Significance
In the hippocampus, the recurrent excitatory circuit established by reciprocal connections between dentate granule cells and mossy cells is dynamically regulated by activity and has been implicated in pattern separation and epilepsy. The extensive projections of mossy cells make this recurrent circuit particularly prone to runaway activity. Here, we identified multiple mechanisms by which activation of cannabinoid receptors, which are highly expressed in mossy cell axons, powerfully dampen the activity of this circuit by selectively suppressing both mossy cell to granule cell basal synaptic transmission and LTP induction. These inhibitory actions likely contribute to a sparse network of quiet granule cells, which is critical to pattern separation, precludes epilepsy, and may explain some of the marijuana-mediated effects in hippocampal-dependent memory.
Keywords: dentate gyrus, LTP, presynaptic, metaplasticity, CB1R
Abstract
Recurrent excitatory neural networks are unstable. In the hippocampus, excitatory mossy cells (MCs) receive strong excitatory inputs from dentate granule cells (GCs) and project back onto the proximal dendrites of GCs. By targeting the ipsi- and contralateral dentate gyrus (DG) along the dorsoventral axis of the hippocampus, MCs form an extensive recurrent excitatory circuit (GC-MC-GC) whose dysregulation can promote epilepsy. We recently reported that a physiologically relevant pattern of MC activity induces a robust form of presynaptic long-term potentiation (LTP) of MC-GC transmission which enhances GC output. Left unchecked, this LTP may interfere with DG-dependent learning, like pattern separation—which relies on sparse GC firing—and may even facilitate epileptic activity. Intriguingly, MC axons display uniquely high expression levels of type-1 cannabinoid receptors (CB1Rs), but their role at MC-GC synapses is poorly understood. Using rodent hippocampal slices, we report that constitutively active CB1Rs, presumably via βγ subunits, selectively inhibited MC inputs onto GCs but not MC inputs onto inhibitory interneurons or CB1R-sensitive inhibitory inputs onto GCs. Tonic CB1R activity also inhibited LTP and GC output. Furthermore, brief endocannabinoid release from GCs dampened MC-GC LTP in two mechanistically distinct ways: during induction via βγ signaling and before induction via αi/o signaling in a form of presynaptic metaplasticity. Lastly, a single in vivo exposure to exogenous cannabinoids was sufficient to induce this presynaptic metaplasticity. By dampening excitatory transmission and plasticity, tonic and phasic CB1R activity at MC axon terminals may preserve the sparse nature of the DG and protect against runaway excitation.
The dentate gyrus (DG) of the hippocampus is a key relay station that transfers information from the entorhinal cortex into the hippocampus (1). This area is classically associated with pattern separation, a process by which similar patterns of information from the entorhinal cortex are transformed into distinct neuronal representations and transferred to area CA3 (2, 3). This computation minimalizes the overlap between similar neural patterns to be stored, thereby allowing them to be recalled without interference. How the DG performs this task is poorly understood, but it is believed to rely on the unique properties of dentate granule cells (GCs), including their large number and connectivity, as well as their sparse activity in vivo. The DG also contains hilar mossy cells (MCs), glutamatergic neurons that form a recurrent excitatory GC-MC-GC circuit (4, 5). Remarkably, a single MC establishes ∼35,000 excitatory synapses onto proximal GC dendrites (6) and innervates as much as 75% of the septotemporal axis (7), targeting functionally diverse areas of the hippocampus. Thus, a single MC can have a large impact on DG-dependent information transfer. Moreover, given the extensive projections of MCs and the large proportion of these cells that are active during exploratory behaviors (8, 9), and even during sleep (10), these cells have a great potential to destabilize the recurrent GC-MC-GC excitatory circuit.
We recently reported that physiologically relevant patterns of MC activity (8–10) elicit robust presynaptic long-term potentiation at MC-GC synapses (MC-GC LTP) without changing feed-forward inhibition onto GCs (11). By shifting the excitatory/inhibitory balance onto GCs and allowing MC inputs to overcome powerful feed-forward inhibition, MC-GC LTP can dynamically shape DG output, allowing silent GCs to fire action potentials (11). Therefore, the long-lasting strengthening of MC-GC transmission could further destabilize the GC-MC-GC recurrent network, thus interfering with pattern separation (4) and even promoting epileptic activity (5, 12). MC axon terminals express uniquely high levels of type-1 cannabinoid receptors (CB1Rs) (13–15), whose activation by endogenous cannabinoids (endocannabinoids or eCBs) suppress neurotransmitter release (16). CB1Rs are G protein–coupled receptors that canonically signal via the βγ subunit (which inhibits voltage-gated calcium channels [VGCCs]) and the αi/o subunit (which inhibits cAMP/PKA signaling) to mediate transient and long-lasting suppression of transmitter release, respectively (17). We therefore hypothesized that CB1R activation could play an important role in stabilizing the recurrent GC-MC-GC circuit. CB1Rs are absent in the GCs of the mature brain (15) but are presynaptically expressed and suppress GABA release from a subset of inhibitory inputs onto GCs (18, 19). Unlike other brain areas expressing presynaptic CB1Rs (20), no eCB-mediated long-term depression has been identified at MC-GC synapses (16). Whether and how activation of CB1Rs regulates MC-GC LTP is unknown.
Here, we examined the role of CB1Rs as regulators of the GC-MC-GC recurrent circuit. We report that CB1Rs are tonically active in the absence of eCB release at MC inputs onto GCs specifically, and this tonic activity robustly inhibits MC-GC synaptic transmission, LTP, and GC output. In addition, transient eCB release from GCs during LTP induction, by activating presynaptic CB1Rs, greatly dampened MC-GC LTP magnitude, while eCB-mediated activation of CB1Rs prior to LTP induction triggered presynaptic metaplasticity. Moreover, a single in vivo exposure to exogenous cannabinoids also induced metaplasticity that reduced MC-GC LTP. By regulating MC-GC synaptic transmission and plasticity via multiple signaling cascades, cannabinoids may contribute to DG-dependent forms of learning and also protect the DG from runaway activity in a circuit implicated in seizure generation.
Results
MC-GC Synapses Are Tonically Inhibited by CB1Rs.
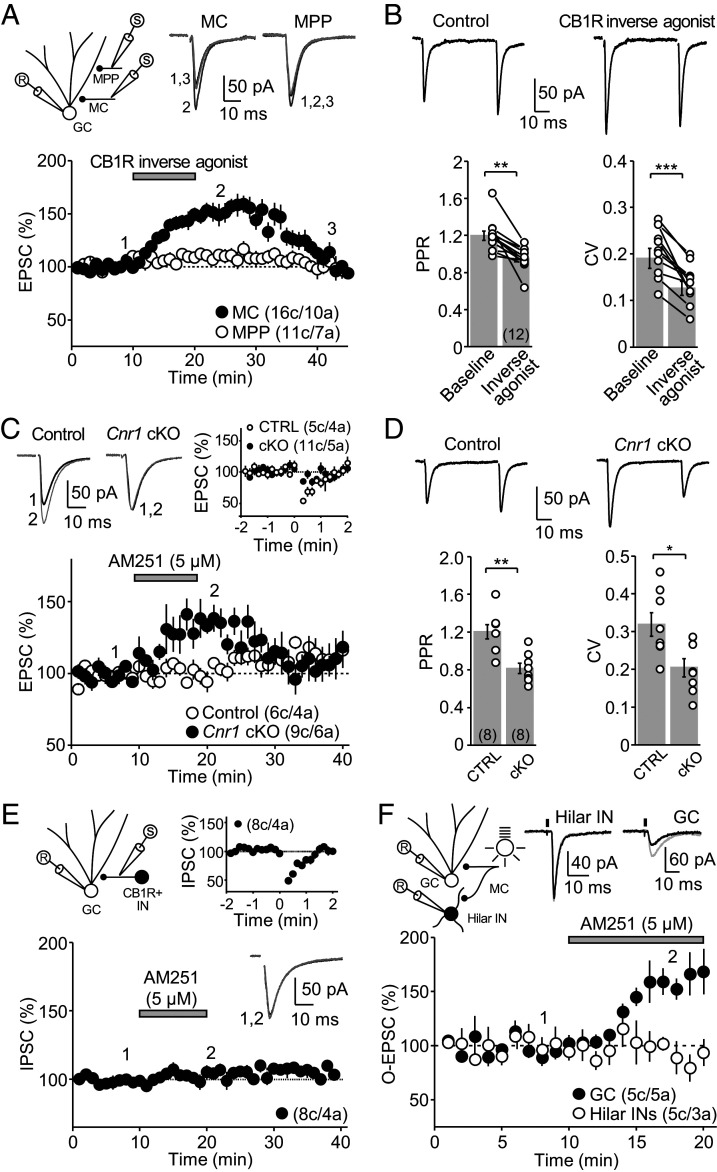
CB1Rs can be tonically active to continuously suppress neurotransmitter release (21–23). To examine whether CB1Rs are tonically active at MC-GC synapses, we evoked excitatory postsynaptic currents (EPSCs) in GCs by electrically stimulating MC axons in the inner molecular layer (IML) and bath applied the CB1R inverse agonists AM251 or SR141716A (5 μM for 10 min). We also monitored medial perforant path (MPP) synaptic inputs onto the same GC. Both AM251 and SR141716A similarly and reversibly increased MC-GC synaptic transmission, and for this reason, data were pooled (Fig. 1A and SI Appendix, Fig. S1 A and B). Moreover, this tonic CB1R activity was also observed at near physiological temperature (SI Appendix, Fig. S1C). This enhancement was accompanied by a decrease in paired-pulse ratio (PPR) and coefficient of variation (CV) (Fig. 1B), suggesting an increase in glutamate release probability. While recent evidence indicates that MPP axon terminals express functional CB1Rs (24), the CB1R inverse agonist–mediated potentiation was input-specific, as MPP-GC synaptic transmission was unaffected (Fig. 1A and SI Appendix, Fig. S1).
Fig. 1.
CB1Rs tonically inhibit MC-GC transmission in an input-specific manner. (A) Bath application of the CB1R inverse agonists AM251 (5 μM) and SR141716A (5 μM) reversibly increased MC-GC but not MPP-GC synaptic transmission (pool data; MC-GC 143 ± 6% of baseline, n = 16, P < 0.001, paired t test; MPP-GC 106 ± 4% of baseline, n = 11, P = 0.1, Wilcoxon signed rank test). (Top Left) Recording configuration, (Top Right) representative traces and (Bottom) time-course summary plot. (B) The enhancement of MC-GC transmission by CB1R inverse agonists (AM251 and SR141716A) was accompanied by a significant reduction in PPR (baseline: 1.20 ± 0.05%; CB1R inverse agonist: 0.95 ± 0.04%, n = 12; P < 0.01, Wilcoxon signed rank test) and CV (baseline: 0.19 ± 0.02%; CB1R inverse agonist: 0.13 ± 0.02%, n = 12, P < 0.001, paired t test). (Top) Representative traces and (Bottom) summary plots. (C) AM251-mediated enhancement of MC-GC transmission was absent in Cnr1 cKO mice (Cnr1 cKO: 99 ± 4% of baseline, n = 10; Control: 134 ± 8% of baseline, n = 6; Cnr1 cKO versus Control: P < 0.01, unpaired t test). (Top Left) Representative traces and (Bottom) summary plots. Cnr1fl/fl mice were injected with AAV5.CamKII.mCherry (Control) or AAV5.CamKII.mCherry-Cre (Cnr1 cKO). DSE was virtually abolished in Cnr1 cKO mice as compared to controls (Top Right; Cnr1 cKO: 90 ± 14% of baseline, n = 11; Control: 65 ± 17% of baseline, n = 5; Cnr1 cKO versus Control: P < 0.01, unpaired t test). (D) PPR and CV were reduced in Cnr1 cKO mice as compared to controls (PPR: Control: 1.20 ± 0.08%, n = 8; Cnr1 cKO: 0.82 ± 0.06%, n = 8; Control versus Cnr1 cKO: P < 0.01, unpaired t test; CV: Control: 0.32 ± 0.03%, n = 8; Cnr1 cKO: 0.20 ± 0.02%, n = 8; Control versus Cnr1 cKO: P < 0.05, unpaired t test). (Top) Representative traces and (Bottom) summary plots. (E) Unlike MC inputs, bath application of 5 μM AM251 did not alter IPSCs recruited by stimulating within the IML in the presence of 100 nM DAMGO, 50 μM D-APV, and 10 μM NBQX (101 ± 3% of baseline, n = 8, P = 0.2909, paired t test). These inhibitory inputs expressed robust DSI (Top Right, 63 ± 3% of baseline, n = 8, P < 0.05, Wilcoxon signed rank test). Recording configuration (Top Left): GC; CB1R+ IN, CB1R-sensitive interneuron. (F) AM251 increased MC-GC but not MC-hilar interneuron synaptic transmission. (Top Right) Sample traces showing O-EPSC recorded from GCs and hilar interneurons (INs). (Bottom) A time-course summary plot showing that bath application of AM251 (5 µM) potentiated synaptic transmission at MC-GC synapses (161 ± 13% of baseline, n = 5, P < 0.01, paired t test) but not at MC-INs (93 ± 4% of baseline, n = 5, P = 0.1776, paired t test). O-EPSCs were recorded in the continuous presence of 100 μM picrotoxin. Here and in all figures, data are presented as mean ± SEM; ***P < 0.001; **P < 0.01; *P < 0.05; numbers between brackets indicate the number of recorded cells and animals, and representative traces correspond to the time points indicated by numbers on the time-course plots.
To determine whether CB1Rs expressed in MC axons mediated the potentiation induced by the CB1R inverse agonists, we used a genetic approach in mice which would also allow us to test the generalizability of our initial observation in rats. To selectively delete CB1Rs from excitatory neurons, Cnr1-floxed (Cnr1fl/fl) mice were bilaterally injected into the hilus with an adenovirus containing Cre-recombinase and mCherry under the neuron-specific promoter CaMKII (AAV5.CamKII.mCherry-Cre). A Cre-empty virus (AAV5.CamKII.mCherry) served as control (SI Appendix, Fig. S2). Of note, mature GCs do not express CB1Rs (13, 15). Consistent with CB1Rs being deleted from MC axons, we found that depolarization-induced suppression of excitation (DSE), a transient CB1R-mediated suppression of MC-GC synaptic transmission (16), was significantly reduced in Cnr1fl/fl mice injected with the Cre virus (Cnr1 cKO) compared to Cnr1fl/fl mice injected with the control virus (Fig. 1C). AM251 (5 µM for 10 min) had no effect in Cnr1 cKO as compared to controls (Fig. 1C). Moreover, both PPR and CV (Fig. 1D) were significantly reduced in Cnr1 cKO mice, suggesting an increased release probability. Together, these findings indicate that neuronal, presumably presynaptic CB1Rs, tonically regulate glutamate release from MC axon terminals of both rat and mouse.
Tonic CB1R activity could also suppress GABA release from CB1R-expressing inhibitory inputs that largely impinge on the proximal dendrites of GCs (18, 19). To test this possibility, we monitored inhibitory postsynaptic currents (IPSCs) elicited by focal stimulation in the IML in the presence of NBQX (10 µM) and D-APV (50 µM) to block excitatory synaptic transmission and the µ-opioid receptor agonist DAMGO (100 nM) to suppress CB1R-negative inhibitory inputs (25). Under these recording conditions, inhibitory responses were eCB-sensitive (i.e., CB1R-expressing inputs), as indicated by a robust depolarization-induced suppression of inhibition (DSI) (26), but unlike MC-GC synapses, adding AM251 (5 µM for 10 min) had no effect (Fig. 1E). In addition to GCs, MCs also target GABAergic interneurons in the DG (27, 28). To test whether MC-interneuron excitatory synapses could be modulated by tonic CB1R activity, we took advantage of the MC commissural projection (1) and optogenetically activated MC axons (11) using the fast version of channelrhodopsin, ChIEF. Accordingly, we injected a ChIEF-expressing virus (AAV1/2.Syn.ChIEF-citrine) into the DG of one hippocampus and recorded optically-evoked EPSCs (O-EPSC), in both hilar interneurons and GCs of the contralateral DG. Bath application of the CB1R selective agonist WIN 55,212-2 (5 µM for 15 min) reduced O-EPSC amplitude recorded in hilar interneurons (SI Appendix, Fig. S3), indicating that functional CB1Rs are present at MC-hilar interneuron synapses. Bath application of AM251 (5 µM for 10 min) significantly increased O-EPSC amplitude recorded from GCs but not from hilar interneurons (Fig. 1F), revealing a target-selective effect. Together, these results indicate that tonically active CB1Rs inhibit glutamate release at MC-GC synapses specifically.
Constitutively Active CB1Rs Inhibit MC-GC Transmission via βγ but Not the αi/o Limb.
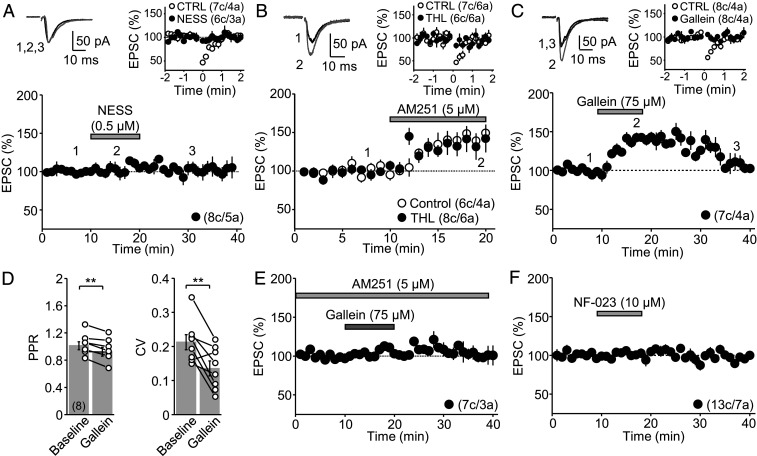
Most evidence indicates that tonic regulation of neurotransmitter release by presynaptic CB1Rs results from continuous release of eCBs (21–23, 29–31). However, CB1Rs could also be constitutively active in the absence of mobilized eCBs (22, 32, 33). To distinguish between these possibilities, we bath applied the CB1R competitive antagonist NESS0327 (22, 34) (0.5 μM for 10 min). NESS0327 had no effect on MC-GC transmission but did block DSE in interleaved experiments (Fig. 2A). In addition, to examine the involvement of eCB release, we also inhibited diacylglycerol lipase-α, the enzyme responsible for synthesizing the eCB 2-arachidonoylglycerol (2-AG), and loaded GCs with the calcium chelator BAPTA (20 mM), a manipulation that interferes with mobilization of the eCBs anandamide and 2-AG. We found that the diacylglycerol lipase-α inhibitor tetrahydrolipstatin (THL, 10 μM, preapplied for at least 1 h) had no effect on the AM251-mediated potentiation of MC-GC transmission but, as expected (35), did block DSE in interleaved experiments (Fig. 2B). Likewise, loading GCs with BAPTA blocked DSE but not the AM251-mediated potentiation (SI Appendix, Fig. S4). Altogether, these results strongly suggest that CB1Rs on MC axons are constitutively active and regulate MC-GC transmission in the absence of mobilized eCBs.
Fig. 2.
Tonic inhibition of MC-GC synapses is ligand independent and involves the βγ but not the αi/o limb of the CB1R. (A) Bath application of the CB1R competitive antagonist NESS0327 (0.5 μM) did not increase MC-GC transmission (Bottom, 106 ± 5% of baseline, n = 8, P = 0.1945, paired t test) but blocked DSE (Top, Control: 62 ± 3% of baseline, n = 7; NESS0327: 93 ± 5% of baseline, n = 6; Control versus NESS0327: P < 0.01, Mann–Whitney U test). (B) Rat hippocampal slices were incubated (>1 h) and perfused with the diacylglycerol lipase-α inhibitor THL (10 µM), while incubation and perfusion with the vehicle DMSO served as Control. THL had no effect on the AM251-mediated enhancement of MC-GC synaptic transmission (Bottom, Control: 143 ± 8% of baseline, n = 6; THL: 137 ± 15% of baseline, n = 8; Control versus THL: P = 0.5090, unpaired t test) but blocked DSE (Top, Control: 54 ± 7% of baseline, n = 7; THL: 91 ± 4% of baseline, n = 6; Control versus THL: P < 0.01, Mann–Whitney U test). (C) Bath application of the βγ limb inhibitor gallein (75 μM) reversibly increased MC-GC transmission (Bottom, 140 ± 4.3% of baseline, n = 7, P < 0.001, paired t test). DSE was blocked by gallein (Top, Control: 67 ± 4% of baseline, n = 8; Gallein: 99 ± 4% of baseline, n = 8; Control versus Gallein: P < 0.001, unpaired t test). (D) Gallein-mediated enhancement of MC-GC transmission was accompanied by a significant reduction in PPR (baseline: 1.01 ± 0.06; Gallein: 0.92 ± 0.06%, n = 8; P < 0.01, paired t test) and CV (baseline: 0.21 ± 0.02; Gallein: 0.14 ± 0.02, n = 8; P < 0.01, paired t test). (E) Gallein had no effect on MC-GC transmission when applied in the presence of 5 μM AM251 (106 ± 3% of baseline, n = 7; P = 0.0760, paired t test). (F) The specific αi/o inhibitor NF-023 (10 μM) had no effect on MC-GC transmission (99 ± 4% of baseline, n = 13, P = 0.7147, paired t test). **P < 0.01.
To investigate the mechanism downstream of CB1R responsible for the tonic inhibition of MC-GC synaptic transmission, we bath applied the βγ limb inhibitor gallein (75 μM for 10 min). Like CB1R inverse agonists, gallein transiently increased MC-GC transmission (Fig. 2C), and this enhancement was also accompanied by a decrease in PPR and CV (Fig. 2D), suggesting a presynaptic increase in glutamate release probability. In addition, in interleaved experiments, gallein blocked DSE (Fig. 2C), consistent with the notion that this phenomenon is mediated by direct inhibition of presynaptic VGCCs by Gβγ subunits (36, 37). The gallein-mediated enhancement of MC-GC transmission was significantly reduced in the presence of AM251 (5 μM, preapplied) (Fig. 2E), suggesting that AM251 and gallein likely increase MC-GC transmission via a common mechanism. Bath application of the αi/o inhibitor NF-023 (10 μM for 10 min) had no effect on MC-GC transmission (Fig. 2F); for a positive control, see Fig. 6F and SI Appendix, Fig. S6. These findings support the notion that the βγ, but not the αi/o, limb of the CB1R mediates tonic inhibition of MC-GC synapses.
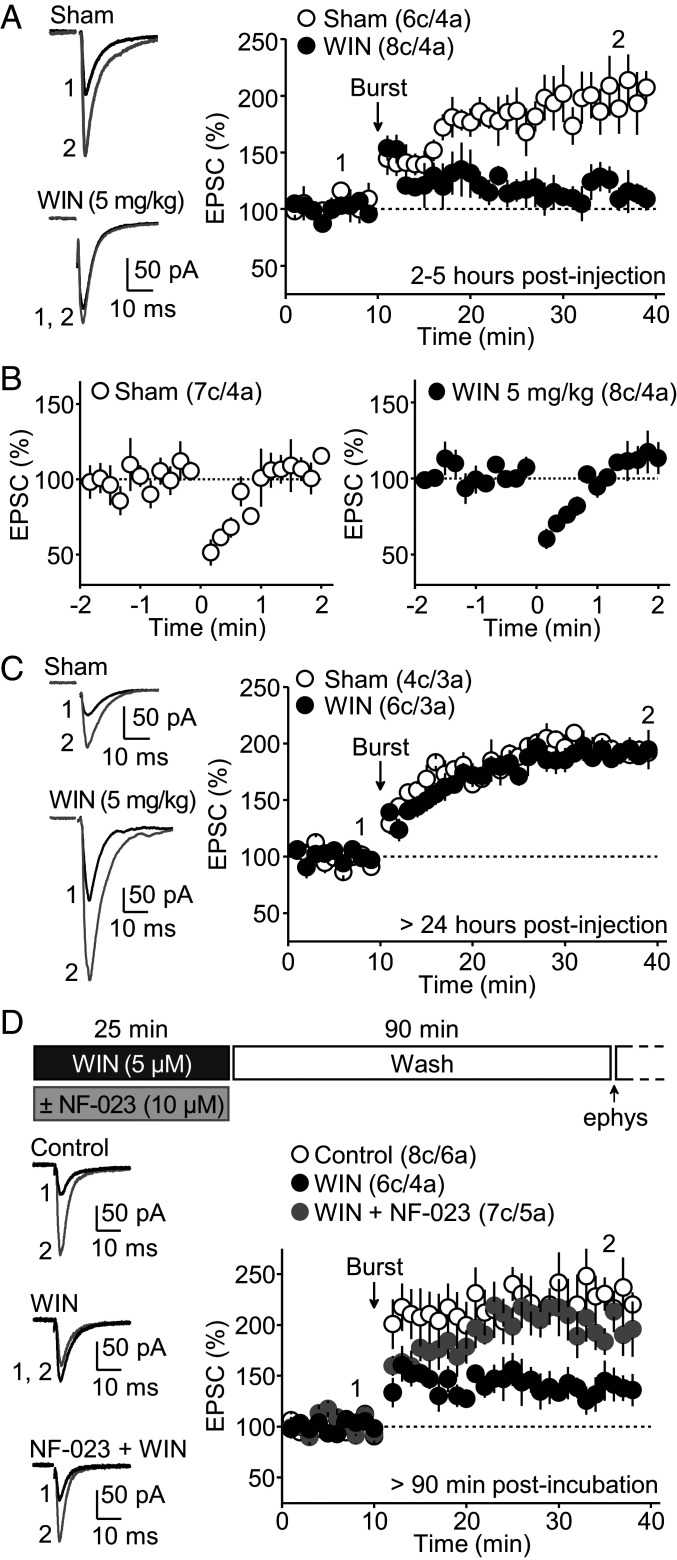
Fig. 6.
Single in vivo injection of a CB1R agonist impairs MC-GG LTP. (A) Hippocampal slices were prepared from rats that received a single injection of the cannabinoid WIN 55,212–2 (5 mg/kg, intraperitoneally, 1 h before slice preparation) or DMSO (Sham). Recordings were performed 2 to 5 h postinjection. MC-GC LTP was markedly reduced in WIN-injected animals as compared to vehicle (DMSO)-injected animals (Sham: 201 ± 19% of baseline, n = 6; WIN: 107 ± 8% of baseline, n = 8; Sham versus WIN: P < 0.001, unpaired t test). (B) DSE was normally elicited in both WIN and Sham conditions (Sham: 60 ± 5% of baseline, n = 7; WIN: 68 ± 3% of baseline, n = 8; Sham versus WIN: P = 0.1441, unpaired t test). (C) In a separate group of animals, MC-GC LTP was fully recovered 24 h post WIN-injection (Sham: 194 ± 7% of baseline, n = 4; WIN: 188 ± 9% of baseline, n = 6; Sham versus WIN: P = 0.6553, unpaired t test). (D) MC-GC LTP was assessed in rat hippocampal slices that were incubated with the cannabinoid WIN 55,212–2 (5 µM, 25 min) and washout in artificial cerebrospinal fluid. Recordings were performed > 90 min after washout. The timeline is shown on top. Representative traces (Left) and the time-course summary plot (Right) show that MC-GC LTP was reduced in WIN-incubated slices as compared to vehicle (DMSO)-incubated slices (Control), and this effect was abolished by co-application with the αi/o inhibitor NF-023 (10 µM) (Control: 228 ± 27% of baseline, n = 8; WIN: 139 ± 15% of baseline, n = 6; WIN + NF-023: 203 ± 16% of baseline, n = 7; Control versus WIN: P < 0.01, WIN versus WIN + NF-023: P < 0.05, Control versus WIN + NF-023: P = 0.8851, one-way ANOVA).
Tonically Active CB1Rs Decrease GC Output.
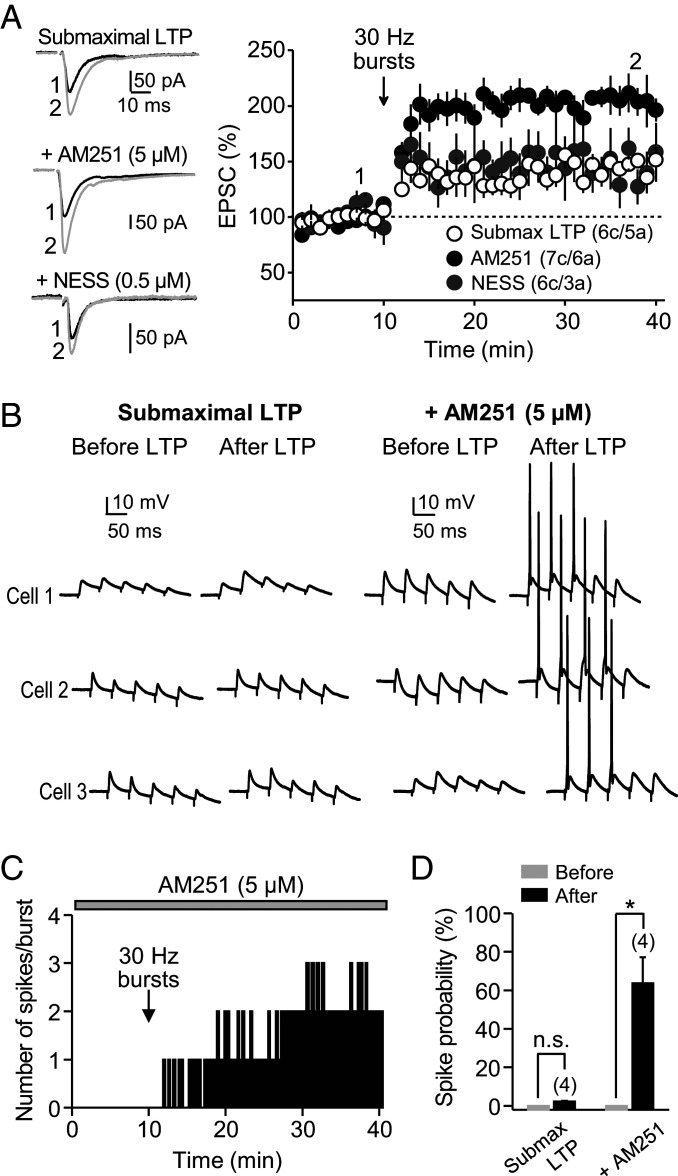
To determine whether tonic CB1R activity could also regulate the induction of MC-GC LTP, we delivered a weak protocol that triggers submaximal LTP (e.g., 30-Hz instead of 100-Hz bursts) (11), which allows us to measure potential increases in LTP magnitude. We found that the inverse agonist AM251 (5 μM), but not competitive antagonist NESS0327 (0.5 μM), robustly and significantly increased MC-GC LTP (Fig. 3A), suggesting that tonic, ligand-independent CB1R activity at MC terminals also dampens MC-GC LTP induction. Tonically active CB1Rs selectively suppress MC-GC transmission but not inhibitory inputs onto MCs (Fig. 1E) or MC inputs onto inhibitory interneurons (Fig. 1F). To determine the functional impact of this selective suppression, we monitored MC-induced burst firing in GCs before and after submaximal LTP induction, in the absence or presence of 5 μM AM251, while both excitatory and inhibitory synaptic transmissions were intact. Under normal conditions (i.e., no drugs in the bath), repetitive activation of MC axons (five pulses at 30 Hz, repeated every 20 s) failed to induce action potentials in GCs before and after submaximal LTP induction (Fig. 3 B and D). However, in the presence of 5 μM AM251, the MC-GC submaximal LTP induction protocol triggered a long-lasting increase in burst firing as indicated by the appearance of one or more spikes per burst (Fig. 3 B–D). Taken together, these results indicate that by selectively suppressing MC-GC transmission, tonic CB1R activity can negatively regulate LTP magnitude and GC output.
Fig. 3.
CB1R tonic inhibition can negatively regulate MC-GC LTP and GC output. (A) A 30-Hz induction protocol induced submaximal MC-GC LTP that was significantly enhanced when AM251 (5 μM), but not NESS0327 (0.5 μM), was included (Submaximal LTP: 146 ± 4% of baseline, n = 6; AM251: 197 ± 8% of baseline, n = 7; NESS: 139 ± 21% of baseline, n = 6; Submaximal LTP versus AM251: P < 0.01; Submaximal LTP versus NESS: P = 0.95553; AM251 versus NESS: P < 0.01 one-way ANOVA). (B) Sample traces of three representative experiments showing burst stimulation before and after application of the submaximal LTP induction protocol (5 pulses, 30 Hz, repeated 50 times every 0.5 s) in the absence (Left) or presence of 5 µM AM251 (Right). (C) Time-course plot (representative experiment) of the number of spikes per burst in the presence of 5 μM AM251 before and after LTP induction. (D) Summary data showing the spike probability before and after delivering the submaximal LTP induction protocol, in the presence (AM251: 64 ± 14%, n = 4, P < 0.05, Mann–Whitney U test) or absence of AM251. *P < 0.05. n.s., nonsignificant.
Endocannabinoid Release from Granule Cells during Induction Dampens MC-GC LTP.
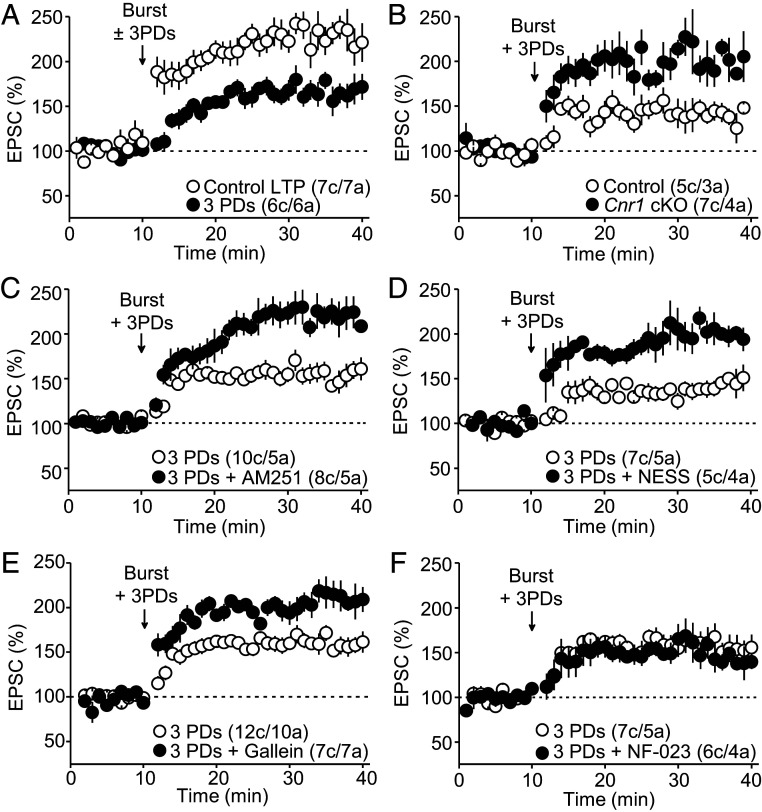
To test whether eCBs could negatively regulate the induction of MC-GC LTP, we elicited eCB release from GCs by delivering three postsynaptic depolarization (PD) steps (from −60 mV to 0 mV for 5 s, 10-s period; 25 s total) to match the duration of the LTP induction protocol. We first confirmed that this manipulation triggered a robust, transient (<2 min) DSE at MC-GC synapses (SI Appendix, Fig. S5A) (16). We found that pairing the LTP induction protocol (burst stimulation consisting of five pulses at 100 Hz × 50 every 0.5 s; 25 s total) with three PDs significantly reduced the magnitude of MC-GC LTP as compared to control experiments with no GC depolarizations (Fig. 4A). The eCB-mediated dampening of MC-GC LTP was abolished in Cnr1 cKO mice as compared to controls (Fig. 4B), indicating that neuronal, presumably presynaptic, CB1Rs mediate the dampening of MC-GC LTP. This MC-GC LTP dampening was abolished (i.e., LTP was rescued) by bath application of 5 μM AM251 (Fig. 4C) and the CB1R competitive antagonist NESS0327 (0.5 μM) (Fig. 4D). These findings indicate that both eCBs and CB1Rs are involved in the PD-mediated dampening of MC-GC LTP.
Fig. 4.
Endocannabinoid release during LTP induction dampens MC-GC LTP. (A) Pairing burst-stimulation with three PDs significantly decreased MC-GC LTP magnitude (Control LTP: 233 ± 12% of baseline, n = 7; three PDs: 160 ± 12% of baseline, n = 6; Control LTP versus three PDs: P < 0.01, unpaired t test). (B) Delivering three PDs to GCs during induction dampened MC-GC LTP in Control but not in Cnr1 cKO mice (Control: 143 ± 10% of baseline, n = 5; Cnr1 cKO: 208 ± 13% of baseline, n = 7; Control versus Cnr1 cKO: P < 0.01, unpaired t test). Cnr1fl/fl mice were injected with AAV5.CamKII.mCherry (Control) or AAV5.CamKII.mCherry-Cre (Cnr1 cKO). (C) The dampening of MC-GC LTP was not observed when the CB1R inverse agonist AM251 (5 μM) was included in the bath (three PDs: 156 ± 9% of baseline, n = 10; three PDs + AM251: 220 ± 14% of baseline, n = 8; three PDs versus three PDs + AM251: P < 0.01, unpaired t test). (D) Bath application of the CB1R competitive antagonist NESS0327 (0.5 μM) also blocked the depolarization-mediated dampening of MC-GC LTP (three PDs: 135 ± 8% of baseline, n = 7; three PDs + NESS0327: 189 ± 10% of baseline, n = 5; three PDs versus three PDs + NESS0327: P < 0.01, unpaired t test). (E) Bath application of the βγ-subunit inhibitor gallein (75 μM) prevented the depolarization-induced dampening of LTP (three PDs: 155 ± 7% of baseline, n = 12; three PDs + Gallein: 206 ± 10% of baseline, n = 7; three PDs versus three PDs + Gallein: P < 0.001, unpaired t test). (F) The dampening of MC-GC LTP was still observed in the presence of the αi/o inhibitor NF-023 (10 μM) (three PDs: 161 ± 13% of baseline, n = 7; three PDs + NF-023: 153 ± 13% of baseline, n = 6; three PDs versus three PDs + NF-023: P = 0.6171, Mann–Whitney U test).
MC-GC LTP induction does not require activation of type 1 and 5 metabotropic glutamate receptors (11), and we also found that it can be elicited in the absence of glutamatergic synaptic transmission (SI Appendix, Fig. S5B). The nonselective ionotropic glutamate receptor antagonist kynurenic acid (Kyn, 10 mM) transiently abolished fast excitatory transmission but not MC-GC LTP induction. It is therefore unlikely that the eCB-mediated dampening could be due to CB1R-mediated suppression of glutamate release. To determine the downstream signaling cascade involved, we used αi/o and βγ subunit inhibitors. We found that the βγ subunit inhibitor gallein (75 μM) abolished LTP dampening (Fig. 4E), whereas the αi/o subunit inhibitor NF-023 (10 μM) had no effect (Fig. 4F). Collectively, these results suggest that eCB release from GCs during induction by activating presynaptic CB1Rs and presumably engaging the βγ, but not the αi/o, limb can dampen MC-GC LTP.
Endocannabinoids Mediate Presynaptic Metaplasticity.
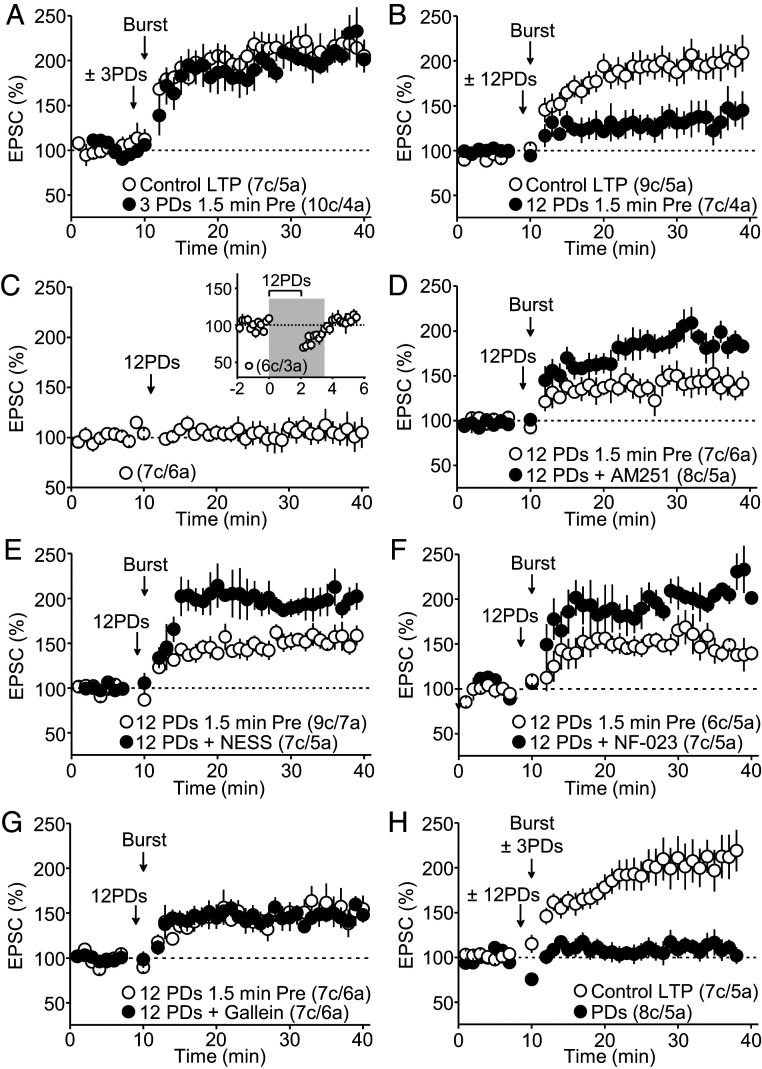
Synapses can incorporate previous activity into their cellular and molecular structure so that they are more or less apt to undergo subsequent plasticity in a process known as metaplasticity. Because eCBs can mediate metaplasticity (38, 39), we sought to determine whether eCB release prior to LTP induction could affect MC-GC LTP. To this end, we released eCBs briefly from GCs as before (SI Appendix, Fig. S5A) and allowed MC transmission to recover (1.5 min) before delivering the MC-GC LTP induction protocol. This manipulation did not significantly change the amplitude of MC-GC LTP as compared to control conditions (Fig. 5A). Since eCB-mediated phenomena often depend on the duration of CB1R activation (40, 41) and presynaptic activity (42, 43), we increased the duration of eCB mobilization by delivering 12 PDs (2 min) and paired them with brief, repetitive stimulation of MC axons (see Methods Summary). We found that this manipulation significantly diminished MC-GC LTP as compared to control conditions (Fig. 5B). Delivering the 12 PDs protocol alone (paired with presynaptic activity) did not cause a long-term change in basal transmission (Fig. 5C) but presumably changed the state of the synapse so that LTP could be less readily induced. Both AM251 (5 μM) and NESS0327 (0.5 μM) rescued MC-GC LTP (i.e., abolished the 12 PDs–induced dampening of MC-GC LTP) when compared to control experiments (Fig. 5 D and E), implicating eCBs and CB1Rs in this phenomenon. These results strongly suggest that eCBs released from GCs, presumably by activating CB1Rs on MC axon terminals, mediate presynaptic metaplasticity.
Fig. 5.
Endocannabinoids mediate presynaptic metaplasticity. (A) Delivering three PDs (25 s total) 1.5 min prior to LTP induction did not alter the magnitude of MC-GC LTP (Control LTP: 200 ± 9% of baseline, n = 7; three PDs 1.5 min pre: 218 ± 13% of baseline, n = 10; Control versus three PDs 1.5 min pre: P = 0.3234, unpaired t test). (B) Delivering 12 PDs (2 min total) 1.5 min prior to LTP induction caused a significant reduction in MC-GC LTP (Control LTP: 195 ± 15% of baseline, n = 9; 12 PDs 1.5 min pre: 127 ± 14% of baseline, n = 7; Control LTP versus 12 PDs 1.5 min pre: P < 0.01, unpaired t test). (C) The 12 PDs protocol did not trigger long-term changes in MC-GC synaptic transmission (98 ± 9% of baseline, n = 7, P = 0.4605, paired t test). (Inset) MC-GC transmission is fully recovered from the 12 PDs protocol by the time of MC-GC LTP induction (gray shadow). (D) The CB1R inverse agonist AM251 (5 μM) blocked the MC-GC LTP dampening induced by 12 PDs (12 PDs 1.5 min pre: 138 ± 11% of baseline, n = 7; AM251: 194 ± 6% of baseline, n = 8; 12 PDs versus AM251: P < 0.05, Mann–Whitney U test). (E) The CB1R competitive antagonist NESS0327 (0.5 μM) also abolished the metaplastic dampening of MC-GC LTP (12 PDs 1.5 min pre: 148 ± 8% of baseline, n = 9; NESS0327: 208 ± 14% of baseline, n = 7; 12 PDs versus NESS0327: P < 0.01, unpaired t test). (F) Bath application of the specific αi/o inhibitor NF-023 (10 μM) prevented the metaplastic dampening of MC-GC LTP via eCB release prior to LTP induction (12 PDs 1.5 min pre: 158 ± 12% of baseline, n = 6; NF-023: 205 ± 8% of baseline, n = 7; 12 PDs versus NF-023: P < 0.05, Mann–Whitney U test). (G) The dampening of MC-GC LTP was still observed in the presence of the βγ-subunit inhibitor gallein (75 μM) (12 PDs 1.5 min pre: 147 ± 10% of baseline, n = 7; Gallein: 148 ± 4% of baseline, n = 7; 12 PDs versus Gallein: P = 0.9587, unpaired t test). (H) Pairing 12 PDs 1.5 min before LTP induction with three PDs during LTP induction abolishes MC-GC LTP (Control LTP: 204 ± 17% of baseline, n = 7; combined PDs: 112 ± 11% of baseline, n = 8; Control LTP versus combined PDs: P < 0.001, unpaired t test).
Longer-lasting CB1R-mediated effects likely involve the αi/o but not the βγ limb (37). Consistent with this notion, presynaptic metaplasticity was blocked by the αi/o inhibitor NF-023 (10 μM) as compared with the robust dampening of MC-GC LTP in control experiments (Fig. 5F), whereas the βγ subunit inhibitor gallein (75 μM) had no effect (Fig. 5G). Because NF-023 is also an antagonist of purinergic P2X receptors, we tested the effect of a selective P2X receptor antagonist PPADS on the 12 PD–induced dampening of MC-GC LTP. We found that bath application of 10 µM PPADS had no effect on the 12 PDs–induced dampening (SI Appendix, Fig. S6A). We also confirmed that 10 µM NF-023 blocks eCB-mediated inhibitory LTD in CA1, which involves the αi/o limb and inhibition of adenylate cyclase (44), whereas 10 µM PPADS had no effect (SI Appendix, Fig. S6B). It is unlikely that NF-023 effects we described in our study could be mediated by P2X receptors. Thus, eCBs reduces MC-GC LTP via two mechanistically distinct processes (i.e., via βγ or αi/o signaling). We, therefore, tested whether these processes could be additive and, when combined, lead to a greater reduction of MC-GC LTP. We found that releasing eCBs both before and during induction abolished MC-GC LTP as compared to control experiments (Fig. 5H). This observation underscores the profound impact eCB release from GCs can have in regulating presynaptic LTP.
CB1Rs Powerfully Regulate the Induction but Not the Expression of MC-GC LTP.
To determine the duration of the metaplastic effect, we delivered the 12 PDs protocol 5 min before LTP induction. This manipulation still generated a significant reduction of LTP (SI Appendix, Fig. S7A). We did not attempt to release eCBs any longer than 5 min before LTP induction, given that MC-GC LTP washes out during whole-cell experiments (unpublished observations). Taken together, these results strongly suggest that the eCB system powerfully regulates MC-GC LTP in two separate ways, via the βγ or the αi/o limb, likely depending on the timing and duration of eCB release.
Given that MC-GC LTP requires presynaptic PKA activation (11), and that the CB1R is a Gi/o protein-coupled receptor whose activation inhibits PKA activity (45), we tested whether eCB release after LTP induction could interfere with LTP expression. To this end, we delivered the 12 PDs protocol, which dampens LTP via the αi/o limb of the CB1R (Fig. 5), right after induction (e.g., 1.5 min postinduction), or once LTP was established (e.g., 15 min postinduction). We found that eCB release right after (SI Appendix, Fig. S7B) or 15 min after LTP induction (SI Appendix, Fig. S7C) did not significantly affect MC-GC LTP. These observations indicate that eCB release from GCs primarily affects the induction but not the expression of MC-GC LTP. Lastly, eCB-mediated presynaptic metaplasticity was also observed at near physiological recording temperature (SI Appendix, Fig. S7D), suggesting that such regulation may also occur in vivo.
Exogenous Cannabinoids Delivered In Vivo Dampen MC-GC LTP.
To test whether exogenous cannabinoids, which are increasingly used for recreational and medical purposes, could also regulate MC-GC synaptic transmission and plasticity in vivo, rats received a single injection of WIN-55,212-2 (5 mg/kg, intraperitoneally), and hippocampal slices were prepared 1 h later and analyzed 2 to 5 h postinjection. We found that MC-GC LTP was severely impaired in WIN-injected rats, whereas vehicle-injected (Sham) animals showed normal LTP (Fig. 6A). It is unlikely that this effect could be due to the presence of WIN in the slices given that both DSE (Fig. 6B) and PPR were indistinguishable in WIN- or Sham-injected animals (PPR − WIN: 1.09 ± 0.03%, n = 10; sham: 1.10 ± 0.03%, n = 8; P = 0.816, unpaired t test). The WIN-induced dampening of LTP fully recovered 24 h postinjection (Fig. 6C).
We next determined whether WIN-mediated presynaptic metaplasticity recruits αi/o signaling through minutes of CB1R activation. Because systemic application of the αi/o inhibitor NF-023 would block αi/o signaling of all GPCRs throughout the brain and the periphery, we used a more controlled in vitro approach. To induce presynaptic metaplasticity, hippocampal slices were preincubated with WIN (5 μM for 25 min) and MC-GC LTP was tested at least 90 min after WIN washout to ensure full recovery of MC-GC synaptic transmission (16). We found that WIN preincubation dampened MC-GC LTP, and this effect was rescued by coincubation with 10 μM NF-023 (Fig. 6D). Altogether, these findings indicate that in vivo exposure to exogenous cannabinoids can also regulate the inducibility of MC-GC LTP through αi/o signaling.
Discussion
Three main findings arise from this study. First, CB1Rs on MC axons impinging on GCs are constitutively active (i.e., in the absence of eCB release from GCs), and this tonic activity is absent at other CB1R-sensitive synapses in the DG (SI Appendix, Fig. S8A). Tonic CB1R activity is likely mediated by βγ subunits (SI Appendix, Fig. S8B) which, by inhibiting both basal MC-GC synaptic transmission and LTP induction, may control GC output. Second, eCBs released from GCs, by activating presynaptic CB1Rs, not only suppress MC-GC synaptic transmission but also strongly inhibit the induction of presynaptically expressed MC-GC LTP via two distinct mechanisms downstream from CB1Rs. While transient eCB release during LTP induction inhibits plasticity via βγ signaling, a more sustained eCB-mediated activation of CB1Rs before LTP induction triggers a form of presynaptic metaplasticity that inhibits LTP generation via αi/o signaling (SI Appendix, Fig. S8B). Third, in vivo activation of CB1Rs by a single exposure to exogenous cannabinoids inhibits LTP induction by triggering presynaptic metaplasticity, which could be relevant to marijuana-mediated effects in hippocampal-dependent memory. Together, our findings indicate that MC-GC synaptic efficacy is under the control of both tonic and phasic CB1R activity. Tonic CB1R activity acts as a continuous brake of MC-GC synaptic function, whereas phasic CB1R activity, which is mediated by eCBs acting as negative feedback, may fine-tune GC firing and effectively prevent runaway activity in the GC-MC-GC recurrent circuit. The robust CB1R-mediated reduction of MC-GC synaptic strength may play important roles in DG-dependent computations, such as pattern separation, which relies on a sparse network of quiet GCs.
Anatomical studies reveal that MC axons express uniquely high levels of CB1Rs (13–15, 46), whose activation by endogenous and exogenous cannabinoids suppresses glutamate release (16). However, the functional relevance for the high expression levels of CB1Rs in MC axons has remained elusive. Here, we provide evidence that these receptors play a critical role in regulating not only basal transmission but also the inducibility of MC-GC LTP, a presynaptic form of plasticity whose induction does not require CB1R activation (11). Although most studies have shown that tonic CB1R activity in the hippocampus is due to continuous eCB release (21–23, 31), there is also evidence that constitutively active CB1Rs inhibit GABA release, but the downstream mechanism was not identified (22). We found similar tonic activity inhibiting glutamate release from MC axons onto GCs, but not GABA release from CB1R-sensitive inputs onto GCs, or MC inputs onto hilar interneurons (SI Appendix, Fig. S8A). Moreover, this target-specific inhibition is likely mediated via βγ signaling and presumably relies on different CB1R conformational states and interacting proteins, both of which may contribute to tonically suppress VGCCs (33, 47, 48). Tonic CB1R activity likely adds a further point of regulation of MC-GC function and GC output. In addition, CB1R activation by eCBs released from GCs transiently inhibited MC-GC LTP induction via βγ subunits, which presumably inhibit presynaptic VGCCs (36). Because MC-GC LTP induction requires BDNF signaling (11) but not glutamatergic transmission (SI Appendix, Fig. S5B), it is conceivable that eCB-mediated activation of presynaptic CB1Rs, by reducing presynaptic calcium influx through VGCCs, decreases BDNF release from MC axons during LTP induction.
Remarkably, pairing eCB release and presynaptic activity for a few minutes, a manipulation that is expected to engage αi/o-mediated suppression of PKA activity (42, 44), dampened subsequent induction of LTP, which constitutes one of the few examples of presynaptic metaplasticity. Previous studies identified presynaptic metaplasticity in the CA3 area and the hypothalamus (49, 50). In these cases, however, the underlying mechanism relies on activity-dependent internalization of presynaptic receptors, including CB1Rs (49), which are critically involved in the induction of long-term forms of presynaptic plasticity. Unlike heterosynaptic eCB-mediated metaplasticity (39), the presynaptic metaplasticity we described here is homosynaptic as it likely occurs in the same presynaptic compartment (i.e., MC axon terminal) where CB1Rs are activated by both eCBs and exogenous cannabinoids. Although we were unable to determine the duration of this form of metaplasticity, our experiments using the CB1R agonist WIN 55,212–2 (Fig. 6) suggests that in vivo–induced metaplasticity may last several hours. Our findings are reminiscent of previous studies showing that eCBs released either by heterosynaptic activation of climbing fibers (51) or by pharmacological activation of muscarinic acetylcholine receptors (52) inhibits presynaptically-expressed, PKA-dependent LTP at parallel fiber synapses, presumably by inhibiting the activation of adenylyl cyclase. To our knowledge, whether eCBs can trigger metaplasticity in the cerebellum remains unexplored. Our study directly shows that distinct signaling cascades downstream from CB1Rs can modulate presynaptic long-term synaptic plasticity, with the activated pathway likely depending on the timing and duration of eCB release. Our findings also indicate that the βγ subunit of the CB1R is involved in both tonic and phasic inhibition of transmitter release from MCs, suggesting a large dynamic range of signaling that can accommodate both tonic and phasic effects. An alternative scenario is that MC axons express two functionally distinct types of CB1Rs, which predominantly signal in a tonic or phasic manner. At present, we cannot distinguish between these two possibilities.
The main computational function of the DG is thought to be pattern separation (53, 54). The sparse activity of GCs (55) is proposed to be critical for pattern separation (2). In addition to targeting GCs locally, as well as distally along the longitudinal axis of the hippocampus, MCs contact GABAergic interneurons, which mediate local feed-forward inhibition onto GCs (27, 28). Local inhibition and distal excitation of GCs by MCs could effectively modulate the DG network to promote pattern separation by GCs (4). MC-GC LTP could contribute to pattern separation by more reliably driving GCs to fire action potentials (11), thereby orthogonalizing patterns of neural activity throughout the DG. If left unchecked, however, MCs could drive GCs to fire more frequently and thus diminish the sparse nature of the DG. Activity-dependent mobilization of 2-AG from GCs, by suppressing glutamate release from MC axon terminals, may down-regulate GC firing and even the induction of LTP at perforant path inputs (56). By dampening both MC-GC synaptic transmission and multiple forms of LTP, tonic and phasic CB1R activity may fine-tune and reduce excitatory drive onto GCs (SI Appendix, Fig. S8C). Presumably, this is the main reason why CB1Rs are so highly expressed in MC axons and why diverse, complementary mechanisms—that is, phasic (mediated by eCB release upon activity) and tonic (mediated by constitutively active CB1Rs)—have emerged to robustly reduce MC-GC transmission and LTP. Of note, MC-GC LTP can be induced by MC activity patterns that occur in vivo and the putative activation of a single MC axon in vitro (11), strongly suggesting that this LTP may occur often and readily in vivo. The combined tonic and phasic dampening of MC-GC transmission and LTP mediated by CB1Rs may play key roles in regulating GC firing in vivo. Assessing these roles will require the development of new molecular tools that acutely and selectively delete CB1Rs from MCs.
Lastly, the DG acts as a gate for incoming excitatory signals, which regulates the propagation of epileptiform activity (57). Because of their extensive connectivity, MCs may facilitate the spread of epileptic activity, and the exact function likely varies in early and late stages of epilepsy (5, 58, 59). In early stages of epilepsy (58), MC-GC synaptic strengthening could open the DG gate and allow the spread of epileptic activity. Under these conditions, eCB release from hyperactive GCs would inhibit MC-GC transmission and LTP induction, thereby controlling GC firing and epileptic activity. This scenario is consistent with previous studies demonstrating a role for eCB signaling in controlling hyperexcitability (15, 60) as well as growing evidence indicating that CB1Rs, acting via endogenous or exogenous cannabinoids, can protect against runaway excitation and seizure generation (61). A deeper mechanistic understanding on how exactly CB1R-mediated signaling inhibits excitatory transmission at the powerful MC-GC synaptic connection may help develop novel strategies to treat epilepsy, as well as provide insights into how the DG performs many of its complex computations that facilitate memory formation and spatial navigation.
Methods Summary
Postnatal day 19 (P19) to P30 Sprague-Dawley rats or P56–P79 Cnr1 floxed (Cnr1fl/fl) mice of either sex were used for electrophysiological experiments. Except for two experiments reported in Figs. 1 C and D and 4B, all other experiments in this study were performed on rats. All animals were group housed in a standard 12-h light/12-h dark cycle. Animal handling and use followed a protocol approved by the Animal Care and Use Committee at the Albert Einstein College of Medicine in accordance with NIH guidelines.
For more details, refer to SI Appendix, Supplementary Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to all the Castillo laboratory members for their invaluable suggestions and feedback on the experimental design and Shivani Kharod for her assistance with the confocal microscope. We thank Dr. Joseph F. Cheer (University of Maryland School of Medicine) for sharing Cnr1fl/fl mice and Dr. Yoav Ben-Simon (Tel Aviv University) for donating AAV1/2.Syn.ChIEF-citrine. We also thank the Einstein Neural Cell Engineering and Imaging Core (supported by The Rose F. Kennedy Intellectual Disabilities Research Center and a shared instrument grant 1S10OD25295) for advice and assistance with Airyscan confocal microscopy acquisition and analysis. This research was supported by NIH grants R01-NS113600, R01-DA17392, R01-MH125772, and R01-MH116673 to P.E.C.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2017590118/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Amaral D. G., Scharfman H. E., Lavenex P., The dentate gyrus: Fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 163, 3–22 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolls E. T., The mechanisms for pattern completion and pattern separation in the hippocampus. Front. Syst. Neurosci. 7, 74 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knierim J. J., Neunuebel J. P., Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiol. Learn. Mem. 129, 38–49 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers C. E., Scharfman H. E., A role for hilar cells in pattern separation in the dentate gyrus: A computational approach. Hippocampus 19, 321–337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scharfman H. E., The enigmatic mossy cell of the dentate gyrus. Nat. Rev. Neurosci. 17, 562–575 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckmaster P. S., Wenzel H. J., Kunkel D. D., Schwartzkroin P. A., Axon arbors and synaptic connections of hippocampal mossy cells in the rat in vivo. J. Comp. Neurol. 366, 271–292 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Amaral D. G., Witter M. P., The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 31, 571–591 (1989). [DOI] [PubMed] [Google Scholar]
- 8.Danielson N. B., et al. , In Vivo imaging of dentate gyrus mossy cells in behaving mice. Neuron 93, 552–559.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GoodSmith D., et al. , Spatial representations of granule cells and mossy cells of the dentate gyrus. Neuron 93, 677–690.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senzai Y., Buzsáki G., Physiological properties and behavioral correlates of hippocampal granule cells and mossy cells. Neuron 93, 691–704.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimotodani Y., et al. , LTP at hilar mossy cell-dentate granule cell synapses modulates dentate gyrus output by increasing excitation/inhibition balance. Neuron 95, 928–943.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratzliff Ad., Santhakumar V., Howard A., Soltesz I., Mossy cells in epilepsy: Rigor mortis or vigor mortis? Trends Neurosci. 25, 140–144 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Katona I., et al. , Molecular composition of the endocannabinoid system at glutamatergic synapses. J. Neurosci. 26, 5628–5637 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchigashima M., et al. , Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J. Neurosci. 31, 7700–7714 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monory K., et al. , The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51, 455–466 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu C. Q., Castillo P. E., Input-specific plasticity at excitatory synapses mediated by endocannabinoids in the dentate gyrus. Neuropharmacology 54, 68–78 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo P. E., Presynaptic LTP and LTD of excitatory and inhibitory synapses. Cold Spring Harb. Perspect. Biol. 4, a005728 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y. C., Cheng J. K., Lien C. C., Rapid dynamic changes of dendritic inhibition in the dentate gyrus by presynaptic activity patterns. J. Neurosci. 34, 1344–1357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hefft S., Jonas P., Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat. Neurosci. 8, 1319–1328 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Heifets B. D., Castillo P. E., Endocannabinoid signaling and long-term synaptic plasticity. Annu. Rev. Physiol. 71, 283–306 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Losonczy A., Biró A. A., Nusser Z., Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc. Natl. Acad. Sci. U.S.A. 101, 1362–1367 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S. H., et al. , Multiple forms of endocannabinoid and endovanilloid signaling regulate the tonic control of GABA release. J. Neurosci. 35, 10039–10057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neu A., Földy C., Soltesz I., Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J. Physiol. 578, 233–247 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peñasco S., et al. , Endocannabinoid long-term depression revealed at medial perforant path excitatory synapses in the dentate gyrus. Neuropharmacology 153, 32–40 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Glickfeld L. L., Atallah B. V., Scanziani M., Complementary modulation of somatic inhibition by opioids and cannabinoids. J. Neurosci. 28, 1824–1832 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isokawa M., Alger B. E., Retrograde endocannabinoid regulation of GABAergic inhibition in the rat dentate gyrus granule cell. J. Physiol. 567, 1001–1010 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larimer P., Strowbridge B. W., Nonrandom local circuits in the dentate gyrus. J. Neurosci. 28, 12212–12223 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharfman H. E., Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. J. Neurophysiol. 74, 179–194 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Oliet S. H., Baimoukhametova D. V., Piet R., Bains J. S., Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J. Neurosci. 27, 1325–1333 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hentges S. T., Low M. J., Williams J. T., Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J. Neurosci. 25, 9746–9751 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S. H., Földy C., Soltesz I., Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J. Neurosci. 30, 7993–8000 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pertwee R. G., Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 76, 1307–1324 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Pan X., Ikeda S. R., Lewis D. L., SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol. Pharmacol. 54, 1064–1072 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Ruiu S., et al. , Synthesis and characterization of NESS 0327: A novel putative antagonist of the CB1 cannabinoid receptor. J. Pharmacol. Exp. Ther. 306, 363–370 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Tanimura A., et al. , The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Mackie K., Hille B., Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc. Natl. Acad. Sci. U.S.A. 89, 3825–3829 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castillo P. E., Younts T. J., Chávez A. E., Hashimotodani Y., Endocannabinoid signaling and synaptic function. Neuron 76, 70–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards D. A., Zhang L., Alger B. E., Metaplastic control of the endocannabinoid system at inhibitory synapses in hippocampus. Proc. Natl. Acad. Sci. U.S.A. 105, 8142–8147 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chevaleyre V., Castillo P. E., Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron 43, 871–881 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Younts T. J., Chevaleyre V., Castillo P. E., CA1 pyramidal cell theta-burst firing triggers endocannabinoid-mediated long-term depression at both somatic and dendritic inhibitory synapses. J. Neurosci. 33, 13743–13757 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chevaleyre V., Castillo P. E., Heterosynaptic LTD of hippocampal GABAergic synapses: A novel role of endocannabinoids in regulating excitability. Neuron 38, 461–472 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Heifets B. D., Chevaleyre V., Castillo P. E., Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc. Natl. Acad. Sci. U.S.A. 105, 10250–10255 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singla S., Kreitzer A. C., Malenka R. C., Mechanisms for synapse specificity during striatal long-term depression. J. Neurosci. 27, 5260–5264 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chevaleyre V., Heifets B. D., Kaeser P. S., Südhof T. C., Castillo P. E., Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron 54, 801–812 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howlett A. C., Efficacy in CB1 receptor-mediated signal transduction. Br. J. Pharmacol. 142, 1209–1218 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawamura Y., et al. , The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 26, 2991–3001 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niehaus J. L., et al. , CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol. Pharmacol. 72, 1557–1566 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Smith T. H., et al. , Cannabinoid receptor-interacting protein 1a modulates CB1 receptor signaling and regulation. Mol. Pharmacol. 87, 747–765 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bains J. S., Wamsteeker Cusulin J. I., Inoue W., Stress-related synaptic plasticity in the hypothalamus. Nat. Rev. Neurosci. 16, 377–388 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Pelkey K. A., Lavezzari G., Racca C., Roche K. W., McBain C. J., mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron 46, 89–102 (2005). [DOI] [PubMed] [Google Scholar]
- 51.van Beugen B. J., Nagaraja R. Y., Hansel C., Climbing fiber-evoked endocannabinoid signaling heterosynaptically suppresses presynaptic cerebellar long-term potentiation. J. Neurosci. 26, 8289–8294 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rinaldo L., Hansel C., Muscarinic acetylcholine receptor activation blocks long-term potentiation at cerebellar parallel fiber-Purkinje cell synapses via cannabinoid signaling. Proc. Natl. Acad. Sci. U.S.A. 110, 11181–11186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Treves A., Rolls E. T., Computational analysis of the role of the hippocampus in memory. Hippocampus 4, 374–391 (1994). [DOI] [PubMed] [Google Scholar]
- 54.Yassa M. A., Stark C. E., Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung M. W., McNaughton B. L., Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3, 165–182 (1993). [DOI] [PubMed] [Google Scholar]
- 56.Sugaya Y., Cagniard B., Yamazaki M., Sakimura K., Kano M., The endocannabinoid 2-arachidonoylglycerol negatively regulates habituation by suppressing excitatory recurrent network activity and reducing long-term potentiation in the dentate gyrus. J. Neurosci. 33, 3588–3601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krook-Magnuson E., et al. , In vivo evaluation of the dentate gate theory in epilepsy. J. Physiol. 593, 2379–2388 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Botterill J. J., et al. , An excitatory and epileptogenic effect of dentate gyrus mossy cells in a mouse model of epilepsy. Cell Rep. 29, 2875–2889.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bui A. D. et al., Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science 359, 787–790 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marsicano G., et al. , CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Katona I., Cannabis and endocannabinoid signaling in epilepsy. Handb. Exp. Pharmacol. 231, 285–316 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.