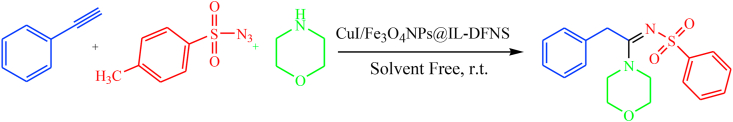
Abstract
Wrinkled fibrous nanosilica (WFNS) which functionalized by ionic liquid modified Fe3O4 NPs and CuI salts has been synthesized and characterized with FE-SEM, TEM, FT-IR, FAAS, EDX, and, XRD, VSM, and BET-BJH analysis. This new and effective magnetic ceramic nanocatalyst has been applied towards rapid synthesis of N-sulfonylamidines using reaction of phenyl acetylene, substituted sulfonyl azide and various amines under solvent-free conditions in very short reaction time. Higher catalytic activity CuI/Fe3O4NPs@IL-DFNS in the reaction is because of special structure of DFNS and existence of ionic liquids on its pores which act as a robust anchors to the loaded various nano-particles. So, this lead to no leaching of them from the pore of the composite. Shorter reaction time, higher yield, recovery of the catalyst using an external magnet and its reusability for 8 series without noteworthy reduction in its activity are the advantages of newly synthetic catalyst toward efficient synthesis of N-sulfonylamidines.
Keywords: Wrinkled fibrous nanosilica, Ceramic nanocatalyst, Advanced nanomaterials, N-sulfonylamidines
Wrinkled fibrous nanosilica, Ceramic nanocatalyst, Advanced nanomaterials, N-sulfonylamidines.
1. Introduction
In recent years, silica materials with porous structure and excellent properties like high thermal, hydrothermal, and mechanical stability, low density and toxicity, good biocompatibility, cost effectiveness, and simple surface functionalization, have been received a lot of attentions [1, 2, 3, 4, 5, 6]. A new class of silica nanospheres with wrinkled, dendritic and fibrous morphology is DFNS that at the first time introduced by Polshettiwar and co-workers. These materials have exceptional activities in several field of research such as catalysis, synthesis, sensors, energy harvesting, and biomedical applications [7,8].
DFNS is an exceptional nanomaterial with accessibility from all sides in contrast with various silica mesoporous nano-materials like SBA-15 and MCM-41 with tube-shaped or cylindrical pores [9, 10, 11]. In other hand, numerous useful materials like organometallics, some metal nanoparticles, metal oxides, and organic functional groups, can be hosted in the channels of the DFNS without the padlocking of its pores and channels the and above all, availability of freshly produced active positions were improved. Although above mentioned silica mesoporous nanomaterials have higher geometric surface area than DFNS, but the outcomes display that, its honesty is appropriate. Moreover, thin silica walls of DFNS with high mechanical and thermal stability. Also, singular structure and morphology permit the better dispersion and loading of the active sites and functional groups in contrast with MCM-41 and SBA-15.
Recently publications exhibit that functionalization of the various silica nano materials with ionic liquids (ILs) can increase their catalytic activity because of specific futures of ILs such as eco-friendly, good stability, appropriate ionic conductivity, extensive liquid temperature range gap, and high thermal stability [12,13]. Until now, various type of ionic liquids which have been attached to the various supports, they have acted as a catalyst, reaction medium and as a stabilizer of numerous nanomaterials according to their ability in coordination of various metal atoms and ions based on electrostatic interaction [14]. Also, the supported ionic liquids can afford a suitable hydrophobic media, and enhance the activity of the catalyst during the reaction procedures. Then, numerous heterocyclic compound synthesis have been generated in the presence of loaded ILs as a nanocatalysts [15].
One of the significant structural motifs in biologically active compounds and natural products are amidines [16, 17, 18]. They have been broadly used in synthetic, coordination, medicinal, and supramolecular chemistry because of their exceptional chemical properties [19, 20, 21, 22]. N-sulfonylamidines as a new versatile class of compounds are one of the useful synthetic intermediates and efficient ligands with important pharmaceutical and biological activities [16, 17, 18,21, 22, 23, 24, 25, 26, 27, 28].
Till now diverse copper containing catalysts such as CuI [29, 30, 31, 32, 33], cellulose functionalized with aminomethylpyridine and copper iodide nanoparticles [34], Cu(OTf)2 [35] and, (MOF-Cu2I2(BTTP4) [36] have been widely used for the efficient production of these important active N-sulfonylamidines. Lower yields, longer reaction times and importantly no reusability and recoverability of the used catalyst are disadvantages of the previously reported methods. Hence, developing a green and efficient procedure using the reusable heterogeneous nanocatalysts for the synthesis of these important biological compounds with higher yields is extremely demanded.
Following of previous research work on the application of DFNS as a new heterogeneous nanocatalyst [37, 38, 39, 40], dendritic nanosilica modified with ionic liquid loaded CuI salts and Fe3O4 NPs is the best candidate towards synthesis of N-sulfonylamidines. In this study, DFNS functionalized with biimidazole as a strong anchors to trap CuI salts and Fe3O4 NPs. In the next step, this green and magnetic nanocatalyst has been used in the synthesis of N-sulfonylamidines derivatives. It is noteworthy that, higher catalytic activity of this catalyst is because of its exceptional dendritic and wrinkled structure of DFNS and its alteration by ionic liquids which perform as vigorous anchors to the loaded nano-particles. So, this lead to no leaching of them from the pore of the composite (Scheme 1).
Scheme 1.
Synthesis of N-sulfonylamidines derivatives in the presence of magnetic nanocatalyst CuI/Fe3O4 NPs@IL-DFNS.
2. Experimental section
2.1. Methods and materials
All of chemicals and materials were purchased from Merck Co.
2.2. Synthesis of N-sulfonylamidines derivatives: a general procedure
A blend of phenyl acetylene (1 mmol), sulfunyl azide (1 mmol), secondary amines (1.2 mmol) and, (CuI/Fe3O4 NPs@IL-DFNS) (1.02 mol %), were involved to the test-tube and stirred for under solvent-free conditions. Surveying of reaction process/completing were performed by TLC (Thin-layer chromatography). Finally, external magnet was used for the separation of nano-catalyst. Also, to increase its purity, obtained material was washed by water/acetone solution several times which the pure products were obtained using preparative layer chromatography in EtOAc/n-Hexan (1:3) as solvent.
2.3. Synthesis of CuI/Fe3O4 NPs@IL-DFNS
After synthesis of dendritic fibrous nanosilica (DFNS) according to our previous report [38]. The obtained precipitate in the previous step was separated using external magnet, washed by deionized water, and dried at 60 °C for 6 h. In the next step, dried precipitate (1 g, Fe3O4 NPs@IL-DFNS) was included to the solution of CuI (0.190 g in 50 ml methanol), and stirred at 30 °C for 24h. Then, the obtained magnetic nanocatalyst was separated by external magnet, washed with ethanol, acetone, and deionized water and dried in air. The schematic of the synthesised nanocatalyst is shown in Scheme 2.
Scheme 2.
Preparation of the CuI/Fe3O4NPs@IL-DFNS nanocatalyst.
3. Results and discussions
3.1. Characterization of the nanocatalyst
After synthesis and modification of the magnetic nanocatalyst, the morphology and structure of CuI/Fe3O4NPs@IL-DFNS were investigated by FAAS, FT-IR, SEM, TEM, EDX, Mapping, XRD, BET, BJH and VLS analysis.
The amount of copper and iron which loaded on the fibrous dendritic silica was determined by FAAS (flame atomic absorption spectroscopy) which the amount of iron and copper doped in the CuI/Fe3O4NPs@IL-DFNS catalyst were 5.91 and 22.54 wt%, respectively. Hence, according to FAAS the amount of Cu loaded on the catalyst is 0.35 mol.
In the FT-IR spectrum (Figure 1), the peaks appear in 1628 and 1428 cm−1 are related to the stretching vibrations of C=N and C=C in the imidazolium rings, respectively. The peak at 593 cm−1 is belonging to the vibration of Fe–O in Fe3O4. Also, the weak peak at 670 cm−1 can be attributed to copper iodide bond and the sharp peak at 3426 cm−1 is assigned to O–H bonds of nanocatalyst [45].
Figure 1.
FT-IR spectra of the prepared CuI/Fe3O4 NPs@IL-DFNS nanocatalyst.
In order to study the structure and morphology of the nanocatalyst, TEM and FE-SEM analyses were used. Uniform spheres with dendritic and fibrous structure of DFNS were confirmed by FE-SEM and TEM images (Figure 2). Also, based on TEM images, the average size of particle was obtained as 30 nm and existence of MNPs on the structure was approved.
Figure 2.
FESEM (a) and TEM (b) images of the CuI/Fe3O4 NPs@IL-DFNS.
To determine the successful coating of Fe3O4 and CuI on the surface of CuI/Fe3O4NPs@IL-DFNS, EDX was employed (Fig. S1A). The EDX analysis paved the presence of Cu, N, C, Si, and O characteristic peaks. Also, Map analysis was applied to confirm the all mentioned results (Fig. S1B).
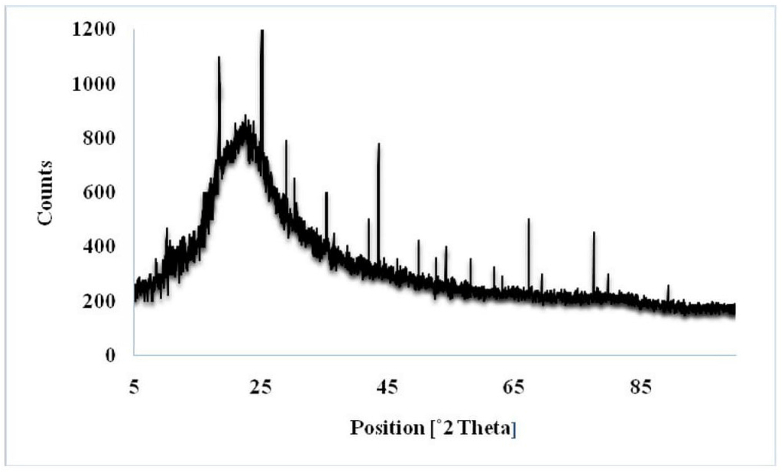
In the following stage, to assess the crystal structure of the CuI/Fe3O4 NPs@IL-DFNS nanocatalyst, X-ray diffraction (XRD) analysis was applied (Figure 3). Results shown that, the peaks at 2θ = 63.08°, 58.09°, 54.14°, 43.53°, 35.24° and 30.39° are associated for cubic CuI and the peaks at 89.21°, 79.30°, 77.49°, 69.30°, 67.24°, 61.76°, 52.60°, 49.85°, 42.01°, 29.33°, 25.20°, and 12.46° are related to cubic Fe3O4. Moreover, a wide peak in the range of 2θ = 19–30° is belong to amorphous silica.
Figure 3.
XRD pattern of CuI/Fe3O4 NPs@IL-DFNS nanocatalyst.
The porous nature of CuI/Fe3O4NPs@IL-DFNS and bare DFNS was investigated using BJH-BET analysis (Fig. S2). Using these methods, surface area and porosity of the nanocatalyst were measured. In addition, the pore volume of the CuI/Fe3O4 NPs@IL-DFNS and DFNS were calculated by using BJH analysis and compared in Table 1. According to these data, the pore volumes changed from 1.52 to 0.77 cm3.g−1 for DFNS (bare material) and CuI/Fe3O4 NPs@IL-DFNS, respectively. Also, surface area was obtained as 225 m2/g, 617 m2/g for CuI/Fe3O4 NPs@IL-DFNS and bare materials (DFNS), respectively. In addition, the mean pore diameter of CuI/Fe3O4 NPs@IL-DFNS and DFNS was obtained as 13.76 and 9.9 nm, respectively.
Table 1.
Porosity evaluation of CuI/Fe3O4 NPs@IL-DFNS and its comparision with DFNS as bare material.
| Material type | Pore size (nm)a | Pore volume (cm3 g−1)b | Surface Area (m2 g−1) |
|---|---|---|---|
| DFNS | 9.9 | 1.5 | 617 |
| CuI/Fe3O4 NPs@IL-DFNS | 13.76 | 0.77 | 225 |
Pore size was calculated by BET method.
Pore volume determined from nitrogen physicosorption isotherm.
The VSM magnetization curve of prepared nanaocatalyst is shown in Fig. S3. The saturation magnetization of the prepared catalyst is obtained as 22.47 emu g−1. All of these results was confirmed by FESEM (Fig. S3) and TEM (Fig. S4) images of nanocatalyst.
3.2. Catalytically synthesis of N-sulfonylamidines derivatives in the presence CuI/Fe3O4 NPs@IL-DFNS
To the obtain of optimum reaction conditions towards synthesis of N-sulfonylamidines derivatives, a mixture of phenyl acetylene (1 mmol), 4-toluenesulfunyl azide (1 mmol), morpholine (1.2 mmol) were designated as a model reaction and the effect of the various solvents, different catalysts and also amount of the catalysts has been investigated (Scheme 3).
Scheme 3.
A model reaction for the catalytically synthesis of N-sulfonylamidines derivatives in the presence CuI/Fe3O4 NPs@IL-DFNS.
Various amount of magnetic nanocatalyst (CuI/Fe3O4 NPs@IL-DFNS) was used for the optimization of synthesis of N-sulfonylamidines derivatives in the presence of DMF at room temperature. Based on obtained results, yield of the reaction has association with the amount of applied nanocatalyst which increasing of this nanocomposite from 1.73 to 6.93 mol% lead to increasing of reaction yield. However, after increment the amount of CuI/Fe3O4 NPs@IL-DFNS to 5.2 mol% has not effect on the yield of the reaction (synthesis of N-sulfonylamidines derivatives). So, 5.2 mol% of the synthesized magnetic nano-catalyst was selected as an optimized amount for the reaction (synthesis of N-sulfonylamidines derivatives) under solvent-free condition and rt (Table 1, entry 1).
In continue, different solvents such as DMF, THF, CH3CN, CH2Cl2, and, CH3Cl in the presence of CuI/Fe3O4 NPs@IL-DFNS (5.2 mol%) were investigated as a solvent for the model reaction (Table 1, entries 1–5). In these solvents, the yields of the reaction were obtained as 95%, 87%, 84%, 83% and 83%, respectively at 100 min. Surprisingly, when the model reaction was done in the solvent-free conditions, the yield of the reaction was increased. Therefore, the reaction time was significantly reduced. Also, solvent-free condition was preferred towards synthesis of N-sulfonylamidines in the presence of candidate magnetic nanocatalyst (5.2 mol%) as an efficient magnetic nanocatalyst and all of the reactions were performed in this condition (Table 1, entry 6).
In addition, various Cu sources like CuI, CuBr, CuCl (10% mol) were tested for the model reaction under solvent-free conditions (Table 1, entries 8–10). The results show that among the copper halides, CuI is an effective catalyst for this reaction, but it is not a recoverable and reusable catalyst in various coupling reactions. Hence, by supporting of this useful catalyst by appropriate substrate such as dendritic fibrous nanosilica, its catalytic efficiency can be improved significantly. It is found that, in the presence of Fe3O4 NPs and Fe3O4 NPs@IL-DFNS as a nanocatalyst, no product was formed (Table 1, entries 11–12). But, by the incorporation of copper iodide on the surface and pores of the fibrous nanosilica the favorite products achieved with short reaction times in high yield. Hence, CuI/Fe3O4 NPs@IL-DFNS was preferred as an effective and reusable nanocatalyst towards synthesis of N-sulfonylamidines in solvent-free conditions. The effectiveness of the catalyst is because of the uniform distribution of copper iodide on the substrate and simple diffusion of the reactants on the channels and so on their interactions with the active sites of the magnetic candidate nanocatalyst.
Based on Table 1, CuI/Fe3O4 NPs@IL-DFNS (with the amount of 5.2 mol%) is an active nanocatalyst for the reaction of the phenylacetylene (1 mmol), sulfunyl azide (1 mmol), and secondary amines (1.1 mmol), under solvent-free conditions to generate N-sulfonylamidines derivatives in high yields.
In order to show the efficiency of CuI/Fe3O4 NPs@IL-DFNS as innovative nanocatalyst, several types of secondary amines were applied to react with phenylacetylene and sulfunyl azide at r.t., and solvent-free conditions (See Table 2).
Table 2.
Optimization of the type of solvent and time of reaction for the synthesis of N-sulfonylamidines.
| Entry | Catalyst (mol %) | Solvent | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | CuI/Fe3O4 NPs@IL-DFNS(1.73, 3.46, 5.2) | DMF | 100 | 80, 86, 95 |
| 2 | CuI/Fe3O4 NPs@IL-DFNS (5.2) | THF | 100 | 87 |
| 3 | CuI/Fe3O4 NPs@IL-DFNS (5.2) | CH3CN | 100 | 84 |
| 4 | CuI/Fe3O4 NPs@IL-DFNS (5.2) | CH2Cl2 | 100 | 83 |
| 5 | CuI/Fe3O4 NPs@IL-DFNS (5.2) | CHCl3 | 100 | 83 |
| 6 | CuI/Fe3O4NPs@IL-DFNS (5.2) | Solvent-free | 5 | 96 |
| 7 | CuI/Fe3O4 NPs@IL-DFNS (6.93) | Solvent-free | 5 | 96 |
| 8 | CuI (10% mol) | 40 | 81 | |
| 9 | CuBr (10% mol) | 60 | 78 | |
| 10 | CuCl (10% mol) | 60 | 73 | |
| 11 | Fe3O4 NPs (40 mg) | 500 | - | |
| 12 | Fe3O4 NPs@IL-DFNS | 500 | - |
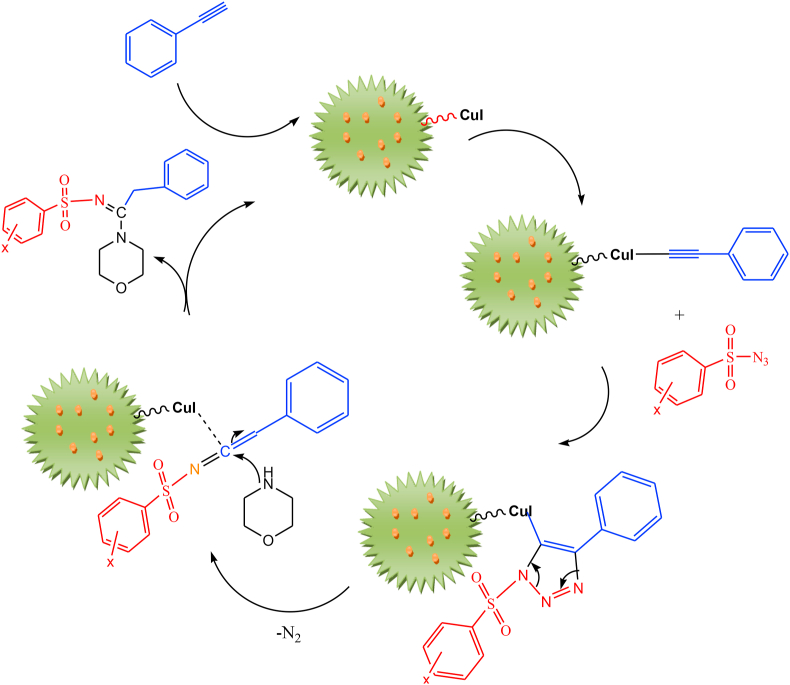
Suggested mechanism for the synthesis procedure of N-sulfonylamidines in the existence of candidate magnetic nano-catalyst (CuI@Fe3O4 NPs-IL-DFNS) is illustrated in Scheme 4. As seen, in the presence of nanocatalyst functionalized with ionic liquid and trapped Fe3O4 NPs and CuI salts, the triazole was formed by the cycloaddition reaction between phenylacetylene and sulfonyl azide. In the next step, after the elimination of the nitrogen, the ketenimine intermediate is created with nucleophilic addition of morphine gives the major product with high yields.
Scheme 4.
Planned mechanism towards efficient synthesis of N-sulfonylamidines derivatives in the existence of magnetic nanocatalyst (CuI@Fe3O4 NPs-IL-DFNS).
Table 3, compare catalytically activity of CuI@Fe3O4 NPs-IL-DFNS on the synthesis of N-sulfonylamidines derivatives with previous reports. According to the obtained results, engineered magnetic nanocatalyst is an appropriated candidate nanocomposite/nanocatalyst towards synthesis of N-sulfonylamidines because of its eco-friendliness, simple recovery and reusability. Also, higher yields, shorter reaction times and solvent free conditions are another advantages of using of this catalyst (see Table 4).
Table 3.
Synthesis of N-sulfonylamidines derivatives in the presence of CuI/Fe3O4 NPs@IL-DFNS.
| Entry | Product | Time (Min) | Yield∗ (%) |
|---|---|---|---|
| 4a |  |
4 | 96 |
| 4b | 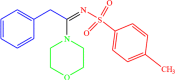 |
4 | 96 |
| 4c | 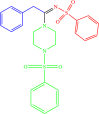 |
10 | 84 |
| 4d | 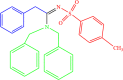 |
5 | 90 |
| 4e | 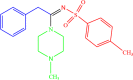 |
4 | 91 |
| 4f | 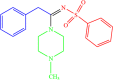 |
4 | 91 |
| 4g | 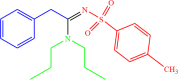 |
4 | 95 |
| 4h | 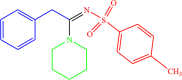 |
4 | 92 |
| 4i | 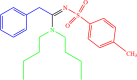 |
5 | 94 |
| 4j | 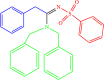 |
7 | 91 |
Isolated yields.
Table 4.
Comparison of prepared nanocatalyst with other reported catalysts in N-sulfonylamidine synthesis.
| Entry | Catalyst | Conditions | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | CuI | r.t., THF | 120 | 89 | [41] |
| 2 | Cu@C | r.t., CH3CN | 180 | 92 | [42] |
| 3 | MOF-Cu2I2 (BTTP4) | r.t., CH3CN | 120 | 88 | [36] |
| 4 | CuI/Fe3O4NPs@AMPCs | r.t., solvent-free | 5 | 94 | [34] |
| 5 | Cu-scolecite | r.t., THF | 80 | 90 | [43] |
| 6 | CuI@Fe3O4 NPs-g-CS | r.t., solvent-free | 10 | 92 | [44] |
| 7 | CuI@Fe3O4 NPs-IL-DFNS | r.t., solvent-free | 5 | 97 | This work |
3.3. Re-usability of the candidate magnetic catalyst
In industrial applications the simple recovery and reusability of nanocatalysts is too important. In the present work, reusability of the proposed magnetic nanocatalyst was investigated by running in various number. For this purpose, after the completion of the reaction, an external magnet was used for the easily separation of the CuI@Fe3O4 NPs-IL-DFNS from the reaction mixture. Then the separated catalyst was washed with acetone and water and as can be seen in Figure 1, proposed magnetic nano-catalyst (CuI@Fe3O4 NPs-IL-DFNS) is recyclable eight times with slightly decrease in its catalytic intrinsic (Figure 4).
Figure 4.
A) Effective separation of nanocatalyst (CuI/Fe3O4 NPs@IL-DFNS) from the reaction mixture by means of external magnet. B) Histogram of catalyst reusability (Yield %) versus number of use.
4. Conclusion
In conclusion, CuI/Fe3O4NPs@IL-DFNS was used as a green magnetic nanocatalyst towards efficient catalytically synthesis of N-sulfonylamidines by one-pot reaction of sulfonyl azides, phenyl acetylene, and secondary amines. Higher surface area and porous structure of the nanocatalyst with its exceptional catalytic activity, magnetically recoverable of the catalyst, shorter reaction times, and excellent yields in comparison with previous reported methods, and no using of toxic solvents are the benefits of this work.
Declarations
Author contribution statement
Sajjad Azizi: Performed the experiments; Wrote the paper.
Nasrin Shadjou: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Beller M., Renken A., Van Santen R.A. Wiley-VCH; 2012. Catalysis: from Principles to Applications. [Google Scholar]
- 2.Azizi S., Soleymani J., Shadjou N. CuI/Fe3O4 NPs@Biimidazole IL-KCC-1 as a leach proof nanocatalyst for the synthesis of imidazo[1,2-a]pyridines in aqueous medium. Appl. Organomet. Chem. 2020 [Google Scholar]
- 3.Rothenberg G. Wiley-VCH; 2008. Catalysis: Concepts and Green Applications. [Google Scholar]
- 4.Polshettiwar V., Asefa T. Wiley; 2013. Nanocatalysis: Synthesis and Applications. [Google Scholar]
- 5.Anvari Gharabaghlou M., Shadjou N., Poursattar Marjani A. Cu@KCC-1-NH-CS2 as a new and highly efficient nanocatalyst for the synthesis of 2-amino-4H-chromene derivatives. Appl. Organomet. Chem. 2020;34(10) [Google Scholar]
- 6.Azizi S., Shadjou N., Hasanzadeh M. KCC-1 aminopropyl-functionalized supported on iron oxide magnetic nanoparticles as a novel magnetic nanocatalyst for the green and efficient synthesis of sulfonamide derivatives. Appl. Organomet. Chem. 2020;34(1) [Google Scholar]
- 7.Polshettiwar V., Cha D., Zhang X., Basset J.M. High-surface-area silica nanospheres (KCC-1) with a fibrous morphology. Angew. Chem. Int. Ed. 2010;49:9652. doi: 10.1002/anie.201003451. [DOI] [PubMed] [Google Scholar]
- 8.V. Polshettiwar, J.M. Basset, High surface area fibrous silica nanoparticles. US20110253643, 2010. [DOI] [PubMed]
- 9.Polshettiwar V., Maity A. Dendritic fibrous nanosilica for catalysis, energy harvesting, carbon dioxide mitigation, drug delivery, and sensing. Chem. Sus. Chem. 2017;10:3866. doi: 10.1002/cssc.201701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maity A., Belgamwar R., Polshettiwar V. Facile synthesis to tune size, textural properties and fiber density of dendritic fibrous nanosilica for applications in catalysis and CO2 capture. Nat. Protoc. 2019;14:2177. doi: 10.1038/s41596-019-0177-z. [DOI] [PubMed] [Google Scholar]
- 11.Maity A., Sandra U.S., Kolthur-Seetharam U., Polshettiwar V. Dendritic fibrous nanosilica (DFNS) for RNA extraction from cells. Langmuir. 2020;36(42):12755. doi: 10.1021/acs.langmuir.0c02520. [DOI] [PubMed] [Google Scholar]
- 12.Dhiman M., Maity A., Das A., Belgamwar R., Chalke B., Lee Y., Sim K., Nam J.-M., Polshettiwar V. Plasmonic colloidosomes of black gold for solar energy harvesting and hotspots directed catalysis for CO2 to fuel conversion. Chem. Sci. 2019;10:6694. doi: 10.1039/c9sc02369k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plechkova N.V., Seddon K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008;37:123. doi: 10.1039/b006677j. [DOI] [PubMed] [Google Scholar]
- 14.Yang H., Han X., Li G., Wang Y. N-Heterocyclic carbene palladium complex supported on ionic liquid-modified SBA-16: an efficient and highly recyclable catalyst for the Suzuki and Heck reactions. Green Chem. 2009;11:1184. [Google Scholar]
- 15.Sadeghzadeh S.M. Ionic liquid immobilized onto fibrous nano-silica: A highly active and reusable catalyst for the synthesis of quinazoline-2,4(1 H,3 H)-diones. Catal. Commun. 2015;72:91. [Google Scholar]
- 16.Ishikawa T. Wiley; Chippenham: 2009. Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Related Organocatalysts. [Google Scholar]
- 17.Patai S., Rappoport Z. Wiley; New York: 1991. The Chemistry of Amidines and Imidates. [Google Scholar]
- 18.Lee M.Y., Kim M.H., Kim J., Kim S.H., Kim B.T., Jeong I.H., Chang S., Kim S.H., Chang S.Y. Synthesis and SAR of sulfonyl- and phosphoryl amidine compounds as anti-resorptive agents. Bioorg. Med. Chem. Lett. 2010;20:541. doi: 10.1016/j.bmcl.2009.11.104. [DOI] [PubMed] [Google Scholar]
- 19.Sienkiewich P., Bielawski K., Bielawska A., Palka J. Effect of probiotic supplement on aflatoxicosis and gene expression in the liver of broiler chicken. Environ. Toxicol. Pharmacol. 2005;20:118. doi: 10.1016/j.etap.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Katagiri H., Tanaka Y., Furusho Y., Yashima E. Multicomponent cylindrical assemblies driven by amidinium-carboxylate salt-bridge formation. Angew. Chem. Int. Ed. 2007;46:2435. doi: 10.1002/anie.200603921. [DOI] [PubMed] [Google Scholar]
- 21.Barker J., Kilner M. The coordination chemistry of the amidine ligand. Coord. Chem. Rev. 1994;133:219. [Google Scholar]
- 22.Graham S.L., Shepard K.L., Anderson P.S., Baldwin J.J., Best D.B., Christy M.E., Freedman M.B., Gautheron P., Habecker C.N., Hoffman J.M., Lyle P.A., Michelson S.R., Ponticello G.S., Robb C.M., Schwam H., Smith A.M., Smith R.L., Sondey J.M., Strohmaier K.M., Sugrue M.F., Varga S.L. Topically active carbonic anhydrase inhibitors. 2. Benzo[b]thiophenesulfonamide derivatives with ocular hypotensive activity. J. Med. Chem. 1989;32:2548. doi: 10.1021/jm00132a009. [DOI] [PubMed] [Google Scholar]
- 23.Scholz T.H., Sondey J.M., Randall W.C., Schwam H., Thompson W.J., Mallorga P.J., Sugrue M.F., Graham S.L. A potent new class of .kappa.-receptor agonist: 4-substituted 1-(arylacetyl)-2-[(dialkylamino)methyl]piperazines. J. Med. Chem. 1993;36:2134. doi: 10.1021/jm00067a004. [DOI] [PubMed] [Google Scholar]
- 24.Deprez P., Heckmann B., Corbier A., Vevert J.P., Fortin M., Guillaume J. Balanced AT1 and AT2 angiotensin II antagonists. I. New orally active 5-carboxyl imidazolyl biphenyl sulfonylureas. Bioorg. Med. Chem. Lett. 1995;5:2605. [Google Scholar]
- 25.Heitsch H., Becker R.H.A., Kleemann H.W., Wagner A. 3N-Methylbiphenylsulfonylurea and -carbamate substituted imidazo[4,5-b]pyridines. Potent antagonists of the ANG II AT1 receptors. Bioorg. Med. Chem. 1997;5:673. doi: 10.1016/s0968-0896(97)00012-6. [DOI] [PubMed] [Google Scholar]
- 26.Bekhit A.A., Ashour H.M.A., Ghany Y.S.A., Bekhit A.E.D.A., Baraka A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Eur. J. Med. Chem. 2008;43:456. doi: 10.1016/j.ejmech.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Vernier W., Chong W., Rewolinski D., Greasley S., Pauly T., Shaw M., Dinh D., Ferre R.A., Nukui S., Ornelas M., Reyner E. Thioether benzenesulfonamide inhibitors of carbonic anhydrases II and IV: Structure-based drug design, synthesis, and biological evaluation. Bioorg. Med. Chem. 2010;18:3307. doi: 10.1016/j.bmc.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Andersen N.K., Chandak N., Brulikova L., Kumar P., Jensen M.D., Jensen F., Sharma P.K., Nielsen P. Efficient RNA-targeting by the introduction of aromatic stacking in the duplex major groove via 5-(1-phenyl-1,2,3-triazol-4-yl)-2′-deoxyuridines. Bioorg. Med. Chem. 2010;18:4702. doi: 10.1016/j.bmc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Yavari I., Ahmadian S., Ghazanfarpur-Darjani M., Solgi Y. Formation of N-sulfonylamidines by copper-catalyzed coupling of sulfonyl azides, terminal alkynes, and trialkylamines. Tetrahedron Lett. 2011;52:668. [Google Scholar]
- 30.He X.W., Shang Y.J., Jinsong H.U., Kai J.U., Jiang W., Wang S.F. Syntheses of N-sulfonyl-N,N-disubstituted amidines via a three-component free-radical coupling reaction of tertiary amines and arenesulfonyl azides with terminal alkynes. Sci. China Chem. 2012;55:214. [Google Scholar]
- 31.Bae I., Han H., Chang S. Highly efficient one-pot synthesis of N-Sulfonylamidines by Cu-Catalyzed three-component coupling of sulfonyl azide, alkyne, and amine. J. Am. Chem. Soc. 2005;27:2038. doi: 10.1021/ja0432968. [DOI] [PubMed] [Google Scholar]
- 32.Yoo E.J., Ahlquist M., Bae I., Sharpless K.B., Fokin V.V., Chang S. Mechanistic studies on the Cu-Catalyzed three-component reactions of sulfonyl azides, 1-Alkynes and amines, alcohols, or water: dichotomy via a common pathway. J. Org. Chem. 2008;73:5520. doi: 10.1021/jo800733p. [DOI] [PubMed] [Google Scholar]
- 33.Kim J., Lee S.Y., Lee J., Do Y., Chang S. Synthetic utility of ammonium salts in a Cu-Catalyzed three-component reaction as a facile coupling partner. J. Org. Chem. 2008;73:9454. doi: 10.1021/jo802014g. [DOI] [PubMed] [Google Scholar]
- 34.Ghasemi Z., Shojaei S., Shahrisa A. Copper iodide nanoparticles supported on magnetic aminomethylpyridine functionalized cellulose: a new heterogeneous and recyclable nanomagnetic catalyst for facile access to N-sulfonylamidines under solvent free conditions†. RSC Adv. 2016;6:56213. [Google Scholar]
- 35.Kim J., Stahl S.S. Cu-Catalyzed aerobic oxidative three-component coupling route to N-Sulfonyl amidines via an ynamine intermediate. J. Org. Chem. 2015;80:2448. doi: 10.1021/jo5029198. [DOI] [PubMed] [Google Scholar]
- 36.Yang T., Cui H., Zhang C., Zhang L., Su C.Y. Porous metal–organic framework catalyzing the three-component coupling of sulfonyl azide, alkyne, and amine. Inorg. Chem. 2013;52:9053. doi: 10.1021/ic4012229. [DOI] [PubMed] [Google Scholar]
- 37.Abbasvash L., Shadjou N. Synthesize of β-cyclodextrin functionalized dendritic fibrous nanosilica and its application for the removal of organic dye (malachite green) J. Mol. Recogn. 2020 doi: 10.1002/jmr.2850. [DOI] [PubMed] [Google Scholar]
- 38.Soleymani J., Hasanzadeh M., Somi M.H., Shadjou N. Highly sensitive and specific cytosensing of HT 29 colorectal cancer cells using folic acid functionalized-KCC-1 nanoparticles. A. Jouyban. Biosens. Bioelectron. 2019;132:122. doi: 10.1016/j.bios.2019.02.052. [DOI] [PubMed] [Google Scholar]
- 39.Azizi S., Shadjou N., Hasanzadeh M. KCC-1-NH2-DPA: an efficient heterogeneous recyclable nanocomposite for the catalytic synthesis of tetrahydrodipyrazolopyridines as a well-known organic scaffold in various bioactive derivatives. Nanocomposites. 2019;5:124. [Google Scholar]
- 40.Khantan N., Shadjou N., Hasanzadeh M. Synthesize of dendritic fibrous nano-silica functionalized by cysteine and its application as advanced adsorbent. Nanocomposites. 2019;5(4):104. [Google Scholar]
- 41.Bae I., Han H., Chang S. Highly efficient one-pot synthesis of N-Sulfonylamidines by Cu-Catalyzed three-component coupling of sulfonyl azide, alkyne, and amine. J. Am. Chem. Soc. 2005;127:2038. doi: 10.1021/ja0432968. [DOI] [PubMed] [Google Scholar]
- 42.Kim M.J., Kim B.R., Lee C.Y., Kim J. N-Sulfonyl amidine synthesis via three-component coupling reaction using heterogeneous copper catalyst derived from metal-organic frameworks. Tetrahedron Lett. 2016;57:4070. [Google Scholar]
- 43.Jagadale M., Bhange P., Salunkhe R., Bhange D., Rajmane M., Rashinkar G. A modular approach for multicomponent synthesis of amidines using modified Scolecite. Appl. Catal. A. 2016;511:95. [Google Scholar]
- 44.Shojaei S., Ghasemi Z., Shahrisa A. Cu(I)@Fe3O4 nanoparticles supported on imidazolium-based ionic liquid-grafted cellulose: green and efficient nanocatalyst for multicomponent synthesis of N-sulfonylamidines and N-sulfonylacrylamidines. Appl. Organomet. Chem. 2017:e3788. [Google Scholar]
- 45.Tajbakhsh M., Farhang M., Hosseinzadeh R., Sarrafi Y. Nano Fe3O4 supported biimidazole Cu(i) complex as a retrievable catalyst for the synthesis of imidazo[1,2-a]pyridines in aqueous medium. RSC Adv. 2014;4:23116. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.