Significance
Neurons in the dorsomedial hypothalamus that express TrkB (DMHTrkB) have been implicated in the regulation of appetite; however, their relevance to energy expenditure is less clear. This study reveals that activation of DMHTrkB neurons potently induce thermogenesis and locomotor activity. Notably, increased energy expenditure mediated by DMHTrkB neurons is not accompanied by increased heart rate or blood pressure, which have traditionally complicated weight loss therapies that target thermogenesis. Furthermore, our results reveal separate populations of DMHTrkB neurons that form diverging neurocircuits to either the raphe pallidus or the paraventricular hypothalamus and preoptic area to independently regulate thermogenesis or feeding, respectively. These findings support a mechanism whereby DMHTrkB neurons act through multiple pathways to promote weight loss without adverse cardiovascular consequences.
Keywords: TrkB, neurocircuitry, dorsomedial hypothalamus, energy expenditure, feeding
Abstract
Mutations in the TrkB neurotrophin receptor lead to profound obesity in humans, and expression of TrkB in the dorsomedial hypothalamus (DMH) is critical for maintaining energy homeostasis. However, the functional implications of TrkB-fexpressing neurons in the DMH (DMHTrkB) on energy expenditure are unclear. Additionally, the neurocircuitry underlying the effect of DMHTrkB neurons on energy homeostasis has not been explored. In this study, we show that activation of DMHTrkB neurons leads to a robust increase in adaptive thermogenesis and energy expenditure without altering heart rate or blood pressure, while silencing DMHTrkB neurons impairs thermogenesis. Furthermore, we reveal neuroanatomically and functionally distinct populations of DMHTrkB neurons that regulate food intake or thermogenesis. Activation of DMHTrkB neurons projecting to the raphe pallidus (RPa) stimulates thermogenesis and increased energy expenditure, whereas DMHTrkB neurons that send collaterals to the paraventricular hypothalamus (PVH) and preoptic area (POA) inhibit feeding. Together, our findings provide evidence that DMHTrkB neuronal activity plays an important role in regulating energy expenditure and delineate distinct neurocircuits that underly the separate effects of DMHTrkB neuronal activity on food intake and thermogenesis.
Impairments in energy homeostasis resulting from the compound effects of overeating and sedentary lifestyles have led to a profound increase in the rate of obesity around the world (1). Therapeutic strategies aimed at combating obesity by increasing energy expenditure or decreasing appetite have commonly failed due to counterregulatory mechanisms (2) and adverse side effects on cardiovascular physiology (3–5). To achieve safe and sustained weight loss, it will be essential to understand the mechanisms that govern and coordinate discrete physiological processes that contribute to energy homeostasis.
Adaptive thermogenesis is the process by which energy is converted into heat and occurs primarily in brown adipose tissue (BAT) in response to environmental cues (6). BAT has a particularly high capacity for dissipating energy from fat and thus represents an important component of energy homeostasis. The dorsomedial hypothalamus (DMH) in the brain is centrally positioned in an established thermoregulatory neurocircuit, receiving inputs from the preoptic area (POA) (7–9) and sending excitatory projections to preautonomic neurons in the raphe pallidus (RPa) (10–13) that promote sympathetic activity in BAT, leading to increased thermogenesis. Direct chemical stimulation of the DMH (14) or activation of select populations of thermogenic DMH neurons (9, 11, 12, 15) leads to increased body temperature and energy expenditure but also significantly increases heart rate and blood pressure (12, 13, 15, 16). An inability to target increased sympathetic tone specifically in BAT without affecting other target tissues has greatly hampered strategies to treat obesity by targeting thermogenesis (4, 5).
In addition to its influence on energy expenditure, the DMH also represents an important brain region in the regulation of feeding (17–19). Lesioning studies support an orexigenic role for the DMH (17), which can promote food intake through inhibitory projections to either the paraventricular hypothalamus (PVH) (18) or the arcuate nucleus (ARC) (20). Despite these early findings, evidence has also emerged that demonstrates the importance of anorexigenic populations of DMH neurons (19, 21, 22). We previously established that the activity of DMH neurons expressing the neurotrophin receptor TrkB (DMHTrkB) is important for regulating feeding, showing that activation of DMHTrkB neurons suppresses feeding and that deletion of the TrkB-encoding Ntrk2 gene in the DMH results in hyperphagia and obesity (21). Furthermore, humans with mutations in the TrkB-encoding NTRK2 gene exhibit severe obesity and impaired thermoregulation (23). However, it is unclear whether activation of DMHTrkB neurons has a direct influence on adaptive thermogenesis. Additionally, the neurocircuitry through which DMHTrkB neurons govern feeding or energy expenditure is unknown.
Here, we demonstrate that DMHTrkB neuronal activity potently promotes energy expenditure by elevating thermogenesis and physical activity with a notable lack of influence on heart rate and blood pressure. We further reveal that DMHTrkB neurons send diverging projections to the RPa or the POA and PVH to differentially regulate energy expenditure and food intake, respectively.
Results
DMHTrkB Neurons Are Sensitive to Environmental Temperature.
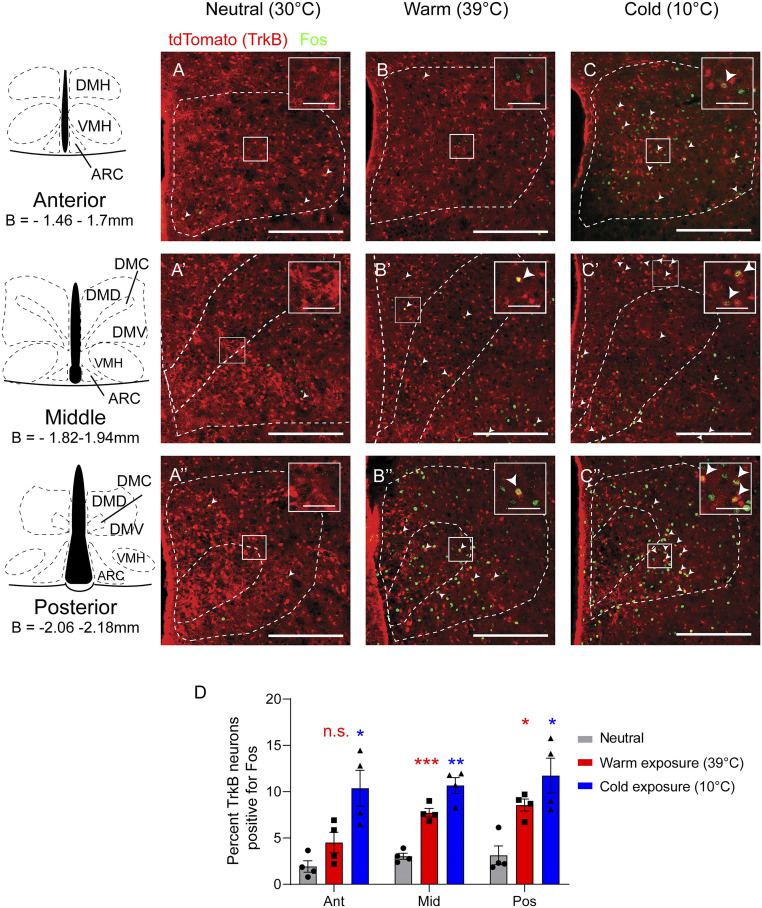
Since DMH neurons that respond to alterations in environmental temperature promote thermogenesis (9, 24), we first sought to determine whether DMHTrkB neurons are temperature sensitive. We crossed Ntrk2CreER/+ mice (25) with the Cre-dependent tdTomato reporter mouse line (Ai9) (26) to generate Ntrk2CreER/+;Ai9 mice in which TrkB neurons are genetically labeled with tdTomato upon translocation of Cre-ERT2 protein to the nucleus induced by tamoxifen administration. Following the labeling of TrkB neurons with tamoxifen, Ntrk2CreER/+;Ai9 mice were exposed to cold (10 °C) or warm (39 °C) temperatures, and immunostaining for Fos, a marker of activated neurons (27), was performed (Fig. 1 A–C). We found that exposure to cold activated 10.4 to 11.8% of TrkB-expressing neurons throughout the anterior to posterior extent of the DMH (Bregma −1.46 to −2.18 mm), while warmth only activated 7.7 to 8.6% of TrkB neurons, predominantly in the middle and posterior DMH (versus 1.9 to 3.1% activated DMHTrkB neurons under the 30 °C thermoneutral condition) (Fig. 1D). Our results suggest that DMHTrkB neurons respond to both increased and decreased ambient temperatures; however, statistical analysis indicates that there is no significant interaction between temperature sensitivity and anatomical location (repeated measures [RM] two-way ANOVA: interaction F(4,18) = 1.58 and P = 0.2225). Thus, cold- and warm-sensitive DMHTrkB neurons may not represent discrete populations.
Fig. 1.
DMHTrkB neurons are sensitive to changes in environmental temperature. Representative images of Fos staining (green) in the DMH of Ntrk2CreER/+;Ai9 reporter mice after exposure for 2 h to (A–A”) thermoneutral (30 °C), (B–B”) warm (39 °C), or (C–C”) cold (10 °C) temperatures. tdTomato (red) marks TrkB-expressing cells in reporter mice. (Scale bars, 250 µm; inset scale bars, 50 µm.) (D) Quantification of Fos induction in TrkB-expressing neurons in the anterior (Ant), middle (Mid), and posterior (Pos) DMH after mice were exposed to different temperatures. Two-way ANOVA: temperature, F(2, 9) = 18.68; P = 0.0006; n = 4 mice per condition; Dunnett’s posttest versus neutral; n.s. = not significant, *P < 0.05, **P < 0.01, and ***P < 0.001. Values represent mean ± SEM. DMH, dorsomedial hypothalamus; DMD, DMH dorsal division; DMC, DMH central division; DMV, DMH ventral division; VMH, ventromedial hypothalamus; ARC, arcuate nucleus; B, bregma.
Chemogenetic Manipulation of DMHTrkB Neurons Alters Thermogenesis and Physical Activity.
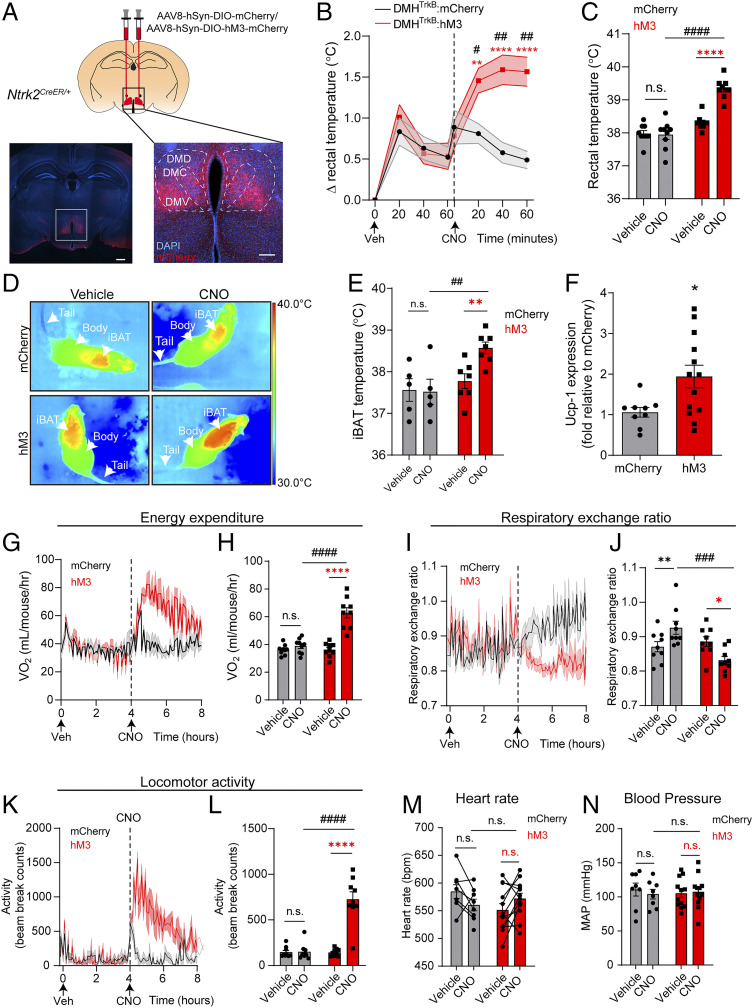
The observed increase in Fos+ neurons in response variation in temperature is consistent with a potential function for DMHTrkB neurons in regulating body temperature through thermogenesis. To test how DMHTrkB neuronal activity influences thermogenesis, we selectively expressed designer receptors exclusively activated by designer drugs (DREADD) in DMHTrkB neurons (28). We delivered the adeno-associated virus (AAV) expressing the excitatory DREADD, hM3Dq (AAV8-hSyn-DIO-hM3-mCherry), into the DMH of Ntrk2CreER/+ mice to selectively activate DMHTrkB neurons using the ligand clozapine N-oxide (CNO) (Fig. 2A and SI Appendix, Fig. S1A) (28). Mice expressing either control mCherry or hM3 in DMHTrkB neurons (DMHTrkB:mCherry or DMHTrkB:hM3 mice) were first acclimated to thermoneutrality (30 °C) prior to measuring the effects of CNO administration on body temperature and energy expenditure in order to mitigate the influence of basal thermogenesis that occurs under normal housing temperatures (22 °C) (29). Stimulation of DMHTrkB:hM3 mice with CNO resulted in an acute and robust increase in body temperature compared with either vehicle (Veh) stimulation in the same mice or CNO treatment in DMHTrkB:mCherry mice (Fig. 2 B and C).
Fig. 2.
DMHTrkB neuronal activity drives negative energy balance. (A) Schematic of bilateral stereotactic delivery of AAV expressing Cre-dependent hM3-mCherry (AAV8-hSyn-DIO-hM3-mCherry) or mCherry (AAV8-hSyn-DIO-mCherry) into the DMH of Ntrk2CreER/+ mice. Low-magnification scale bar, 500 µm; high-magnification scale bar, 200 µm. Mice housed at thermoneutrality expressing either mCherry (gray, n = 9) or hM3-mCherry (red, n = 9) in DMHTrkB neurons were treated with Veh or CNO during the light cycle. (B) Rectal temperature. Two-way RM ANOVA: mCherry versus hM3, F(1, 16) = 10.11, and P = 0.0058. (C) Average rectal temperatures 60 min after treatment. Two-way RM ANOVA: mCherry versus hM3, F(1, 16) = 36.55, and P < 0.0001. (D) Representative thermal images. (E) iBAT temperature of mice housed at thermoneutrality expressing mCherry (n = 5) or hM3 (n = 7) in DMHTrkB neurons 60 min postinjection with either vehicle or CNO. Two-way RM ANOVA: mCherry versus hM3, F(1, 10) = 6.350, and P = 0.0304. (F) Levels of Ucp1 mRNA in iBAT from mice expressing hM3 or mCherry in DMHTrkB neurons 2 h following treatment with CNO (n= 9 mCherry and n= 13 hM3; unpaired, two-tailed t test, and P = 0.0207). (G) Oxygen consumption (VO2) over 4 h after treatment with Veh (0 to 4 h) and CNO (4 to 8 h). Mixed-effects model: mCherry versus hM3 (post-CNO), F(1, 16) = 26.89, and P < 0.0001. (H) VO2 for the duration of the first 4 h after treatment. Two-way RM ANOVA: mCherry versus hM3, F(1, 16) = 17.64, and P = 0.0007. (I) RER over 4 h following treatment. Mixed-effects model: mCherry versus hM3 expression (post-CNO), F(1, 16) = 16.65, and P = 0.0009. (J) Average RER for the duration of the first 4 h after treatment. Two-way RM ANOVA: mCherry versus hM3 expression, F(1, 16) = 5.072, and P = 0.0387. (K) Locomotor activity over 4 h after treatment. Mixed-effects model: mCherry versus hM3 (post-CNO), F(1, 16) = 39.78, and P < 0.0001. (L) Average locomotor activity for the duration of the first 4 h after treatment. Two-way RM ANOVA: mCherry versus hM3, F(1, 16) = 30.86, and P < 0.0001. (M and N) Heart rate and mean arterial pressure (MAP) in mice expressing mCherry or hM3 in DMHTrkB neurons 1 h after vehicle or CNO treatment. Two-way RM ANOVA for heart rate: mCherry versus hM3 expression, F(1, 18) = 0.6447, and P = 0.4325. Two-way RM ANOVA for MAP: mCherry versus hM3 expression, F(1, 18) = 0. 01210, and P = 0.9136. n = 8 mCherry mice and 12 hM3 mice. Values represent mean ± SEM. (B, C, E, and G–N) Sidak posttest (red * for hM3-Veh versus hM3-CNO, black * for mCherry-Veh versus mCherry-CNO, and # for mCherry versus hM3 post-CNO: n.s. = not significant; * and #, P < 0.05; ** and ##, P < 0.01; *** and ###, P < 0.001; **** and ####, P < 0.0001).
Activation of thermogenesis in BAT represents the predominant mechanism for increasing body temperature in small mammals such as mice (29, 30). We asked whether the increase in core body temperature mediated by DMHTrkB neurons was due to activation of BAT. Due to the proximity of BAT to the surface of the skin, thermal imaging has emerged as a useful tool for evaluating thermogenesis (30). Therefore, we employed infrared thermography to further investigate the effect of stimulating DMHTrkB neurons on promoting thermogenesis locally in BAT (Fig. 2D). Following chemogenetic activation of DMHTrkB neurons, we observed an increase in the subcutaneous temperature of the region above interscapular BAT (iBAT; Fig. 2E). Furthermore, we found an increase in the transcript level of the thermogenic marker, UCP-1, in iBAT 2 h after CNO stimulation in mice expressing hM3Dq in DMHTrkB neurons but not mCherry (Fig. 2F). Thermogenic activity in BAT requires lipolysis, which is mediated in part by phosphorylation of hormone-sensitive lipase (HSL) to produce free fatty acids and glycerol (6). We observed that administration of CNO in mice expressing hM3, but not mCherry, in DMHTrkB neurons led to an increase in both levels of p-HSL (SI Appendix, Fig. S1B) and glycerol (SI Appendix, Fig. S1C) in iBAT.
Thermogenesis is a metabolically demanding process that demands significant energy expenditure. Consistent with this requirement, we also found that chemogenetic activation of DMHTrkB neurons drove a strong increase in energy expenditure (Fig. 2 G and H) while reducing respiratory exchange ratio (RER) (VCO2/VO2) (Fig. 2 I and J). A reduction in RER is indicative of a switch to preferential utilization of fat as a metabolic substrate.
In contrast to the reduction in RER that was observed after CNO treatment in DMHTrkB:hM3 mice, CNO treatment increased RER in control DMHTrkB:mCherry mice (Fig. 2 I and J). This nonspecific effect can likely be attributed to the conversion of CNO to clozapine in vivo (31). Clozapine is an atypical antipsychotic that has been documented as inhibiting lipolysis (32) and impairing thermogenesis (33). We found a significant interaction between the expression of DREADD virus and treatment with CNO (two-way RM ANOVA: interaction F(1,16) = 20.88; P = 0.0003), indicating that DMHTrkB neurons expressing hM3 with CNO can overcome the endogenous repressive effects of clozapine and further decrease RER beyond baseline levels. Together, our data indicate that DMHTrkB neurons promote lipolysis in BAT to fuel thermogenesis, which results in increased energy expenditure and a lower RER. An additional effect of DMHTrkB activation was a robust increase in locomotor activity, which could also contribute to the observed increase in total energy expenditure (Fig. 2 K and L).
Neurons in the DMH that stimulate thermogenesis through an increase in sympathetic nerve activity (SNA) also elicit increases in heart rate and blood pressure (12, 15, 16). Additionally, activation of SNA by cold or stress has been shown to promote subcutaneous vasoconstriction, resulting in a decrease in tail temperature in mice (8, 30, 34). Intriguingly, we found that stimulation of DMHTrkB neurons in mice did not alter heart rate (Fig. 2M) or mean arterial pressure (Fig. 2N). Additionally, we did not observe a change in tail temperature upon activation of DMHTrkB neurons (SI Appendix, Fig. S1D). These data support a mechanism through which DMHTkrB neurons elevate energy expenditure and body temperature by selectively activating thermogenesis in BAT.
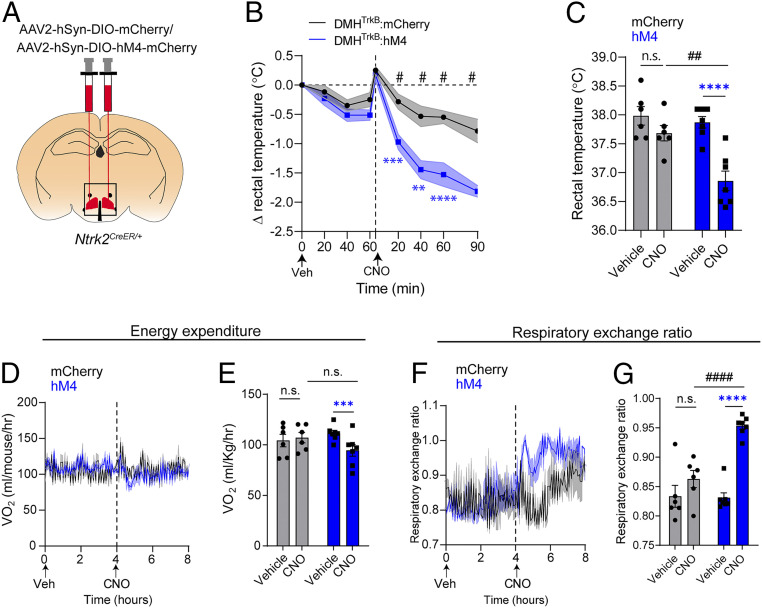
Next, we tested the necessity of DMHTrkB neuronal activity for adaptive thermogenesis induced by cold exposure. We inactivated DMHTrkB neurons by treating mice expressing the inhibitory chemogenetic receptor, hM4Di, in DMHTrkB neurons (Fig. 3A) with CNO. Silencing DMHTrkB neurons in cold-housed (10 °C) mice led to a reduction in body temperature (Fig. 3 B and C). We also observed a small decrease in energy expenditure in mice expressing hM4 in DMHTrkB neurons following treatment with CNO compared to vehicle (two-way RM ANOVA: Veh versus CNO, F(1, 11) = 8.574, and P = 0.0137); however, this effect was not significant when compared to oxygen consumption in control CNO-treated mice expressing mCherry in DMHTrkB neurons (two-way RM ANOVA: mCherry versus hM4, F(1, 11) = 0.1502, and P = 0.7058) (Fig. 3E). We also observed a significant increase in RER (Fig. 3 F and G), which could be attributed either to an increase in feeding that results from silencing DMHTrkB neurons during the day (21) or inhibition of lipolysis that might be expected to occur concomitantly with the inhibition of BAT activity. Together, these findings support a necessary role for DMHTrkB neuron activity in the maintenance of body temperature in response to cold exposure.
Fig. 3.
DMHTrkB neurons are necessary for cold-induced thermogenesis. (A) Schematic of bilateral stereotactic delivery of AAV expressing Cre-dependent inhibitory hM4-mCherry (AAV2-hSyn-DIO-hM4-mCherry) or mCherry (AAV2-hSyn-DIO-mCherry) into the DMH of Ntrk2CreER/+. (B–G) Mice housed at 10 °C expressing either mCherry (gray, n = 6) or hM4-mCherry (blue, n = 7) in DMHTrkB neurons were treated with vehicle (Veh) or CNO during the light cycle. (B) Rectal temperature of mice following treatment with Veh (0 to 60 min) or CNO (0 to 90 min). Two-way RM ANOVA: mCherry versus hM4, F(1, 11) = 20.40, and P = 0.0009. (C) Average rectal temperatures 60 min after treatment. Two-way RM ANOVA: mCherry versus hM4, F(1, 11) = 7.577, and P = 0.0188. (D) Oxygen consumption over 4 h after treatment. Mixed-effects model: mCherry versus hM4 expression (post-CNO), F(1, 11) = 3.066, and P = 0.1078. (E) Average VO2 for the duration of the first 4 h after treatment. Two-way RM ANOVA: mCherry versus hM4 expression, F(1, 11) = 0.1502, and P = 0.7058). (F) RER over 4 h after treatment. Mixed-effects model: mCherry versus hM4 (post-CNO), F(1, 11) = 39.28, and P < 0.0001. (G) RER for the duration of the first 4 h after treatment. Two-way RM ANOVA: mCherry versus hM4, F(1, 11) = 8.633, and P = 0.0135. Values represent mean ± SEM (B–G). Sidak posttest (blue * for hM4-Veh versus hM4-CNO, black * for mCherry-Veh versus mCherry-CNO, and # for mCherry versus hM4 post-CNO: n.s. = not significant; #P < 0.05; ** and ##, P < 0.01; ***P < 0.001; **** and ####, P < 0.0001).
Separate populations of sympathetic neurons have been shown to innervate either white adipose tissue or BAT, indicating selectivity in sympathetic output at the level of the peripheral nervous system; however, the mechanisms governing this selectivity have not been defined (35). Since activation of DMHTrkB neurons can induce thermogenesis without affecting heart rate, we hypothesized that these neurons may form a selective neurocircuit with sympathetic nerves that innervate BAT, but not the heart. To test this possibility, we performed polysynaptic anterograde tracing in DMHTrkB neurons using Cre-dependent HSV129ΔTK-tdTomato (SI Appendix, Fig. S1E). Four days after infection, we observed connections between DMHTrkB neurons and iBAT as evident by dense tdTomato labeling in UCP-1 expressing iBAT (SI Appendix, Fig. S1F). In contrast, we did not see tdTomato labeling in the heart or the sympathetic fibers innervating the heart (SI Appendix, Fig. S1G). Our findings suggest that DMHTrkB neurons form selective neurocircuits with sympathetic neurons that innervate BAT tissue.
A DMHTrkB → RPa Neurocircuit Regulates Thermogenesis and Energy Expenditure.
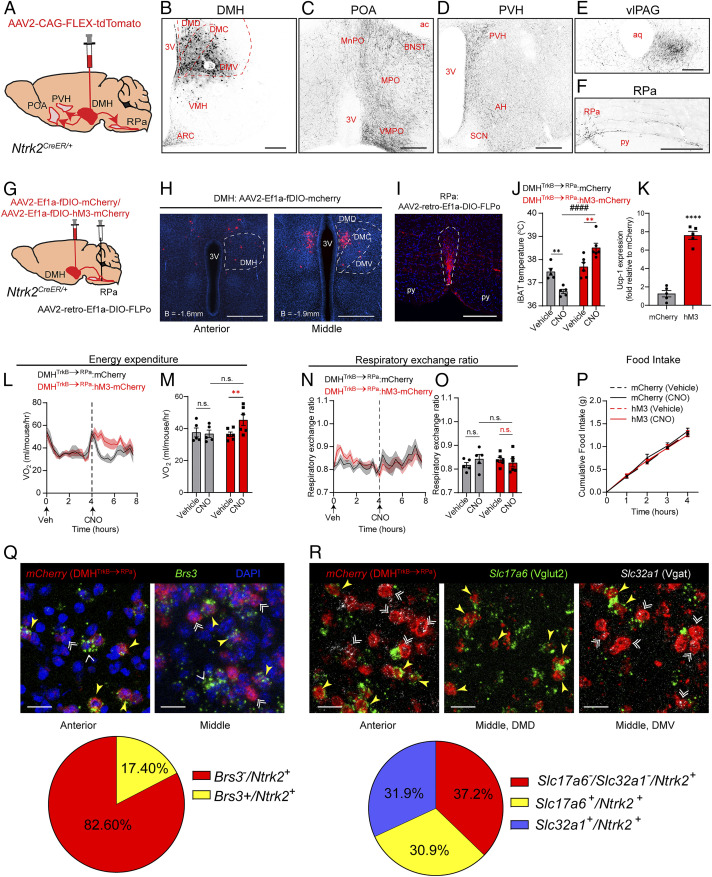
Activation of DMHTrkB neurons promotes negative energy balance by both inhibiting feeding (21) and increasing energy expenditure (Fig. 2). We performed anterograde tracing of DMHTrkB neurons using AAV expressing Cre-dependent tdTomato (AAV2-CAG-FLEX-tdTomato) to determine the efferent targets responsible for mediating DMHTrkB neuron functions (Fig. 4A). Following viral delivery to the DMH of Ntrk2CreER/+ mice and induction with tamoxifen, DMHTrkB neurons at the injection site were labeled with tdTomato (Fig. 4B), and their projections were observed in multiple brain regions, including the POA (Fig. 4C), PVH (Fig. 4D), ventrolateral periaqueductal gray matter (vlPAG) (Fig. 4E), and RPa (Fig. 4F). We also observed projections to the bed nucleus of the stria terminalis (BNST), anterior hypothalamus (AH), and ARC. Together, these data indicate that DMHTrkB neurons have a broad projection field which may underly their functional diversity.
Fig. 4.
A DMHTrkB → RPa neurocircuit regulates energy expenditure and body temperature. (A) Diagram of anterograde tracing of DMHTrkB neurons in Ntrk2CreER/+ mice unilaterally injected with AAV2-CAG-FLEX-TdTomato. (B) Injection site. (C–F) Projection targets: (C) POA including the median preoptic area, MPO, and VMPO and the BNST; (D) PVH and AH; (E) vlPAG; and (F) RPa. (Scale bars, 200 µm.) (G) Schematic of stereotactic delivery of retrograde AAV expressing Cre-dependent FLPo (AAV2-retro-Ef1a-DIO-FLPo) and AAV2-CMV-GFP to the RPa, and FLP-dependent (fDIO) mCherry or hM3-mCherry expressing virus to the DMH in Ntrk2CreER/+ mice. (H) Expression of mCherry (red) in DMHTrkB→RPa neurons. (Scale bar, 500 µm.) (I) mCherry-labeled DMHTrkB→RPa terminals are detected in the RPa (outlined). (Scale bar, 200 µm.) (J–O) Mice housed at thermoneutrality and expressing hM3-mCherry (red, n = 6) or mCherry (gray, n = 5) in DMHTrkB→RPa neurons were treated with Veh or CNO during the light cycle. (J) BAT temperature 60 min after Veh or CNO treatment. Two-way RM ANOVA: mCherry versus hM3, F(1, 9) = 29.24, and P = 0.0004. (K) Relative levels of Ucp1 mRNA in BAT 2 h post-CNO treatment. Unpaired, two-tailed t test, P < 0.0001. (L) Oxygen consumption after treatment. Two-way RM ANOVA: mCherry versus hM3 (post-CNO), F(1, 9) = 4.695, and P = 0.0584. (M) Average oxygen consumption for the duration of the first 4 h after treatment. Two-way RM ANOVA: mCherry versus hM3, F(1, 9) = 1.227, and P = 0.2967. (N) RER after treatment with vehicle or CNO. Two-way RM ANOVA: mCherry versus hM3 (post-CNO), F(1, 9) = 0.2985, and P = 0.5985. (O) Average RER for the duration of the first 4 h after treatment. Two-way RM ANOVA: mCherry versus hM3, F(1, 9) = 0.03257, and P = 0.8608. (P) Nocturnal food intake of mice expressing mCherry or hM3 in DMHTrkB→RPa neurons after treatment with vehicle or CNO. Two-way RM ANOVA: mCherry versus hM3, F(1, 16) = 0.02664, and P = 0.8724. (Q) Detection of Brs3 (green) and mCherry (red) expression by ISH in the anterior (Left) and middle (Right) DMH of DMHTrkB→RPa:mCherry expressing mice. Yellow arrowheads indicate Brs3+ DMHTrkB→RPa neurons. White double arrowheads indicate Brs3− DMHTrkB→RPa neurons. Single white arrowheads indicate Brs3+ mCherry−. (Scale bars, 20 µm.) (R) In situ detection of Slc17a6 (Vglut2, green) and Slc32a1 (Vgat, white) in the anterior and middle-dorsal and middle-ventral DMH of DMHTrkB→RPa:mCherry mice. Yellow arrowheads indicate Vglut+ DMHTrkB→RPa neurons. Double white arrowheads indicate Vgat+ DMHTrkB→RPa neurons. (Scale bars, 20 µm.) (Q and R) Representative images are from n = 3 animals. Values represent mean ± SEM. Sidak posttest (red * for hM3-Veh versus hM3-CNO, black * for mCherry-Veh versus mCherry-CNO, and # for mCherry versus hM3 post CNO; n.s. = not significant; **P < 0.01; **** and ####, P < 0.0001). 3V, third ventricle; ac, anterior commissure; py, pyramid; SCN, suprachiasmatic nucleus.
Sympathetic premotor neurons in the RPa are an established target of DMH neurons that activate thermogenesis (12, 13, 36). Since we observed that DMHTrkB neurons send projections to the RPa, we first reasoned that a DMHTrkB → RPa neurocircuit might be important for regulating thermogenesis and energy expenditure. To test this possibility, we developed a viral strategy to express the excitatory DREADD, hM3, in DMHTrkB neurons in a projection specific manner. We simultaneously delivered retrograde AAV expressing Cre-dependent codon-optimized flippase (FLPo) (AAV2-retro-Ef1a-DIO-FLPo) to the RPa and AAV expressing FLPo-dependent hM3-mCherry or control mCherry (AAV2-Ef1a-fDIO-hM3-mCherry or AAV2-Ef1a-fDIO-mCherry) to the DMH in Ntrk2CreER/+ mice (Fig. 4G). TrkB-expressing neurons in the DMH that project to the RPa (DMHTrkB→RPa neurons) become infected with AAV2-retro-Ef1a-DIO-FLPo, and Cre expressed in TrkB neurons in Ntrk2CreER/+ mice can induce expression of FLP recombinase, allowing for subsequent expression of hM3-mCherry or mCherry.
We confirmed expression of mCherry in neurons in the DMH (Fig. 4H) and in axon terminals in the RPa (Fig. 4I). When AAV2-Ef1a-fDIO-mCherry/hM3-mCherry were injected into the DMH simultaneously with AAV2-retro-Ef1a-DIO-FLPo into the RPa of wild-type mice that lack Cre expression in TrkB-expressing neurons, we saw no mCherry signal (SI Appendix, Fig. S2 A–C). Additionally, injection of AAV2-Ef1a-fDIO-mCherry/hM3-mCherry alone into the DMH of Ntrk2CreER/+ mice without a second injection of AAV2-retro-Ef1a-DIO-FLPo into the RPa was insufficient to induce expression of mCherry (SI Appendix, Fig. S2 D–F). Treatment with CNO did not result in increased Fos expression in DMHTrkB→RPa neurons expressing mCherry compared with hM3 (SI Appendix, Fig. S2 G and H). These results support our ability to target specific DMHTrkB neurons for chemogenetic activation based on their projection target.
We then tested the effect of activating the DMHTrkB → RPa neurocircuit on thermogenesis and energy expenditure. Treatment with CNO, but not vehicle, increased iBAT temperature (Fig. 4J) and expression of Ucp1 (Fig. 4K) in Ntrk2CreER/+ mice expressing hM3 in DMHTrkB→RPa neurons compared with mCherry expressing control mice. Treatment of control mCherry mice with CNO led to a decrease in iBAT temperature, which we hypothesize is attributed to the nonspecific antithermogenic effect of clozapine. Consistent with the activation of thermogenesis that was observed upon stimulation of DMHTrkB→RPa neurons, we saw a trend toward increased oxygen consumption in mice treated with CNO and expressing hM3 compared with mice expressing mCherry in DMHTrkB→RPa neurons (P = 0.0584) (Fig. 4L). Furthermore, treatment with CNO resulted in an increase in oxygen consumption compared with vehicle treatment in mice expressing hM3 in DMHTrkB→RPa neurons (Fig. 4M). In contrast to the effect of activating all DMHTrkB neurons, stimulation of DMHTrkB→RPa neurons did not alter RER (Fig. 4 N and O) or physical activity (SI Appendix, Fig. S3 A and B). We previously showed that activation of DMHTrkB neurons suppresses nocturnal feeding (21); however, stimulation of a DMHTrkB → RPa circuit had no effect on food intake (Fig. 4P). Although activation of DMHTrkB→RPa neurons increased thermogenesis in BAT to a similar level as previously observed, the DMHTrkB → RPa neurocircuit was not sufficient to drive total energy expenditure to the same extent or duration as that observed for activation of all DMHTrkB neurons (Figs. 2G and 4L). Nevertheless, DMHTrkB → RPa neurons contribute to the net negative effect of activating DMHTrkB neurons on energy balance without contributing to alterations in fat metabolism or feeding.
Glutamatergic neurons in the anterior and dorsal compartments of the DMH that express bombesin receptor subtype 3 (Brs3) or the leptin receptor (Lepr) have been shown to project to the RPa and promote thermogenesis (15, 24, 37). We previously showed that there is very little overlap between Lepr and TrkB expression in the DMH (21), and, in contrast to DMHTrkB neurons that do not affect cardiovascular function, both DMHBrs3 and DMHLepR neurons influence heart rate in addition to body temperature (15, 16). To determine whether there is any overlap between RPa-projecting DMHTrkB and DMHBrs3 neurons, we performed fluorescent in situ hybridization (ISH) for Brs3 and mCherry expression in the DMH of DMHTrkB→RPa:mCherry mice (Fig. 4Q). Although we observed partial overlap between mCherry (representing DMHTrkB→RPa neurons) and Brs3 expression, not all Brs3-expressing neurons expressed mCherry. Conversely, we observed many mCherry-positive, Brs3-negative neurons, indicating that DMHBrs3 and DMHTrkB→RPa neurons are not identical populations. We also analyzed the expression of Slc17a6 (Vglut2) and Slc32a1 (Vgat) in DMHTrkB→RPa neurons, which are markers of excitatory and inhibitory neurotransmission, respectively (Fig. 4R). Other DMH → RPa neurons have been characterized as predominantly glutamatergic (Vglut2 expressing) (12). Surprisingly, we found that DMHTrkB→RPa neurons are a heterogeneous population consisting of both Vglut2- and Vgat-expressing neurons, as well as some neurons that do not appear to express either neurotransmitter at levels detectable by ISH.
DMHTrkB Neurons Collateralize to the PVH and POA to Regulate Feeding and Metabolism.
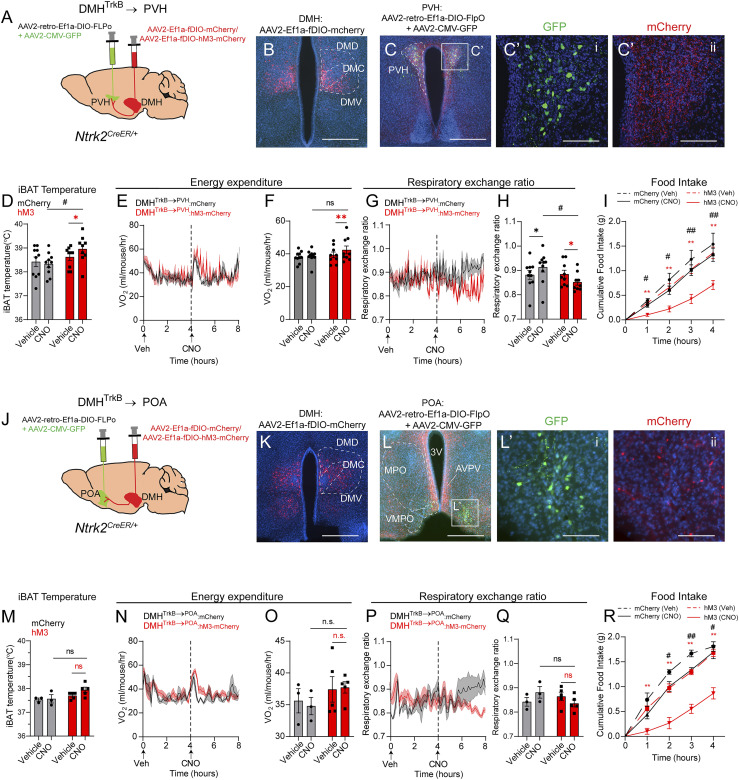
Activation of DMHTrkB neurons drives high levels of oxygen consumption concurrent with stimulation of lipolysis (Fig. 2 and SI Appendix, Fig. S1) and inhibition of homeostatic feeding (21). Since DMHTrkB→RPa neurons do not influence RER or feeding, we aimed to determine the identity of a DMHTrkB neurocircuit that regulates these other components of energy balance. Neurons in the PVH that express brain-derived neurotrophic factor (BDNF—a high-affinity TrkB ligand) have been implicated in regulating food intake, energy expenditure, and locomotor activity; however, the identity of their presynaptic partners is unknown (38). Our anterograde tracing results indicate that the PVH receives projections from DMHTrkB neurons (Fig. 4D); thus, we hypothesized that a DMHTrkB→PVH pathway might account for the effect of DMHTrkB neuronal activity on feeding. Using the same projection-specific viral strategy described earlier, we expressed mCherry or hM3-mCherry in PVH-projecting DMHTrkB neurons (Fig. 5A). DMHTrkB→PVH:mCherry neurons are mainly present in the middle-to-posterior ventral DMH (DMV) and the medial-ventral part of the central DMH (DMC) (Fig. 5B). We confirmed proper targeting of the PVH by coinjection of AAV-GFP with AAV-retro-DIO-FLPo. PVH neurons at the injection site expressed GFP and received dense innervation by mCherry+ DMHTrkB→PVH fibers (Fig. 5 C and C’). These results indicate successful targeting of a DMHTrkB → PVH neurocircuit for chemogenetic manipulation.
Fig. 5.
DMHTrkB neurons project to the PVH and POA to regulate feeding and metabolism. (A) Strategy for projection-specific targeting of mCherry or hM3-mCherry expression in DMHTrkB neurons. Retrograde AAV expressing Cre-dependent FLPo (AAV2-retro-Ef1a-DIO-FLPo) and AAV-GFP are delivered to the PVH, while Flp-dependent mCherry or hM3-mCherry–expressing virus is injected into the DMH in Ntrk2CreER/+ mice. (B) Expression of mCherry in DMHTrkB→PVH neurons. (Scale bar, 500 µm.) (C and C’) Expression of GFP marking the injection site of retrograde AAV2-retro-Ef1a-DIO-FLPo in the PVH (C’, i) and mCherry in axonal terminals of DMHTrkB neurons in the PVH (C’, ii). Scale bars, 500 µm in B and 100 µm in C’. (D–H) Mice housed at thermoneutrality and expressing mCherry (gray, n = 10) or hM3-mCherry (red, n = 9) in DMHTrkB→PVH neurons were treated with Veh and then CNO during the light cycle. (D) iBAT temperature. Two-way RM ANOVA: mCherry versus hM3, F(1, 17) = 3.178, and P = 0.0925. (E) Oxygen consumption. Mixed-effects model: mCherry versus hM3 (post-CNO), F(1, 17) = 0.4561, and P = 0.5085. (F) Average oxygen consumption for the duration of 4 h following treatment. Two-way RM ANOVA: mCherry versus hM3, F(1, 17) = 1.252, and P = 0.2787. (G) RER. Mixed-effects model: mCherry versus hM3 (post-CNO), F(1, 17) = 5.311, and P = 0.0341. (H) Average RER for the duration of 4 h following treatment. Two-way RM ANOVA: mCherry versus hM3, F(1, 17) = 1.614, and P = 0.008168. (I) Nocturnal food intake of mice expressing mCherry or hM3 in DMHTrkB→PVH neurons postinjection of vehicle or CNO. Two-way RM ANOVA: mCherry versus hM3, F(1, 17) = 4.913, and P = 0.0406. (J) Schematic for projection-specific targeting of DMHTrkB→POA neurons. (K) Expression of mCherry in DMHTrkB→POA neurons. (Scale bar, 500 µm.) (L and L’) Expression of GFP marking the injection site of AAV2-retro-Ef1a-DIO-FLPo in the POA and mCherry in axonal terminals of DMHTrkB neurons in the POA. (Scale bars, 500 µm in L and 100 µm in L’. (M–O) Mice housed at thermoneutrality and expressing mCherry (gray, n = 3) or hM3-mCherry (red, n = 5) in DMHTrkB→POA neurons were treated with Veh and then CNO during the light cycle. (M) iBAT temperature. Two-way RM ANOVA: mCherry versus hM3, F(1, 6) = 5.031, and P = 0.0661. (N) Oxygen consumption. Two-way RM ANOVA: mCherry versus hM3 (post-CNO), F(1, 6) = 3.557, and P = 0.1083. (O) Average oxygen consumption for the duration of 4 h following treatment. Two-way RM ANOVA: mCherry versus hM3, F(1, 6) = 1.475, and P = 0.2701). (P) RER. Two-way RM ANOVA: mCherry versus hM3 (post-CNO), F(1, 6) = 2.985, and P = 0.1348. (Q) Average respiratory ratio for the duration of 4 h following treatment. Two-way RM ANOVA; mCherry versus hM3, F(1, 6) = 0.2390, and P = 0.6423. (R) Nocturnal food intake of mice expressing mCherry or hM3 in DMHTrkB→POA neurons. Two-way RM ANOVA: mCherry versus hM3, F(1, 6) = 24.98, and P = 0.0025. Values represent mean ± SEM. Sidak posttest (* for Veh versus CNO, # for mCherry versus hM3; * and #, P < 0.05; ** and ##, P < 0.01; n.s. = not significant).
Activation of DMHTrkB→PVH neurons increased iBAT temperature 1 h after treatment with CNO (Fig. 5D); however, only a small increase in energy expenditure, mainly restricted to the first hour, was evident (Fig. 5 E and F). Similar to DMHTrkB→RPa neurons, the DMHTrkB → PVH neurocircuit was not sufficient to drive oxygen consumption to the same extent as activation of all DMHTrkB neurons, nor did it have any influence on physical activity (SI Appendix, Fig. S3 C and D). Stimulation of the DMHTrkB → PVH circuit lowers RER despite the nonspecific effect of CNO which increases RER in control DMHTrkB→PVH:mCherry animals (Fig. 5 G and H) and inhibits nocturnal feeding in mice (Fig. 5I). Thus, DMHTrkB→PVH neurons contribute to negative energy balance predominantly by regulating nutrient consumption and fatty acid utilization, but not thermogenesis.
Analysis of Slc17a6 (Vglut2) and Slc32a1 (Vgat) expression in DMHTrkB→PVH neurons labeled with mCherry revealed a slight bias in the number of inhibitory Vgat-expressing DMHTrkB→PVH neurons (40%) over those that express Vglut2 (24.6%) (SI Appendix, Fig. S5 A–D). However, similar to DMHTrkB→RPa neurons, we found many DMHTrkB→PVH neurons that did not appear to express either Vglut2 or Vgat (SI Appendix, Fig. S5 A–D).
BDNF-expressing neurons that project to the DMH are also located in the POA (POABDNF) (8). However, as POABDNF neurons negatively regulate body temperature by inhibiting BAT activity, DMHTrkB neurons projecting to the POA could contribute to increased energy expenditure by inhibiting POABDNF neurons. Thus, we targeted expression of mCherry or hM3-mCherry to DMHTrkB→POA neurons (Fig. 5 J and K) to evaluate the function of a DMHTrkB → POA neurocircuit on energy expenditure. Expression of GFP at the injection site was predominantly restricted to the ventromedial preoptic area (VMPO) (Fig. 5 L and L’, i), where warm-sensitive BDNF-expressing neurons have previously been reported (8). While mCherry+ DMHTrkB→POA axon terminals were evident in the VMPO (Fig. 5 L and L’, ii), we also noted collateralization to the medial preoptic area (MPO) as well as the anteroventral periventricular nucleus (Fig. 5L). These data indicate that DMHTrkB→POA neurons have a large projection field within the POA, which is likely not restricted to POABDNF neurons. Activation of DMHTrkB→POA neurons expressing hM3-mCherry with CNO had no significant effect on BAT temperature (Fig. 5M), oxygen consumption (Fig. 5 N and O), RER (Fig. 5 P and Q), or physical activity (SI Appendix, Fig. S3 E and F). However, we noted a slight trend toward a lower RER in the fourth hour following CNO treatment in mice expressing hM3-mCherry compared with mCherry (Fig. 5P). As was observed for the activation of the DMHTrkB → PVH circuit, we saw that treatment with CNO inhibited nocturnal feeding in mice expressing hM3 compared with mCherry expression in DMHTrkB→POA neurons or compared with vehicle treatment (Fig. 5R). Together, these results demonstrate that DMHTrkB→POA and DMHTrkB→PVH neurons have a redundant function in the regulation of food intake.
DMHTrkB→PVH and DMHTrkB→POA neurons also appeared to have a similar anatomical distribution within the DMH (Fig. 5 B and K). These results raise the possibility that a single population of DMHTrkB neurons sends projections to both the PVH and the POA. Indeed, we observed that DMHTrkB→PVH neurons send collaterals to the POA, including both the MPO and VMPO, and to the BNST (SI Appendix, Fig. S4 A–C and C′), but not the RPa (SI Appendix, Fig. S4D). Similarly, we observed DMHTrkB→POA collaterals in the PVH (SI Appendix, Fig. S4 E–G and G′), but DMHTrkB→RPa collaterals were not apparent in either the POA (SI Appendix, Fig. S4 H–J) or the PVH (SI Appendix, Fig. S4K).
To further confirm the overlap between DMHTrkB→PVH and DMHTrkB→POA populations, we simultaneously injected cholera toxin B subunit (CTB) labeled with two different fluorophores, Alexa Fluor 488 or 647 (CTB-488 or CTB-647), into the PVH and the POA of Ntrk2CreER/+;Ai9/+ mice induced with tamoxifen (SI Appendix, Fig. S4 L–N). Neurons in the DMH that project to the PVH were evident by labeling with CTB-488, while those that project to the POA were labeled with CTB-647 (SI Appendix, Fig. S4O), and TrkB-expressing neurons were labeled with tdTomato (SI Appendix, Fig. S4P). PVH- and POA-projecting DMH neurons were concentrated in the ventral part of the middle-to-posterior DMH. Upon further inspection, we found that most DMH neurons that send collateral projections to both the POA and PVH express TrkB (SI Appendix, Fig. S4 Q–T, arrowheads), supporting the existence of a population of DMHTrkB neurons that send projections to both the POA and the PVH.
Discussion
Expression of TrkB in the brain is essential for maintaining normal body weight in both humans and rodents (23, 39, 40). We have previously reported that TrkB-expressing neurons in the DMH (21) and the PVH (41) are essential for regulating food intake, and DMHTrkB neuronal activity modulates homeostatic feeding. Although loss of TrkB signaling in the DMH also leads to reduced oxygen consumption and physical activity (21), these consequences could be secondary to excessive weight gain. In the present study, we establish that DMHTrkB neuronal activity has a direct influence on energy expenditure and body temperature that does not extend to influence components of cardiovascular physiology, which are typically affected by other DMH neuron populations. Furthermore, we reveal diverging DMHTrkB neurocircuits are responsible for regulating adaptive thermogenesis, physical activity, fat metabolism, and appetite. Together, our findings support an integral role for DMHTrkB neurons in coordinating multiple arms of energy balance and provide insight into the neural mechanisms underlying their functions, which may be expected to inform efforts to target these neurons for the development of antiobesity therapies.
The DMH is recognized as a brain center that promotes energy expenditure in part through the activation of thermogenesis in BAT (14, 42). Interestingly, we observed that DMHTrkB neurons can be activated by either warm or cold temperatures. However, statistical analysis revealed that there is no interaction between temperature and location, indicating that there is not a region-specific sensitivity of DMHTrkB neurons to a particular temperature. Furthermore, previous studies have shown that stress can activate separate populations of neurons in the dorsal and central/ventral DMH compartments that project to the RPa and the PVH, respectively (12). Given the extreme temperatures that mice were exposed to during this experiment, it is reasonable to assume that some Fos+ DMHTrkB neurons we detected in our experiment are nonspecifically responsive to stress rather than temperature. Thus, we cannot conclude that DMHTrkB neurons can be definitively divided into warm- and cold-sensitive populations.
Neurons located in the anterior and dorsal compartments of the DMH that project to the RPa mediate increased sympathetic tone in BAT, leading to thermogenesis (12, 15, 43). Consistent with these reports, we found that activation of DMHTrkB→RPa neurons induces heat production and Ucp1 expression in BAT. However, DMHTrkB→RPa neurons are more broadly distributed than previously characterized DMH→RPa neurons in the dorsal DMH. Although cell body fluorescent signals in DMHTrkB→RPa:mCherry mice appear concentrated in the dorsal DMH, detection of mCherry expression by ISH reveals numerous DMHTrkB→RPa neurons in the central and ventral compartments of the DMH. Additionally, activation of DMHTrkB neurons has no obvious effect on cardiovascular physiology, which is in contrast to other DMH → RPa sympathoexcitatory projections that lead to increased heart rate and blood pressure (12, 15, 16). The specificity of the DMHTrkB→RPa neuron function may be reflective of the selectivity of DMHTrkB neurocircuitry, which we see forms connections with BAT, but not the heart; however, it is unclear how autonomic selectivity for peripheral tissues is retained at the level of the RPa.
Previously described populations of RPa-projecting DMH neurons have predominantly been glutamatergic. Our ISH results reveal that DMHTrkB→RPa neurons are heterogeneous, comprising almost equally of excitatory and inhibitory populations as well as those that do not appear to express either Vglut2 or Vgat. Inhibitory input to RPa neurons has only been implicated in the negative regulation of thermogenesis and has not been described to increase body temperature (11). Thus, we speculate that subsets of DMHTrkB→RPa neurons might target different populations of RPa neurons and act in concert to promote thermogenesis while inhibiting increases in heart rate or blood pressure. Future investigations aimed at characterizing the molecular diversity of DMHTrkB neurons by single-cell RNA sequencing methods to find more specific markers that would distinguish the Vgat, Vglut2, and other populations are necessary. Dissecting the neurocircuitry from the DMH to targets in the RPa will provide useful insight into the mechanisms of functional autonomic selectivity that hold therapeutic value for treating cardiovascular disease.
Food intake represents the positive arm of energy balance, which shares equal importance with energy expenditure for regulating body weight. Our previous studies indicate that TrkB expression in the DMH and DMHTrkB neuron activity are necessary for regulating homeostatic feeding that is dependent on the time of day (21). In this study, we identify the PVH and POA as specific brain regions that are targeted by DMHTrkB neurons to inhibit feeding. Inhibitory input to Bmal1-expressing neurons in the PVH has recently been implicated in maintaining normal rhythmicity in feeding and metabolism (44); however, the source of this inhibition is unclear. It is possible that the inhibitory population of DMHTrkB→PVH neurons we identified could suppress the activity of PVHBmal1 neurons to maintain normal metabolic and feeding rhythmicity. We also see that many DMHTrkB→PVH neurons express the excitatory marker, Vglut2. These glutamatergic DMHTrkB→PVH neurons might contribute to the suppression of feeding by acting on established anorexigenic neuron populations in the PVH, including those that express BDNF (38, 45, 46). BDNF signaling through TrkB is critical for neural circuit development and synaptic plasticity (47); thus, PVHBDNF neurons represent an attractive potential neural substrate for DMHTrkB→PVH neurons that govern feeding.
Given that activation of DMHTrkB→RPa neurons does not drive energy expenditure to the full extent seen by stimulating the global DMHTrkB population, other DMHTrkB neurocircuits likely contribute to increased total energy expenditure. DMHTrkB→PVH neurons might help facilitate BAT activity by inhibiting nucleus tractus solitarius (NTS)-projecting PVH neurons that negatively regulate the RPa (48, 49) or by exciting PVHBDNF neurons that are polysynaptically connected to BAT (38). This possibility is in line with the subtle thermogenic effect of activating a DMHTrkB → PVH neurocircuit. Despite significant overlap in retrogradely labeled DMHTrkB populations that project to the PVH and the POA, activation of DMHTrkB→POA neurons was not sufficient to significantly increase BAT temperature or energy expenditure as was observed for activation of DMHTrkB→PVH neurons. This discrepancy may be due to an incomplete activation of all DMHTrkB→POA neurons, attributed to the limited expression of retrograde FLPo-expressing virus at the VMPO. Alternatively, DMHTrkB→PVH neurons that drive thermogenesis and energy expenditure could represent a subpopulation that does not send collaterals to the POA. A yet unknown DMHTrkB neurocircuit that promotes physical activity could also contribute to the increase in total energy expenditure observed upon activating DMHTrkB neurons.
Materials and Methods
Mice.
The Ai9/+ mouse strain [Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J; stock number 007909] was acquired from Jackson Laboratory. The Ntrk2CreER/+ (also known as TrkBCreER) mouse strain was generously provided by Dr. David Ginty (Harvard Medical School). Male and female mice aged 6 to 12 wk old were used for initial investigations of chemogenetic activation or inhibition of global DMHTrkB neurons. Both males and females showed similar phenotypes; thus, only female mice were used for subsequent projection specific manipulation of DMHTrkB neuronal activity. Details on animal housing and drug treatments can be found in SI Appendix, Supplementary Materials and Methods. All experiments were performed in accordance with relevant guidelines and regulations regarding the use of experimental animals. The Animal Care and Use Committees at The Scripps Research Institute Florida approved all animal procedures used in this study.
Viruses and Plasmid Construction.
AAV2-CMV-GFP (4.4 × 1012 vg/mL; The University of North Carolina at Chapel Hill [UNC] Vector Core), AAV2-CAG-FLEX-tdTomato (4.8 × 1012 vg/mL, UNC Vector Core), AAV8-hSyn-DIO-hM3-mCherry (4 × 1012 vg/mL, Addgene 44362), AAV8-hSyn-hM4-mCherry (6 × 1012 vg/mL, UNC Vector Core), and AAV8-hSyn-mCherry (2.1 × 1013 vg/mL, Addgene) were used. AAV2-retro-EF1a-DIO-FLPo (2.6 × 1013 vg/mL) was packaged by Vigene Biosciences from pAAV-EF1a-DIO-FLPo-WPRE-hGHpA (a gift from Li Zhang, Addgene 87306). AAV2-fDIO-mCherry was previously described (41) (4.2 × 1012 vg/mL, packaged by Vigene), and AAV2-fDIO-hM3-mCherry (6.64 × 1012 vg/mL, packaged by Vigene) was similarly constructed; pAAV-hSyn-DIO-hM3-mCherry (a gift from Brian Roth, Addgene 44361) and pAAV-EF1a-fDIO-EYFP (a gift from Karl Deisseroth, Addgene 55461) were digested with AscI and NheI, and the hM3-mCherry fragment was ligated into pAAV-EF1a-fDIO backbone. The resulting pAAV-EF1a-fDIO-hM3-mCherry plasmid was confirmed by sequencing and additional digestion with XmaI prior to commercial packaging. Herpes simplex virus (HSV)129ΔTK-tdTomato was obtained from the Center for Neuroanatomy with Neurotropic Viruses.
Immunohistochemistry.
For Fos staining, mice were perfused with 4% paraformaldehyde (PFA), and the brain was dissected and postfixed overnight in 4% PFA. Brains were cryoprotected, and 40-µm-thick sections were collected with a sliding microtome (Leica) and immunostained before fluorescent imaging and quantification. For analysis of anterograde tracing in BAT and heart tissues, 20-µm frozen sections were collected with a cryostat (Leica). Details are available in SI Appendix, Supplementary Materials and Methods.
Stereotaxic Surgery.
Ntrk2CreER/+ mice at 6 to 8 wk of age underwent stereotactic delivery of AAV viruses followed by 1 wk of recovery and 1 wk of tamoxifen treatment. Mice injected with HSV129ΔTK were pretreated with tamoxifen 2 d before viral delivery and continued to be treated on postoperative days 1 through 4. Injection coordinates (anterior-posterior [AP], mediolateral [ML], and dorsoventral [DV]) were as follows: POA (AP, +0.60 mm; ML, ±0.35 mm; and DV, −5.70 mm), PVH (AP, −0.50 mm; ML, ±0.35 mm; and DV, −5.30 mm), DMH (AP, −1.50 mm; ML, ±0.35 mm; and DV, −5.70 mm), and RPa (AP, −6.00 mm; ML, 0.00 mm; and DV, −6.35 mm). Additional information is available in SI Appendix, Supplementary Materials and Methods.
Temperature Measurements.
Core body temperature was determined with a digital thermometer (Fisher Traceable Type K thermometer) with rodent rectal temperature probe (World Precision Instruments, RET-3).
For thermal imaging experiments, the fur on the back of mice was shaved, and a thermal camera (FLIR E53sc) was mounted above a cage with mice. Thermal images were collected 1 h after injection of vehicle or CNO, and regions above the shoulders (above interscapular BAT), lumbar area (body), and tail were analyzed using ResearchIR software (FLIR). Details are available in SI Appendix, Supplementary Materials and Methods.
Physiological Measurements.
Locomotor activity and oxygen consumption in individual mice were measured using a Columbus Instruments’ Comprehensive Lab Animal Monitoring System. Mice received a vehicle intraperitoneal (i.p.) injection in the morning (between 8 and 9 AM) and a second i.p. injection with CNO (between 12 and 1 PM). Physical activity and metabolic data were collected for 4 h following treatment with either vehicle or CNO. Heart rate and blood pressure were recorded using a tail cuff system (MC4000, Hatteras Instruments), and nocturnal food intake studies were performed as previously described (21). Additional details can be found in SI Appendix, Supplementary Materials and Methods.
Quantitative RT-PCR.
BAT tissue was dissected 2 h after injection of CNO and snap-frozen in liquid nitrogen prior to extraction of total RNA. Following reverse transcription (New England Biolabs, M0253), qPCR was carried out using SYBR green mix (Roche) in an StepOne cycler (Applied Biosystems). Additional details and primer sequences are available in SI Appendix, Supplementary Materials and Methods.
Immunoblotting.
Protein was extracted from BAT samples, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene difluoride membrane. Membranes were blocked in 5% milk and immunoblotted for p-HSL, HSL, and β-actin. Densitometric analysis of bands was performed using ImageJ software. Further details are available in SI Appendix, Supplementary Materials and Methods.
Glycerol Measurement.
Glycerol content of BAT from mice expressing either mCherry or hM3 in DMHTrkB neurons and treated with CNO (1.5 mg/kg) was determined using a Glycerol-Glo Assay (Promega, J3150) according to the manufacturer’s instructions. Details are available in SI Appendix, Supplementary Materials and Methods.
In Situ Hybridization.
Brains from DMHTrkB→RPa:mCherry and DMHTrkB→PVH:mCherry mice were dissected and immediately frozen on dry ice and stored at −80 °C. Prior to sectioning, samples were embedded in optimal cutting temperature compound (OCT) (Tissue Tek). Fresh frozen sections (14 µm) were collected with a cryostat (Leica) onto Superfrost Plus slides (Fisherbrand, 12-550-15). Fluorescent ISH was carried out according to the manufacturer’s instructions (ACD, RNAScope Multiplex Fluorescent detection kit v2, 323110). More details are available in SI Appendix, Supplementary Materials and Methods.
Statistical Analyses.
All data are presented as means ± SEM. Graphs and statistical analyses were generated using GraphPad Prism software. Statistical significance was determined using either two-tailed unpaired Student’s t test or two-way ANOVA when comparing more than two groups. All experiments were performed at least three times independently or in three separate animals. All statistical analyses are listed in SI Appendix, Table S1.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH to B.X. (R01 DK105954 and R01 DK103335) and J.H. (F32 NS106810). HSV129ΔTK-TT was provided by the Center for Neuroanatomy with Neurotropic Viruses, which was supported by an NIH grant (P40 RR018604).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2017218118/-/DCSupplemental.
Data Availability.
All study data are included in the article and/or SI Appendix.
References
- 1.Hruby A., Hu F. B., The epidemiology of obesity: A big picture. Pharmacoeconomics 33, 673–689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leibel R. L., Rosenbaum M., Hirsch J., Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 332, 621–628 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Lotfi K., Palmer K., Apovian C. M., Case Study: Weight loss in a patient with type 2 diabetes: Challenges of diabetes management. Obesity (Silver Spring) 23 (suppl. 1), S11–S12 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Chen K. Y., et al. , Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. J. Biol. Chem. 295, 1926–1942 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalil G. Z., Haynes W. G., Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens. Res. 35, 4–16 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowell B. B., Spiegelman B. M., Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y., et al. , Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur. J. Neurosci. 22, 3137–3146 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan C. L., et al. , Warm-sensitive neurons that control body temperature. Cell 167, 47–59.e15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Z. D., et al. , A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. U.S.A. 114, 2042–2047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura K., et al. , Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J. Neurosci. 24, 5370–5380 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneeberger M., et al. , Regulation of energy expenditure by brainstem GABA neurons. Cell 178, 672–685.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kataoka N., Hioki H., Kaneko T., Nakamura K., Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 20, 346–358 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Cao W. H., Morrison S. F., Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology 51, 426–437 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Zaretskaia M. V., Zaretsky D. V., Shekhar A., DiMicco J. A., Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 928, 113–125 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Piñol R. A., et al. , Brs3 neurons in the mouse dorsomedial hypothalamus regulate body temperature, energy expenditure, and heart rate, but not food intake. Nat. Neurosci. 21, 1530–1540 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simonds S. E., et al. , Leptin mediates the increase in blood pressure associated with obesity. Cell 159, 1404–1416 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellinger L. L., Bernardis L. L., The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: Lessons learned from lesioning studies. Physiol. Behav. 76, 431–442 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Otgon-Uul Z., Suyama S., Onodera H., Yada T., Optogenetic activation of leptin- and glucose-regulated GABAergic neurons in dorsomedial hypothalamus promotes food intake via inhibitory synaptic transmission to paraventricular nucleus of hypothalamus. Mol. Metab. 5, 709–715 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garfield A. S., et al. , Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 19, 1628–1635 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rau A. R., Hentges S. T., GABAergic inputs to POMC neurons originating from the dorsomedial hypothalamus are regulated by energy state. J. Neurosci. 39, 6449–6459 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao G. Y., Kinney C. E., An J. J., Xu B., TrkB-expressing neurons in the dorsomedial hypothalamus are necessary and sufficient to suppress homeostatic feeding. Proc. Natl. Acad. Sci. U.S.A. 116, 3256–3261 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J., Scott K. A., Zhao Z., Moran T. H., Bi S., Characterization of the feeding inhibition and neural activation produced by dorsomedial hypothalamic cholecystokinin administration. Neuroscience 152, 178–188 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonoyama T., et al. , Human BDNF/TrkB variants impair hippocampal synaptogenesis and associate with neurobehavioural abnormalities. Sci. Rep. 10, 9028 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., et al. , Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J. Neurosci. 31, 1873–1884 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutlin M., et al. , The cellular and molecular basis of direction selectivity of Aδ-LTMRs. Cell 159, 1640–1651 (2014).Corrected in: Cell160, 1027 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madisen L., et al. , A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg M. E., Ziff E. B., Greene L. A., Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science 234, 80–83 (1986). [DOI] [PubMed] [Google Scholar]
- 28.Alexander G. M., et al. , Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer A. W., Cannon B., Nedergaard J., Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol. Metab. 7, 161-170 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer C. W., Ootsuka Y., Romanovsky A. A., Body temperature measurements for metabolic phenotyping in mice. Front. Physiol. 8, 520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez J. L., et al. , Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vestri H. S., Maianu L., Moellering D. R., Garvey W. T., Atypical antipsychotic drugs directly impair insulin action in adipocytes: Effects on glucose transport, lipogenesis, and antilipolysis. Neuropsychopharmacology 32, 765–772 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Blessing W. W., Zilm A., Ootsuka Y., Clozapine reverses increased brown adipose tissue thermogenesis induced by 3,4-methylenedioxymethamphetamine and by cold exposure in conscious rats. Neuroscience 141, 2067–2073 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T. M., et al. , A discrete neuronal circuit induces a hibernation-like state in rodents. Nature 583, 109–114 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Brito N. A., Brito M. N., Bartness T. J., Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1445–R1452 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Tupone D., Madden C. J., Morrison S. F., Autonomic regulation of brown adipose tissue thermogenesis in health and disease: Potential clinical applications for altering BAT thermogenesis. Front. Neurosci. 8, 14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rezai-Zadeh K., et al. , Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol. Metab. 3, 681–693 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An J. J., Liao G. Y., Kinney C. E., Sahibzada N., Xu B., Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell Metab. 22, 175–188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeo G. S., et al. , A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 7, 1187–1189 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Xu B., et al. , Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 6, 736–742 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.An J. J., et al. , TrkB-expressing paraventricular hypothalamic neurons suppress appetite through multiple neurocircuits. Nat. Commun. 11, 1729 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang N., Yang L., Guo L., Bi S., Activation of dorsomedial hypothalamic neurons promotes physical activity and decreases food intake and body weight in zucker fatty rats. Front. Mol. Neurosci. 11, 179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu S., et al. , Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J. Neurosci. 36, 5034–5046 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim E. R., et al. , Paraventricular hypothalamus mediates diurnal rhythm of metabolism. Nat. Commun. 11, 3794 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M. M., et al. , The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102, 653–667.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y., et al. , Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab. 18, 860–870 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang E. J., Reichardt L. F., Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong D., et al. , GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151, 645–657 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madden C. J., Morrison S. F., Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R831–R843 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.