whether endogenous IRS1 expression was modulated by miR-664 in BC, we applied RT-qPCR and Western blot analyses to detect IRS1 expression in MCF-7 and MDA-MB-231 cells after transfection with miR-664 mimic or miR-NC. Resumption of the expression of miR-664 obviously decreased IRS1 expression in MCF-7 and MDA-MB-231 cells at both mRNA (p < 0.05) (Fig. 3B) and protein (p < 0.05) (Fig. 3C) levels. Afterward, the luciferase reporter assays were carried out to evaluate whether miR-664 can directly bind to the 3′-UTR of IRS1. As illustrated in Figure 3D, upregulation of miR-664 suppressed the luciferase activities of pMIR-Wt-IRS1-3′-UTR compared with the miR-NC group (p < 0.05) but had no effect on the pMIR-Mut-IRS1-3′-UTR in MCF-7 and MDA-MB-231 cells. These results suggest that miR-664 negatively regulates IRS1 expression in BC by directly targeting its 3′-UTR.
Figure 3.
miR-664 directly targets the 3′-untranslated region (3′-UTR) of insulin receptor substrate 1 (IRS1) and reduces IRS1 expression in BC cells. (A) Schematic of a putative wild-type (Wt) and mutant (Mut) miR-664 binding site in the 3′-UTR of IRS1. miR-664 mimic or miR-NC was transfected into MCF-7 and MDA-MB-231 cells. At 48 h posttransfection, (B) RT-qPCR analysis was conducted to measure IRS1 mRNA expression. (C) Western blot was used to determine IRS1 protein level at 72 h posttransfection. *p < 0.05 compared with miR-NC. (D) MCF-7 and MDA-MB-231 cells were cotransfected with miR-664 mimic or miR-NC and pMIR-Wt-IRS1-3′-UTR or pMIR-Mut-IRS1-3′-UTR. After 72 h of transfection, luciferase reporter assays were adopted to assess luciferase activity. *p < 0.05 compared with miR-NC.
Inhibition of IRS1 Simulates the Suppressive Roles of miR-664 in BC Cells
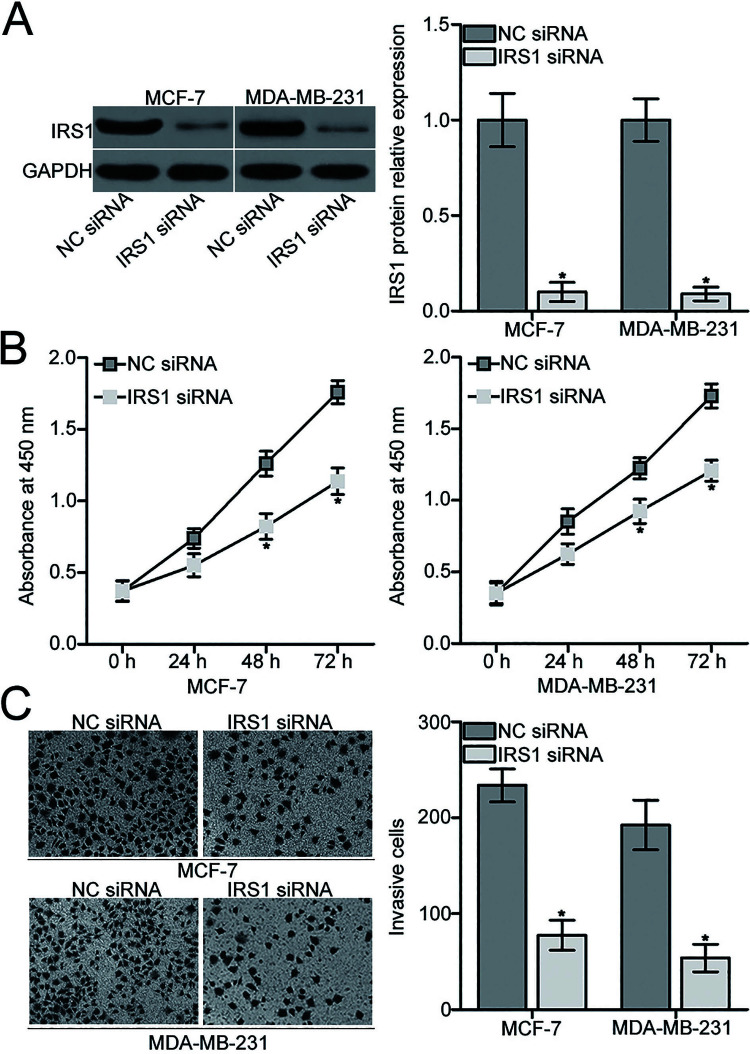
Considering that IRS1 was validated as a direct target of miR-664 in BC, we hypothesized that the suppressive effects of miR-664 in BC cell proliferation and invasion are exhibited by IRS1 knockdown. To verify this speculation, IRS1 siRNA was introduced into MCF-7 and MDA-MB-231 cells to knock down IRS1 expression. Western blot analysis indicated that IRS1 protein was effectively downregulated in MCF-7 and MDA-MB-231 cells after transfection with IRS1 siRNA compared with those transfected with NC siRNA (p < 0.05) (Fig. 4A). We then performed functional assays and observed that inhibition of IRS1 attenuated proliferation (p < 0.05) (Fig. 4B) and invasion (p < 0.05) (Fig. 4C) of MCF-7 and MDA-MB-231 cells, which was similar to those caused by miR-664 overexpression. These results further suggest that IRS1 is a functional downstream target of miR-664 in BC.
Figure 4.
IRS1 knockdown inhibits proliferation and invasion of MCF-7 and MDA-MB-231 cells. (A) MCF-7 and MDA-MB-231 cells were transfected with IRS1 small interfering RNA (siRNA) or NC siRNA. Western blot analysis was conducted to detect IRS1 protein expression. *p < 0.05 compared with NC siRNA. (B) Effect of IRS1 knockdown on MCF-7 and MDA-MB-231 cell proliferation was analyzed by the CCK-8 assay. *p < 0.05 compared with NC siRNA. (C) IRS1 siRNA- or NC siRNA-transfected MCF-7 and MDA-MB-231 cells were subjected to Transwell invasion assay, and the number of invasive cells was counted. *p < 0.05 compared with NC siRNA.
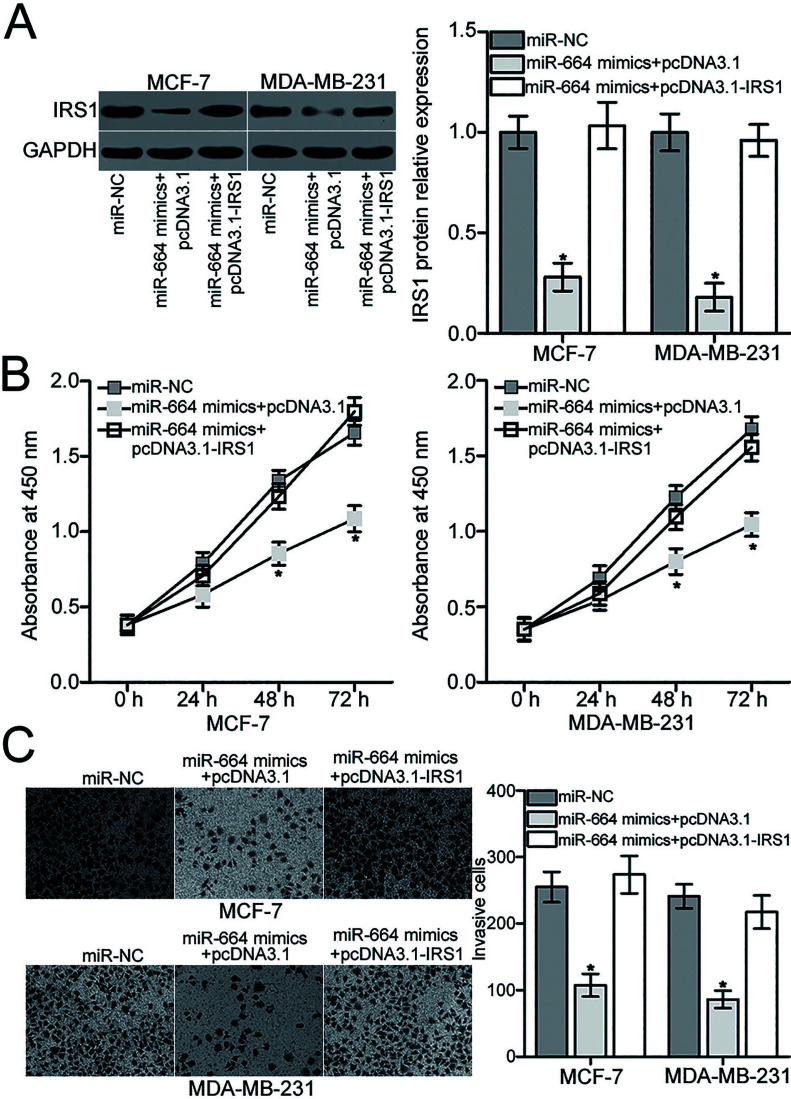
Restored IRS1 Expression Partially Rescues the Inhibitory Effects of miR-664 on the Malignant Phenotypes of BC Cells
Rescue experiments were used to further examine whether the effects of miR-664 overexpression on BC cell proliferation and invasion are mediated by inhibiting IRS1. To confirm this hypothesis, miR-664-overexpressing MCF-7 and MDA-MB-231 cells were transfected with empty pcDNA3.1 plasmid or IRS1 overexpression plasmid pcDNA3.1-IRS1 lacking the respective 3′-UTR. The IRS1 protein level was detected by Western blot analysis, which confirmed that the reduced IRS1 protein level induced by miR-664 overexpression was restored in MCF-7 and MDA-MB-231 cells after cotransfection with pcDNA3.1-IRS1 (p < 0.05) (Fig. 5A). Subsequent MTT and Transwell invasion assays demonstrated that restoration of IRS1 expression partially counteracted the suppressive effects of miR-664 overexpression on the proliferation (p < 0.05) (Fig. 5B) and invasion (p < 0.05) (Fig. 5C) of MCF-7 and MDA-MB-231 cells. Overall, miR-664 may serve tumor suppressive roles in BC, partly by downregulating IRS1 expression.
Figure 5.
IRS1 mediates the suppressive roles of miR-664 in MCF-7 and MDA-MB-231 cells. (A) Western blot analysis was applied to examine the IRS1 protein expression in MCF-7 and MDA-MB-231 cells cotransfected with miR-664 mimics and pcDNA3.1 plasmid or IRS1 overexpression plasmid pcDNA3.1-IRS1. *p < 0.05 compared with miR-NC, and miR-664 mimics + pcDNA3.1-IRS1. (B) CCK-8 and (C) Transwell invasion assays were conducted to determine the proliferation and invasion of MCF-7 and MDA-MB-231 cells after cotransfection with miR-664 mimic and pcDNA3.1 plasmid or pcDNA3.1-IRS1. *p < 0.05 compared with miR-NC, and miR-664 mimic + pcDNA3.1-IRS1.
DISCUSSION
A large number of miRNAs have been previously demonstrated to be dysregulated in BC, and alterations in miRNA expression may affect the initiation and progression of BC26,27. Therefore, in-depth understanding of the regulatory mechanisms of miRNAs in BC will provide new approaches for the identification of novel and effective therapeutic targets for patients with this disease. In this study, we found that the expression of miR-664 was underexpressed in BC tissues and cell lines compared with that in adjacent relatively normal tissues and in the normal mammary epithelial cell line MCF-10A, respectively. Enforced expression of miR-664 prohibited BC cell proliferation and invasion in vitro. In addition, IRS1 was confirmed as a direct target gene of miR-664 in BC. Furthermore, downregulation of IRS1 can mimic the suppressive roles of miR-664 in BC cells. Moreover, recovered IRS1 expression partially rescued the inhibitory roles of miR-664 on the proliferation and invasion of BC cells. Thus, our current study provides evidence that miR-664 restricts proliferation and invasion of BC cells and reveals the specific regulatory mechanism of miR-664 in BC, suggesting that miR-664 may serve as a potential therapeutic target for the treatment of BC patients.
Dysregulation of miR-664 has been identified in several cancer types. For example, miR-664 was downregulated in cervical cancer tissues and cell lines.
Decreased miR-664 expression was correlated with lymphatic invasion, distant metastasis, FIGO stage, and histological grade. Cervical cancer patients with low miR-664 exhibited poorer overall survival than those patients with high miR-664 expression. Multivariate analysis identified miR-664 expression as an independent prognostic factor for overall survival in cervical cancer15. miR-664 expression was also reduced in cutaneous malignant melanoma tissues and cell lines. Downregulation of miR-664 was strongly associated with a shorter overall survival time of cutaneous malignant melanoma patients16. However, miR-664 was observed to be highly expressed in osteosarcoma17,18, lung cancer19, and T-cell acute lymphoblastic leukemia20. These conflicting studies imply that the expression pattern of miR-664 has tissue specificity and suggest that miR-664 may represent a diagnosis and prognosis biomarker for these human cancer types.
Emerging studies have previously reported that miR-664 plays crucial roles in multiple biological and pathological processes of human cancers. For instance, resumption of miR-664 expression decreased cell migration and increased chemosensitivity of cervical cancer cells to cisplatin28. Ding et al. revealed that miR-664 overexpression suppressed cell proliferation and anchorage-independent growth in cutaneous malignant melanoma in vitro, induced G1/S transitional cell cycle arrest, and decreased tumor growth in vivo16. Nevertheless, miR-664 was found to serve oncogenic roles in osteosarcoma by promoting cell growth and metastasis in vitro as well as tumor growth in vivo17,18. Zhu et al. found that miR-664 reexpression enhanced cell proliferation and motility in lung cancer19. A study by Zhu et al. showed that enforced expression of miR-664 increased cell proliferation and migration in T-cell acute lymphoblastic leukemia20. These studies evidentially demonstrate that the biological roles of miR-664 exhibit tissue specificity and suggest that miR-664 may be investigated as a potential therapeutic target for human cancer intervention.
Previously, multiple direct targets of miR-664, including PLP216 in cutaneous malignant melanoma and SOX717 and FOXO418 in osteosarcoma, have been validated. In our current study, IRS1 was identified as a novel target of miR-664 in BC. IRS1 transmits signals from the insulin and insulin-like growth factor-1 receptors to intracellular pathways, such as the PI3K/Akt and MAPK pathways29. In BC, IRS1 was overexpressed and closely related with poor differentiation and lymph node metastasis22. BC patients with high IRS1 expression had shorter disease-free survival than those with low IRS1 expression23. Dysregulated IRS1 contributed to the onset and development of BC by affecting various cellular processes, including cell proliferation, cycle, apoptosis, angiogenesis, migration, invasion, metastasis, and chemosensitivity24,25. Hence, targeting IRS1 may be an effective strategy to delay or stop the progression of BC.
In conclusion, to the best of our knowledge, our current study is the first to demonstrate that miR-664 is downregulated in BC tissues and cell lines. In addition, miR-664 restricts proliferation and migration of BC cells. Moreover, IRS1 is validated as a direct target of miR-664 in BC. These findings suggest that miR-664 may function as a suppressor in the progression of BC, and the miR-664/IRS1 axis may be a potential therapeutic target for BC patients.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Martin HL, Smith L, Tomlinson DC. Multidrug-resistant breast cancer: Current perspectives. Breast Cancer (Dove Med Press) 2014;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perri F, Longo F, Giuliano M, Sabbatino F, Favia G, Ionna F, Addeo R, Della Vittoria Scarpati G, Di Lorenzo G, Pisconti S. Epigenetic control of gene expression: Potential implications for cancer treatment. Crit Rev Oncol Hematol. 2017;111:166–72. [DOI] [PubMed] [Google Scholar]
- 4. Beckmann MW, Niederacher D, Schnurch HG, Gusterson BA, Bender HG. Multistep carcinogenesis of breast cancer and tumour heterogeneity. J Mol Med. (Berl) 1997;75:429–39. [DOI] [PubMed] [Google Scholar]
- 5. Nakamura S, Yagata H, Ohno S, Yamaguchi H, Iwata H, Tsunoda N, Ito Y, Tokudome N, Toi M, Kuroi K, Suzuki E. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer 2010;17:199–204. [DOI] [PubMed] [Google Scholar]
- 6. Weigelt B, Peterse JL, van ‘t Veer LJ. Breast cancer metastasis: Markers and models. Nat Rev Cancer 2005;5:591–602. [DOI] [PubMed] [Google Scholar]
- 7. Alonso DF, Ripoll GV, Garona J, Iannucci NB, Gomez DE. Metastasis: Recent discoveries and novel perioperative treatment strategies with particular interest in the hemostatic compound desmopressin. Curr Pharm Biotechnol. 2011;12:1974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muenst S, Daster S, Obermann EC, Droeser RA, Weber WP, von Holzen U, Gao F, Viehl C, Oertli D, Soysal SD. Nuclear expression of snail is an independent negative prognostic factor in human breast cancer. Dis Markers 2013;35:337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. [DOI] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell 2009;136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–63. [DOI] [PubMed] [Google Scholar]
- 12. Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 14. Ambros V. The functions of animal microRNAs. Nature 2004;431:350–5. [DOI] [PubMed] [Google Scholar]
- 15. Zhang YX, Qin LL, Yang SY. Down-regulation of miR-664 in cervical cancer is associated with lower overall survival. Eur Rev Med Pharmacol Sci. 2016;20:1740–4. [PubMed] [Google Scholar]
- 16. Ding Z, Jian S, Peng X, Liu Y, Wang J, Zheng L, Ou C, Wang Y, Zeng W, Zhou M. Loss of miR-664 expression enhances cutaneous malignant melanoma proliferation by upregulating PLP2. Medicine (Baltimore) 2015;94:e1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bao Y, Chen B, Wu Q, Hu K, Xi X, Zhu W, Zhong X, Chen J. Overexpression of miR-664 is associated with enhanced osteosarcoma cell migration and invasion ability via targeting SOX7. Clin Exp Med. 2017;17:51–8. [DOI] [PubMed] [Google Scholar]
- 18. Chen B, Bao Y, Chen X, Yi J, Liu S, Fang Z, Zheng S, Chen J. Mir-664 promotes osteosarcoma cells proliferation via downregulating of FOXO4. Biomed Pharmacother. 2015;75:1–7. [DOI] [PubMed] [Google Scholar]
- 19. Zhu X, Ju S, Yuan F, Chen G, Shu Y, Li C, Xu Y, Luo J, Xia L. microRNA-664 enhances proliferation, migration and invasion of lung cancer cells. Exp Ther Med. 2017;13:3555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu H, Miao MH, Ji XQ, Xue J, Shao XJ. miR-664 negatively regulates PLP2 and promotes cell proliferation and invasion in T-cell acute lymphoblastic leukaemia. Biochem Biophys Res Commun. 2015;459:340–5. [DOI] [PubMed] [Google Scholar]
- 21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 22. Koda M, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Kisielewski W, Baltaziak M, Wincewicz A, Sulkowski S. Expression of the insulin receptor substrate 1 in primary tumors and lymph node metastases in breast cancer: Correlations with Bcl-xL and Bax proteins. Neoplasma 2005;52:361–3. [PubMed] [Google Scholar]
- 23. Rocha RL, Hilsenbeck SG, Jackson JG, VanDenBerg CL, Weng C, Lee AV, Yee D. Insulin-like growth factor binding protein-3 and insulin receptor substrate-1 in breast cancer: Correlation with clinical parameters and disease-free survival. Clin Cancer Res. 1997;3:103–9. [PubMed] [Google Scholar]
- 24. Wang Y, Zhang X, Zou C, Kung HF, Lin MC, Dress A, Wardle F, Jiang BH, Lai L. miR-195 inhibits tumor growth and angiogenesis through modulating IRS1 in breast cancer. Biomed Pharmacother. 2016;80:95–101. [DOI] [PubMed] [Google Scholar]
- 25. Liu MM, Li Z, Han XD, Shi JH, Tu DY, Song W, Zhang J, Qiu XL, Ren Y, Zhen LL. MiR-30e inhibits tumor growth and chemoresistance via targeting IRS1 in breast cancer. Sci Rep. 2017;7:15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joyce DP, Kerin MJ, Dwyer RM. Exosome-encapsulated microRNAs as circulating biomarkers for breast cancer. Int J Cancer 2016;139:1443–8. [DOI] [PubMed] [Google Scholar]
- 27. Asghari F, Haghnavaz N, Baradaran B, Hemmatzadeh M, Kazemi T. Tumor suppressor microRNAs: Targeted molecules and signaling pathways in breast cancer. Biomed Pharmacother. 2016;81:305–17. [DOI] [PubMed] [Google Scholar]
- 28. Yang Y, Liu H, Wang X, Chen L. Up-regulation of microRNA-664 inhibits cell growth and increases cisplatin sensitivity in cervical cancer. Int J Clin Exp Med. 2015;8:18123–9. [PMC free article] [PubMed] [Google Scholar]
- 29. Burnol AF, Morzyglod L, Popineau L. [Cross-talk between insulin signaling and cell proliferation pathways]. Ann Endocrinol. (Paris) 2013;74:74–8. [DOI] [PubMed] [Google Scholar]