Abstract
Women with congenital amino acid disorders, including maple syrup urine disease (MSUD), are at risk of metabolic crisis at delivery. There are still only a few case reports of maternal MSUD globally, and we are the first to report the successful perinatal management of a woman with classical MSUD in Japan. A healthy baby was delivered by scheduled cesarean section despite the presence of several uterine fibroids. With precise diet therapy and accurate preparation, she completed the postpartum period without metabolic decompensation. Although her clinical outcome was favorable, she experienced hypoproteinemia at delivery because the available branched-chain amino acid-free medical food did not contain sufficient protein to meet the recommended nutrient intake. Therefore, this case also indicates a potential issue regarding a shortage of variations in specific amino acid-free medical food in Japan, which should be addressed to achieve a better nutrient status of adults with MSUD and other amino acid disorders.
Keywords: Diet therapy, Maple syrup urine disease, Perinatal management, Uterine fibroid
1. Introduction
Maple syrup urine disease (MSUD: OMIM#248600) is an amino acid disorder that results from the inability of the branched-chain α-keto acid dehydrogenase (BCKDH) complex to metabolize the branched-chain amino acids (BCAA), namely, leucine, isoleucine, and valine [1,2]. Delayed management is often fatal to the newborn with classical MSUD. Even if well controlled by diet therapy, catabolic events may lead to metabolic crisis. The accumulation of leucine causes irreversible brain damage, adversely affecting the prognosis.
Before newborn screening was expanded to detect MSUD, the prognosis of the disease was extremely poor. Most individuals with MSUD had developmental delays; however, the introduction of screening in 1977 allowed for early diagnosis and intervention to ameliorate high leucine levels. Even so, improvements to neurological outcomes were still not sufficient, as some individuals with classical MSUD continued to have attention deficit and hyperactivity disorder [3]. With precise dietary management and care during a metabolic crisis, outcomes have gradually improved. In addition, many individuals with classical MSUD have been treated with liver transplantation from either living or deceased donors [[4], [5], [6]]. Thus, a new era in which adults with MSUD can work, marry, and give birth, has begun.
Pregnancy is a risk for a woman with classical and uncontrolled intermediate type of MSUD due to its increased energy demand. Uterine reconstruction in the postpartum period is a risk factor for catabolism and subsequent hyperleucinemia. Globally, nine cases of maternal MSUD have been reported since 1992 [[7], [8], [9], [10], [11], [12], [13], [14]]. Seven of these patients were diagnosed with classical MSUD, in which the BCKDH activity was suspected to be less than 2% of normal [2]. Little information on delivery by cesarean section could be found, as the mode of delivery in most cases was spontaneous. Six cases showed leucine elevation during the postpartum period, whereas the other cases involved no complications. The outcomes of maternal MSUD were favorable overall; however, there has been no reports of good outcomes in Japan. The precedent case died suddenly on postpartum day 51 [9]. Although death was not attributed to elevated leucine, the risk associated with this mode of delivery cannot be ignored.
Herein, for the first time, we report the successful delivery and postpartum management of a woman with classical MSUD in Japan. Despite coexisting multiple uterine fibroids, she delivered a healthy baby by cesarean section without metabolic decompensation. We also propose a potential feature of dietary management for MSUD.
2. Case presentation
2.1. Case
The patient was a G0P0 thirty-one-year-old woman who had been diagnosed with MSUD in infancy. The positive result on MSUD was found by a Guthrie test conducted 5 days after birth, which targeted four metabolic diseases, including phenylketonuria, MSUD, homocystinuria, and galactosemia. Her leucine concentration measured by high-performance liquid chromatography using dried blood collected on filter paper was 633.3 μmol/L (cutoff value: 267 μmol/L). At 17 days after birth, her leucine concentration was 899.6 μmol/L (normal value: 57.2–247.0 μmol/L), her isoleucine concentration was 403.3 μmol/L (normal value: 28.2–129.6 μmol/L), and her valine concentration was 793.9 μmol/L (normal value: 112.7–400.5 μmol/L). An allo-isoleucine peak was observed in the serum amino acid analysis. No abnormal elevations in the other amino acids were observed. Urine organic analysis detected an elevation in α-ketoisovaleric acids, and the DNPH test was positive. Then, she was diagnosed with classical MSUD following genetic analysis (reported missense mutations in the BCKDA gene, c.757G > T/ c.1087C > T).
Under strict management involving diet therapy with BCAA-free medical food and a low-protein diet, her leucine concentrations decreased and were adequately controlled throughout childhood (target therapeutic range of leucine: 150–400 μmol/L in Japan, 75–300 μmol/L abroad [15]). With normal physical and mental development, she graduated from college, became employed and was married. She regularly visited the hospital and never experienced severe metabolic decompensation. Her tolerated natural intact protein and leucine intakes before pregnancy were up to 20 g (0.43 g/kg/day) and 1600 mg, respectively. In addition to this low-protein diet, she took 180 g of a BCAA-free amino acid mixture made in Japan containing 12.6 g protein per 100 g powder (Table 1).
Table 1.
Nutrition recommendation and actual intake of this case.
| Prepregnancy |
1st trimester |
2nd trimester |
3rd trimester |
Perioperative period |
Postpartum |
|
|---|---|---|---|---|---|---|
| Nutrition recommendation in Japan for women aged 30–49 years with moderate physical activity (level 2) | ||||||
| Energy (calories) DRI 2015a |
2000 | 2050 | 2250 | 2450 | NA | 2350b |
| Total protein (g) DRI 2015a |
50 | 50 | 55 | 75 | NA | 70b |
| Actual intake of this case | ||||||
| Energy (calories) | 2000 | 2100 | 2200 | 2450 | 3100c | 2500 |
| Natural intact protein (g) | 15–20 | 15–20 | 20 | 20 | ~10 | 15 |
| Leucine (mg) | ~1600 | ~1600 | 1600 | 1600 | ~800 | 1200 |
| BCAA-free protein (g) | 23 | 25 | 29 | 30 | ~23 | 27 |
| Total protein (g) | 43 | 45 | 49 | 50 | ~33 | 42 |
Dietary Reference Intakes (DRI) proposed by the Japanese Ministry of Health, Labor and Welfare in 2015, https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf (Available on November 1st, 2020).
Nutrition recommendation for lactating women.
Calories combined with intravenous hyperalimentation.
2.2. Pregnancy
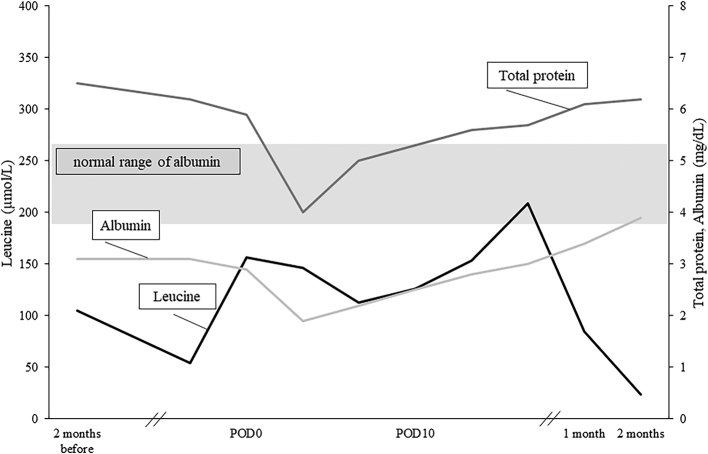
She became pregnant naturally, and the pregnancy was confirmed at 4 gestational weeks. Based on the recommendation of the nutrition management guidelines for MSUD, she attempted to take an additional 60 g of BCAA-free amino acid mixture (total 50.2 g protein) per day to meet the increased protein requirement of pregnancy (Table 1). Although assessments of biochemical status were performed every three months prior to pregnancy, frequent measurements of leucine concentration were conducted twice a month during pregnancy. Given that the treatment range of leucine throughout pregnancy remained within a normal range (150–400 μmol/L in Japan, 75–300 μmol/L abroad [15]), she achieved adequate leucine concentrations (Min 54.1 μmol/L, Max 310.6 μmol/L). In addition, we prescribed Vit B1, folic acid, Fe, zinc, selenium, and carnitine to support her nutritionally. She did not experience morning sickness; however, taking 240 g of the BCAA-free amino acid mixture in total was sometimes hard to achieve. Her serum total protein was as low as 5.8 g/dL (normal value: 6.3–8.1 g/dL) and albumin was 2.8 g/dL (normal value: 3.8–5.3 g/dL) at delivery (Fig. 1), although essential amino acid deficiency was not observed in the amino acid analysis.
Fig. 1.
Serum leucine concentration measured by high-performance liquid chromatography in the perioperative period and postpartum period. Serum total protein and albumin significantly declined during pregnancy and recovered in the postpartum period. POD: postoperative day.
The abdominal ultrasound revealed three uterine fibroids (5.8 × 3.4 cm, 5.8 × 6.1 cm, and 3.9 × 2.9 cm) around her uterus, suggesting the risk of arrested labor and bleeding during delivery. Increased protein loading due to an opportune transfusion for bleeding could cause high leucinemia, which may require hemodialysis for recovery. For safe delivery, all settings were planned for the daytime. Therefore, a cesarean section was conducted at 37 weeks and 4 days of gestation. For fluid intervention and dialysis due to possible excessive bleeding, we decided to place the central venous catheter in advance. Postoperative monitoring in the intensive care unit was planned.
2.3. Intrapartum management
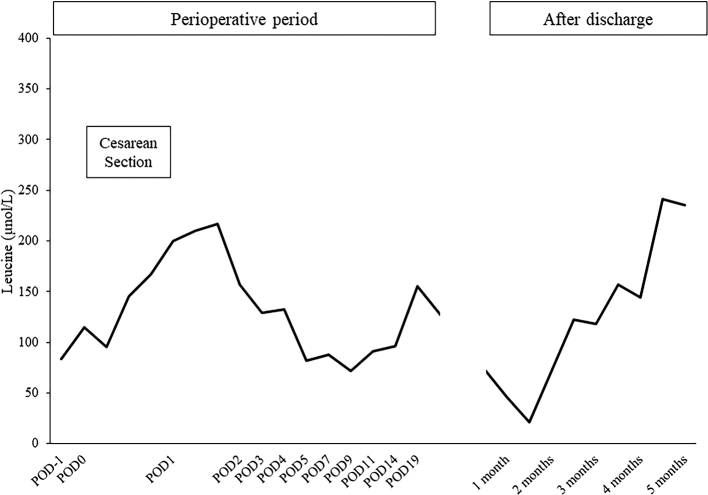
Two days before the cesarean section, a central venous catheter was placed. The infusion of 10% dextrose was started at 80 mL/h beginning at six hours before the surgery. The same infusion continued during surgery and added glucose-free infusion as needed. As uncontrolled pain could cause hyperglycemia and catabolism, epidural analgesia was chosen. Since the myomas were located in front of the abdomen, precluding the usual approach, the skin section was enlarged to safely deliver a neonate. Despite the technical difficulty, the surgery was finished in two hours. The patient was hemodynamically stable with acceptable arterial oxygenation and urination. Since leucine concentrations could not be measured immediately in our institution, blood gas analysis and ketone-checker detection of 3-hydroxybutyrate were performed every hour to monitor the signs of catabolism. These signs indicated no metabolic acidosis, ketosis or hyper/hypoglycemia during the surgery. Leucine concentrations, which were measured on filter paper every 4 h, did not increase (Fig. 2), and no subsequent neurological symptoms were observed through the intrapartum period.
Fig. 2.
Leucine concentration measured by liquid chromatography-mass spectrometry using blood dried collected on filter paper in the perioperative period and postpartum period. Leucine concentrations were within the target therapeutic range. POD: postoperative day.
2.4. Postpartum management
The blood gas analysis detected slightly elevated lactate 12 h after the surgery at 2.7 mmol/L, but it gradually recovered to normal. Beginning at 13 h after the surgery, intravenous insulin therapy was started for hyperglycemia (11 mmol/L). Oral intake was started with clear water beginning at 19 h after the surgery. The patient began to take BCAA-free medical food starting on postoperative day 1.
Her starting diet consisted of 33 g total protein, 800 mg leucine, and a total of 3100 kcal to prevent an increase in leucine due to postpartum uterine involution and was supported by 20% dextrose-based intravenous hyperalimentation (Table 1). The recovery of her oral intake was relatively slow because the invasiveness of surgery due to myoma would impair intestinal motility. Therefore, intravenous hyperalimentation was continued and gradually decreased over two weeks. Although lactation was not restricted, only colostrum was supplied to the baby because of the low supply of breast milk. The leucine concentration remained within the normal range during postoperative day one, peaked at 217.1 μmol/L and did not increase through the postpartum period due to the sufficient combination of infusion and intake of energy and BCAA-free medical food (Fig. 1).
She was discharged from the hospital on the 19th postpartum day with a normal leucine concentration of 154.9 μmol/L. Her serum total protein and albumin concentrations gradually recovered to her prepregnancy levels within two months after discharge.
2.5. Neonate
Her baby was born with an Apgar score of 5 at 1 min. Due to rapid resuscitation, the Apgar score at 5 min increased to 9. The birth weight was 2673 g, and the length was 46.5 cm. As her initial glucose concentration was 1.8 mmol/L, glucose infusion was started immediately after transfer to the neonatal intensive care unit. Liquid chromatography-mass spectrometry analysis performed on day 0 detected normal concentration of leucine (106.7 μmol/L) and a slight elevation of the allo-isoleucine concentration. However, all of the BCAAs were normalized at the second analysis on day 1, suggesting that the initial abnormal result was due to a mixture of mother-derived amino acids. Sufficient body weight gain and normal physical development were observed on routine assessment at 4 months.
3. Discussion
Pregnancy in women with MSUD carries a high risk because it has several potential triggers of metabolic crisis. According to previous case reports, the outcomes vary among individuals [14]. Six cases involved acute elevations in leucine [7,8,10,11], and one patient died during the postpartum period [9]. However, the other cases were well controlled without any complications [[12], [13], [14]]. Based on these prior observations, precise diet therapy and risk management were crucial for successful pregnancy outcome in women with MSUD.
Increased protein and energy intake are required to support the proliferation of maternal tissues and growth of the fetus [15]. Addition of vitamins and minerals is also recommended. In cases of morning sickness, an adequate caloric intake becomes difficult, and rapid intervention is necessary to avoid delayed fetal growth and maternal metabolic crisis. In this case, we modified her dietary protocol by increasing protein intake and prescribed vitamins and minerals. With regular assessment of free carnitine and trace element levels, carnitine supplementation was also prescribed, although this strategy has not yet been supported by strong evidence. Although she had not experienced morning sickness in early pregnancy, she was unable to take the proper amount of BCAA medical food, causing hypoproteinemia. Since this problem lasted until just before delivery, there was a concern that malnutrition could impair the growth of the fetus.
Hypoproteinemia in maternal MSUD should be a focus as a potential issue of dietary management in Japan. One of the reasons is the low-protein amount in the Japanese BCAA-free amino acid mixture, which contains 12.6 g protein per 100 g powder. Other BCAA-free medical foods for MSUD are unavailable in Japan; therefore, a considerable amount of the only available medical food must be consumed during pregnancy to provide the desired amount of protein. For example, 396 g BCAA-free amino acid mixture should be taken to ensure consumption of 50 g BCAA-free protein according to the recommended nutrient intake in early pregnancy [15]. If the BCAA-free amino acid mixture is made at a 15% standard concentration, the amount of liquid would be greater than 2640 mL per day. During pregnancy in particular, it is difficult to consume such a large amount of liquid medical food, and this difficulty results in a shortage of total protein intake. In other countries, Vilactin AA plus (Cambrooke-Ajinomoto, USA) contains 20 g protein in 250 mL ready-to-drink medical food, and MSUD Express 20 (Vitaflo, Australia) contains 20 g protein in 34 g powder type medical food. These products would make it much easier to achieve the nutrient goal for maternal MSUD. As described in the Introduction, a new era has begun in which MSUD patients no longer have mental delays. Given that the number of maternal MSUD cases is increasing, the availability of high protein medical food for MSUD needs to be addressed immediately in Japan and other countries with no appropriate medical products for congenital amino acid disorders.
For intrapartum management, risk management is important to avoid accidental accumulation of leucine due to delayed intervention. In this case, as rapid analysis of leucine was unavailable, all staff were apprised of important clinical signs to monitor. Scheduled cesarean section with epidural anesthesia was efficient in anticipating and addressing catabolism in advance because uterine fibroids may cause active bleeding. In addition to the peripartum period, careful monitoring should be taken during the postpartum period. After delivery, catabolism due to postpartum uterine involution occurred. Our case showed the importance of continuing glucose infusion until sufficient energy can be obtained orally. The patient successfully completed the postpartum period without hyperleucinemia. Importantly, if the period of inadequate oral energy intake is prolonged, the administration of lipids and even parental amino acids free of BCAA should be considered. In the representative case, 3-in-1 parenteral nutrition containing BCAA-free amino acids and the intravenous fat emulsion was used during 48 h of labor and subsequent cesarean section [11].
Phenylketonuria is a well-known teratogenic disorder that causes developmental delay and microcephaly of the fetus upon exposure to high phenylalanine concentrations [16]. In MSUD, a few reports suggest that non-treated maternal hyperleucinemia may be harmful to offspring in a rat model [17,18]; however, no reports have demonstrated teratogenicity due to hyperleucinemia in humans. In this case, her leucine concentration was not elevated during pregnancy. Her baby lacked surface malformations and exhibited normal physical development.
Under maternal management, infants are at risk for reactive hypoglycemia due to high maternal glucose infusion. Therefore, a rapid examination of glucose and intervention should be conducted. Breastfeeding is recommended for all infants and is effective in aiding postpartum maternal uterine contraction; however, adequate maternal energy and protein consumption is necessary to support lactation. In this case, we did not restrict her lactation because the amount was relatively small. If she produced large volumes of breast milk, she required considerably more energy and protein for lactation, and the current Japanese BCAA-free medical food might not have provided adequate nourishment for her body for the reason described above, possibly leading to catabolism.
In conclusion, we present the first successful perinatal management of a woman with classical MSUD in Japan. The favorable clinical course during pregnancy and the perinatal and postpartum periods indicated that metabolic decompensation was avoided even with her obstetric complications. Our case also showed that the outcome of pregnancy with MSUD would depend on precise diet therapy and accurate preparation as well as the amount of residual BCKDH. As technical developments in liver transplantation or regenerative medicine would increase the number of successful pregnancies in women with MSUD, the potential issue of dietary management for adult patients, including a shortage of variations in specific amino acid-free medical food, needs to be addressed. The accumulative case reports would also be informative in terms of the deliveries of cases with other congenital metabolic diseases.
Author contributions
Takano C, Ishige M and Ogawa E: Provided internal medical care and nutritional management. Kawakami K, Komatsu A, and Kawana K: Provided obstetric management. Nagano N and Okahashi A: Provided neonatal management. Takano C: Drafted the manuscript. Ishige M and Ogawa E: Provided conceptual advice. Morohashi T, Urakami T and Morioka I: Supervised. All authors read and approved the final manuscript.
Ethics statement
Informed consent was obtained from the patient with written agreement.
Declaration of Competing Interest
None.
Acknowledgments
We thank all of the physicians and medical staff as follows: Department of Pediatrics and Child Health, Department of Obstetrics and Gynecology, Department of Anesthesiology, and Division of Emergency and Critical Care Medicine, Department of Acute Medicine, Nihon University Itabashi Hospital; Department of Pediatrics and Child Health, Department of Obstetrics and Gynecology, Nihon University Hospital. We also thank Tokyo Health Service Association (Tokyo, Japan) for amino acid analysis.
This research was partially supported by the Practical Research Project for Rare/Intractable Diseases from Japan Agency for Medical Research and Development, AMED under Grant Number JP19ek0109276 and the MHLW Research Program on Rare and Intractable Diseases under Grant Number JPMH20FC1025.
References
- 1.David V.E.S., Chuang T. Maple syrup urine disease (branched-chain ketoaciduria) the online metabolic&molecular bases of inherited disease part 8. Amino Acids. 2020:1–74. [Google Scholar]
- 2.Blackburn P.R., Gass J.M., Vairo F.P.E., Farnham K.M., Atwal H.K., Macklin S., Klee E.W., Atwal P.S. Maple syrup urine disease: mechanisms and management. Appl. Clin. Genet. 2017;10:57–66. doi: 10.2147/TACG.S125962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couce M.L., Ramos F., Bueno M.A., Diaz J., Meavilla S., Boveda M.D., Fernandez-Marmiesse A., Garcia-Cazorla A. Evolution of maple syrup urine disease in patients diagnosed by newborn screening versus late diagnosis. Eur. J. Paediatr. Neurol. 2015;19:652–659. doi: 10.1016/j.ejpn.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Mazariegos G.V., Morton D.H., Sindhi R., Soltys K., Nayyar N., Bond G., Shellmer D., Shneider B., Vockley J., Strauss K.A. Liver transplantation for classical maple syrup urine disease: long-term follow-up in 37 patients and comparative united network for organ sharing experience. J. Pediatr. 2012;160:116–121. doi: 10.1016/j.jpeds.2011.06.033. (e111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feier F.H., Miura I.K., Fonseca E.A., Porta G., Pugliese R., Porta A., Schwartz I.V.D., Margutti A.V.B., Camelo J.S., Jr., Yamaguchi S.N., Taveira A.T., Candido H., Benavides M., Danesi V., Guimaraes T., Kondo M., Chapchap P., Neto J.S. Successful domino liver transplantation in maple syrup urine disease using a related living donor. Braz. J. Med. Biol. Res. 2014;47:522–526. doi: 10.1590/1414-431X20143830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takano C., Ishige M., Ogawa E., Usui H., Kagawa R., Tajima G., Fujiki R., Fukao T., Mizuta K., Fuchigami T., Takahashi S. A case of classical maple syrup urine disease that was successfully managed by living donor liver transplantation. Pediatr. Transplant. 2017;21:e12948. doi: 10.1111/petr.12948. [DOI] [PubMed] [Google Scholar]
- 7.Van Calcar S.C., Harding C.O., Davidson S.R., Barness L.A., Wolff J.A. Case reports of successful pregnancy in women with maple syrup urine disease and propionic acidemia. Am. J. Med. Genet. 1992;44:641–646. doi: 10.1002/ajmg.1320440523. [DOI] [PubMed] [Google Scholar]
- 8.Grunewald S., Hinrichs F., Wendel U. Pregnancy in a woman with maple syrup urine disease. J. Inherit. Metab. Dis. 1998;21:89–94. doi: 10.1023/a:1005396823030. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida S., Tanaka T. Postpartum death with maple syrup urine disease. Int. J. Gynaecol. Obstet. 2003;81:57–58. doi: 10.1016/s0020-7292(02)00446-0. [DOI] [PubMed] [Google Scholar]
- 10.Tchan M., Westbrook M., Wilcox G., Cutler R., Smith N., Penman R., Strauss B.J., Wilcken B. The management of pregnancy in maple syrup urine disease: experience with two patients. JIMD Rep. 2013;10:113–117. doi: 10.1007/8904_2013_212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wessel A.E., Mogensen K.M., Rohr F., Erick M., Neilan E.G., Chopra S., Levy H.L., Gray K.J., Wilkins-Haug L., Berry G.T. Management of a woman with maple syrup urine disease during pregnancy, delivery, and lactation. JPEN J. Parenter. Enteral Nutr. 2015;39:875–879. doi: 10.1177/0148607114526451. [DOI] [PubMed] [Google Scholar]
- 12.Heiber S., Zulewski H., Zaugg M., Kiss C., Baumgartner M. Successful pregnancy in a woman with maple syrup urine disease: case report. JIMD Rep. 2015;21:103–107. doi: 10.1007/8904_2014_401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunert S.C., Rosenbaum-Fabian S., Schumann A., Schwab K.O., Mingirulli N., Spiekerkoetter U. Successful pregnancy in maple syrup urine disease: a case report and review of the literature. Nutr. J. 2018;17:51. doi: 10.1186/s12937-018-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown J., Tchan M., Nayyar R. Maple syrup urine disease: tailoring a plan for pregnancy. J. Matern. Fetal Neonatal Med. 2018;31:1663–1666. doi: 10.1080/14767058.2017.1323328. [DOI] [PubMed] [Google Scholar]
- 15.Frazier D.M., Allgeier C., Homer C., Marriage B.J., Ogata B., Rohr F., Splett P.L., Stembridge A., Singh R.H. Nutrition management guideline for maple syrup urine disease: an evidence- and consensus-based approach. Mol. Genet. Metab. 2014;112:210–217. doi: 10.1016/j.ymgme.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Murphy E. Medical problems in obstetrics: inherited metabolic disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2015;29:707–720. doi: 10.1016/j.bpobgyn.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 17.de Franceschi I.D., Rieger E., Vargas A.P., Rojas D.B., Campos A.G., Rech C., Feksa L.R., Wannmacher C.M. Effect of leucine administration to female rats during pregnancy and lactation on oxidative stress and enzymes activities of phosphoryltransfer network in cerebral cortex and hippocampus of the offspring. Neurochem. Res. 2013;38:632–643. doi: 10.1007/s11064-012-0961-4. [DOI] [PubMed] [Google Scholar]
- 18.de Franceschi I.D., da Silva J.D., Nitzke Minuzzi B., de Barros K.C., Fernandes E.K., Bortoluzzi V.T., Rieger E., Preissler T., Feksa L.R., Hahn R.Z., Linden R., Rech C., Casali E.A., Wannmacher C.M.D. Ibuprofen during gestation prevents some changes in physical and reflex development in offspring in a model of hyperleucinemia and maternal inflammation. Int. J. Dev. Neurosci. 2020;00:1–11. doi: 10.1002/jdn.10035. [DOI] [PubMed] [Google Scholar]