Highlights
-
•
Glomangiopericytomas, which arises in the nasal cavity and may extend into the paranasal sinuses, is categorized as a borderline low malignancy tumor by the WHO classification.
-
•
Complete transnasal endoscopic excision is the optimal treatment.
-
•
A regular postoperative follow-up is recommended for early finding of tumor recurrence.
Keywords: Glomangiopericytoma, Inferior nasal turbinate, Endoscopic surgery
Abstract
Introduction
Glomangiopericytoma is defined as a sinonasal tumor with perivascular myoid phenotype, which was first described in 1942 by Stout and Murray as a soft tissue tumor with characteristic proliferation including branching vessels and small vessel perivascular hyalinization.
The tumor accounts for less than 0.5 % of all sinonasal neoplasms. The World Health Organization (WHO) classified this tumor as glomangiopericytoma in 2005.
Case report
A 47-year-women presented with two years history of permanent left nasal obstruction and frequent epistaxis. Rhinoscopy revealed a friable grayish pink polypoidal mass, fully occupying the left anterior naris. Computed tomography showed a lesion involving the left nasal cavity, with a soft tissue density (70 UH) measuring 50 × 16 mm, widely infiltrative.
Endoscopic surgery was performed to remove the mass, considering the size, limited expansion and the accessible location of the tumor. The immunohistochemistry examination showed positive staining b-catenin tumor cells which confirmed the diagnostic of glomangiopericytoma.
After a 2 years follow-up, the patient showed no signs of recurrence.
Conclusion
Glomangiopericytomas generally arises in the nasal cavity and may extend into the paranasal sinuses. It is categorized as a borderline low malignancy tumor by the WHO classification. Complete transnasal endoscopic excision is the optimal treatment.
1. Introduction
Glomangiopericytoma is defined as a sinonasal tumor with perivascular myoid phenotype, which was first described in 1942 by Stout and Murray as a soft tissue tumor with characteristic proliferation including branching vessels and small vessel perivascular hyalinization [1].
Glomangiopericytoma is rare tumor and accounts for less than 0.5 % of all sinonasal neoplasms. The World Health Organization (WHO) classified glomangiopericytoma in 2005 as a borderline and low-malignant-potential soft tissue tumor of the sinonasal tract. Although it has been called sinonasal type-hemangiopericytoma, sinonasal hemangiopericytoma, hemangiopericytoma-like tumor, hemangiopericytoma, and sinonasal glomus tumor [2].
The etiology is not clear although past trauma, hypertension, pregnancy and use of corticosteroids may be involved [3].
We report here a case of a women treated with corticosteroids for sarcoidosis who was admitted for a glomangiopericytoma arising from the left nasal cavity which was treated by endoscopic endonasal resection.
This study has been reported in accordance with the SCARE criteria [4].
2. Case report
A 47 years-old women, with a history of sarcoidosis treated with corticosteroids 40 mg per day 4 months ago and a controlled asthma and without any other personal nor familial relevant medical history, was admitted to otolaryngology department of Ibn Rochd Hospital for permanent left nasal obstruction and frequent epistaxis since 2 years, without anosmia or any other sinonasal symptoms.
Rhinoscopy revealed a friable grayish pink polypoidal mass, fully occupying the left anterior naris, that bleeds on manipulation. The right nasal cavity was normal.
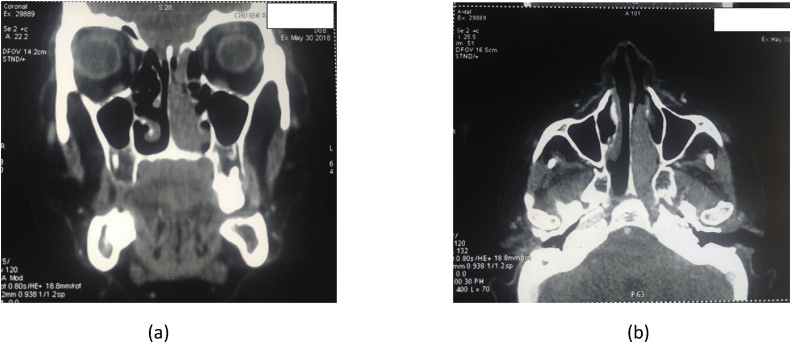
Computed tomography (CT) showed a lesion involving the left nasal cavity, with a soft tissue density (70 UH) measuring 50 × 16 mm, widely infiltrative (left nasal turbinates, uncinate process, left half of nasopharynx and palatine bone), with a posterior left ethmoidal sinus thickening and without any bony destruction (Fig. 1).
Fig. 1.
Computed tomography scan showing soft tissue density in the left nasal cavity (a: coronal, b: axial).
Microscopic examination of the biopsy showed a lobular arrangement of spindle-shaped cells admixed with vascular channels of variable size. The staining of STAT 6 immunohistochemistry (IHC) was heterogeneous suggesting a solitary fibrous tumor.
We decided to perform an endoscopic endonasal surgery by a senior rhinologist surgeon to remove the mass considering its size, limited expansion and accessible location. We explained the type of surgery, its risk and potential complications to patient which gave his informed consent.
During surgery, the tumor was vascular and polypoid in structure, bleeded very frequently and the base of the tumor was located at the anterior part of the left inferior nasal turbinate.
Complete excision of the mass was performed and extemporaneous biopsies revealed that the margins of the excision were disease free.
Histopathological examination of excision specimen showed normal respiratory epithelium. Subepithelial, oval-to-spindle cell proliferation forms short fascicles and sheets that do not infiltrate the overlying epithelium. The cells had cytologically uniform nuclei and indistinct cytoplasmic borders surrounded by thin-walled vessels.
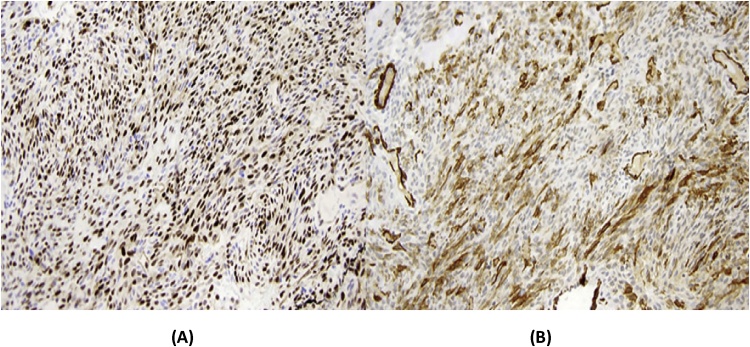
Immunohistochemically, tumor cells showed positive staining for b-catenin, cyclin D1, heterogeneous staining for CD34 and negative staining with STAT 6. These findings were compatible with glomangiopericytoma (Fig. 2).
Fig. 2.
Immunohistochemicall aspect of glomangiopericytoma. A. Tumor cells expressing muscle actine. B. Tumor cells expressing Cycline D1.
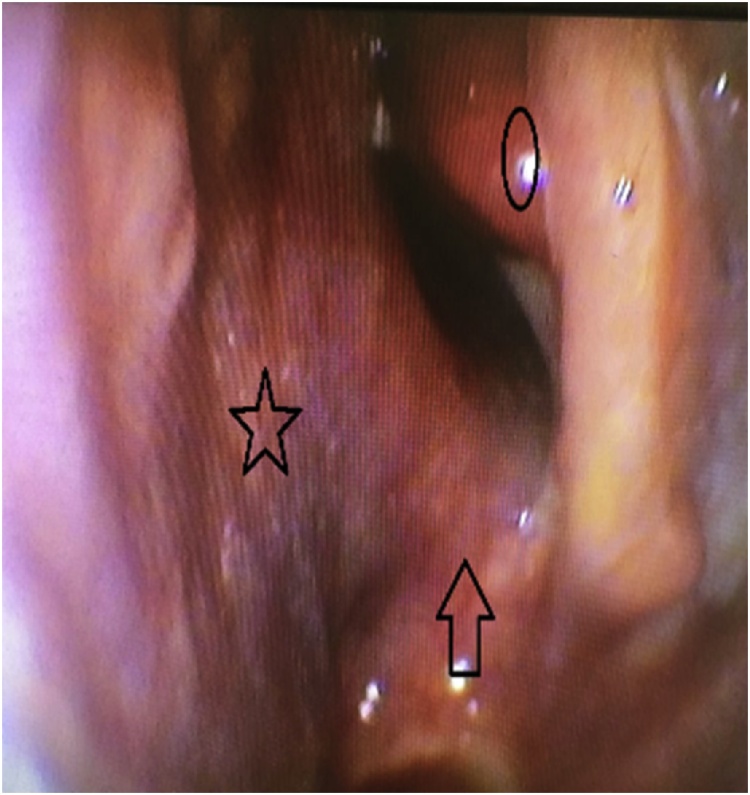
After a 2 years follow-up the patient remained free of symptoms and nasal endoscopy showed no abnormalities (Fig. 3).
Fig. 3.
Postoperative endoscopy (star : nasal septum, circle : middle turbinate, arrow : nasal floor).
3. Discussion
Glomangiopericytoma, previously known as hemangiopericytoma is defined as a tumor arising from pericytes which can occur in any location with capillaries. It is a rare tumor with a peak of incidence at the seventh decade with a slight female predominance [5].
The concept of hemangiopericytoma was first described in 1942 by Stout and Murray [1]. This lesion was thought to fall in the spectrum between glomus tumors and capillary hemangioma and hence the term hemangiopericytoma was chosen [6] now known as glomangiopericytoma, which is different from the traditional soft-tissue hemangiopericytoma [5].
It was defined by the WHO in 2005 as a sinonasal tumor demonstrating a perivascular myoid phenotype, categorized as a borderline and low malignant potential soft tissue tumors of the nose and paranasal sinuses [2]. The majority of findings are unilateral nasal obstruction and/or recurrent epistaxis. Clinically, glomangiopericytoma is polypoid, beefy red to grayish pink, soft, fleshy to friable and edematous to hemorrhagic in appearance which bleeds easily on clinical examination [6].
The etiology is still unclear, although past trauma, hypertension, pregnancy and use of corticosteroids may be the causative factors [3].
Imaging via CT or MRI is useful to precise the extent of the tumor which can be aggressive and invasive. For larger tumors, angiography may be used to determine the vascular supply of the tumor and to allow preoperative embolization [1].
Histological examination is important for the diagnosis. Hematoxylin–eosin staining shows a subepithelial well delineated but unencapsulated cellular tumor, surrounding the normal respiratory epithelium, characterized by diffuse growth of closely packed cells, forming short fascicles and sometimes exhibiting storiform, whorled or palisaded pattern, interspersed with numerous thin-walled, branching staghorn vessels. The neoplastic cells are uniform, and oval to spindle-shaped [6].
Immunohistochemically, glomangiopericytoma can be distinguished from soft tissue hemangiopericytoma by the characteristic diffuse reactivity for actin, factor XIIIA and vimentin without strong diffuse staining for CD34 [3]. The challenge is most commonly differentiation from lobular capillary hemangioma (pyogenic granuloma), solitary fibrous tumor, leiomyoma, and angiofibroma [7,8].
In our case the glomangiopericytoma was confused with solitary fibrous tumor at the biopsy examination, but the diagnosis was confirmed at histopathological examination of excision specimen.
Due to the low morbidity and the lack of external scars associated with endoscopic surgery and the fact that there is no difference in recurrence between endoscopic and open procedures, endoscopic endonsal resection represents the current preferred primary treatment modality. Radiotherapy could thus represent an adjunct therapy when complete surgical resection is not possible [9].
Patients with glomangiopericytoma have long overall survival and metastasis is rare. The recurrence rate is approximatively 10 %. Complete surgical resection can enhance disease-free survival and adjuvant radiotherapy/ chemotherapy could be helpful to prolong disease-survival time when complete resection is impossible [10].
In our case, the patient showed no signs of recurrence after 2 years follow-up.
4. Conclusion
Glomangiopericytomas arise in the nasal cavity and may extend into the paranasal sinuses and is categorized as a borderline low malignancy tumor by the WHO classification. Complete endonasal endoscopic excision is the optimal treatment. The regular postoperative follow-up with an endoscopic examination each 3 months for at least 2 years is recommended for early finding of tumor recurrence.
Declaration of Competing Interest
The authors declare no conflict of interest.
Funding
This study did not receive any sources of funding.
Ethical approval
This type of study does not require any ethical approval by our institution.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
A. Chaouki: drafting the article
Z. Najib: acquisition of data
A. Mkhatri: study design
S. Rouadi: revising the article
M. Mahtar: final approval
Registration of research studies
Not applicable.
Guarantor
A. Chaouki.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Stout A.P., Murray M.R. Hemangiopericytoma: a vascular tumor featuring Zimmermann’s pericytes. Ann. Surg. 1942;116(1):26–33. doi: 10.1097/00000658-194207000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson L.D., Fanburg-Smith J.C., Wenig B.M. Borderline and low malignant potential tumours of soft tissues. In: Barnes L., Eveson J.W., Reichart P., Sidransky D., editors. Pathology and Genetics of Head and Neck Tumours. 1st ed. IARC Press; Lyon, France: 2005. pp. 43–45. [Google Scholar]
- 3.Roy N.P., Desai D.P., Jain S.A. Glomangiopericytoma of nasal cavity. Indian J. Pathol. Microbiol. 2015;58:554–556. doi: 10.4103/0377-4929.168864. [DOI] [PubMed] [Google Scholar]
- 4.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Thompson L.D. Sinonasal tract glomangiopericytoma (hemangiopericytoma) Ear Nose Throat J. 2004;83:807. [PubMed] [Google Scholar]
- 6.Nunnery E.W., Kahn L.B., Reddick R.L. Hemangiopericytoma: a light microscopic and ultrastructural study. Cancer. 1981;47 doi: 10.1002/1097-0142(19810301)47:5<906::aid-cncr2820470516>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Higashi Kenjiro. Glomangiopericytoma of the nasal cavity. Auris Nasus Larynx. 2011;38:415–417. doi: 10.1016/j.anl.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Duval Melanie. Systematic review of treatment and prognosis of sinonasal hemangiopericytoma. Head Neck. 2012 doi: 10.1002/hed.23074. [DOI] [PubMed] [Google Scholar]
- 9.Dandekar M., McHugh J.B. Sinonasal glomangiopericytoma: case report with emphasis on the differential diagnosis. Arch. Pathol. Lab. Med. 2010;134 doi: 10.5858/2010-0233-CR.1. [DOI] [PubMed] [Google Scholar]
- 10.Park Eun Su, Kim Jiyoung, Jun Sun-Young. Characteristics and prognosis of glomangiopericytomas: a systematic review. Head Neck. 2017:1–13. doi: 10.1002/hed.24818. [DOI] [PubMed] [Google Scholar]