Abstract
Helminths born diseases are related to pitiable management practices and improper control strategies. The medicinal plants contain various phytoconstituents that are liable for their anthelmintic activity. The aerial parts of the Chenopodium album were successively extracted with microwaves assisted extraction using petroleum ether, ethyl acetate, methanol, hydroalcoholic and aqueous solvents to get respective extracts (CAPE, CAEE, CAME, CAHE, and CAAE). All the extracts were analyzed for preliminary phytochemical screening for the identification of phytoconstituents. The anthelmintic activity was analyzed on Indian adult earthworms Eisenia foetida using piperazine citrate (PCT) as a standard drug. All the extracts (apart from CAAE) lead to paralysis and fatality of the earthworms. CAEE extract exhibits highly significant anthelmintic activity at a 10 mg/ml concentration by causing paralysis and fatality of earthworms and was more potent than PCT suspension. At a concentration of 10 mg/ml, paralysis and death time for CAEE was recorded as (10.08 ± 1.11) and (65.28 ± 2.09) respectively, while for standard piperazine citrate, it was recorded as (22.96 ± 1.12) and (65.09 ± 1.23). The CAEE exhibits two major compounds by LC-MS, i.e., NG and DG, that are mainly accountable for the Chenopodium album anthelmintic activity. The plant possesses GABA-mimetic action and thereby leads to flaccid, reversible paralysis of the body wall muscle.
Keywords: Chenopodium album, Eisenia foetida, Piperazine citrate, Anthelmintic activity, LC-MS
Chenopodium album, Eisenia foetida, Piperazine citrate, Anthelmintic activity, LC-MS
1. Introduction
Helminths contaminations or infections influence a vast number of global populations, especially in rising countries [1]. Approximately 819 million people globally affected with Ascaris, 438 million by means of hookworm, and 464 million by Trichuris [2, 3]. Helminths infection occurs due to the warm, humid equatorial regions and inadequate hygiene sanitation facilities [4]. Helminths are classified into various categories, i.e., Tapeworms, roundworms, and flukes, and predominantly affect humans and animals, thereby causing physiological damage. Helminths born diseases are related to pitiable management practices and deprived and improper control strategies [5, 6, 7]. Medicinal plants are vital in managing parasitic infections and possess several advantages over available synthetic medicine. The medicinal plants contain various secondary metabolites that exhibit anthelmintic activity [8, 9, 10].
The Chenopodium album Linn. is commonly recognized as bathua classified into the Chenopodiaceae family, widely spread across the globe, and contains various bioactive constituents that help treat various anticancer diseases hepatoprotective, antioxidant, antinociceptive activity, etc. [11, 12, 13]. The plant was reported for its anthelmintic activity [14, 15, 16]. Nevertheless, the responsible secondary metabolites and their mechanism for anthelmintic activity have never been explored. Therefore, the current research attempts to estimate the in vitro anthelmintic effect of various extracts obtained from the aerial parts of Chenopodium album in earthworm Eisenia foetida to estimate and authenticate the ethnobotanical use in a scientific manner.
2. Materials and methods
2.1. Collection and authentication of plant material
Air-dried aerial parts of Chenopodium album were collected from Ludhiana's local areas (Punjab). They were authenticated by Dr. Sunita Garg, Emeritus Scientist, Department of Raw Material Herbarium & Museum, National Institute of Sciences Communication and Information Resources, New Delhi, under the voucher specimen number Ref. No. NISCAIR/RHMD/Consult/2018/3227/28-1.
2.2. Extraction of plant material
The aerial parts of the Chenopodium album were shade dried at room temperature and crushed by a pulverizer. The powder was sequentially extracted by microwaves assistant technique [17, 18] with slight modifications using petroleum ether, ethyl acetate, methanol, hydroalcoholic, and aqueous solvents to get respective extracts. The solvents were recovered by distillation under reduced pressure by a rotary evaporator. The petroleum ether extract (CAPE), ethyl acetate extract (CAEE), methanol extract (CAME), hydroalcoholic extract (CAHE), and aqueous extract (CAAE) were placed in desiccators till further use. All these extracts were used to evaluate anthelmintic activity.
2.3. Phytochemical screening
All the extracts CAPE, CAEE, CAME, CAHE and CAAE were analyzed for preliminary phytochemical screening for the identification of various phytoconstituents such as flavonoids, tannins, and phenolic compounds, terpenoids, alkaloids, steroid, glycosides, saponins, proteins, amino acids, and carbohydrates [19, 20, 21, 22].
2.3.1. Test for alkaloids
To the dried extracts, add dilute HCL dropwise and filter it. The resulting filtrate was screened for the preliminary tests of alkaloids.
Test with Dragendorff's reagent: The reagent was mixed with the filtrate on a watch glass to observe orange, brown precipitates that reveal the alkaloids' occurrence.
Test with Mayer's reagent: The reagent was mixed with the filtrate on a watch glass to observe cream-colored precipitates that reveal the alkaloids' occurrence.
Test with Wagner's reagent: The reagent was mixed with the filtrate on a watch glass to observe reddish-brown precipitates that reveal the alkaloids' occurrence.
Test with Hager's reagent: The reagent was mixed with the filtrate on a watch glass to observe yellow precipitates that reveal the alkaloids' occurrence.
2.3.2. Test for tannins & phenolic compounds
All the dried extracts were mixed with ethanol and filtered for performing the various preliminary tests for the tannins.
5% FeCl3 solution: To the filtrate, FeCl3 solution was mixed, monitored for the appearance of a dark blue-black color that reveals the tannins' occurrence.
Lead acetate solution: The lead acetate was mixed with the filtrate, monitored for white precipitates' appearance that reveals the tannins' occurrence.
Gelatin solution: The gelatin solution was mixed with the filtrate, monitored for white precipitates' appearance revealing the tannins' occurrence.
Iodine solution test: The dilute iodine solution was mixed with the filtrate, monitored for red color appearance reveals the tannins' occurrence.
2.3.3. Test for flavonoids
All the dried extracts were mixed with ethanol, filtered for performing the various preliminary identifications for the flavonoids.
Shinoda test: The 95% ethanol (5 ml) and HCL was added to the filtrate and after that, magnesium turning was added, monitored for the presence of pink color reveals the occurrence of flavonoids.
Lead acetate solution: The solution was mixed with the filtrate, monitored for the appearance of yellow-colored precipitates reveal the occurrence of flavonoids.
2.3.4. Test for steroids
All the dried extracts were mixed with chloroform and filtered for performing the various preliminary tests for the steroids.
Salkowski test: The chloroform (2 ml) and conc. H2SO4 were mixed with filtrate. Afterward, it was monitored for red and greenish-yellow fluorescence for the CHCl3 and acid layer, respectively, which reveals the occurrence of steroids.
Liebermann Burchard test: The chloroform (2 ml), acetic anhydride (1–2 ml), and conc. H2SO4 were mixed with filtrate, monitored for red, blue, and green color, revealing the steroids' occurrence.
2.3.5. Test for saponins
All the dried extracts were mixed with water and filtered for performing the various preliminary tests for the saponins.
Foam test: The alcoholic and aqueous filtrate was vigorously shaken with water, monitored for the appearance of tenacious foam, revealing the saponins' occurrence.
2.3.6. Test for amino acids and proteins
Biuret test: Reagent was added to the test solution, monitored for the appearance of violet or pink color, revealing the proteins' occurrence.
Million's reagent test: The reagent was added to the test solution, observed for white precipitates' appearance. After that, white precipitates were warmed and kept aside to form a dark red color solution that reveals the proteins' occurrence.
Ninhydrin reagent test: The 5% ninhydrin solution was mixed with the test sample and kept for 10 min on a water bath, monitored for the presence of purple or bluish color reveals the occurrence of the proteins.
2.3.7. Test for carbohydrates
All the extracts were mixed with water and filtered to perform the various preliminary tests for the carbohydrates.
Test with Molisch's reagent: The α-naphthol solution prepared in alcohol and concentrated sulfuric acid was mixed with the filtrate, monitored for the development of the violet ring between the two layers test tube reveals the occurrence of the carbohydrates and glycosides.
Test with Fehling solution: Both A and B Fehling solution was mixed, boiled for a minute, and then added to the filtrate and further kept for 5–10 min on a water bath, monitored for yellow, dark red precipitates reveal the occurrence of carbohydrates and glycosides.
Benedict's reagent: To the filtrate, add reagent and kept for 5 min on a water bath, monitored for a green, yellow and red color solution. The color of the solution depends on the availability of the reducing sugar in the sample.
Barfoed test: The reagent was mixed with the filtrate and kept for 1–2 min on a water bath, monitored for the presence of red-colored precipitates, which exhibits the occurrence of the monosaccharides.
2.3.8. Test for triterpenoid
All the extracts were mixed with ethanol and filtered for performing the various preliminary tests for the triterpenoid.
Thionyl chloride test: Tin metal bead and thionyl chloride solution were mixed with the filtrate, monitored for the appearance of pink color with effervescence exhibiting the triterpenoid occurrence.
2.4. Anthelmintic activity
The anthelmintic activity was analyzed on Indian adult earthworms (Eisenia foetida) that possess similarity with human intestinal parasites. The earthworms were purchased and authenticated by Mahavir Organic Manure, Phillaur, Punjab, India, having reference No 82 and were kept under standard conditions of living.
2.5. Chemicals
Piperazine citrate (PCT) was purchased from (GlaxoSmithKline Pharmaceuticals), Sodium carboxymethyl cellulose (CMC), and Sodium chloride from (Loba Chemicals, Mumbai).
2.6. Experimental design
The anthelmintic activity was assessed on Eisenia foetida with slight modifications [23, 24, 25]. The adult earthworms (E. foetida) with 6–12 cm length and 0.2–0.4 mm diameter were separated into 35 groups consisting of five earthworms in each group. The vehicle control (Group I–V) was administrated with normal saline and 1% CMC at different concentrations, i.e., 2, 4, 6, 8, and 10 mg/ml, respectively. The standard groups (Group VI- X) received the PCT suspension at concentrations of 2, 4, 6, 8, and 10 mg/ml, respectively. All the test samples CAPE (Group XI-XV), CAEE (Group XVI-XX), CAME (Group XXI-XXV), CAHE (XXVI-XXX), and CAAE (Group XXXI- XXXV) were also administrated with different concentrations, i.e., 2, 4, 6, 8 and 10 mg/ml respectively. The time taken by each group for causing paralysis and fatality to Eisenia foetida was observed separately for the individual worm. The paralysis was confirmed when there is the loss of movement and contraction in earthworms when it is pressed by a finger, whereas the fatality of the earthworm is represented by the loss of their motility with the fading of their body color. The experiment was carried out in triplicate.
2.7. Preparation of standard
The oral suspension of piperazine citrate (PCT) was dissolved in normal saline to prepare the stock solution (20 mg/ml). The various dilutions (2–10 mg/ml) were prepared with normal saline from the stock solution. These dilutions were administrated as a standard drug in the earthworm.
2.8. Preparation of test sample
The stock solution (200 mg/ml) was prepared from all the extracts (CAPE, CAEE, CAME, CAHE) using carboxymethylcellulose (1% CMC), and normal saline was used for preparing the CAAE extract. Further various dilutions (2–10 mg/ml) were prepared from the stock solutions for all the extracts.
2.9. LC-MS of the bioactive extract
The bioactive extract (CAEE) obtained from the aerial parts of the plant was characterized by LC-MS using Micromass Q-Tof Micro (waters) instrument for the phytoconstituents' presence responsible the activity. It is equipped with electrospray ionization (ESI) and atmospheric pressure chemical ionization (APcI) sources having a mass Range of 4000 amu in quadruple and 20000 amu in Time of Flight. A hexapole collision cell between the two mass analyzers is used to induce fragmentation to study the structural investigations while using an MS/MS mode instrument. The Mass Spectrometer is coupled with Waters 2795 HPLC having quaternary pumping configured for flow rates from 0.05- 5.0 ml/Min, and water + 0.1 formic acid/Acetonitrile was used as a mobile phase. The autosampler is configured with a 100 micro-liter syringe. The identified components were correlated with the compounds' mass fragments reported in available literature [26, 27].
2.10. Molecular docking studies
For this molecular modeling software, Autodock-vina [28, 29, 30] was used by the pdb or protein selected 4MS3 as a GABA (gamma-aminobutyric acid) receptor of (human) due to the unavailability of nematode GABA protein crystal structure and were downloaded from the protein data bank. The GABA receptor is a well-known target for piperazine exhibits weak GABA-mimetic and causes reversible paralysis of body wall muscle [31, 32, 33]. The structures of these molecules were drawn by ChemDraw and changed to 3D. Further minimizations of energy were carried out using the MM2 Interface program on ChemBio3D Ultra 12.0, and molecules were saved in pdb format (Cambridge Soft). To identify the most active molecule, removing the internal ligand was initially done, and docking was carried out in a similar pattern to an actual ligand.
3. Results
3.1. Extraction of the plant material
The aerials parts of the plant Chenopodium album was successfully extracted with different solvents. The % yield of the various extracts CAPE, CAEE, CAME, CAHE, and CAAE were found to be 6.83, 8.58, 12.15, 10.12, and 14.56 (w/w), respectively (Table 1).
Table 1.
Color, consistency and % yield of Chenopodium album extracts.
| Extract | Color observed in Day light | Consistency | % Yield (w/w) |
|---|---|---|---|
| CAPE | Light Green | Semisolid | 6.83 |
| CAEE | Dark Green | Semisolid | 8.58 |
| CAME | Dark Green | Semisolid | 12.15 |
| CAHE | Dark Green | Semisolid | 10.12 |
| CAAE | Light Green | Semisolid | 14.56 |
CAPE: Chenopodium album petroleum ether extract; CAEE: Chenopodium album ethyl acetate extract; CAME: Chenopodium album methanolic extract; CAHE: Chenopodium album hydro alcoholic extract; CAAE: Chenopodium album aqueous extract.
3.2. Phytochemical screening
All the extracts of the plant were analyzed for the occurrence of various secondary metabolites. The phytochemical screening exhibits triterpenoid and steroids in CAPE extract, Flavonoids, Alkaloids, steroids, and tannins in CAEE extract. Furthermore, the flavonoids, alkaloids, tannins, saponins, carbohydrates, and proteins were present in CAME and CAHE, whereas the CAAE showed carbohydrates and proteins (Table 2).
Table 2.
Phytochemical screening of the various extracts from Chenopodium album.
| S. No | Chemical Test | CAPE | CAEE | CAME | CAHE | CAAE |
|---|---|---|---|---|---|---|
| 1 | Alkaloids | --- | + | ++ | + | --- |
| 2 | Flavonoids | --- | ++ | +++ | + | --- |
| 3 | Tannins and phenolic compounds | --- | ++ | ++ | + | --- |
| 4 | Saponins | --- | --- | ++ | ++ | --- |
| 5 | Triterpenoid | ++ | --- | --- | --- | --- |
| 6 | Steroids | ++ | + | --- | --- | --- |
| 7 | Carbohydrates | --- | --- | ++ | ++ | ++ |
| 8 | Protein and amino acids | --- | --- | ++ | ++ | ++ |
+: Weak positive test; ++: Low positive test; +++: Strong positive test; -: Negative test. CAPE: Chenopodium album petroleum ether extract; CAEE: Chenopodium album ethyl acetate extract; CAME: Chenopodium album methanolic extract; CAHE: Chenopodium album hydro alcoholic extract; CAAE: Chenopodium album aqueous extract.
3.3. Anthelmintic activity
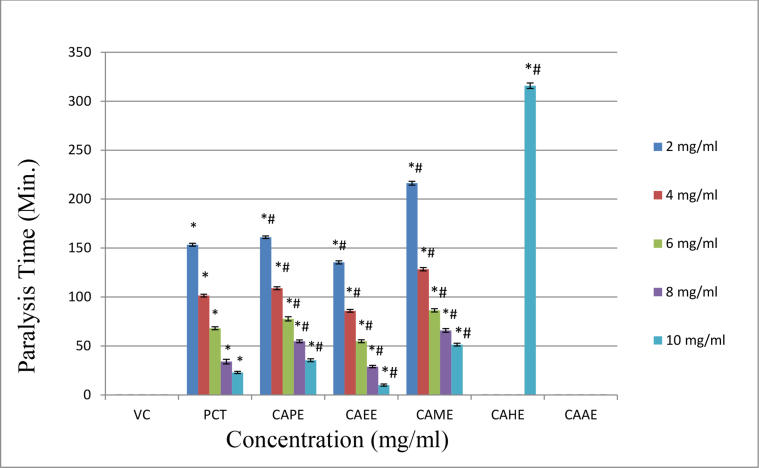
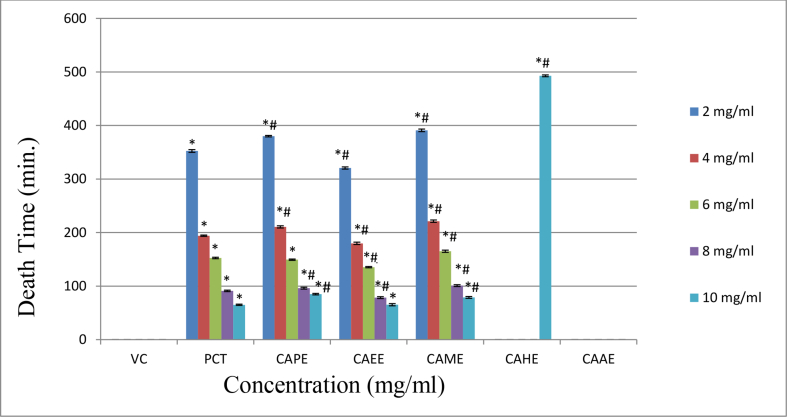
The aerial parts of the Chenopodium album showed potent anthelmintic activity when evaluated on Indian earthworm Eisenia foetida. The vehicle control does not produce any effect on the earthworm. However, all the extracts (apart from CAAE extract) lead to the earthworm's paralysis and fatality. The CAEE extract posse's highly significant potent anthelmintic activity compared to the standard PCT at a concentration of 10 mg/ml. The paralysis and death time for CAEE at 10 mg/ml concentration was recorded as (10.08 ± 1.11) and (65.28 ± 2.09) respectively, while for standard PCT, it was recorded as (22.96 ± 1.12) and (65.09 ± 1.23). The CAPE and CAME also showed significant anthelmintic activity but less than that of standard PCT. However, the CAHE showed very mild anthelmintic activity at 10 mg/ml concentration only, while the CAAE did not possess anthelmintic activity (Table 3, Figures 1 and 2). The results represent that CAEE extract showed a highly significant anthelmintic effect by causing paralysis and death to the earthworm compared to the standard drug. Therefore, CAEE extract was further explored for the identification of the responsible phytoconstituents.
Table 3.
Represents the anthelmintic activity of the different extracts of Chenopodium album.
| Conc. (mg/ml) | Paralysis Time (Minutes) Mean ± SD |
Death Time (Minutes) Mean ± SD |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VC | PCT | CAPE | CAEE | CAME | CAHE | CAAE | VC | PCT | CAPE | CAEE | CAME | CAHE | CAAE | |
| 2 | --- | 153.43 ± 1.49∗ | 161.10 ± 1.34∗# | 135.45 ± 1.61∗# | 216.25 ± 1.99∗# | --- | --- | --- | 352.49 ± 2.46∗ | 380.23 ± 1.34∗# | 320.66 ± 2.09∗# | 390.89 ± 2.33∗# | --- | --- |
| 4 | --- | 101.52 ± 1.43∗ | 109.10 ± 1.50∗# | 85.95 ± 1.36∗# | 128.40 ± 1.67∗# | --- | --- | --- | 194.16 ± 1.31∗ | 210.72 ± 2.01∗# | 179.86 ± 2.24∗# | 221.29 ± 2.30∗# | --- | --- |
| 6 | --- | 68.19 ± 1.53∗ | 77.74 ± 2.20∗# | 54.86 ± 1.47∗# | 86.45 ± 1.76∗# | --- | --- | --- | 152.45 ± 1.35∗ | 149.42 ± 1.15∗ | 135.35 ± 1.24∗# | 165.02 ± 2.05∗# | --- | --- |
| 8 | --- | 34.14 ± 2.35∗ | 54.84 ± 1.39∗# | 29.02 ± 1.23∗# | 65.86 ± 1.83∗# | --- | --- | --- | 90.98 ± 1.36∗ | 96.25 ± 1.82∗# | 78.48 ± 1.66∗# | 101.06 ± 1.76∗# | --- | --- |
| 10 | --- | 22.96 ± 1.12∗ | 35.52 ± 1.55∗# | 10.08 ± 1.11∗# | 51.45 ± 1.43∗# | 315.78 ± 2.72∗# | --- | --- | 65.09 ± 1.23∗ | 84.94 ± 1.43∗# | 65.28 ± 2.09∗ | 78.93 ± 171∗# | 492.88 ± 1.81∗# | --- |
∗All the Values are expressed as Mean ± SD; N = 5; Statistical analysis was performed using one way ANOVA followed by Turkey's multiple comparison test, ∗ represents p < 0.05 vs. Vehicle Control, # represents p < 0.05 vs. Standard(PCT); PCT: Piperazine citrate; CAPE: Chenopodium album petroleum ether extract; CAEE: Chenopodium album ethyl acetate extract; CAME: Chenopodium album methanolic extract; CAHE: Chenopodium album hydro alcoholic extract; CAAE: Chenopodium album aqueous extract.
Figure 1.
Bar chart represents the time (minutes) taken for the paralysis of Eisenia foetida. Statistical analysis was performed using one way ANOVA followed by Turkey's multiple comparison test, ∗ represents p < 0.05 vs. Vehicle Control, # represents p < 0.05 vs. Standard (PCT); PCT: Piperazine citrate, CAPE: Chenopodium album petroleum ether extract, CAEE: Chenopodium album ethyl acetate extract, CAME: Chenopodium album methanolic extract, CAHE: Chenopodium album hydro alcoholic extract, CAAE: Chenopodium album aqueous extract.
Figure 2.
Bar chart represents the time (minutes) taken for the death of Eisenia foetida. Statistical analysis was performed using one way ANOVA followed by Turkey's multiple comparison test, ∗ represents p < 0.05 vs. Vehicle Control, # represents p < 0.05 vs. Standard (PCT); PCT: Piperazine citrate, CAPE: Chenopodium album petroleum ether extract, CAEE: Chenopodium album ethyl acetate extract, CAME: Chenopodium album methanolic extract, CAHE: Chenopodium album hydro alcoholic extract, CAAE: Chenopodium album aqueous extract.
3.4. LC-MS characterization of CAEE
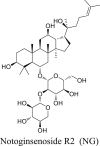
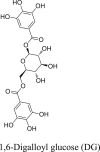
The bioactive extract CAEE was analyzed by LC-MS spectra and predicted two significant compounds, i.e., notoginsenoside R2 (NG) having retention time 7.14 with a mass of m/z 770.98 (49%) and 1,6-Digalloyl glucose (DG) at 9.13 retention time with a mass of m/z 484.36 (18.53%) (Table 4). This represents that both these compounds are the primary active constituents of the CAEE extract and are primarily responsible for the plant's anthelmintic activity.
Table 4.
LC-MS identification of major phytoconstituents in the CAEE extract.
3.5. Molecular docking
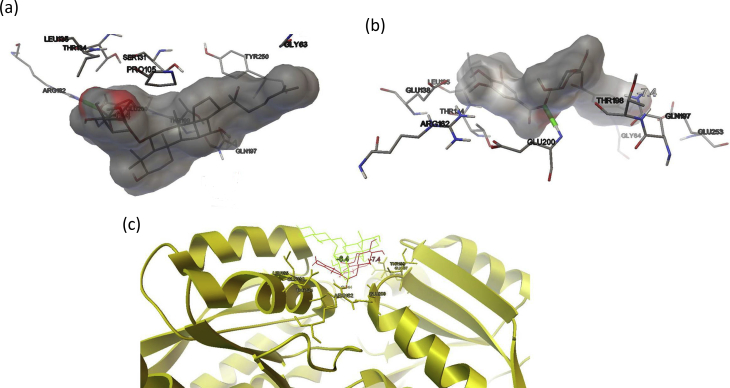
Both the phytochemical NG and DG were found to be most active with binding affinities of -6.4 kcal/mol, -7.4 kcal/mol, respectively, in comparison to internal ligand gamma-aminobutyric acid (-4.2 kcal/mol) and also from piperazine (-3.3 kcal/mol) on GABA receptor (Table 5). A detailed interaction for NG and DG was represented in Figure 3a and b respectively at the GABA receptor binding site. An overlay of NG and DG was also represented in Figure 3c at the GABA receptor binding site. NG showed hydrogen bonds (green color cylinder shown in Figure 3a) to the amino group of ARG162 at the binding site while other hydrophobic interactions were observed with LEU135, polar electrostatic interaction with THR134, SER131, THR199, and PRO105 (Figure 3a). DG also showed similar interactions and found to be better than NG. It showed hydrogen bond GLU200, while hydrophobic interaction with GLY64 and LEU195. Different amino acid residues like GLU138, THR134, ARG162, THR198, and GLU253 are involved in electrostatic polar interaction with DG (Figure 3b).
Table 5.
Molecular docking results of the major phytoconstituents in CAEE.
| S. No. | Molecule | Binding affinity (kcal/mol) GABA receptor (4MS3) |
|---|---|---|
| 1 | NG | -6.4 |
| 2 | DG | -7.4 |
| 3 | Gamma Aminobutyric acid | -4.2 |
| 4 | Piperazine | -3.3 |
Figure 3.
(a) Interaction of NG (molecular surface) in the binding site of GABA receptor (b) Interaction of DG (molecular surface) in the binding site of GABA receptor (c) Ribbon structure of GABA receptor (yellow color) and overlay of NG (green color) and DG (red color).
4. Discussion
Helminths infection occurs due to the warm, humid equatorial regions and inadequate hygiene sanitation facilities [34, 35]. The medicinal plants contain various secondary metabolites that exhibit anthelmintic activity [36, 37, 38]. The plant Chenopodium album plant was earlier reported for its anthelmintic activity [14, 15, 16]. Nevertheless, the responsible secondary metabolites and their mechanism for anthelmintic activity have never been explored. In this context, the Chenopodium album aerial parts were extracted using the microwave-assisted technique with different solvents, and the preliminary phytochemical screening of the extracts represents the occurrence of various secondary metabolites. The plant extracts CAPE, CAEE, CAME, CAHE and CAAE were screened for their anthelmintic activity on adult Indian earthworm Eisenia foetida. All the extracts (apart from CAAE extract) lead to paralysis and fatality of the earthworm. CAEE extract exhibits potent anthelmintic activity at a 10 mg/ml concentration by causing paralysis and death time of earthworms and was more potent than PCT suspension. At a concentration of 10 mg/ml, paralysis and death time for CAEE was recorded as (10.08 ± 1.11) and (65.28 ± 2.09) respectively, while for standard PCT, it was recorded as (22.96 ± 1.12) and (65.09 ± 1.23).
Further, LC-MS spectra analyzed the bioactive extract CAEE and predicted two significant compounds, i.e., NG and DG. It is predicted from LC-MS that both these compounds are the plant's primary active constituents, i.e., primarly responsible for the plant's anthelmintic activity. Moreover, we explored the molecular docking studies to predict the mechanism of action of the identified compounds. Both the phytochemical NG and DG were most active with binding affinities of -6.4 kcal/mol, -7.4 kcal/mol, respectively, compared to internal ligand gamma-aminobutyric acid (4.2 kcal/mol) and also from piperazine (3.3 kcal/mol) on GABA receptor. The PCT possess weak GABA-mimetic action that leads to reversible paralysis of body wall muscle [33, 39, 40]. The NG and DG are the two primary compounds present in the plant Chenopodium album primarily responsible for its GABA-mimetic action, causing reversible paralysis of body wall muscle in the earthworm. This finding supports the potential role of the Chenopodium album as an anthelmintic agent.
5. Conclusion
All the extracts (apart from CAAE extract) lead to paralysis and fatality of the earthworm. CAEE extract showed potent anthelmintic activity at a concentration of 10 mg/ml by causing paralysis and death time of earthworms and was more potent than PCT suspension. The CAEE exhibits two significant compounds, i.e., NG and DG, mainly responsible for the plant's anthelmintic activity. The NG act and DG possess GABA-mimetic action and thereby leads to reversible paralysis of body wall muscle.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are thankful to the management of Lovely Professional University, Phagwara, and PCTE Group of Institutes for providing the necessary support and research facility for conducting the research work.
References
- 1.Jourdan P.M., Lamberton P.H.L., Fenwick A., Addiss D.G. Soil-transmitted helminth infections. Lancet. 2018;391:252–265. doi: 10.1016/S0140-6736(17)31930-X. [DOI] [PubMed] [Google Scholar]
- 2.Brooker S., Bethony J., Hotez P.J. Human hookworm infection in the 21st century. Adv. Parasitol. 2004:198–286. doi: 10.1016/S0065-308X(04)58004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collender P.A., Kirby A.E., Addiss D.G., Freeman M.C., Remais J.V. Methods for quantification of soil-transmitted helminths in environmental media: current techniques and recent advances. Trends Parasitol. 2015:1–15. doi: 10.1016/j.pt.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salam N., Azam S. Prevalence and distribution of soil-transmitted helminth infections in India. BMC Publ. Health. 2017;17:1–12. doi: 10.1186/s12889-017-4113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mascarini-Serra L. Prevention of soil-transmitted helminth infection. J. Glob. Infect. Dis. 2011;3:175–182. doi: 10.4103/0974-777X.81696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira L.E., Castro P.M.N., Chagas A.C.S., França S.C., Beleboni R.O. In vitro anthelmintic activity of aqueous leaf extract of Annona muricata L. (Annonaceae) against Haemonchus contortus from sheep. Exp. Parasitol. 2013;134:327–332. doi: 10.1016/j.exppara.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Rehman A., Ullah R., Uddin I., Zia I., Rehman L., Abidi S.M.A. In vitro anthelmintic effect of biologically synthesized silver nanoparticles on liver amphistome, Gigantocotyle explanatum. Exp. Parasitol. 2019;198:95–104. doi: 10.1016/j.exppara.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho C.O., Chagas A.C.S., Cotinguiba F., Furlan M., Brito L.G., Chaves F.C.M., Stephan M.P., Bizzo H.R., Amarante A.F.T. The anthelmintic effect of plant extracts on Haemonchus contortus and Strongyloides venezuelensis. Vet. Parasitol. 2012;183:260–268. doi: 10.1016/j.vetpar.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 9.Wink M. Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules. 2012;17:12771–12791. doi: 10.3390/molecules171112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozan E., Küpeli Akkol E., Süntar I. Potential anthelmintic activity of Pelargonium endlicherianum Fenzl. J. Ethnopharmacol. 2016;187:183–186. doi: 10.1016/j.jep.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 11.Jain N., Singhai A. Protective effects of Chenopodium album (L.) on ethanol - mediated hepatotoxicity and oxidative stress. Planta Med. 2012;78:34–39. [Google Scholar]
- 12.Nowak R., Szewczyk K., Gawlik-Dziki U., Rzymowska J., Komsta Ł. Antioxidative and cytotoxic potential of some Chenopodium L. species growing in Poland. Saudi J. Biol. Sci. 2016;23:15–23. doi: 10.1016/j.sjbs.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhary N., Prabhu K.S., Prasad S.B., Singh A., Agarhari U.C., Suttee A. Phytochemistry and Pharmacological exploration of Chenopodium album: current and future perspectives. Res. J. Pharm. Technol. 2020;13:3933–3940. [Google Scholar]
- 14.Kumar R.R., Vatsya S., Yadav C.L. Anthelmintic activity and phytochemical analysis of Chenopodium. Res. Rev. J. Vet. Sci. 2016;2:53–57. [Google Scholar]
- 15.Lone B.A., Chishti M.Z., Bhat F.A., Tak H., Bandh S.A., Khan A. Evaluation of anthelmintic antimicrobial and antioxidant activity of Chenopodium album. Trop. Anim. Health Prod. 2017;49:1597–1605. doi: 10.1007/s11250-017-1364-y. [DOI] [PubMed] [Google Scholar]
- 16.Jabbar A., Zaman M.A., Iqbal Z., Yaseen M., Shamim A. Anthelmintic activity of Chenopodium album (L.) and Caesalpinia crista (L.) against trichostrongylid nematodes of sheep. J. Ethnopharmacol. 2007;114:86–91. doi: 10.1016/j.jep.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Chan C.H., Yusoff R., Ngoh G.C., Kung F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A. 2011;1218:6213–6225. doi: 10.1016/j.chroma.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 18.Florez N., Conde E., Domínguez H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 2015:1–18. [Google Scholar]
- 19.Choudhary N., Bijjem K.R.V., Kalia A.N. Antiepileptic potential of flavonoids fraction from the leaves of Anisomeles malabarica. J. Ethnopharmacol. 2011;135:238–242. doi: 10.1016/j.jep.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Kaur H., Amini M.H., Prabhakar P.K., Singh A., Suttee A. Phytochemical screening and antimicrobial activity of caesalpinia sappan L. Leaves. Int. J. Pharmacogn. Phytochem. Res. 2016;8:1040–1045. [Google Scholar]
- 21.Rana S., Suttee A. Phytochemical investigation and evaluation of free radical scavenging potential of Benincasa hispida peel extracts. Int. J. Curr. Pharm. Rev. Res. 2012;3:43–48. [Google Scholar]
- 22.Gul R., Jan S.U., Faridullah S., Sherani S., Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from ephedra intermedia indigenous to Balochistan. Sci. World J. 2017:1–7. doi: 10.1155/2017/5873648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh A., Das S., Dey M. Determination of anthelmintic activity of the leaf and bark extract of Tamarindus Indica linn. Indian J. Pharm. Sci. 2011;73:104. doi: 10.4103/0250-474X.89768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harjit K., Amini M.H., Suttee A. Evaluation of antioxidant and anthelmintic properties of Caesalpinia sappan L. Leaves. Int. J. Pharmacogn. Phytochem. Res. 2016;8:362–368. [Google Scholar]
- 25.Singh G., Suttee A., Barnwal R.P., Singla N., Sharma A., Chatterjee M., Kaura G., Chanana V., Mishra V.K. Investigation of in vitro anthelmintic activity of Caesalpiniapulcherrimaleaves. Plant Arch. 2019;19:4527–4530. [Google Scholar]
- 26.Mena P., Calani L., Dall’Asta C., Galaverna G., García-Viguera C., Bruni R., Crozier A., Del Rio D. Rapid and comprehensive evaluation of (Poly)phenolic compounds in pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules. 2012;17:14821–14840. doi: 10.3390/molecules171214821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S., Liu L., Wang L., Hu Y., Zhang W., Liu R. Structural characterization and identification of major constituents in jitai tablets by high-performance liquid chromatography/diode-array detection coupled with electrospray ionization tandem mass spectrometry. Molecules. 2012;17:10470–10493. doi: 10.3390/molecules170910470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trott O., Olson A.J. Software news and update AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:454–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiametis A.S., Silva M.A., Romeiro L.A.S., Martins J.B.L., Gargano R. Potential acetylcholinesterase inhibitors: molecular docking, molecular dynamics, and in silico prediction. J. Mol. Model. 2017;67:23–69. doi: 10.1007/s00894-017-3228-9. [DOI] [PubMed] [Google Scholar]
- 30.Bashary R., Khatik G.L. Design, and facile synthesis of 1,3 diaryl-3-(arylamino)propan-1-one derivatives as the potential alpha-amylase inhibitors and antioxidants. Bioorg. Chem. 2019;82:156–162. doi: 10.1016/j.bioorg.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Page S.W. Antiparasitic drugs. Small Anim. Clin. Pharmacol. 2008:198–260. [Google Scholar]
- 32.Abiramalatha T., Mehndiratta S., Rajeshwari K., Dubey A. Piperazine citrate induced myoclonus in a child. Indian J. Pharmacol. 2013;45:640–649. doi: 10.4103/0253-7613.121391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holden-Dye L., Walker R.J. WormBook; 2014. Anthelmintic Drugs and Nematicides: Studies in Caenorhabditis elegans; pp. 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abossie A., Seid M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BMC Publ. Health. 2014;14:1–8. doi: 10.1186/1471-2458-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdeltawabi M.S., El Seddik N., Salem H.K. World wide epidemiology of helminths infection. Hum. Helminthiasis. 2017:3–16. [Google Scholar]
- 36.Mohit C., Rakesh P., Rohit S. A review on medicinal plants having anthelmintic activity. Int. J. Curr. Adv. Res. 2019;8:17177–17180. [Google Scholar]
- 37.Zaman M.A., Iqbal Z., Sindhu Z.U.D., Abbas R.Z., Qamar M.F. An overview of plants with acaricidal and anthelmintic properties. Int. J. Agric. Biol. 2017;19:957–968. [Google Scholar]
- 38.Waterman C., Smith R.A., Pontiggia L., DerMarderosian A. Anthelmintic screening of Sub-Saharan African plants used in traditional medicine. J. Ethnopharmacol. 2010;127:755–759. doi: 10.1016/j.jep.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Hondebrink L., Hermans E.J., van Kleef R.G., Meulenbelt J. The role of GABA receptors during intoxications with designer drugs: a mechanism-based approach for piperazine derivatives. Clin. Toxicol. 2014;52:395–396. [Google Scholar]
- 40.Hondebrink L., Hermans E.J.P., Schmeink S., van Kleef R.G.D.M., Meulenbelt J., Westerink R.H.S. Structure-dependent inhibition of the human α1β2γ2 GABAA receptor by piperazine derivatives: a novel mode of action. Neurotoxicology. 2015;51:1–9. doi: 10.1016/j.neuro.2015.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.