Abstract
The global COVID-19 pandemic is currently underway. In December 2020, the European Agency of Medicine (EMA) licensed the first Sars-CoV-2 vaccine. Therapeutic management of the COVID-19 positive patient should primarily aim to avoid the severe complications and organ injury caused by generalized inflammation caused by a cytokine storm and occurring in the most severe stages of viral infection. Current knowledge of the pathophysiological mechanisms of SARS- CoV-2 suggests a central role for exaggerated activation of the innate immune system as an important contributor to the adverse outcomes of COVID-19. Several studies have shown that blocking the cytokine storm or acting early with prevention of it can be effective; studies are underway to evaluate agents that may be able to reduce this hyperinflammatory state. The search for effective management strategies for COVID-19 continues to evolve. The actions of colchicine, one of the oldest anti-inflammatory therapies, target multiple targets associated with excessive COVID-19 inflammation. Colchicine is easily administered, generally well tolerated, and inexpensive. This article reports the scientific and molecular rationale for the use of colchicine as monotherapy or in combination in the various stages of SARS-CoV-2 infection to modulate and control the inflammatory state. Low-dose colchicine may be considered safe and effective for the treatment and prevention of cytokine storm in patients with SARS-CoV-2 infection, particularly as an adjunctive remedy to other therapeutic agents. Well-organized clinical studies are needed in this direction.
Keywords: Colchicine, Sars-cov-2, Cytokine, Interleukin, Coronavirus, Pneumonia
1. Introduction
An abnormal number of cases occurred in Wuhan, China In December 2019, an abnormal number of pneumonia caused by a new coronavirus named SARS-CoV-2 was identified. This coronavirus has shown rapid spread in China and also in other countries causing a global pandemic. To date, there are 2.04 Mln deaths and 95.6 Mln people infected. The genome sequence of SARS-CoV-2 has been found to be 79.5% of that of SARS-CoV.[1,2]. In most cases SARS-CoV-2 infection can have an asymptomatic or mildly symptomatic course that does not require hospitalization, however in a percentage of cases it can have a severe course with high viral load present, a generalized hyperinflammatory state causing organ damage that in some cases can be fatal. In the most severe stages of infection, the generalized hyperinflammatory state is caused by a sudden release of cytokines into the circulation referred to as a “cytokine storm” [3,4]. SARS-CoV-2 enters through ACE2 into cells [[5], [6], [7]]. Clinical experience and data to date emphasize the role of excessive inflammation [8,9] as a cause of organ injury in the disease, and suggest a potential role for colchicine, an anti-inflammatory drug with pleiotropic effects.
2. SARS-CoV-2 infection
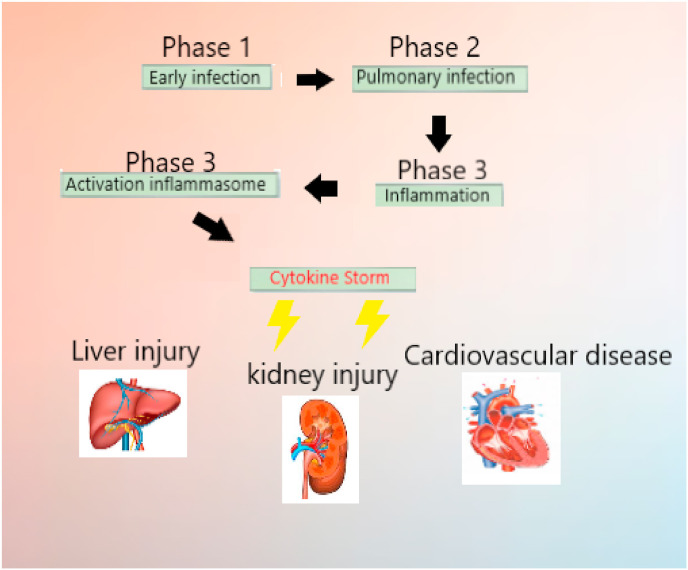
The progression of COVID-19 viral infection can be divided into three distinct phases (Fig. 1 ), specifically [10]: early infection phase, in which the virus infiltrates host lung cells [11]; pulmonary phase, in which viral replication and spread causes lung injury; and cytokin storm, generalized inflammatory response causes multi-organ injury [12]. In particular, this third phase may be responsible for severe COVID-19 complications. Modulation and arrest of this generalized inflammatory state may be of therapeutic benefit in avoiding the most severe complications (Fig. 1).
Fig. 1.
The progression of COVID-19 viral infection can be divided into three distinct phases, specifically: early infection phase, pulmonary phase, cytokin storm phase. The third phase can cause generalized inflammatory response and multi-organ injury.
2.1. Phase 1 or early infection phase
The patient has contracted SARS-CoV-2, infection begins, and the immune system reacts against the virus. Initial symptoms may include cough, fatigue, fever, nausea, and diarrhea. During this nonsevere phase, a specific adaptive immune response is required to knock down the virus and prevent disease progression into the severe phases. Therefore, strategies to boost immune responses could certainly be important. An antiviral may be useful at this stage to inhibit viral load and avoid complications with prevention of virus replication [[13], [14], [15]]. Arguably, an antiviral might be effective in stimulating the immune system even more at this stage, avoiding the use of steroidal or nonsteroidal anti-inflammatory drugs, and being able to consider the administration of immunostimulants or plasma derived from cured patients[16,17]. Currently, there is evidence of efficacy as antivirals for remdesivir drugs. If the infection is contained at this stage and the host immune system response is adequate, the patient will recover without serious complications.
2.2. Phase 2 or pulmonary phase
The second phase of infection is characterized by a compromised protective immune response; the immune system was unable to defeat the infection. The virus has reproduced and invaded the deep respiratory tract, such as the lungs. The hypoxic phase begins; hospitalization and oxygen administration may be required during this phase. In addition there may be cardiac involvement and a hyperactive coagulation system with risk of thrombus formation, a greater risk of entering the serious clinical picture. Laboratory tests may show a decrease in lymphocytes, an increase in transaminases, and a moderate increase in pro-inflammatory markers [18]. The most indicated treatment at this stage may be use of antiviral drugs, use of anti-inflammatory drugs, and administration of LMWH-(Low-molecular-weight-heparin) to prevent thromboembolic events.
2.3. Phase 3 or inflammatory phase
The third stage is the most severe, which can lead to patient death in some cases. In this stage there is a hyperactive, systemic (not just pulmonary) inflammatory state caused by a cytokine storm and which can lead to multi-organ dysfunction and organ injury. At this stage, inflammation marker values are very high The patient may have severe respiratory failure and cardiac, liver, and kidney injury. There may also be neurological damage. Administration of immunomodulatory agents (corticosteroids, anti-interleukin-6 such as tocilizumab and sarilumab, IL-1 receptor antagonists such as anakinra or canakinumab, JAK inhibitors, convalescent plasma) [19] may be necessary at this stage to attempt to reduce an overactive inflammatory state. In addition, there may be fibrotic tissue formation, particularly pulmonary fibrosis that may require antifibrotic therapy [20]. The prognosis for patients at this stage of the disease can be very serious[21].
3. The role of inflammation
In the most severe COVID-19 cases, an abnormal inflammatory/immune response of the host organism is responsible for multi-organ injury and COVID-19-induced severe complications. In particular, the elevated inflammatory response is characterized by a massive production of cytokines, the so-called “cytokine storm.” Cytokines are very powerful mediators in inducing an adequate inflammatory/immune response directed to fight the virus, however during a cytokine storm an abnormal inflammatory response is the cause of organ injury. A key role is played by the NLRP3 inflammasome. The NLRP3 inflammasome is a critical component of the innate immune system that mediates caspase-1 activation and the secretion of proinflammatory cytokines in response to microbial infection and cellular damage. However, the aberrant activation of the NLRP3 inflammasome has been linked with several inflammatory disorders [22,23] induced by COVID-19 and causing lung injury, cardiac injury, renal injury [24].
4. Colchicine
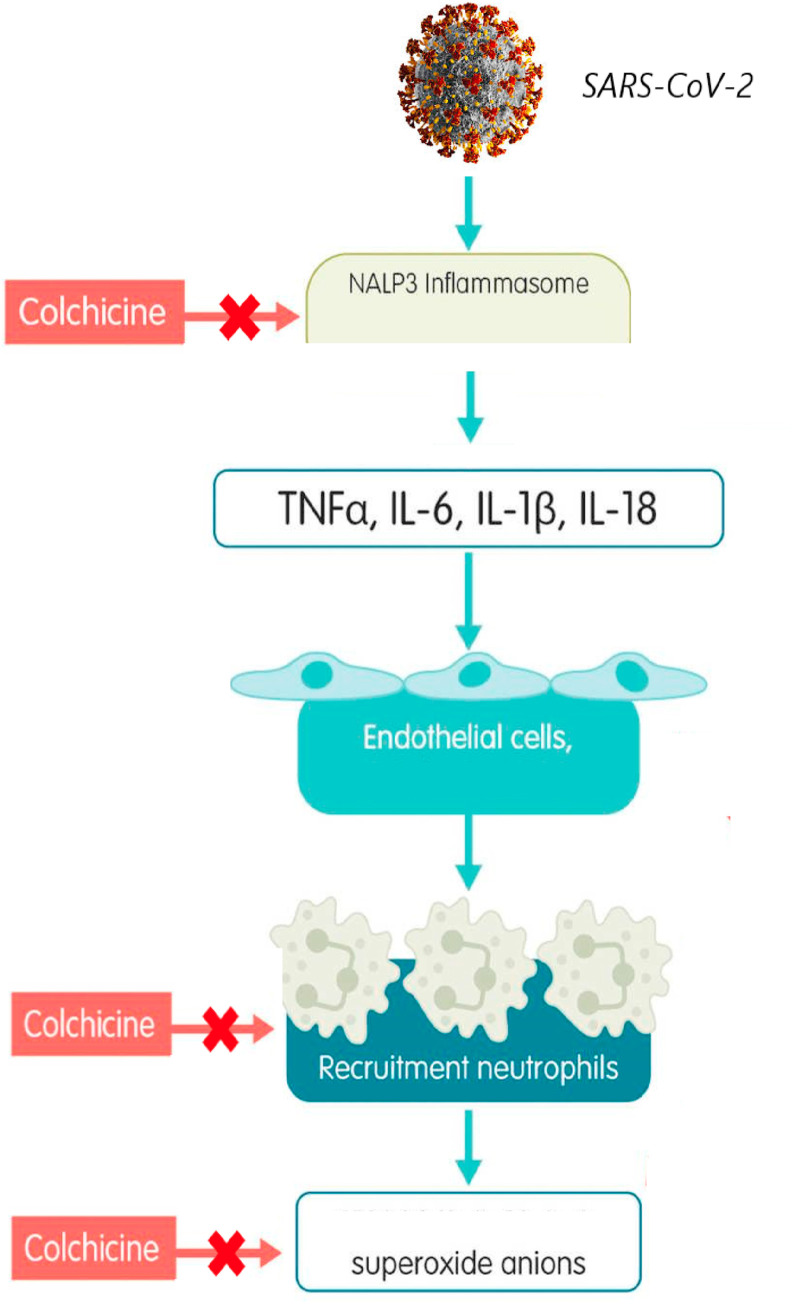
Colchicine is a pharmacological agent that has been used for a long time and with different therapeutic indications. Today it is used effectively in the treatment of gout, Behçet's disease, for the prevention of pericarditis, Familial Mediterranean Fever (FMF), Sweet's syndrome. Probably in recent years, the drug has achieved the greatest clinical success in the treatment of Familial Mediterranean Fever (FMF) prophylaxis, in addition to its traditional use as a first-line anti-gout treatment. Recently, a very important study has also been published supporting the use of colchicine in post-MI (Acute Myocardial Infarction) secondary prevention [25] and many new articles exploring the potential role of colchicine as an anti-atherothrombotic/inflammatory drug are available. Colchicine modulates multiple antiinflammatory pathways. Colchicine prevents microtubule assembly and thereby disrupts inflammasome activation, microtubule-based inflammatory cell chemotaxis, generation of leukotrienes and cytokines, and phagocytosis. Many of these cellular processes can be found in many diseases involving chronic inflammation. The multitarget mechanism of action of colchicine suggests potential efficacy of colchicine in other comorbid conditions associated with gout, such as osteoarthritis and cardiovascular disease. The scientific hypothesis of the use of colchicine in SARS-CoV-2 infection is based on the anti-inflammatory properties of the drug [26]. Recent evidence on colchicine seem to suggest a potential synergy in the treatment of the cytokine cascade at different levels. Indeed, colchicine acts by decreasing inflammation through multiple mechanisms. The main mechanism of action is to bind the tubulin molecule and thus inhibit its polymerization as microtubules in neutrophils, resulting in inhibition of migration [27]. In addition, colchicine can alter the distribution of adhesion molecules on the surface of neutrophils and of endothelial cells, leading to a significant inhibition of the interaction between white blood cells and endothelial cells by interfering with their transmigration(Fig. 2 ). However, the main mechanism of action for the reduction of cytokine storm in patients with SARS-CoV-2 is probably the inhibition of IL-1, IL-6, and IL-18 production because of their ability to interfere with the NLRP3 inflammatory protein complex that plays a central role in cytokine storm [28]. In addition, colchicine inhibits superoxide anion production and inhibits mast cell degranulation. Importantly, studies have shown that viroporin E, a component of the SARS-associated coronavirus (SARS-CoV), creates Ca-permeable ion channels and activates NLRP3 inflammation [29,30]. In addition, another viroporin 3a induces activation of NLRP3 inflammation. The mechanisms are unclear. NLRP3 inflammation can be activated through several mechanisms and plays an important role in the development of phase three cytokinin storm from SARS-CoV-2. Upstream inhibition of NLRP3 inflammation may be considered as a novel approach for the prevention or treatment of SARS-CoV-2 infection [31,32]. Several clinical trials are currently moving to study the efficacy of colchicine in patients with SARS-CoV-2 infection. Since 1972, colchicine (at a dose of 0.5–2 mg/day) has been the drug used in the prophylaxis of FMF attacks. The idea and hypothesis of extending the use of colchicine in SARS-Cov2 infection is closely related to its use in FMF, i.e., an inherited autoinflammatory disease characterized by recurrent febrile episodes (attacks) and acute inflammation. Due to these considerations, the mechanism of action is similar; in fact, the use of colchicine up to a maximum dose of 3 mg/day is effective in preventing the onset of inflammatory attacks in 60–65% of FMF cases. Considering the pharmacodynamic properties of colchicine and based on knowledge of its tolerability profile (derived from drug use over many years), the use of this drug could be considered as monotherapy or in combination in all three phases of coronavirus infection [34]. (Fig. 2).
Fig. 2.
Pathophysiology of Sars-CoV-2-induced inflammation and molecular targets of colchicine. Macrophage inflammation leads to inflammatory activity, cytokine production, and neutrophil activation/transmigration, with surface expression of selectins, integrins, and intercellular adhesion molecules that promote neutrophil adhesion to the vasculature. Colchicine inhibits the NLRP3 inflammasome, with the potential to prevent the development of cytokine storms and limit neutrophil transmigration.
5. Pharmacological rationale to management inflammation, colchicine in COVID-19 infection
Based on the large number of data available on the efficacy of colchicine as monotherapy in the prevention of FMF and in the prevention of recurrent pericarditis, we believe that the drug can be used at common doses used for these diseases. In this phase 1, a practical approach could be to use low initial doses (0.5 mg/day) as a preventive method to avoid moving to phase two and/or three and at the same time give the possibility to use this therapy also in combination with antivirals to decrease the viral load and wait for the immune system reaction against the infection. Used at standard doses, colchicine shows a good tolerability profile and no immunosuppressive effect is expected [35]. This is very important to fight the first phase. In addition, in this phase, the non-administration of immunosuppressants or glucocorticoids may be useful to avoid a decrease of the immune system [33]. The second phase is a crucial time for therapy. It may be important to continue treatment with antivirals even at this stage, monitoring the patient's condition and avoiding adverse reactions due to drug interactions. Based on clinical and laboratory parameters and inflammatory markers, a change in colchicine doses is considered [36]. During phase 2, a practical approach could be based on the use of colchicine increasing up to 0.5 mg twice daily if the patient is an adult with a body weight greater than 70 kg. Attention is needed to avoid the accumulation of toxic doses by monitoring liver and kidney health conditions and considering all possible interactions between colchicine and other agents in use [37]. Another therapeutic approach at this stage could be the use of a 0.5 mg dose of colchicine (as step 1) in combination with hydroxychloroquine [38]. From a pharmacodynamic point of view, colchicine and hydroxychloroquine can act in sinergism modulating two fundamental objectives of inflammation. Hydroxychloroquine reduces the secretion of proinflammatory cytokines and in particular TNF-alpha by stimulated monocytes-macrophages and in addition to having antiviral effects, colchicine acts instead on inflammatory NLP3 as described above. Evidence is showing that the use of hydroxychloroquine is dubiously effective and with a low safety profile. Further studies are needed. The initiation of the use of anti IL-6 or anti IL-1 or glucocorticoids, in particulary dexamethasone [39,40], or other specific treatments able to interrupt the progression of the cytokine storm, including the right time to start LMWH or administer antibiotics, should be considered according to the patient's clinical condition. The third phase is characterized by cytokine storm and generalized inflammation. This phase influences the patients state of health and it makes the clinical picture severe. At this stage it is evident that the most important therapeutic strategy to be implemented is to slow down or block the uncontrolled inflammatory response. Antiviral treatments continue to be important. However, the anti-inflammatory therapy can help prevent further complications, multi-organ dysfunction and patient death. As we know, there is a variety of anti-inflammatory drugs, including non-steroidal anti-inflammatory drugs, glucocorticoids, immunomodulators. The use of glucocorticoids is still a matter of discussion, in particular the doses to be used and the time when can be used. Alcune evidenze cliniche associano una buona efficacia al desametasone [41].
The use of cytokine inhibitors such as tocilizumab (IL-6 inhibitor) or anakinra (IL-1 receptor antagonist) has shown good efficacy and several studies are underway to test them [42]. However, as with glucocorticoids, there are still many open questions, when to use immunomodulators, what doses, to which patients? Only valid clinical trial protocols can answer these questions. All these questions are still the subject of intense debate and an uncommon answer in scientific opinion. The main concern, of course, is that immunomodulatory drugs can delay the elimination of the virus by the immune system and, worse still, increase the risk of secondary infections, especially of the respiratory tract. The biological agents that have shown good efficacy, and for which several trials are underway, are the inhibitors IL-6 tocilizumab and sarilumab, which are indicated for the treatment of rheumatoid arthritis. In addition, on August 30, 2017, Tocilizumab was approved in the United States for life-threatening cytokine release syndrome caused by chimeric T cell antigen receptor immunotherapy (CAR-T) [43], and studies are now underway to evaluate its efficacy in the treatment of FMF and pericarditis, just like colchicine. At this stage 3, it may be useful to administer colchicine (0.5 mg once or twice daily), in monotherapy or in combination with glucocorticoids(dexamethasone) to control Citokine storm. The advantage of colchicine over IL-6 inhibitors is that it acts upstream of the cytokine cascade and not only on one cytokine in particular, it also appears to have a higher safety profile than immunomodulants and glucocorticoids. At this stage we could also consider a triple therapy hydroxychloroquine colchicine and dexamethasone to block the inflammatory cascade on multiple points. Other pharmacologic agents may be considered for the management of severe COVID-19 complications [[44], [45], [46], [47], [48]].
6. Clinical trials
Clinical evidence shows that anti-inflammatory therapy may be beneficial in COVID-19 patients. The potential advantage of colchicine over glucocorticoids such as dexamethasone is that colchicine does not share immunosuppressive effects. Several studies have evaluated the benefit of colchicine in patients with COVID-19. A retrospective study of ICU patients with COVID-19 demonstrated a lower risk of death in patients on colchicine therapy[49]. The GRECO-19 study is demonstrated a significant reduction in the primary clinical outcome of a two-point deterioration on the WHO disease severity scale [50]. Another sudio demonstrated that colchicine given in combination with lopinavir/ritonavir, dexamethasone, or hydroxychloroquine had a significant mortality benefit (84% vs 64% survival) vs controls [51]. Important data may come from the ongoing ColCorona Trial (www.colcorona.net), This is a large placebo-controlled study of the use of colchicine within 2 days of diagnosis of COVID-19, regardless of symptoms, in patients with comorbidities that place patients at increased risk of developing COVID-19-related complications that may provide additional information. Other studies in this direction are ongoing [52].
7. Aspects of clinical pharmacology and safety considerations
Although colchicine at low doses (0.5–1 mg per day) was found to be safe even if administered continuously for decades, there are possible side effects, more common, as the gastrointestinal ones found in 5–10% of cases, less common to consider as the bone marrow suppression, hepatotoxicity, myotoxicity [53]. The dose in the three stages of SARS-CoV-2 infection in patients should however be modified depending on the clinical condition of the patient, especially renal and hepatic function. The simultaneous administration of colchicine and cytochrome P450 3A4 (CYP3A4) or glycoprotein P (P-gp) inhibitors increases the potential toxicity of colchicine. A patient with SARS-CoV-2 infection is a complex patient who may have various organ dysfunctions and take several medications [54]. A patient with SARS-CoV-2 infection could be on therapy with cytochrome P450 3A4 (CYP3A4) inhibitors such as macrolides, this interaction may decrease the metabolization and excretion of colchicine, increasing the risk of severe adverse reactions, currently it seems reasonable to avoid co-administration of colchicine and macrolides. The macrolides as the clarithromycin are P-gp inhibitors, for this, concomitant administration with such as could increase the risk of toxicity. A SARS-CoV-2 patient may be in therapeutic treatment with antivirals such as Ritonavir lopinavir darunavir and ribavirin. Due to inhibition of P-gp and/or CYP3A4 by ritonavir/lopinavir, a reduction in colchicine dose or discontinuation of treatment (in patients with regular renal or hepatic function) would be appropriate to avoid accumulation of toxic doses and serious adverse reactions such as rhabdomyolysis [55,56]. Same interactions also for darunavir. Ribavirin does not inhibit cytochrome P450 enzymes. There is no evidence from toxicity studies that ribavirin induces liver enzymes or interferes with gl-P, so there is a minimal possibility of P450 and gl-P-based interactions with colchicine. The expression of CYP450 liver enzymes is suppressed by cytokines, such as IL-6, which stimulate chronic inflammation. Therefore, the expression of CYP450 can be reversed when used a powerful cytokine inhibition therapy with tocilizumab or sarilumab. In vitro studies with cultured human hepatocytes have shown that IL-6 causes a reduction in expression of the enzymes CYP1A2, CYP2C9, CYP2C19 and CYP3A4. In phase three, IL-6 is present at high levels. Tocilizumab or sarilumab can normalize the expression of these enzymes by inhibiting IL-6. Based on this, if colchicine is administered in phase three in combination with Tocilizumab or sarilumab, a decrease in colchicine concentrations may occur compared to when tocilizumab or sarilumab is not administered. No particular pharmacokinetic interaction seems instead to be found between colchicine and the following drugs, LWHM, immunostimulants, plasma derivatives or hydroxychloroquine. However, in any polytherapy it is always important to refer to the RCP of medicines to avoid unpleasant interactions. The most common adverse reactions with colchicine are related to the gastrointestinal tract, diarrhea is the most commonly reported symptom, followed by vomiting and nausea. Adverse events of the gastrointestinal tract can be a problem for the patient SARS-CoV-2 which can presents symptoms such as diarrhea, nausea and vomiting due to infection, in these patients a decrease in the dose of colchicine could be considered to avoid electrolyte imbalance. In addition, drugs such as hydroxychloroquine, ritonavir/lopinavir or macrolides can cause gastrointestinal symptoms such as diarrhea with common or very common frequency, this could be a problem with possible co-administration between these drugs and colchicine. In addition, concomitant therapy with colchicine hydroxychloroquine and darunavir or lopinavir/ritonavir could increase the risk of serious adverse reactions affecting the musculoskeletal system and connective tissue. Finally, in a polytherapy with colchicine, IL-6 inhibitors, hydroxychloroquine, and possibly glucocorticoids it should be monitored continuously the patient's inflammatory/immune status and to verify laboratory parameters. However, each patient should be carefully monitored for possible side effects, including blood tests (transaminases, serum creatinine, creatin kinase, creatin kinase and blood cell count), renal and liver function and possible drug interactions. The SARS-CoV-2 patient is to be considered a complex patient, the benefit-risk ratio in that specific patient with those specific clinical conditions should always be considered in any politherapy [57]. Colchicine does not have a wide therapeutic window, treatment with this drug should be managed well, however, clinical studies and stronger evidence are needed to validate the use of colchicine in SARS-CoV-2 infection.
8. Discussion
In view of the inhibitory effects of colchicine on neutrophil activity, cytokine production and inflammation in general, in association with a lack of an immunosuppressive effect, colchicine represents a very valuable potential drug treatment for the treatment of the three phases of COVID-19 infection. Colchicine is generally well tolerated and inexpensive, this could be of particular benefit in more resource-poor countries. The optimal dose of colchicine in COVID-19 infection has yet to be established. The largest colchicine study for COVID-19 (ColCorona) tested a dose of 0.5 mg daily, another 0.5 mg twice daily. The duration of treatment also needs to be determined. Finally, the timing of colchicine initiation is still uncertain. Current evidence suggests that the use of colchicine may prevent progression from inflammatory activation (stage 2) to a generalized hyperinflammatory state (stage 3), and that the potential benefits of colchicine are maximized when used early in the infectious process, (ideally in stage 1), such as in non hospitalized patients within days of diagnosis of COVID-19 positivity. However, the optimal timing and dose continues to require further clinical investigation.
9. Conclusion
Evidence has shown that reducing or stopping the hyperinflammatory state that occurs in some infected patients COVID-19 is effective in improving health. The use of colchicine, as well as its proven efficacy in the prophylaxis and treatment of autoinflammatory diseases such as FMF or pericarditis, could be considered in all three stages of SARS-CoV-2 infection, especially in those patients at high risk of developing serious lung complications in a dramatically short time, in monotherapy or in combination, carefully monitoring possible drug interactions.
Funds
None.
Copyright
The authors certify that the manuscript is original, never submitted to other journal for publication before. All authors contributed equally to the manuscript and had the opportunity to revise and approve the final text.
CRediT authorship contribution statement
Antonio Vitiello: Conceptualization, Writing - original draft, Methodology. Francesco Ferrara: Writing - review & editing, Supervision, Validation.
Declaration of competing interest
I, The undersigned, Francesco Ferrara and any other author, declare that:
-
•
The manuscript was written entirely by the authors;
-
•
All authors made an equal contribution in the development of the paper;
-
•
We have no conflict of interest;
-
•
We have not received funding/source;
-
•
There are no sensitive data and no patients were recruited for this study;
-
•
The document does not conflict with ethical legislation.
-
•
The authors accept the full TRANSFER OF COPYRIGHT to the journal.
Acknowledgement
The authors have nothing to say about ethical standards, ethical approval and funding. This manuscript is not a clinical trial and does not violate ethical rules. No funding has been received for its preparation.
References
- 1.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000 May 4;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 May;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggiolini M., Walz A., Kunkel S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Standiford T.J., Rolfe M.W., Kunkel S.L., Lynch J.P., 3rd, Burdick M.D., Gilbert A.R., et al. Macrophage inflammatory protein-1 alpha expression in interstitial lung disease. J. Immunol. 1993 Sep 1;151(5):2852–2863. [PubMed] [Google Scholar]
- 5.Vitiello A., Ferrara F. Correlation between renin-angiotensin system and Severe Acute Respiratory Syndrome Coronavirus 2 infection: what do we know? Eur. J. Pharmacol. 2020;883 doi: 10.1016/j.ejphar.2020.173373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitiello A., Ferrara F. Therapeutic strategies for SARS-CoV-2 acting on ACE-2. Eur. J. Pharmaceut. Sci. 2021 Jan 1;156 doi: 10.1016/j.ejps.2020.105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitiello A., Ferrara F. Pharmacological agents to therapeutic treatment of cardiac injury caused by Covid-19. Life Sci. 2020 Dec 1;262 doi: 10.1016/j.lfs.2020.118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara F., Vitiello A. Potential pharmacological approach in the regulation of ACE-2 and DPP-IV in diabetic COVID-19 patient. Ital. J Med. 2020 doi: 10.4081/itjm.2020.1435. [DOI] [Google Scholar]
- 9.Vitiello A., La Porta R., D'Aiuto V., Ferrara F. Pharmacological approach for the reduction of inflammatory and prothrombotic hyperactive state in COVID-19 positive patients by acting on complement cascade. Hum. Immunol. 2021 doi: 10.1016/j.humimm.2021.01.007. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong E., Du H., Gardner L. An interactive web- based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhmerov A., Marbán E. COVID-19 and the heart. Circ. Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara F., Porta R., D'Aiuto V., Vitiello A. Remdesivir and COVID-19. Ir. J. Med. Sci. 2020 Oct 17:1–2. doi: 10.1007/s11845-020-02401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitiello A., Ferrara F., Porta R. Remdesivir and COVID-19 infection, therapeutic benefits or unnecessary risks? Ir. J. Med. Sci. 2021 Jan 12:1–2. doi: 10.1007/s11845-020-02482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitiello A., Ferrara F. Remdesivir versus ritonavir/lopinavir in COVID-19 patients. Ir. J. Med. Sci. 2020 Nov 18:1–2. doi: 10.1007/s11845-020-02440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suter P.M., Suter S., Girardin E., Roux-Lombard P., Grau G.E., Dayer J.M. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am. Rev. Respir. Dis. 1992 May;145(5):1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- 17.Siler T.M., Swierkosz J.E., Hyers T.M., Fowler A.A., Webster R.O. Immunoreactive interleukin-1 in bronchoalveolar lavage fluid of high-risk patients and patients with the adult respiratory distress syndrome. Exp. Lung Res. 1989;15:881–894. doi: 10.3109/01902148909069633. [DOI] [PubMed] [Google Scholar]
- 18.Goodman R.B., Strieter R.M., Martin D.P., Steinberg K.P., Milberg J.A., Maunder R.J., et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1996 Sep;154(3 Pt 1):602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara F., Vitiello A. Efficacy of synthetic glucocorticoids in COVID-19 endothelites. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021 Jan 14:1–5. doi: 10.1007/s00210-021-02049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara F., Granata G., Pelliccia C., La Porta R., Vitiello A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2: anti-inflammatory and anti-fibrotic therapy could solve the lung complications of the infection? Eur. J. Clin. Pharmacol. 2020 Nov;76(11):1615–1618. doi: 10.1007/s00228-020-02947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park W.Y., Goodman R.B., Steinberg K.P., Ruzinski J.T., Radella F., 2nd, Park D.R., et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001 Nov 15;164(10 Pt 1):1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 22.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20(13):3328. doi: 10.3390/ijms20133328. Published 2019 Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khandkar C., Vaidya K., Patel S. Colchicine for stroke prevention: a systematic review and meta-analysis. Clin. Therapeut. 2019 Mar;41(3):582–590. doi: 10.1016/j.clinthera.2019.02.003. e3. [DOI] [PubMed] [Google Scholar]
- 26.Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine--Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015;45:341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreu J.M., Timasheff S.N. Tubulin bound to colchicine forms polymers different from microtubules. Proc. Natl. Acad. Sci. U. S. A. 1982;79:6753–6756. doi: 10.1073/pnas.79.22.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benhamou J.O., Geva S., Jacobs M., Drew J., Waldman M. The use of colchicine in respiratory diseases or current respiratory medicine. Review. 2013;9(5):300–304. doi: 10.2174/1573398X10666140526235154. [DOI] [Google Scholar]
- 29.Donnelly S.C., Strieter R.M., Reid P.T., Kunkel S.L., Burdick M.D., Armstrong I., et al. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann. Intern. Med. 1996 Aug 1;125(3):191–196. doi: 10.7326/0003-4819-125-3-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Arend W.P., Joslin F.G., Thompson R.C., Hannum C.H. An IL-1 inhibitor from human monocytes: production and characterization of biologic properties. J. Immunol. 1989;143:1851–1858. [PubMed] [Google Scholar]
- 31.Arend W.P. Interleukin-1 receptor antagonist. Adv. Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- 32.Burger D., Chicheportiche R., Giri J.G., Dayer J.M. The inhibitory activity of human interleukin-1 receptor antagonist is enhanced by type II interleukin-1 soluble receptor and hindered by type I interleukin-1 soluble receptor. J. Clin. Invest. 1995 Jul;96(1):38–41. doi: 10.1172/JCI118045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020 March-April;34(2):327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 34.Tsai T.L., Wei J.C., Wu Y.T., Ku Y.H., Lu K.L., Wang Y.H., Chiou J.Y. The association between usage of colchicine and pneumonia: a nationwide, population-based cohort study. Front. Pharmacol. 2019 Aug 16;10:908. doi: 10.3389/fphar.2019.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Society of Health-System Pharmacists. Colchicine Monograph for Professionals. Drugs.Com. Retrieved 27 March 2019.
- 36.Vitiello A., Ferrara F., Pelliccia C., Granata G., La Porta R. Cytokine storm and colchicine potential role in fighting SARS-CoV-2 pneumonia. Ital. J Med. 2020;14(2):88–94. doi: 10.4081/itjm.2020.1284. [DOI] [Google Scholar]
- 37.US Food and Drug Administration . 17 February 2010. Colcrys (colchicine, USP) tablets 0.6 mg. Drug Approval Package.https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022352_colcrys_toc.cfm Retrieved 19 August 2018. [Google Scholar]
- 38.Meo S.A., Klonoff D.C., Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020 Apr;24(8):4539–4547. doi: 10.26355/eurrev_202004_21038.PMID:32373993. [DOI] [PubMed] [Google Scholar]
- 39.Lan S.H., Lai C.C., Huang H.T., Chang S.P., Lu L.C., Hsueh P.R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2020;56(3) doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed M.H., Hassan A. Dexamethasone for the treatment of coronavirus disease (COVID-19): a review [published online ahead of print, 2020 oct 31] SN Compr Clin Med. 2020:1–10. doi: 10.1007/s42399-020-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N. Engl. J. Med. 2020 Jul 17 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. [DOI] [Google Scholar]
- 42.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., et al. BACC bay tocilizumab trial investigators. Efficacy of tocilizumab in patients hospitalized with covid-19. N. Engl. J. Med. 2020 Dec 10;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A., et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncol. 2018 Aug;23(8):943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrara F. Antirheumatic in SARS-CoV-2: benefit or risk? Ital. J Med. 2020;14(2):114–115. doi: 10.4081/itjm.2020.1290. [DOI] [Google Scholar]
- 45.Vitiello A., La Porta R., Ferrara F., Sacubitril, valsartan SARS-CoV-2. BMJ Evid Based Med. 2020 Jul 27 doi: 10.1136/bmjebm-2020-111497. bmjebm-2020. [DOI] [PubMed] [Google Scholar]
- 46.Vitiello A., La Porta R., Ferrara F. Scientific hypothesis and rational pharmacological for the use of sacubitril/valsartan in cardiac damage caused by COVID-19. Med. Hypotheses. 2021 Jan 7;147 doi: 10.1016/j.mehy.2021.110486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vitiello A., Pelliccia C., Ferrara F. Drugs acting on the renin-angiotensin system and SARS-CoV-2. Drug Discov. Today. 2021 Jan 21;(21):4–37. doi: 10.1016/j.drudis.2021.01.010. S1359-6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrara F., Porta R., Santilli P., D'Aiuto V., Vitiello A. Are multiple sclerosis therapies safe in severe acute respiratory syndrome coronavirus 2 times? Indian J. Pharmacol. 2020 Sep-Oct;52(5):441–442. doi: 10.4103/ijp.IJP_417_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyes A.Z., Hu K.A., Teperman J. 08 december 2020. Anti-inflammatory therapy for COVID-19 infection: the case for colchicineAnnals of the rheumatic diseases published online first. [DOI] [Google Scholar]
- 50.Deftereos S.G., Giannopoulos G., Vrachatis D.A., Siasos G.D., Giotaki S.G., Gargalianos P., et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020 Jun 1;3(6) doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scarsi M., Piantoni S., Colombo E. Association between treatment with colchicine and improved survival in a single- centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann. Rheum. Dis. 2020;79:1286–1289. doi: 10.1136/annrheumdis-2020-217712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clinical trials website. https://www.clinicaltrials.gov/
- 53.U.S. Food and Drug Administration. Information for Healthcare Professionals: New Safety Information for Colchicine (Marketed as Colcrys). [Retrieved 19 August 2018].
- 54.Tardif J.C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019 Dec 26;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 55.Wechalekar M.D., Vinik O., Moi J.H., Sivera F., van Echteld I.A., van Durme C., et al. The efficacy and safety of treatments for acute gout: results from a series of systematic literature reviews including Cochrane reviews on intraarticular glucocorticoids, colchicine, nonsteroidal antiinflammatory drugs, and interleukin-1 inhibitors. J. Rheumatol. Suppl. 2014 Sep;92:15–25. doi: 10.3899/jrheum.140458. [DOI] [PubMed] [Google Scholar]
- 56.Stamp L.K. Safety profile of anti-gout agents: an update. Curr. Opin. Rheumatol. 2014 Mar;26(2):162–168. doi: 10.1097/BOR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 57.Lombardi N., Crescioli G., Bettiol A., Marconi E., Vitiello A., Bonaiuti R., et al. Characterization of serious adverse drug reactions as cause of emergency department visit in children: a 5-years active pharmacovigilance study. BMC Pharmacol Toxicol. 2018 Apr 16;19(1):16. doi: 10.1186/s40360-018-0207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]