Highlights
-
•
Amyloid PET not different for those with and without acute stroke.
-
•
Amyloid PET not different for those that developed post-stroke dementia.
-
•
Amyloid PET not different for African American and non-Hispanic white participants.
-
•
African Americans more likely to have microbleeds and small infarcts.
Keywords: Preclinical Alzheimer Disease, Stroke, MRI, African American, Amyloid PET
Abstract
Introduction
Stroke and Alzheimer disease share risk factors and often co-occur, and both have been reported to have a higher prevalence in African Americans as compared to non-Hispanic whites. However, their interaction has not been established. The objective of this study was to determine if preclinical Alzheimer disease is a risk factor for stroke and post-stroke dementia and whether racial differences moderate this relationship.
Methods
This case-control study was analyzed in 2019 using retrospective data from 2007 to 2013. Participants were adults age 65 and older with and without acute ischemic stroke. Recruitment included word of mouth and referrals in Saint Louis, MO, with stroke participants recruited from acutely hospitalized patients and non-stroke participants from community living older adults who were research volunteers. Our assessment included radiologic reads of infarcts, microbleeds, and white matter hyperintensitites (WMH); a Pittsburgh Compound B PET measure of cortical β-amyloid binding; quantitative measures of hippocampal and WMH volume; longitudinal Mini Mental State Examination (MMSE) scores; and Clinical Dementia Rating (CDR) 1 year post-stroke.
Results
A total of 243 participants were enrolled, 81 of which had a recent ischemic stroke. Participants had a mean age of 75, 57% were women, and 52% were African American. Cortical amyloid did not differ significantly by race, stroke status, or CDR post-stroke. There were racial differences in MMSE scores at baseline (mean 26.8 for African Americans, 27.9 for non-Hispanic whites, p = 0.03), but not longitudinally. African Americans were more likely to have microbleeds (32.8% vs 22.6%, p = 0.04), and within the acute stroke group, African Americans were more likely to have small infarcts (75.6% vs 56.8%, p = 0.049).
Conclusion
Preclinical Alzheimer disease did not show evidence of being a risk factor for stroke nor predictive of post-stroke dementia. We did not observe racial differences in β-amyloid levels. However, even after controlling for several vascular risk factors, African Americans with clinical stroke presentations had greater levels of vascular pathology on MRI.
Nomenclature
- AA
African American
- AD
Alzheimer Disease
- ADRC
Alzheimer Disease Research Center Comparison Cohort
- APOE4
Apolipoprotein E ε4
- CDR
Clinical Dementia Rating
- HbA1c
Hemoglobin A1c
- MMSE
Mini Mental State Examination
- NHW
Non-Hispanic White
- NIHSS
National Institutes of Health Stroke Scale
- PIB
[11C]-Pittsburgh Compound B
- TOAST
Trial of Org 10,172 in Acute Stroke Treatment
- WMH
White Matter Hyperintensities
1. Introduction
Medical research on African Americans (AAs) has a fraught history that has resulted in limited knowledge of racial differences in various pathologies, as well as an understandable hesitance for minorities to participate in research (Hooper et al., 2019). Several studies addressing racial disparities in Alzheimer disease (AD) and stroke have found higher incidence rates in the Black population for both diseases as compared with the non-Hispanic white (NHW) population (Benjamin et al., 2017, Manly and Mayeux, 2019). Note that we use both the broader term ‘Black’ and the more specific term ‘African American’ depending on the language used in each previously published study. Our use of ‘African American’ reflects how our participants identify themselves, but this may not be the appropriate term for studies in other regions or outside the United States.
The impact of stroke is severe, with over 140,000 deaths per year caused by stroke in the United States (Yang et al., 2017). Stroke leads to long-term consequences even for those who survive: almost 40% of stroke survivors develop long-term disabilities (Luengo-Fernandez et al., 2013), and stroke is associated with cognitive decline before and after the stroke event (Zheng et al., 2019). Black individuals are twice as likely to have a stroke (Benjamin et al., 2017), and are more likely to die from that stroke (Yang et al., (2017). These racial differences have been attributed to stroke risk factors such as higher rates of hypertension and diabetes, and lower socio-economic status and education (Benjamin et al., 2017).
Similarly, many publications report higher rates of dementia and AD for Black persons compared with NHWs (Manly and Mayeux, 2019, Green, 2002, Mayeda et al., 2016, Tang et al., 2001, Graff-Radford et al., 2016). While there is little doubt of the racial disparities seen in dementia, most of these studies are based on a clinical diagnosis of AD, which corresponds to neuropathological diagnosis in only 83% of cases (Beach et al., 2012) and neuropathological studies are often lacking in racial minorities. Racial differences in AD have been largely attributed to AD risk factors, many of which overlap with stroke risk factors. Higher rates of cardiovascular disease and of Apolipoprotein E ε4 (APOE4) alleles and fewer years of education are examples (Graff-Radford et al., 2016). Regarding APOE4, some studies suggest that higher rates of APOE4 explain much of the racial differences in AD, while others have found that APOE4 alleles in AAs have less associated risk for AD compared to APOE4 in NHW populations (Morris et al., 2019). Additionally, some reports show no racial differences in AD (Fillenbaum et al., 1998, Fitzpatrick et al., 2004, Riudavets et al., 2006, Xiong et al., 2020), but many of these papers controlled for baseline racial differences in education, cognitive scores, and APOE4.
In addition to racial differences, stroke and AD have a complex and poorly understood relationship with each other. Both diseases are highly prevalent and are strongly associated with age, leading to frequent co-occurrence in older adults. Additionally, one possible direct interaction is post-stroke dementia - wherein older adults develop dementia within a year of having a stroke (Pendlebury and Rothwell, 2009). While post-stroke dementia is correlative based on recent history of stroke, it suggests an interaction of AD and stroke. It is possible that preclinical AD predisposes people to have a stroke, or that stroke accelerates the development of dementia when it occurs in persons with preclinical AD. Previous studies have generally found greater amyloid pathology in post-stroke dementia (Chi et al., 2019, Yang et al., 2015, Liu et al., 2015, Hagberg et al., 2020, Thiel et al., 2014, Gamaldo et al., 2006, Mok et al., 2016. However, these studies measure amyloid pathology months to years after stroke, and do not contrast with the high incidence of Alzheimer pathology in the general population. The only study to examine amyloid within a month of stroke found only a small, highly localized area of higher amyloid deposition in the precuneus (Yasuno et al., 2019). Several recent studies showed that various vascular factors did not directly impact amyloid deposition (Bos et al., 2019, Gottesman et al., 2020, Bennett et al., 2020), suggesting pre-existing preclinical AD may be the important factor in the development of post-stroke dementia.
In this study, we examined AA and NHW older adults who were admitted with acute stroke to the stroke service of a tertiary care facility. For comparison, we used participants without acute or subacute stroke from the Knight Alzheimer Disease Research Center (ADRC) at Washington University in St. Louis. We examined whether pre-existing cortical amyloid positivity is a risk factor for stroke and if it increases the likelihood of dementia at 1 year post-stroke. Additionally, we examined the possibility of racial differences in the frequency of preclinical AD, vascular pathology, and in longitudinal cognitive change.
2. Methods
2.1. Participants
This study includes acute stroke patients and retrospectively selected non-stroke participants used as a comparison group. All procedures were approved by Washington University’s Human Research Protection Office. Written informed consent was obtained from all participants and a stipend was provided.
Acute stroke participants were recruited from the Barnes-Jewish Hospital Stroke Registry and from St. John’s Mercy Medical Center in St. Louis between 2009 and 2012. Persons were eligible to enroll if they were 65 years or older, had a recent ischemic stroke of embolic or occlusive origin, and a National Institutes of Health Stroke Scale (NIHSS) (Ortiz, 2014) score of 2–18. Participants were excluded if they had moderate-severe aphasia or pre-stroke cognitive decline, as determined by an informant-reported AD8 (Galvin et al., 2005) score >2 the week prior to the acute stroke.
Non-stroke participants, referred to as the ADRC group, were volunteers in the longitudinal clinical studies at the Knight ADRC from 2007 to 2013. Details of recruitment at the ADRC have been outlined previously (Morris et al., 2019). Due to low enrollment of AAs in the Knight ADRC, the ADRC cohort was not matched 1-to-1 to the stroke cohort. Instead, all AA participants who were 65 years or older, had no known deterministic mutation for AD, and had an MRI were included. The NHW participants from the Knight ADRC who also met these criteria were then matched to the AA ADRC cohort as much as possible on the basis of age and sex.
2.2. Demographics
Self-reported race, age at time of MRI, biological sex at birth, years of education, self-reported family history of dementia in first-degree relatives, body mass index, hemoglobin A1c (HbA1c), APOE4 allele status, blood pressure, history of stroke, and history of diabetes were assessed. The stroke group was further classified by stroke type using The Trial of Org 10,172 in Acute Stroke Treatment (TOAST) and severity using the National Institutes of Health Stroke Scale (NIHSS). All of these measures, along with MMSE discussed below, were collected within the stroke group 1–40 (median 10.5) days from stroke occurrence, except for blood pressure which was collected 1–50 (median 4) days from stroke occurrence. Within the ADRC group, hemoglobin A1c was collected 0–3110 (median 365) days from the rest of the clinical assessment. Hypertension was defined as having systolic blood pressure of at least 140 or diastolic blood pressure of at least 90 mm Hg (Whelton et al., 2018).
2.3. Imaging measures
Participants had a structural, T1-weighted magnetization-prepared, rapid gradient-echo (MPRAGE) MRI collected using either a 1.5T or 3T Siemens scanner and a resolution of either 1 × 1 × 1.25 mm or 1 × 1 × 1 mm. Most participants additionally had a T2w and T2* scan. MR imaging was acquired 0–385 (median 78) days from clinical assessment for the ADRC group, and 0–592 (median 1) days for the stroke group. Experienced radiologists read each set of scans and completed a radiologic report on: A. the number of large infarcts (0,1,2,3+), B. small infarcts (0,1,2,3+), C. microbleeds (0,1–4, 5–10, 11+), and D. the severity of leukoaraiosis/WMH (0 = none, 1 = mild, 2 = moderate, or 3 = severe) according to Fazekas scoring (Fazekas et al., 1987). Scans which passed quality control also had hippocampal volumes obtained using FreeSurfer 5.3 (Fischl, 2012) and WMH volumes with the Lesion Segmentation Tool (Schmidt et al., 2012). Hippocampal volumes were adjusted for head size using a regression scaling approach with total intracranial volume (Buckner et al., 2004).
All acute stroke and many ADRC participants underwent [11C]-Pittsburgh compound B (PIB) PET imaging to assess the level of amyloid plaques in their brain. PET imaging was acquired 2–442 (median 112) days from clinical assessment for the ADRC group. For the stroke group, this interval was 0–29 (median 0) days from clinical assessment and 1–50 (median 14.5) days from stroke occurrence. Data from the 30- to 60-minute post-injection window was processed with an in-house pipeline using FreeSurfer-derived regions (PET Unified Pipeline, github.com/ysu001/PUP) and a cerebellar cortex reference region. The mean cortical binding potential from the precuneus, prefrontal cortex, gyrus rectus, and lateral temporal regions was used both as a continuous variable and as a marker of amyloid positivity (mean cortical binding potential >0.18) (Su et al., 2013, Su et al., 2019).
2.4. Clinical and cognitive measures
The mini mental state examination (MMSE) (Folstein et al., 1975) was a general measure of cognition collected at the same time as the clinical assessment described in section 2.2. Some participants (108 AA and 101 NHW) also had longitudinal MMSE assessed 1–14 (median 4.6) years after the original.
Clinical Dementia Rating® (CDR®) (Morris, 1993) at 1 year follow-up for a subset of acute stroke participants assessed possible decline in cognitive and functional abilities relative to the participant’s previously attained levels. It was determined by experienced clinicians using independent, semi-structured interviews with the participant and a collateral source, and a CDR >0 was used as an indicator of post-stroke dementia (Storandt et al., 2006, Morris, 2012, Jack, 2020). Baseline CDR was not available for the stroke group.
2.5. Statistical analysis
All analyses were performed on SAS 9.4 or R 4.0.2. Each variable was assessed for cross-sectional racial differences and for differences between the acute stroke and ADRC groups. This was accomplished by modeling race, stroke status, and their interaction with logistic regression for categorical variables (binaries used: presence of large infarcts, presence of small infarcts, moderate-severe WMH, presence of microbleeds, and 5 or more microbleeds) and general linear models for continuous. The imaging measures and baseline MMSE were additionally analyzed by an adjusted model that included the covariates age (centered), family history, APOE4, education, sex, hypertension, and the first order interactions of these variables with race. The included covariates were selected by first individually testing each of the covariates in Table 1 with each of the outcomes. Those shown to be significant with most of the outcomes were included in the full model. If it was significant with only one outcome but then not significant in the full model it was dropped from the list.
Table 1.
Demographics.
| AA | NHW | Race Difference (p) | Stroke p-value |
||
|---|---|---|---|---|---|
| Within AAs | Within NHWs | ||||
| Participants, n | NA | NA | |||
| ADRC Group | 84 | 78 | 0.64 | ||
| Stroke Group | 42 | 39 | 0.74 | ||
| Age (y), mean (SD)a | >0.99 | 0.008 | |||
| ADRC Group | 74.7 (7.38) | 73.1 (5.96) | 0.42 | ||
| Stroke Group | 75.0 (6.85) | 77.4 (6.88) | 0.40 | ||
| Male, n (%)a | 0.30 | 0.12 | |||
| ADRC Group | 30 (35.7) | 34 (43.6) | 0.31 | ||
| Stroke Group | 19 (45.2) | 23 (59.0) | 0.22 | ||
| Education (y), mean (SD)a | <0.001 | <0.001 | |||
| ADRC Group | 14.5 (2.76) | 15.3 (2.66) | 0.24 | ||
| Stroke Group | 11.9 (1.66) | 12.8 (3.11) | 0.43 | ||
| Family history of dementia, n (%)b | 0.15 | <0.001 | |||
| ADRC Group | 25 (29.8) | 43 (55.1) | 0.001 | ||
| Stroke Group | 4 (15.4) | 4 (14.8) | 0.95 | ||
| One or two APOE4 Alleles, n (%)c | 0.19 | 0.20 | |||
| ADRC Group | 38 (46.3) | 33 (42.3) | 0.61 | ||
| Stroke Group | 22 (59.5) | 11 (29.7) | 0.01 | ||
| Body mass index (kg/m2), mean (SD)d | >0.99 | 0.99 | |||
| ADRC Group | 29.2 (5.24) | 26.9 (5.55) | 0.03 | ||
| Stroke Group | 29.3 (5.20) | 27.2 (4.24) | 0.27 | ||
| Hemoglobin A1c (%), mean (SD)e | <0.001 | 0.06 | |||
| ADRC Group | 5.87 (0.80) | 5.68 (0.49) | 0.82 | ||
| Stroke Group | 6.77 (1.67) | 6.31 (1.35) | 0.30 | ||
| Mean Arterial Pressure (mm Hg), mean (SD)f | 0.09 | 0.13 | |||
| ADRC Group | 93.9 (11.7) | 92.2 (10.2) | 0.81 | ||
| Stroke Group | 99.2 (15.8) | 93.0 (15.9) | 0.93 | ||
| Hypertension, n (%)f | <0.001 | 0.09 | |||
| ADRC Group | 24 (28.6) | 27 (35.5) | 0.35 | ||
| Stroke Group | 27 (64.3) | 19 (52.8) | 0.30 | ||
| Reported Previous Stroke, n (%)g | 0.006 | <0.001 | |||
| ADRC Group | 5 (6.0) | 2 (2.6) | 0.31 | ||
| Stroke Group | 10 (24.4) | 11 (30.6) | 0.55 | ||
| History of Diabetes, n (%)h | 0.002 | 0.15 | |||
| ADRC Group | 14 (16.9) | 9 (3.8) | 0.35 | ||
| Stroke Group | 18 (43.9) | 8 (3.4) | 0.05 | ||
AA: African American; ADRC: Alzheimer Disease Research Center comparison cohort; APOE4: Apolipoprotein E ε4; NHW: non-Hispanic white
a. Missing data: 0 AA ADRC, 1 AA Stroke, 0 NHW ADRC, 2 NHW Stroke.
b. Missing data: 0 AA ADRC, 16 AA Stroke, 0 NHW ADRC, 12 NHW Stroke.
c. Missing data: 2 AA ADRC, 5 AA Stroke, 0 NHW ADRC, 2 NHW Stroke.
d. Missing data: 1 AA ADRC, 1 AA Stroke, 1 NHW ADRC, 2 NHW Stroke.
e. Missing data: 36 AA ADRC, 3 AA Stroke, 22 NHW ADRC, 9 NHW Stroke.
f. Missing data: 0 AA ADRC, 0 AA Stroke, 3 NHW ADRC, 3 NHW Stroke.
g. Missing data: 1 AA ADRC, 0 AA Stroke, 1 NHW ADRC, 3 NHW Stroke.
h. Missing data: 1 AA ADRC, 1 AA Stroke, 1 NHW ADRC, 3 NHW Stroke.
Longitudinal MMSE data were examined using linear mixed effects models with random slope and random intercept (Laird and Ware, 1982), and the unstructured correlation matrix between the random intercept and slope. Linear mixed models, an extension of simple linear models, were chosen to allow both fixed and random effects. They are used when there is non-independence in the data, such as arises from a hierarchical structure where there are multiple observations per subject, or participants do not enter the study at the same time point or have different length of follow-up. The longitudinal models were adjusted for baseline age and education.
Due to the infarcts observed in MRIs of the ADRC group, analyses were also repeated with participants re-grouped as stroke and comparison group based upon an infarct definition of stroke. The presence of a small or large infarct on MRI defined the infarct stroke group, while lack of small or large infarct defined the new comparison group. These results are reported separately at the end of the results section.
To examine the possibility of post-stroke dementia, a logistic regression was used to predict acute stroke participants with CDR 0 vs. CDR >0 at the 1 year follow-up using baseline demographics and outcome variables. This was not repeated with the infarct definition of stroke.
3. Results
3.1. Participants
A total of 243 participants were included: 84 AA ADRC, 78 NHW ADRC, 42 AA stroke, and 39 NHW stroke. At Barnes-Jewish Hospital, 3880 ischemic stroke patients were examined for eligibility, 226 were found eligible, and 72 were enrolled and completed the study. At St. John’s Mercy Medical Center, 9 participants were enrolled, but we do not have access to detailed information on their total patient pool. The 162 ADRC participants included were those eligible from the pool of 1368 ADRC participants.
3.2. Demographics
Demographic data are summarized in Table 1, and boxplots of the continuous demographics are in eFig. 1 in the supplemental material. The stroke participants had fewer years of education and a more frequent history of stroke than the ADRC group, and the NHW ADRC group had a family history of dementia significantly higher than the other three groups.
Overall there was a high incidence of diabetes and hypertension. HbA1c and hypertension were significantly worse in AA stroke group than in AA ADRC group, with a similar but non-significant pattern seen in NHW group. History of diabetes was more severe only within the AA stroke group as compared to the AA ADRC group. Higher rate of APOE4 alleles in AAs was observed within the stroke group only (Table 1). No differences in APOE4 were seen by race in the ADRC group, or across stroke status, but APOE4 frequencies (30–60%) were higher than the ~14% that has previously been reported for the general population (Liu et al., 2013).
All demographic variables in Table 1 were used as covariates when modeling our outcome variables, except HbA1c and body mass index. Additional demographics specific to the stroke participants indicated that 28.4% had coronary artery disease, 28.4% had prior stroke, and 52.4% were taking statins (missing data n = 4). The TOAST classification (Adams et al., 1993) indicated stroke types were 12.1% large artery atherosclerosis, 27.0% cardioembolism, 36.5% small artery occlusion, and 24.3% undetermined etiology.
3.3. Amyloid PET outcome measures
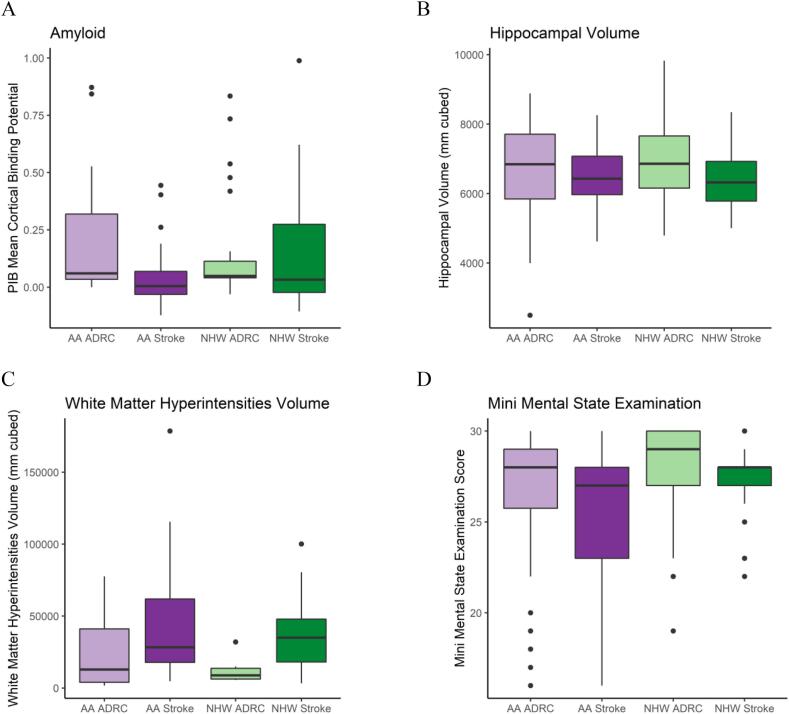
Table 2 lists the results from the statistical models and Fig. 1 displays the amyloid PET results for each group. Amyloid PET, both as a continuous variable and as a binary of amyloid+ and amyloid−, did not show differences by race or by stroke status. This indicates that preclinical AD was not more common in AAs in our cohort, and that preclinical AD does not appear to be a risk factor for stroke. The overall percentage of amyloid positive participants was high relative to other reports, indicating a high rate of preclinical AD both in the community and in the ADRC research volunteers, who may volunteer because they are at higher risk for AD due to family history.
Table 2.
Group Differences By Race and Stroke Status.
| AA | NHW | Race p-value | |||
|---|---|---|---|---|---|
| Unadjusted Models | Adjusted Modelse | ||||
| Amyloid PET | PIB Mean Cortical Binding Potential, mean (SE)a | 0.11 (0.04) | 0.11 (0.03) | 0.57 | 0.96 |
| ADRC Group | 0.20 (0.05) | 0.13 (0.05) | >0.99 | 0.68 | |
| Stroke Group | 0.05 (0.06) | 0.10 (0.05) | 0.98 | 0.90 | |
| PIB Positive, n (%)a | 16 (22.5) | 17 (25.0) | 0.90 | 0.67 | |
| ADRC Group | 10 (31.3) | 5 (16.1) | 0.92 | 0.34 | |
| Stroke Group | 6 (15.4) | 12 (32.4) | 0.09 | 0.94 | |
| Quantitative MRI | Total Hippocampal Volume (normalized), mean (SE)b | 6720 (132) | 6730 (143) | 0.57 | 0.97 |
| ADRC Group | 6610 (128) | 6780 (117) | 0.60 | 0.76 | |
| Stroke Group | 6480 (230) | 6450 (247) | >0.99 | >0.99 | |
| WMH Volume, mean (SE)c | 28,400 (10200) | 49,200 (14500) | 0.32 | 0.25 | |
| ADRC Group | 27,900 (17600) | 55,100 (25000) | 0.85 | 0.81 | |
| Stroke Group | 31,500 (9760) | 52,100 (12100) | 0.91 | 0.56 | |
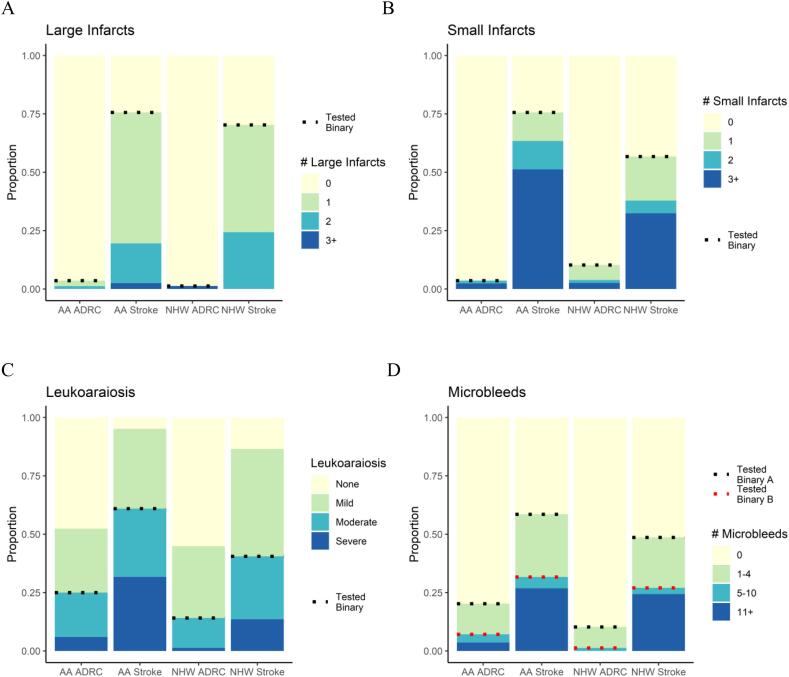
| Radiologic Read of MRI | Presence of Large Infarcts, n (%)d | 34 (27.2) | 27 (23.5) | 0.30 | 0.86 |
| ADRC Group | 3 (3.6) | 1 (1.3) | 0.37 | 0.69 | |
| Stroke Group | 31 (75.6) | 26 (70.3) | 0.60 | 0.91 | |
| Presence of Small Infarcts, n (%)d | 34 (27.2) | 29 (25.2) | 0.75 | 0.48 | |
| ADRC Group | 3 (3.6) | 8 (10.3) | 0.11 | 0.12 | |
| Stroke Group | 31 (75.6) | 21 (56.8) | 0.08 | 0.047 | |
| Leukoaraiosis Moderate-Severe, n (%)d | 46 (36.8) | 26 (22.6) | 0.01 | 0.14 | |
| ADRC Group | 21 (25.0) | 11 (14.1) | 0.09 | 0.28 | |
| Stroke Group | 25 (61.0) | 15 (40.5) | 0.07 | 0.24 | |
| Presence of Microbleeds, n (%)d | 41 (32.8) | 26 (22.6) | 0.07 | 0.04 | |
| ADRC Group | 17 (20.2) | 8 (10.3) | 0.08 | 0.07 | |
| Stroke Group | 24 (58.5) | 18 (48.7) | 0.38 | 0.37 | |
| Microbleeds ≥ 5, n (%)d | 19 (15.2) | 11 (9.6) | 0.10 | 0.93 | |
| ADRC Group | 6 (7.1) | 1 (1.3) | 0.10 | 0.93 | |
| Stroke Group | 13 (31.7) | 10 (27.0) | 0.65 | 0.54 | |
| Neuropsychological | MMSE, mean (SE)d | 26.8 (0.37) | 27.9 (0.37) | <0.001 | 0.03 |
| ADRC Group | 26 (0.38) | 27.4 (0.35) | 0.03 | 0.07 | |
| Stroke Group | 26.8 (0.64) | 28.1 (0.63) | 0.06 | 0.46 | |
AA: African American; ADRC: Alzheimer Disease Research Center comparison cohort; APOE4: Apolipoprotein E ε4; MMSE: mini mental state examination; NHW: non-Hispanic white; PIB: [11C]-Pittsburgh compound B; WMH: white matter hyperintensities
a. Missing data: 52 AA ADRC, 3 AA Stroke, 47 NHW ADRC, 2 NHW Stroke
b. Missing data: 3 AA ADRC, 11 AA Stroke, 0 NHW ADRC, 15 NHW Stroke
c. Missing data: 76 AA ADRC, 6 AA Stroke, 72 NHW ADRC, 11 NHW Stroke
d. Missing data: 0 AA ADRC, 1 AA Stroke, 0 NHW ADRC, 2 NHW Stroke
e. Adjusted models have controlled for baseline age, family history, APOE4, education, sex, and hypertension.
Fig. 1.
Continuous Demographic Measures.
Displays boxplots of the participants’ amyloid PET, hippocampal volume, white matter hyperintensity volume, and mini mental state exam score, with data separated by race (African American or non-Hispanic white) and cohort (acute stroke or ADRC comparison).
AA: African American; ADRC: Alzheimer Disease Research Center comparison cohort; NHW: non-Hispanic white; PIB: [11C]-Pittsburgh compound B.
A. Missing data: 52 AA ADRC, 3 AA Stroke, 47 NHW ADRC, 2 NHW Stroke.
B. Missing data: 3 AA ADRC, 11 AA Stroke, 0 NHW ADRC, 15 NHW Stroke.
C. Missing data: 76 AA ADRC, 6 AA Stroke, 72 NHW ADRC, 11 NHW Stroke.
D. Missing data: 0 AA ADRC, 1 AA Stroke, 0 NHW ADRC, 2 NHW Stroke.
3.4. Quantitative MRI outcome measures
Quantitative MRI measures of hippocampal volume and WMH volume (Fig. 1) did not show an overall effect of race or stroke status (Table 2). Quantitative MRI measures were possible only on a subsample of the participants and so may not be representative of our entire cohort. These results did not differ when left and right hippocampal volumes were assessed separately, nor when amyloid positive and negative participants were assessed separately.
3.5. Radiologic MRI outcome measures
The acute stroke group was more likely to have large infarcts, small infarcts, moderate-severe WMH, and microbleeds than the ADRC group (Fig. 2, Table 2). An interaction of race and APOE4 was observed for large infarcts, such that AA APOE4 carriers and AA APOE4 non-carriers were significantly different, but NHWs were not. Within the acute stroke group, AAs were more likely to have small infarcts than NHWs after adjusting for covariates. Before adjusting for covariates, the combined AA group was more likely to have more severe Fazekas stage 3/4 than the NHW group. The presence of microbleeds was more common in AA in the adjusted model. The cutoff of 5+ microbleeds, used as exclusion criteria in many clinical trials, showed no significant differences.
Fig. 2.
Categorical Outcome Measures.
Displays the participants’ radiologic data results for number of large infarcts, small infarcts, leukoaraiosis, and microbleeds, with data separated by race (African American or non-Hispanic white) and cohort (acute stroke or ADRC comparison).
AA: African American; ADRC: Alzheimer Disease Research Center comparison cohort; NHW: non-Hispanic white.
A-D Missing data: 0 AA ADRC, 1 AA Stroke, 0 NHW ADRC, 2 NHW Stroke.
3.6. Cognitive outcome measures
AA participants had lower baseline MMSE scores (Fig. 1) in both unadjusted and adjusted models (Table 2). Models of longitudinal MMSE included baseline data for all participants and at least one follow-up exam for 108 AAs and 101 NHWs (average follow-up time 4.1 and 5.2 years, respectively). Estimated change in MMSE per year did not differ significantly by race or stroke status: AA ADRC: −0.68 (SE = 0.15), AA Stroke: −1.08 (SE = 0.34), NHW ADRC: −0.58 (SE = 0.15), NHW Stroke: −0.53 (SE = 0.32) (eFig. 1). None of these results differed significantly when controlling for baseline age and education, nor when amyloid positive and negative participants were assessed separately.
3.7. Model covariates
While many covariates were included in the adjusted models for the outcome variables, the only ones commonly found to be significant were age and APOE4 status. Both were significant for PIB mean cortical binding potential, PIB positivity, and hippocampal volume; age was additionally significant for leukoaraiosis and MMSE. No significant interactions of race*age were observed, but WMH volume and large infarcts had a significant race*APOE4 interaction.
3.8. Models using infarct definition of stroke
Demographics when using the infarct definition of stroke are in eTable 1, while eTable 2 lists the results from the statistical models created for each outcome variable. Few differences were observed. The higher rate of APOE4 alleles in AAs observed within the stroke group lost significance. The adjusted model for continuous amyloid showed significantly lower amyloid in the stroke group (p changes from 0.10 to 0.02 in the adjusted model; mean cortical binding potential = 0.180 for ADRC group, 0.067 for stroke group). The presence of microbleeds gained significant racial differences in the unadjusted model and within the ADRC group in the adjusted model. The presence of 5 or more microbleeds gained significance such that the combined AA group had higher rates than the combined NHW group.
3.9. CDR at one year follow-up
Another measure examined only within the acute stroke participants was CDR at 1 year follow-up (median 383 days), for which 55 of the 81 S participants returned. As shown in eTable 3, those without follow up data were more likely to be male (69.6% vs 41.8%, p = 0.03), have an APOE4 allele (81.3% vs 34.6%, p = 0.003), and have a higher NIHSS (7.15 vs 4.58, p = 0.01). Individuals with CDR >0 at follow-up did not have statistically different levels of baseline amyloid than those with CDR 0 (mean cortical binding potential of 0.14 vs. 0.05, p = 0.14, eTable 4), indicating that preclinical AD did not predict post-stroke dementia. This finding was not impacted by stroke TOAST subtype. CDR at follow-up also did not differ significantly by race (57.7% of AA with stroke vs. 42.3% NHW with stroke had CDR >0, p = 0.35). The only significant predictors of a CDR >0 at the 1 year follow-up were a prior history of stroke (43.2% vs 7.41%, p = 0.008) and having 5+ microbleeds (p = 0.046). When all factors in eTable 4 were assessed in a single model, none significantly predicted CDR >0. When history of stroke and 5+ microbleeds were combined into a single model, only history of stroke remained significant (p = 0.01).
4. Discussion:
We examined how MRI measures of stroke and AD biomarkers differed by race and presence of acute stroke. We did not see evidence that preclinical AD is a risk factor for stroke or predicts post-stroke dementia, supporting the idea that vascular disease and amyloid pathology are separate disease mechanisms that each may lead to dementia. However, we found that AAs are more likely to have vascular pathology observable on MRI than NHWs. While outside our original aims, our finding that AAs were more likely to have 5 or more microbleeds suggests clinical trials are likely turning away larger numbers of AA volunteers due to this cutoff.
We did not see a higher risk of post-stroke dementia in African-Americans, in contradistinction to what previous studies have reported (Pendlebury and Rothwell, 2009, Desmond et al., 2000, Douiri et al., 2013, Levine et al., 2015, Stansbury et al., 2005), but this may be due to the sample size and localized recruitment (St. Louis) of this study. Our results also differed from a previous paper (Morris et al., 2019) which observed racial differences in hippocampal volume and used a sample independent from the acute stroke participants in this study. The previously observed lack of racial difference in amyloid PET, however, was replicated in this study (Morris et al., 2019). Previous studies have also strongly suggested that AAs are more likely to be APOE4 carriers (Manly and Mayeux, 2019, Green, 2002, Mayeda et al., 2016, Tang et al., 2001, Graff-Radford et al., 2016). The ADRC group was not representative of this, and the acute stroke group replicated only the racial difference in APOE4. As such, we were unable to test for racial difference mediated by these factors. Similarly, the acute stroke group that had a 1 year follow-up had a lower frequency of APOE4 than the original group, which may have impacted the lack of association we saw between preclinical AD and post-stroke dementia. Although we relate APOE4 and race in this paper, this is not meant to suggest that genetics as opposed to racism is the main driver of racial disparities in health (Boyd et al., 2020). Structural, interpersonal, and internalized racism can be attributed to all of the other risk factors we adjusted for as well as the racial differences that persisted even after this adjustment was made (Williams and Ovbiagele, 2020).
One limitation of this study is the limited statistical power driven by the small number of participants, though our enrollment matched or surpassed similar studies. Another limitation of this study is that we were unable to assess a cardiovascular risk score; all established cardiovascular risk scores require a cholesterol reading, which was not collected at time of the study. We instead examined HbA1c and blood pressure individually. Finally, differences by stroke status, especially in regards to demographic variables, may be due to differences in cohort selection. The acute stroke group came from a community sampling at two local hospitals, while the ADRC group includes volunteers from Alzheimer disease research studies. The low historical inclusion of AAs in research studies means they are particularly pursued as volunteers in the Knight ADRC, and so may better represent the general community than their NHW counterparts who may be self-selecting from a family history of dementia. This idea is supported by the unusually high rate of family history of dementia seen in the NHW ADRC group but not the AA ADRC group.
While the community sampling of stroke patients makes it more difficult to interpret differences by stroke status, it makes our racial comparisons within the acute stroke group more likely to generalize. This analysis is unique as the intersection of stroke and race in biomarkers of preclinical AD has not been previously explored. Our data supports the idea that preclinical AD does not increase the risk for a stroke nor increase the likelihood developing post-stroke dementia. Future studies should attempt to replicate this in a larger cohort. It would be particularly important to assess regional information of the vascular pathologies, which were not examined in this study but have been shown to impact risk of post-stroke dementia (Zhao et al., 2018). Future work should also examine proteinopathies other than amyloid which may be affecting the development of post-stroke dementia.
Acknowledgements
This study was supported by grants from the National Institute on Aging awarded to Dr Morris: P50AG005681, P01AG003991, P01AG026276, U19AG032438. Dr. Benzinger reports grants from the NIH during the conduct of the study; grants and non-financial support from Eli Lilly / Avid Radiopharmaceuticals outside the submitted work. Dr. Benzinger also reports the following relationships: Speakers' Bureau: Biogen, Eisai; clinical trial investigator: Biogen, Eisai, Roche, Lilly; support for radiopharmaceutical production: Avid Radiopharmaceuticals, Cerveau; unpaid consulting: Siemens, Eisai, Lilly.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102553.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Hooper M.W., Mitchell C., Marshall V.J. Understanding multilevel factors related to urban community trust in healthcare and research. Int. J. Environ. Res. Public Health. 2019;16(18) doi: 10.3390/ijerph16183280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., Isasi C.R., Jiménez M.C., Jordan L.C., Judd S.E., Lackland D., Lichtman J.H., Lisabeth L., Liu S., Longenecker C.T., Mackey R.H., Matsushita K., Mozaffarian D., Mussolino M.E., Nasir K., Neumar R.W., Palaniappan L., Pandey D.K., Thiagarajan R.R., Reeves M.J., Ritchey M., Rodriguez C.J., Roth G.A., Rosamond W.D., Sasson C., Towfighi A., Tsao C.W., Turner M.B., Virani S.S., Voeks J.H., Willey J.Z., Wilkins J.T., Wu J.HY., Alger H.M., Wong S.S., Muntner P. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10) doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Mayeux R. Ethnic Differences in Dementia and Alzheimer’ s Disease. In: Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. National Academies Press (US); 2019:1-32.
- Yang Q., Tong X., Schieb L., Vaughan A., Gillespie C., Wiltz J.L., King S.C., Odom E., Merritt R., Hong Y., George M.G. Vital Signs: Recent Trends in Stroke Death Rates — United States, 2000–2015. MMWR Morb. Mortal. Wkly. Rep. 2017;66(35):933–939. doi: 10.15585/mmwr.mm6635e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo-Fernandez R., Paul N.L.M., Gray A.M., Pendlebury S.T., Bull L.M., Welch S.J.V., Cuthbertson F.C., Rothwell P.M. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. 2013;44(10):2854–2861. doi: 10.1161/STROKEAHA.113.001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F., Yan L.i., Zhong B., Yang Z., Xie W. Progression of cognitive decline before and after incident stroke. Neurology. 2019;93(1):e20–e28. doi: 10.1212/WNL.0000000000007716. [DOI] [PubMed] [Google Scholar]
- Green R.C. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- Mayeda E.R., Glymour M.M., Quesenberry C.P., Whitmer R.A. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M.-X., Cross P., Andrews H., Jacobs D.M., Small S., Bell K., Merchant C., Lantigua R., Costa R., Stern Y., Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- Graff-Radford N.R., Besser L.M., Crook J.E., Kukull W.A., Dickson D.W. Neuropathologic differences by race from the National Alzheimer's Coordinating Center. Alzheimer's Dement. 2016;12(6):669–677. doi: 10.1016/j.jalz.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach T.G., Monsell S.E., Phillips L.E., Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J. Neuropathol. Exp. Neurol. 2012;71(4):266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.C., Schindler S.E., McCue L.M., Moulder K.L., Benzinger T.L.S., Cruchaga C., Fagan A.M., Grant E., Gordon B.A., Holtzman D.M., Xiong C. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76(3):264. doi: 10.1001/jamaneurol.2018.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenbaum G.G., Heyman A., Huber M.S., Woodbury M.A., Leiss J., Schmader K.E., Bohannon A., Trapp-Moen B. The prevalence and 3-year incidence of Dementia in older black and white community residents. J. Clin. Epidemiol. 1998;51(7):587–595. doi: 10.1016/s0895-4356(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick A.L., Kuller L.H, Ives D.G. Incidence and prevalence of dementia in the Cardiovascular Health Study. J. Am. Geriatr. Soc. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- Riudavets M.A., Rubio A., Cox C., Rudow G., Fowler D., Troncoso J.C. The prevalence of Alzheimer neuropathologic lesions is similar in blacks and whites. J. Neuropathol. Exp. Neurol. 2006;65(12):1143–1148. doi: 10.1097/01.jnen.0000248548.20799.a3. [DOI] [PubMed] [Google Scholar]
- Xiong C., Luo J., Coble D., Agboola F., Kukull W., Morris J.C. Complex interactions underlie racial disparity in the risk of developing Alzheimer's disease dementia. Alzheimer's Demen. 2020;16(4):589–597. doi: 10.1002/alz.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendlebury S.T., Rothwell P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- Chi N.-F., Chao S.-P., Huang L.-K. Plasma Amyloid Beta and Tau levels are predictors of post-stroke cognitive impairment: a Longitudinal Study. Front. Neurol. 2019;10 doi: 10.3389/fneur.2019.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Wong A., Wang Z., Liu W., Au L., Xiong Y., Chu W.W.C., Leung E.Y.L., Chen S., Lau C., Chan A.Y.Y., Lau A.Y.L., Fan F., Ip V., Soo Y., Leung T., Ho C.L., Wong L.K.S., Mok V.C.T. Risk factors for incident dementia after stroke and transient ischemic attack. Alzheimer's Demen. 2015;11(1):16–23. doi: 10.1016/j.jalz.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Liu W., Wong A., Au L., Yang J., Wang Z., Leung E.Y.L., Chen S., Ho C.L., Mok V.C.T. Influence of amyloid-β on cognitive decline after stroke/transient ischemic attack: three-year Longitudinal Study. Stroke. 2015;46(11):3074–3080. doi: 10.1161/STROKEAHA.115.010449. [DOI] [PubMed] [Google Scholar]
- Hagberg G., Ihle-Hansen H., Fure B., Thommessen B., Ihle-Hansen H., Øksengård A.R., Beyer M.K., Wyller T.B., Müller E.G., Pendlebury S.T., Selnes P. No evidence for amyloid pathology as a key mediator of neurodegeneration post-stroke - a seven-year follow-up study. BMC Neurol. 2020;20(1) doi: 10.1186/s12883-020-01753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel A., Cechetto D.F., Heiss W.-D., Hachinski V., Whitehead S.N. Amyloid burden, neuroinflammation, and links to cognitive decline after ischemic stroke. Stroke. 2014;45(9):2825–2829. doi: 10.1161/STROKEAHA.114.004285. [DOI] [PubMed] [Google Scholar]
- Gamaldo A., Moghekar A., Kilada S., Resnick S.M., Zonderman A.B., O'Brien R. Effect of a clinical stroke on the risk of dementia in a prospective cohort. Neurology. 2006;67(8):1363–1369. doi: 10.1212/01.wnl.0000240285.89067.3f. [DOI] [PubMed] [Google Scholar]
- Mok V, Liu W, Wong A. Detection of amyloid plaques in patients with post-stroke dementia. HKMJ. Published online February 24, 2016. Accessed December 6, 2020. https://www.hkmj.org/abstracts/v22%20Suppl%202n/S40.htm. [PubMed]
- Yasuno F., Kajimoto K., Ihara M., Taguchi A., Yamamoto A., Fukuda T., Kazui H., Iida H., Nagatsuka K. Amyloid β deposition in subcortical stroke patients and effects of educational achievement: A pilot study. Int. J. Geriatr. Psy. 2019;34(11):1651–1657. doi: 10.1002/gps.5178. [DOI] [PubMed] [Google Scholar]
- Bos I., Vos S.J.B., Schindler S.E., Hassenstab J., Xiong C., Grant E., Verhey F., Morris J.C., Visser P.J., Fagan A.M. Vascular risk factors are associated with longitudinal changes in cerebrospinal fluid tau markers and cognition in preclinical Alzheimer's disease. Alzheimer's Demen. 2019;15(9):1149–1159. doi: 10.1016/j.jalz.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman R.F., Mosley T.H., Knopman D.S., Hao Q., Wong D., Wagenknecht L.E., Hughes T.M., Qiao Y.e., Dearborn J., Wasserman B.A. Association of intracranial Atherosclerotic disease With brain β-amyloid deposition: secondary analysis of the ARIC Study. JAMA Neurol. 2020;77(3):350. doi: 10.1001/jamaneurol.2019.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett E.E., Gianattasio K.Z., Hughes T.M., Mosley T.H., Wong D.F., Gottesman R.F., Power M.C. The association between midlife lipid levels and late-life brain amyloid deposition. Neurobiol. Aging. 2020;92:73–74. doi: 10.1016/j.neurobiolaging.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz GA, L. Sacco R. National Institutes of Health Stroke Scale (NIHSS). In: Wiley StatsRef: Statistics Reference Online. John Wiley & Sons, Ltd; 2014. doi:10.1002/9781118445112.stat06823.
- Galvin J.E., Roe C.M., Powlishta K.K., Coats M.A., Muich S.J., Grant E., Miller J.P., Storandt M., Morris J.C. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr, Collins K.J., Dennison Himmelfarb C., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., MacLaughlin E.J., Muntner P., Ovbiagele B., Smith S.C., Jr, Spencer C.C., Stafford R.S., Taler S.J., Thomas R.J., Williams K.A., Sr, Williamson J.D., Wright J.T., Jr 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138(17) doi: 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- Fazekas F., Chawluk J.B., Alavi A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Neuroradiol. 1987;8(3):421–426. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P., Gaser C., Arsic M., Buck D., Förschler A., Berthele A., Hoshi M., Ilg R., Schmid V.J., Zimmer C., Hemmer B., Mühlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. NeuroImage. 2012;59(4):3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Head D., Parker J., Fotenos A.F., Marcus D., Morris J.C., Snyder A.Z. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Su Y, D’Angelo GM, Vlassenko AG, et al. Quantitative Analysis of PiB-PET with FreeSurfer ROIs. Chen K, ed. PLoS ONE. 2013;8(11):e73377. doi:10.1371/journal.pone.0073377. [DOI] [PMC free article] [PubMed]
- Su Y.i., Flores S., Wang G., Hornbeck R.C., Speidel B., Joseph‐Mathurin N., Vlassenko A.G., Gordon B.A., Koeppe R.A., Klunk W.E., Jack C.R., Jr., Farlow M.R., Salloway S., Snider B.J., Berman S.B., Roberson E.D., Brosch J., Jimenez‐Velazques I., Dyck C.H., Galasko D., Yuan S.H., Jayadev S., Honig L.S., Gauthier S., Hsiung G.-Y., Masellis M., Brooks W.S., Fulham M., Clarnette R., Masters C.L., Wallon D., Hannequin D., Dubois B., Pariente J., Sanchez‐Valle R., Mummery C., Ringman J.M., Bottlaender M., Klein G., Milosavljevic‐Ristic S., McDade E., Xiong C., Morris J.C., Bateman R.J., Benzinger T.L.S. Comparison of Pittsburgh compound B and florbetapir in cross‐sectional and longitudinal studies. Alzheimer's Demen. Diag. Assesst. Disease Monit. 2019;11(1):180–190. doi: 10.1016/j.dadm.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. J Psy Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Storandt M., Grant E.A., Miller J.P., Morris J.C. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67(3):467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Morris J.C. Revised criteria for mild cognitive impairment may compromise the diagnosis of Alzheimer Disease Dementia. Arch. Neurol. 2012;69(6):700–708. doi: 10.1001/archneurol.2011.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr Preclinical Alzheimer's disease: a valid concept. Lancet Neurol. 2020;19(1):31. doi: 10.1016/S1474-4422(19)30440-5. [DOI] [PubMed] [Google Scholar]
- Laird N.M., Ware J.H. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963. doi: 10.2307/2529876. [DOI] [PubMed] [Google Scholar]
- Liu C.-C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams H.P., Jr, Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., Marsh E.E., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Desmond D.W., Moroney J.T., Paik M.C., Sano M., Mohr J.P., Aboumatar S., Tseng C.-L., Chan S., Williams J.B.W., Remien R.H., Hauser W.A., Stern Y. Frequency and clinical determinants of dementia after ischemic stroke. Neurology. 2000;54(5):1124–1131. doi: 10.1212/wnl.54.5.1124. [DOI] [PubMed] [Google Scholar]
- Douiri A., Rudd A.G., Wolfe C.D.A. Prevalence of poststroke cognitive impairment: South London stroke register 1995–2010. Stroke. 2013;44(1):138–145. doi: 10.1161/STROKEAHA.112.670844. [DOI] [PubMed] [Google Scholar]
- Levine D.A., Kabeto M., Langa K.M., Lisabeth L.D., Rogers M.A.M., Galecki A.T. Does stroke contribute to racial differences in cognitive decline? Stroke. 2015;46(7):1897–1902. doi: 10.1161/STROKEAHA.114.008156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbury J.P., Jia H., Williams L.S., Vogel W.B., Duncan P.W. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- Boyd RW, Lindo EG, Weeks LD, McLemore MR. On Racism: A New Standard For Publishing On Racial Health Inequities | Health Affairs. Published online July 2, 2020. doi:10.1377/hblog20200630.939347.
- Williams O., Ovbiagele B. Stroking out while black—the complex role of racism. JAMA Neurol. 2020;77(11):1343. doi: 10.1001/jamaneurol.2020.3510. [DOI] [PubMed] [Google Scholar]
- Zhao L., Biesbroek J.M., Shi L., Liu W., Kuijf H.J., Chu W.WC., Abrigo J.M., Lee R.KL., Leung T.WH., Lau A.YL., Biessels G.J., Mok V., Wong A. Strategic infarct location for post-stroke cognitive impairment: a multivariate lesion-symptom mapping study. J. Cereb. Blood Flow Metab. 2018;38(8):1299–1311. doi: 10.1177/0271678X17728162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.