Significance
Alzheimer’s disease (AD) is the leading cause of dementia in the elderly. Although dysregulated hydrogen sulfide (H2S) metabolism has been reported in AD, and H2S donors are beneficial, molecular mechanisms underlying neuroprotective effects of H2S are largely unknown. We now show that H2S confers neuroprotection by sulfhydrating GSK3β to inhibit its activity, thereby preventing hyperphosphorylation of Tau, a key pathogenic event in AD. Administering H2S donors improves motor and cognitive functions in a mouse model of AD.
Keywords: Alzheimer’s disease, Tau, sulfhydration, GSK3beta, hydrogen sulfide
Abstract
Alzheimer’s disease (AD), the most common cause of dementia and neurodegeneration in the elderly, is characterized by deterioration of memory and executive and motor functions. Neuropathologic hallmarks of AD include neurofibrillary tangles (NFTs), paired helical filaments, and amyloid plaques. Mutations in the microtubule-associated protein Tau, a major component of the NFTs, cause its hyperphosphorylation in AD. We have shown that signaling by the gaseous molecule hydrogen sulfide (H2S) is dysregulated during aging. H2S signals via a posttranslational modification termed sulfhydration/persulfidation, which participates in diverse cellular processes. Here we show that cystathionine γ-lyase (CSE), the biosynthetic enzyme for H2S, binds wild type Tau, which enhances its catalytic activity. By contrast, CSE fails to bind Tau P301L, a mutant that is present in the 3xTg-AD mouse model of AD. We further show that CSE is depleted in 3xTg-AD mice as well as in human AD brains, and that H2S prevents hyperphosphorylation of Tau by sulfhydrating its kinase, glycogen synthase kinase 3β (GSK3β). Finally, we demonstrate that sulfhydration is diminished in AD, while administering the H2S donor sodium GYY4137 (NaGYY) to 3xTg-AD mice ameliorates motor and cognitive deficits in AD.
Alzheimer’s disease (AD), the most prevalent neurodegenerative disorder, involves loss of memory and executive functions (1, 2). Currently, no cure exists for AD, and clinical trials of diverse agents have largely failed to demonstrate therapeutic benefit (3, 4). AD may occur sporadically or have a genetic origin, with several mutations linked to a high risk for the disease (5). AD is characterized by aggregation of the microtubule-associated protein Tau and β-amyloid peptides, which are components of neurofibrillary tangles (NFTs) and amyloid plaques, respectively (2, 3, 6). AD belongs to the class of diseases termed tauopathies, which include progressive supranuclear palsy, corticobasal degeneration, Pick’s disease, and frontotemporal lobar degenerative disorders (7, 8). Tau was originally identified as a microtubule-binding protein, which mediates assembly of microtubules (9). Tau undergoes several posttranslational modifications in vivo, including phosphorylation, sumoylation, and acetylation (10–13). Disease progression in AD is closely linked to Tau pathology (14, 15). Hyperphosphorylation of Tau, a hallmark of AD, decreases its binding to microtubules and causes its aggregation and mislocalization, leading to neurotoxicity via multiple mechanisms, including changes in cytoskeletal architecture, axonal transport, and mitochondrial respiration (16–20).
AD is associated with increased oxidative stress, which promotes neurodegeneration (21). The reverse transsulfuration pathway leading to the synthesis of cysteine and glutathione (GSH) helps maintain redox homeostasis in the brain (Fig. 1A) and is dysregulated in neurotoxicity and neurodegeneration (22–26). Cystathionine γ-lyase (CSE) is the biosynthetic enzyme for the gaseous signaling molecule hydrogen sulfide (H2S) as well as its precursor cysteine (27) (Fig. 1A). CSE utilizes cystathionine, which is synthesized from homocysteine by cystathionine β-synthase (CBS), to generate cysteine (28). Both CSE and CBS synthesize H2S in the brain, with CSE expressed in neurons and CBS in astrocytes (29). H2S is formed endogenously in almost all tissues and signals by sulfhydration/persulfidation (27, 30–33). Like nitric oxide (NO) and carbon monoxide (CO), H2S is a gasotransmitter with pleiotropic roles (27, 34). Apart from its role as an endothelial-derived relaxation factor, H2S has neuroprotective functions at physiological concentrations (34–37). We have shown previously that disrupted metabolism of cysteine and H2S may be pathogenic in neurodegenerative conditions such as Parkinson’s disease (PD) and Huntington’s disease (HD) (24, 25, 38). Sulfhydration is an evolutionarily conserved process, which is diminished during aging (39). Depletion of cysteine, a product of the reverse transsulfuration pathway, is also associated with aging and neurodegeneration (40, 41). We now report that the reverse transsulfuration pathway and sulfhydration are dysregulated in AD, while supplementation with H2S donors is beneficial. Moreover, motor and cognitive deficits are mitigated by administration of H2S donors.
Fig. 1.
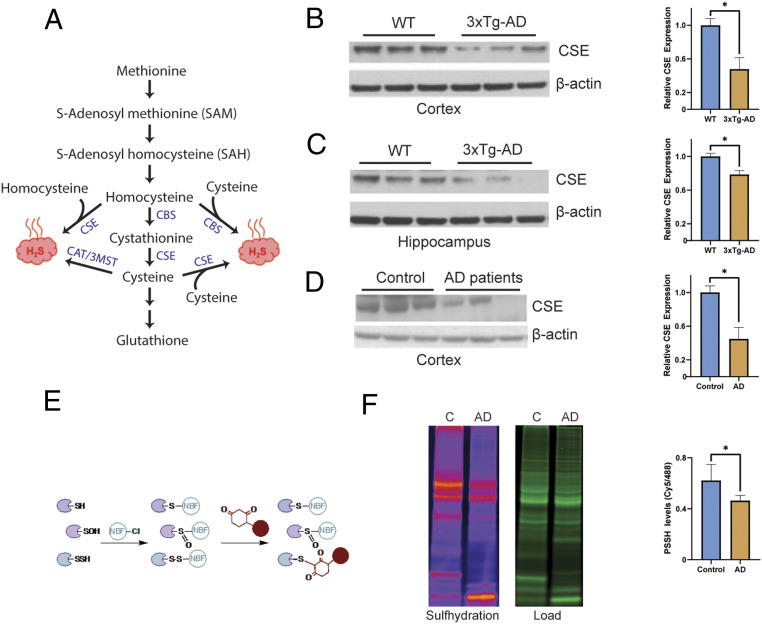
Cystathionine γ-lyase expression is decreased in AD. (A) The reverse transsulfuration pathway in mammals. Homocysteine, generated from dietary methionine, is condensed with serine to generate cystathionine by CBS. Cystathionine is acted on by CSE to produce cysteine. Cysteine can either be utilized to synthesize GSH and other sulfur-containing molecules or used as a substrate to generate hydrogen sulfide (H2S). Both homocysteine and cysteine may be utilized to produce H2S. While CSE may generate H2S from either cysteine or homocysteine, CBS produces H2S using a combination of cysteine and homocysteine. The 3-mercaptopyruvate sulfur transferase (3MST), in conjunction with cysteine aminotransferase (CAT), a third enzyme, also produces H2S from cysteine. (B) CSE is depleted in the cortex of 24-mo 3xTg-AD mice (n = 3, bars indicate SEM, *P < 0.05). (C) CSE is depleted in the hippocampus of 3xTg-AD mice (n = 3, bars indicate SEM, *P < 0.05). (D) CSE is diminished in the cortex of AD patients (Braak stage 6; n = 3, bars indicate SEM, *P < 0.05). (E) The dimedone switch assay. Proteins were reacted with 4-chloro-7-nitrobenzofurazan (NBF-Cl) to label persulfides, thiols, sulfenic acids, and amino groups. Reaction with amino groups specifically results in a characteristic green fluorescence. Next, the NBF tag is switched by a dimedone-based probe, which emits red fluorescence (the Cy5 tag is shown as a red circle), selectively labeling persulfides. The mixture is then run on sodium dodecyl sulfate gels, and signals are detected by fluorescence scanning. (F) Gel scan showing reduced sulfhydration in postmortem human AD brain samples and quantitation (n = 4, bars indicate SEM, *P < 0.05).
Results
Dysregulation of the Reverse Transsulfuration Pathway in AD.
Previously, we reported altered H2S metabolism and sulfhydration patterns in PD, while administering H2S donors proved beneficial in mouse models of PD (9, 10). Similarly, in mouse models of AD, H2S donors reversed disease symptoms and improved spatial and cognitive deficits (42, 43). We analyzed the expression of CSE in AD mouse models as well as human postmortem samples. We utilized the 3xTg-AD mouse model of AD, which harbors the mutations PS1M146V, APPSwe, and Tau P301L and develops both NFTs and amyloid plaques (44). CSE expression was reduced in the cerebral cortex and hippocampus of these mice (Fig. 1 B and C). Moreover, we observed a 50% decrease in CSE expression in the cortex of AD postmortem brain (Fig. 1D). Using the dimedone-switch assay, we observed decreased levels of overall sulfhydration (Fig. 1 E and F).
CSE and CBS Interact with Wild Type but Not Tau P301L.
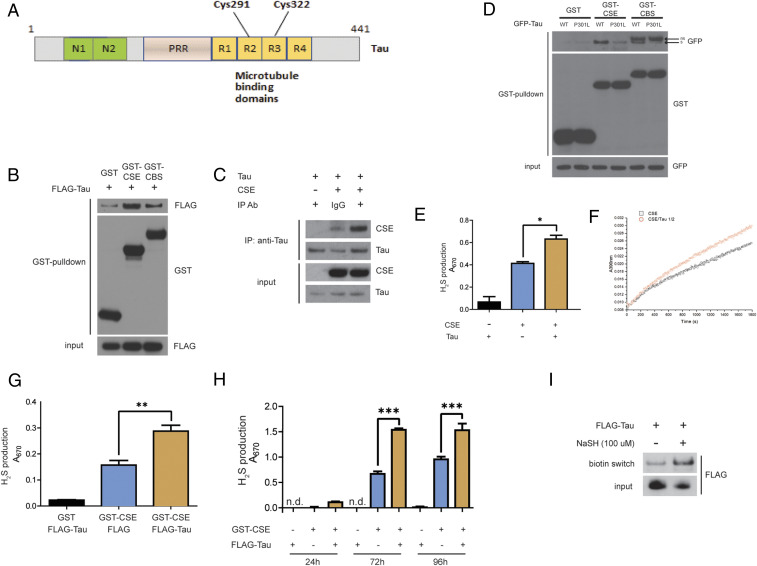
As H2S levels and sulfhydration are decreased in AD patients, we explored the interaction of CSE and CBS, the major H2S-producing enzymes, with Tau and amyloid precursor protein (APP), which constitute the NFTs and amyloid plaques, respectively. Neither CSE nor CBS bound APP (SI Appendix, Fig. S1 A and B). In the adult brain, Tau exists as six isoforms derived by alternative splicing (45). We utilized full-length Tau comprising 441 amino acid residues, which is also present in neurons (46) (Fig. 2A). CSE and CBS bind to wild type Tau in HEK293 cells overexpressing CSE or CBS and Tau (Fig. 2B). Next, we studied the interaction of Tau and CSE purified from bacterial cells (SI Appendix, Fig. S2). Purified CSE and Tau also interacted, indicating that CSE binds Tau directly (Fig. 2C). As the 3xTg-AD mouse model harbors the mutant Tau P301L, we studied the binding of CSE and CBS to this mutant in HEK293 cells. Both CSE and CBS did not bind the P301L mutant of Tau (Fig. 2D). In the case of CBS, using GFP-Tau, we observed additional bands migrating above the band corresponding to GFP-Tau, likely reflecting nonspecific bands (Fig. 2D). In the case of Flag-tagged wild type Tau (Flag-Tau), additional bands were not observed (Fig. 2B). As Tau is a neuronal protein, and CSE, but not CBS, resides in neurons, with CBS being localized to astrocytes, we focused the remainder of our studies on CSE. We analyzed the influence of Tau on CSE activity by measuring H2S production from L-cysteine in the presence of its cofactor, pyridoxal 5-phosphate (PLP). Purified Tau enhanced H2S production from human recombinant CSE in vitro (Fig. 2 E and F). We also measured H2S production (by supplementing with L-cysteine and PLP) from the lysates of HEK293 cells transfected with CSE and Tau (Fig. 2G). CSE activity increased with time, and wild type Tau further augmented H2S generation by CSE (Fig. 2H). As CSE is the biosynthetic enzyme for H2S in neurons and signals by sulfhydration, we assessed whether Tau is sulfhydrated by CSE. Tau contains two cysteine residues, Cys291 and Cys322, which could be sulfhydrated (Fig. 2A). We monitored Tau sulfhydration in transfected HEK293 cells using the modified biotin switch assay (Fig. 2I), as well as the dimedone switch method in conjunction with mass spectrometry, which revealed that Tau is indeed sulfhydrated at C322 (SI Appendix, Fig. S5).
Fig. 2.
CSE binds the microtubule-binding protein Tau. (A) Schematic representation of full-length Tau, which is composed of 441 amino acids. Tau harbors the N-terminal domains N1 and N2, a proline-rich region (PRR), and four repeat domains R1 through R4, which bind microtubules. Two cysteine residues, Cys291 and Cys322, are present in R2 and R3, respectively. (B) Interaction of Tau with CSE and CBS. HEK293 cells were transfected with constructs encoding Flag-Tau and either glutathione S-transferase (GST)-tagged CSE or CBS or GST vector, and GST pulldown assay was conducted. GST-CSE and GST-CBS interact with Flag-Tau. (C) CSE binds Tau directly. In vitro coimmunoprecipitation assay using purified CSE and Tau. Normal IgG control was used as an isotype control for the anti-Tau antibody used in the immunoprecipitation. (D) CSE and CBS do not bind to mutant Tau P301L, as revealed by coimmunoprecipitation assays in HEK293 cells overexpressing GST-CSE or GST-CBS and either wild type Tau (WT) or mutant Tau P301L. Arrow with “s” indicates specific GFP-Tau band; arrow with “ns” indicates nonspecific band. (E) Wild type Tau stimulates activity of CSE in vitro (using purified proteins) as measured by H2S production by the methylene blue assay (n = 3, bars indicate SEM, *P < 0.05). (F) Kinetics of H2S production from human recombinant CSE without (black squares) or with Tau (red dots). CSE/Tau protein molar ratio is 1/2. Wild type Tau stimulates activity of CSE as assayed by a spectrophotometric assay utilizing 0.22 µM purified CSE and 0.44 µM in 100 mM Hepes buffer (pH 7.4) containing 0.4 mM lead acetate at 37 °C for 3 min and absorbance measured at 390 nm, reflecting lead sulfide formed by reaction of H2S with lead acetate. (G) Wild type Tau stimulates activity of CSE in HEK 293 cells in an in vitro reaction containing 10 mM L-cysteine and 250 μM PLP as measured by H2S production by the methylene blue assay (n = 3, bars indicate SEM, **P < 0.01). (H) WT Tau increases the activity of CSE in a time-dependent manner (n = 3, bars indicate SEM, ***P < 0.001). (I) Tau is sulfhydrated by H2S. Flag-Tau was transfected into HEK293 cells and treated with 100 μM NaSH, and sulfhydration analyzed by the modified biotin switch assay.
H2S Generated by CSE Inhibits Phosphorylation of Tau by Glycogen Synthase Kinase 3 β.
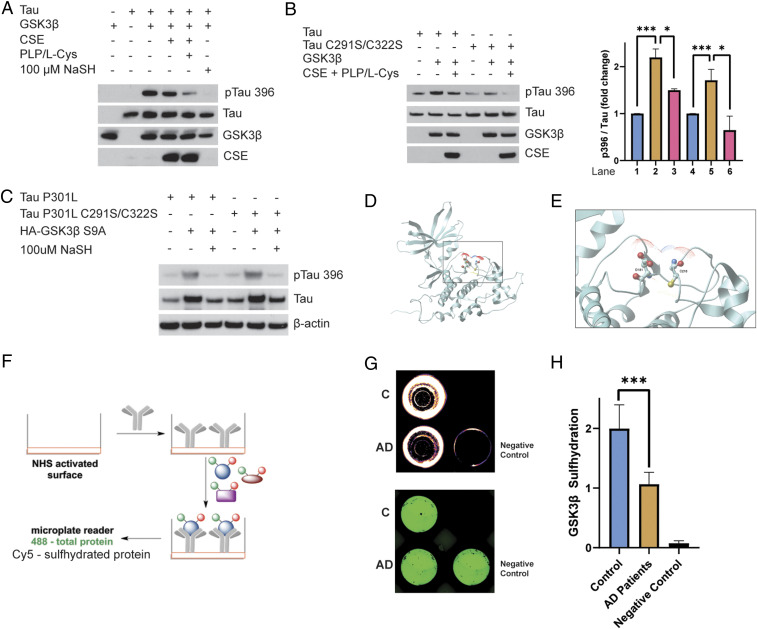
Tau harbors several sites that are phosphorylated by multiple kinases. Hyperphosphorylation of Tau decreases its affinity for microtubules and causes its aggregation. One of the major kinases that phosphorylates Tau is glycogen synthase kinase 3β (GSK3β), a serine/threonine kinase, which modifies several sites on the protein in vivo (47). We wondered whether CSE and H2S modulate Tau phosphorylation by GSK3β. To explore the effect of H2S on Tau phosphorylation, we utilized purified Tau, CSE, and GSK3β in an in vitro assay (Fig. 3A). Phosphorylation of Tau at Ser396 by GSK3β was significantly diminished when CSE in combination with L-cysteine and PLP, the substrate and cofactor for CSE, respectively, were added to the reaction mixture (containing CSE, Tau, and ATP as described in Materials and Methods), indicating a role for H2S. Consistent with this observation, phosphorylation of Tau was reduced when sodium hydrosulfide (NaSH) was added alone to GSK3β, Tau, and ATP in the absence of CSE, L-cysteine, and PLP (Fig. 3A). To determine whether the cysteines in Tau affect its phosphorylation, we mutated these residues to serine and conducted the phosphorylation assays with GSK3β. Phosphorylation of the mutant Tau C291S/C322S was inhibited as well, indicating that absence of cysteine residues does not prevent the inhibition of Tau phosphorylation by GSK3β (Fig. 3B). As GSK3β is inhibited by phosphorylation of its Ser9 residue by the endogenous kinase Akt, we explored whether the inhibitory effect of H2S on phosphorylation of Tau involves Ser9 of GSK3β. We utilized a constitutively active mutant of GSK3β, GSK3β S9A, wherein Ser9 is mutated to Ala (and therefore is not subject to inhibition by Akt), and examined the effect of H2S on phosphorylation of Tau. We analyzed Tau phosphorylation in HEK293 cells using the mutant Tau P301L, which is a mutation present in the 3xTg-AD mouse model of AD (44). NaSH inhibited phosphorylation of Tau P301L even when GSK3β S9A was present, indicating that H2S acts by a mechanism independent of phosphorylation of GSK3β at Ser9 (Fig. 3C). Similarly, H2S also inhibited phosphorylation of the C291S/C322S mutant of Tau P301L in HEK293 cells, further confirming that inhibition of Tau phosphorylation does not require the cysteine residues on Tau (Fig. 3C). In HEK293 cells, phosphorylation of Tau resulted in its slower migration on gels as reported previously (48). Treatment with NaSH inhibited phosphorylation at Ser396 and resulted in faster mobility of Tau P301L on the gel (Fig. 3C). NaSH also inhibited phosphorylation of Tau at Ser202 and Thr205 (SI Appendix, Fig. S3A). Moreover, total Tau levels were increased in the GSK3β-transfected samples, which may reflect stabilization of Tau P301L by GSK3β, which could result in increased accumulation of Tau and neurotoxicity. To further characterize inhibition of GSK3β activity by H2S, we conducted activity assays using radioactive [γ-32P]-ATP, GSK3β, and a peptide substrate of GSK3β, monitoring phosphorylation of the peptide by scintillation counting. Like the assays conducted earlier, NaSH significantly inhibited phosphorylation of the peptide (SI Appendix, Fig. S3B). As HEK293 cells harbor other kinases such as extracellular signal-related kinase-1 and -2, mitogen-activated protein kinases, p38 kinase, and c-Jun N-terminal kinase, which can also phosphorylate Tau, it remains to be determined whether H2S inhibits phosphorylation of Tau by these kinases (48). Thus, it appeared likely that H2S prevents phosphorylation of Tau by inhibiting GSK3β, possibly by sulfhydrating it. Therefore, we examined the sulfhydration of GSK3β using mass spectrometry, revealing that GSK3β was indeed modified by H2S at Cys218 (SI Appendix, Fig. S4). A closer analysis of the sequence of GSK3β revealed that Cys218 lies close to Tyr216, which is phosphorylated in the kinase domain. Moreover, 3-dimensional modeling showed that Cys218 lies close to Asp181 in the active site, which is involved in hydrogen bond formation for catalysis. Sulfhydration of Cys218 could disrupt the active site conformation (Fig. 3 D and E). We analyzed sulfhydration of GSK3β in human AD samples using the dimedone switch assay in combination with an antibody array method we previously developed (39). In this method, a GSK3β antibody is immobilized on a 96-well plate with an N-hydroxysuccinimide–activated surface as described previously (Fig. 3F) (39). Considering that proteins are labeled with 4-chloro-7-nitrobenzofurazan (NBF; green), reflecting total load and with cyanine-5 (Cy5; red) for sulfhydration, the ratio of these two signals would yield the observed levels of GSK3β sulfhydration (Fig. 3G). As a negative control, 488-labeled albumin (instead of antibody) was used to block the available surface and then incubated with control lysates. The assay revealed that sulfhydration of GSK3β was significantly diminished in the cortex of AD patients compared to normal subjects (Fig. 3 G and H). Sulfhydration of GSK3β was decreased almost twofold in the cerebral cortex of AD patients, further confirming our observation that sulfhydration is decreased in AD.
Fig. 3.
CSE and H2S inhibit phosphorylation of Tau by GSK3β. (A) Phosphorylation assays with purified Tau, GSK3β, and CSE in vitro in the presence or absence of L-cysteine (L-Cys) and PLP or treated with 100 μM NaSH. Phosphorylation of Tau was assessed by Western blotting using antibodies against phosphorylated Tau (pTau 396). Tau phosphorylation was significantly diminished when CSE, L-cysteine, and PLP were added. Addition of NaSH alone in the absence of CSE also prevented Tau phosphorylation. (B) Cysteine residues do not play a role in phosphorylation of Tau by GSK3β. Purified Tau or Tau C291S/C322S and GSK3β were incubated in the presence or absence of L-cysteine and PLP and analyzed for phosphorylation of Tau at Ser396. Western blot analysis revealed that mutation of cysteine residues Cys291 and Cys322 does not affect phosphorylation of Tau at Ser396 (n = 3, bars indicate SEM, *P < 0.05, ***P < 0.001). (C) H2S inhibits phosphorylation of P301L Tau by GSK3β. HEK293 cells were transfected with Tau P301L or Tau P301L C291S/C322S and GSK3β S9A, treated with 100 μM NaSH for 24 h, and analyzed for phosphorylation of Tau at Ser396 by Western blotting. While GSK3β phosphorylated Tau, NaSH prevented this phosphorylation. (D) Ribbon model of GSK3β (Protein Data Bank ID code 1J1B; DOI: 10.1107/S090744490302938x). Intercept: (E) thiolate side chain of Cys218 (ball-and-stick model) that we found to be sulfhydrated is already in close proximity to Asp181 in the active site of GSK3β, so the presence of an additional sulfur atom will inevitably alter the conformation of the active site, which would inhibit its kinase activity. Oxygen atoms are shown in red, sulfur in yellow, and nitrogen in blue. Dots around the atoms represent expected water surface accessibility. (F) Schematic representation of the antibody array-like approach to study sulfhydration status of GSK3β in AD brains. Anti-GSK3β antibody was immobilized on a 96-well plate with N-hydroxysuccinimide–activated surface. Brain cortical lysates from normal and AD postmortem tissues were added to the wells to allow recognition of GSK3β from lysates by the antibody. The bound protein was labeled with NBF (green) for a total load and with Cy5 (red) for sulfhydration, and the ratio of the two signals was measured to yield sulfhydration levels. As a negative control, 488-labeled albumin (instead of antibody) was used to block the available surface and then incubated with control lysates. (G) Readout from a representative experiment showing decreased sulfhydration (red) in cortex of AD patients, while the negative control shows no signals. The plate was recorded on a Typhoon FL9500 at 488 nm (NBF fluorescence signal in green represents total load) and 635 nm (Cy5 signal in red represents sulfhydration). (H) Quantitation of H (n = 4, bars indicate SEM, ***P < 0.001).
H2S Donors Alleviate Behavioral Symptoms in the 3xTg-AD Mouse Model.
To examine the neuroprotective effects of H2S in vivo, we administered NaGYY, a synthetic sodium salt derivative of Lawesson’s reagent, N-benzoylthiobenzamide, GYY4137, and a slow-releasing H2S donor to 3xTg-AD mice (49–52). Commercially available GYY4137 is synthesized as a morpholine salt (morpholine is toxic and biologically active) and also contains undisclosed amounts of the carcinogenic solvent (dichloromethane) that is metabolized to CO, potentially complicating the interpretation of effects obtained. Accordingly, we utilized in-house ultrapure NaGYY (Materials and Methods provides additional details), which is devoid of these confounding effects and has been well characterized, with the additional advantage of being water-soluble (52, 53).
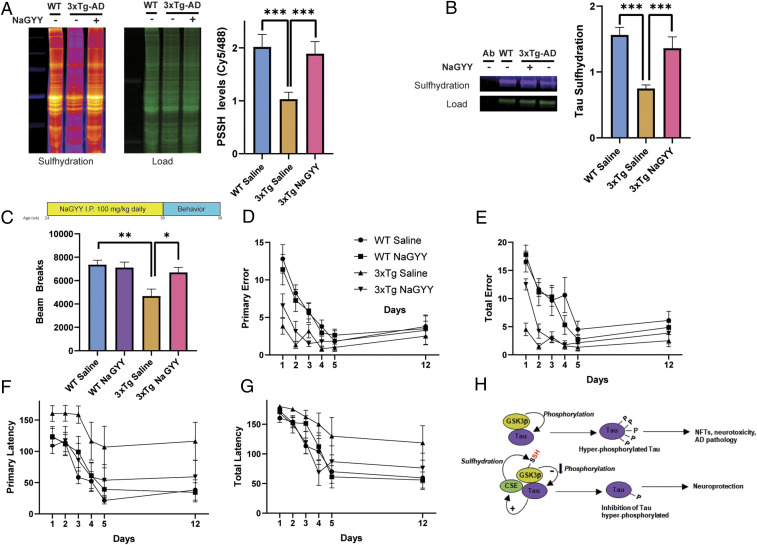
Mice were treated either with NaGYY or saline (vehicle) at 6 mo via daily intraperitoneal injections (100 mg/kg in saline) for 12 wk. Levels of sulfhydration and behavioral studies were conducted 3 mo after treatment with NaGYY at 9 mo. Overall levels of sulfhydration were decreased in the 3xTg-AD mice, which was rescued in the 3xTg-AD mice treated with NaGYY (Fig. 4A). In addition, we observed that sulfhydration of immunoprecipitated Tau is decreased in AD mice and restored in NaGYY-treated animals (Fig. 4B). Next, we studied the effects of the H2S donor on motor and cognitive functions of AD mice. We used an open-field test to study the overall locomotor activity of 3xTg-AD mice treated with the H2S donor. The AD mice had a reduced locomotor activity as compared to the wild type mice. NaGYY treatment enhanced overall locomotor activity of the AD mice (Fig. 4C). The most studied features of AD are memory impairments and cognitive deficits, although noncognitive deficits, such as motor dysfunction, are also present and may even precede classical clinical symptoms (54). Motor symptoms have been observed in patients with autosomal-dominant AD that correlate with disease progression (55). Treatment with NaGYY partially rescued memory deficits of 3xTg-AD mice in the Barnes maze memory tests at 9 mo as compared to their vehicle (saline)-treated controls. The primary latency in the Barnes maze test was significantly improved, but there was no significant change in the primary error, total error, or total latency in these mice (Fig. 4 D–G). Thus, the H2S donor NaGYY elicits beneficial effects on motor and cognitive deficits of AD mice.
Fig. 4.
The H2S donor NaGYY ameliorates AD symptoms. (A) Overall sulfhydration is decreased in the hippocampus of 3xTg-AD mice, which is rescued by NaGYY treatment in 3xTg-AD mice as revealed by the dimedone switch method (n = 3, bars indicate SEM, ***P < 0.001). (B) Sulfhydration of Tau is decreased in the hippocampus of 3xTg-AD mice as revealed by immunoprecipitation assays in combination with the dimedone switch assay. Treatment with NaGYY rescues sulfhydration of Tau. (C) Treatment regimen for 3xTg-AD mice with the H2S donor NaGYY. Mice were treated at 6 mo with 100 mg/kg NaGYY by intraperitoneal injection daily for 12 wk, and behavioral analyses were conducted at 9 mo. The open-field test revealed significant deficits in locomotor activity in the male 3xTg-AD mice, which were rescued by NaGYY (n = 6 to 10, bars indicate SEM, **P < 0.01 and *P < 0.05). (D–G) NaGYY partially rescues memory deficits in the 3xTg-AD mice. These mice do not exhibit significant differences in primary error and total error in the Barnes maze test (D and E). NaGYY treatment partially rescues primary and total latency (F and G; n = 6 to 10, bars indicate SEM, *P < 0.05 for comparison between primary latency of 3xTg-AD saline and 3xTg-AD NaGYY by one-way ANOVA followed by a post hoc Tukey test). (H) Model depicting a possible mode of neuroprotection afforded by H2S. GSK3β (yellow-ochre) binds Tau (purple) and phosphorylates it (marked as “P”), which leads to the formation of NFTs and AD pathology in the 3xTgAD mice. H2S produced by CSE (green) sulfhydrates GSK3β (“SH” in red text) and inhibits phosphorylation of Tau. Tau binds to CSE and enhances its activity (arrow with a plus sign), forming part of a virtuous cycle that decreases Tau phosphorylation and confers neuroprotection.
Discussion
The principal finding of this study is that the gasotransmitter H2S is neuroprotective in AD by inhibiting phosphorylation of Tau via sulfhydration of GSK3β, the kinase for Tau. In addition, by sulfhydrating cysteine residues on target proteins, H2S prevents irreversible oxidation of cysteine residues as demonstrated previously (39). Earlier, we reported decreased H2S signaling by sulfhydration in PD and HD and during aging (24, 25, 39). Neuronal H2S produced by CSE mediates stress responses, which are compromised in neurodegenerative diseases (38, 56).
H2S levels are tightly regulated in cells. Excess H2S deranges mitochondria and has been implicated in a state of suspended animation attributed to inhibition of complex IV of the electron transport chain (57, 58). The major H2S-producing enzymes are spatially compartmentalized in the adult brain, with CBS concentrated in astrocytes and CSE in neurons (29, 59). In amyotrophic lateral sclerosis (ALS) caused by the G93A mutation in superoxide dismutase 1 (SOD1) and in Down’s syndrome, excess H2S is neurotoxic (60–63). H2S donors are therapeutic in several AD models; however, direct links to sulfhydration have not been established (43, 64–70).
In this study, we detected diminished expression of CSE and sulfhydration in the AD brain. The 3xTg-AD mouse model, as well as postmortem cortex samples of AD patients, display reduced sulfhydration. Supplementation with the slow-releasing H2S donor NaGYY rescues the diminished sulfhydration levels in the brains of 3xTg-AD mouse model and alleviates motor and cognitive deficits. Our findings concur with reports of diminished H2S levels in serum of AD patients and confirm the neuroprotective role of H2S donors in rodent models of AD (42, 43, 64, 65, 69, 71, 72). Treatment with H2S donors ameliorated several deficits, including those in learning and memory.
How might sulfhydration be neuroprotective? We propose that H2S sulfhydrates GSK3β, thereby inhibiting phosphorylation of Tau and preventing neurotoxicity (Fig. 4H). As H2S participates in multiple signaling cascades, additional neuroprotective pathways may be involved (37). For example, the Nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway, which regulates response to oxidative stress, may be enhanced by H2S. Under basal conditions, Nrf2 is sequestered in the cytosol of cells by the kelch-like ECH-associated protein (Keap1), which targets it for proteasomal degradation (73). Keap1 has reactive cysteine residues, which, when sulfhydrated, cause its dissociation from Nrf2, which then translocates to the nucleus to transcribe genes involved in stress responses (42, 74). Similarly, H2S modulates transcriptional regulatory networks that are disrupted in neurodegeneration (38, 75). Stimulating the reverse transsulfuration pathway may be beneficial in AD. This pathway also leads to the production of GSH, a cellular antioxidant, which regulates redox homeostasis and neurotransmission (76, 77). As the reverse transsulfuration pathway is a central hub in several neuroprotective signaling networks, its stimulation may afford therapeutic benefits by restoring redox balance and H2S metabolism (28, 41). This pathway is disrupted in several neurodegenerative diseases exhibiting impaired redox homeostasis. Thus, in PD and HD, stimulating the production of cysteine and H2S via CSE is neuroprotective (24, 25, 38, 56). Aging is associated with diminished transsulfuration and sulfhydration as well as elevated oxidative stress. We have shown previously that decreased sulfhydration and increased oxidation of cysteine residues on proteins occur across evolutionary boundaries during aging (39). Additionally, aging is the greatest risk factor for developing neurodegenerative diseases, including AD (78). Accordingly, targeting the reverse transsulfuration pathway may afford therapeutic benefits for aging and neurodegenerative diseases involving suboptimal H2S signaling.
Materials and Methods
Cell Cultures and Reagents.
HEK293 cells were from the American Tissue Culture Type Collection. All chemicals were from Sigma unless mentioned otherwise. In this study, we used a sodium salt derivative of the slow-releasing H2S donor GYY4137 (NaGYY). Use of this compound was necessary, as commercial preparations of GYY4137 are morpholine salts complexed with unstated quantities of the carcinogenic solvent methylene chloride. Morpholine and dichloromethane (methylene chloride) are highly toxic and are not biologically inert, with the latter well documented to be metabolized to carbon monoxide. Since sodium salts are pharmaceutically acceptable and nontoxic, we therefore synthesized NaGYY in house as described previously by us to avoid these contaminants and impurities (51, 52). Lipofectamine 2000 (Invitrogen) was used for all transfection studies. The pRK5-eGFP-Tau (no. 46904), pRK5-eGFP-Tau P301L (no. 4690), pcDNA3-HA-GSK3β (no. 14753), and pcDNA3-HA-GSK3β S9A (no. 14754) constructs were obtained from Addgene.
Immunoprecipitation Assays and Western Blot Analysis.
HEK293 cells were transfected with indicated plasmids 24 h prior to lysis of the cells. Additional details of reagents and methods are provided in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by US Public Health Service Grant DA044123 (to S.H.S.); American Heart Association (AHA)–Allen Initiative in Brain Health and Cognitive Impairment (to S.H.S. and associates); Medical Research Council, United Kingdom (MR/S002626/1 to M.W.); the Brian Ridge Scholarship (R.T.); and the European Research Council under the European Union’s Horizon 2020 research and innovation programme (864921 to M.R.F.). We are grateful to Olga Pletnikova, Brain Resource Center at Johns Hopkins University, for providing postmortem AD brain samples. The Johns Hopkins University Mass Spectrometry Core is acknowledged for analysis of protein sulfhydration. We especially thank the reviewers for their valuable comments and suggestions, which have greatly strengthened the study.
Footnotes
Competing interest statement: M.W., R.T., and the University of Exeter have patents (awarded/pending) on hydrogen sulfide delivery molecules and their therapeutic use.
See online for related content such as Commentaries
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2017225118/-/DCSupplemental.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.Lane C. A., Hardy J., Schott J. M., Alzheimer’s disease. Eur. J. Neurol. 25, 59–70 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Masters C. L., et al. , Alzheimer’s disease. Nat. Rev. Dis. Primers 1, 15056 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Long J. M., Holtzman D. M., Alzheimer disease: An update on pathobiology and treatment strategies. Cell 179, 312–339 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings J. L., Morstorf T., Zhong K., Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res. Ther. 6, 37 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karch C. M., Goate A. M., Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry 77, 43–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballatore C., Lee V. M., Trojanowski J. Q., Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Lee V. M., Goedert M., Trojanowski J. Q., Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Arendt T., Stieler J. T., Holzer M., Tau and tauopathies. Brain Res. Bull. 126, 238–292 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W., A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. U.S.A. 72, 1858–1862 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avila J., Lucas J. J., Perez M., Hernandez F., Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 84, 361–384 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Arakhamia T., et al. , Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell 180, 633–644.e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Mandelkow E., Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Martin L., Latypova X., Terro F., Post-translational modifications of tau protein: Implications for Alzheimer’s disease. Neurochem. Int. 58, 458–471 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Braak H., Braak E., Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 16, 271–278, discussion 278–284 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Isla T., et al. , Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann. Neurol. 41, 17–24 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Medina M., Hernández F., Avila J., New features about tau function and dysfunction. Biomolecules 6, 21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jouanne M., Rault S., Voisin-Chiret A. S., Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents. Eur. J. Med. Chem. 139, 153–167 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Lindwall G., Cole R. D., Phosphorylation affects the ability of tau protein to promote microtubule assembly. J. Biol. Chem. 259, 5301–5305 (1984). [PubMed] [Google Scholar]
- 19.Grundke-Iqbal I., et al. , Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U.S.A. 83, 4913–4917 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoover B. R., et al. , Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sbodio J. I., Snyder S. H., Paul B. D., Redox mechanisms in neurodegeneration: From disease outcomes to therapeutic opportunities. Antioxid. Redox Signal. 30, 1450–1499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitvitsky V., Thomas M., Ghorpade A., Gendelman H. E., Banerjee R., A functional transsulfuration pathway in the brain links to glutathione homeostasis. J. Biol. Chem. 281, 35785–35793 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Diwakar L., Ravindranath V., Inhibition of cystathionine-gamma-lyase leads to loss of glutathione and aggravation of mitochondrial dysfunction mediated by excitatory amino acid in the CNS. Neurochem. Int. 50, 418–426 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Paul B. D., et al. , Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington’s disease. Nature 509, 96–100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandiver M. S., et al. , Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 4, 1626 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohl J. B., Mellis A. T., Schwarz G., Homeostatic impact of sulfite and hydrogen sulfide on cysteine catabolism. Br. J. Pharmacol. 176, 554–570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sen N., et al. , Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell 45, 13–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sbodio J. I., Snyder S. H., Paul B. D., Regulators of the transsulfuration pathway. Br. J. Pharmacol. 176, 583–593 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morikawa T., et al. , Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc. Natl. Acad. Sci. U.S.A. 109, 1293–1298 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul B. D., Snyder S. H., H2S: A novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 40, 687–700 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul B. D., Snyder S. H., Protein sulfhydration. Methods Enzymol. 555, 79–90 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Paul B. D., Snyder S. H., H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 13, 499–507 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Filipovic M. R., Zivanovic J., Alvarez B., Banerjee R., Chemical biology of H2S signaling through persulfidation. Chem. Rev. 118, 1253–1337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R., Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 92, 791–896 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Wang R., Hydrogen sulfide: A new EDRF. Kidney Int. 76, 700–704 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Yang G., et al. , H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 322, 587–590 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul B. D., Snyder S. H., Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem. Pharmacol. 149, 101–109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sbodio J. I., Snyder S. H., Paul B. D., Transcriptional control of amino acid homeostasis is disrupted in Huntington’s disease. Proc. Natl. Acad. Sci. U.S.A. 113, 8843–8848 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zivanovic J., et al. , Selective persulfide detection reveals evolutionarily conserved antiaging effects of S-sulfhydration. Cell Metab. 30, 1152–1170.e13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dröge W., Oxidative stress and ageing: Is ageing a cysteine deficiency syndrome? Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 2355–2372 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul B. D., Sbodio J. I., Snyder S. H., Cysteine metabolism in neuronal redox homeostasis. Trends Pharmacol. Sci. 39, 513–524 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y., et al. , Hydrogen sulfide ameliorates learning memory impairment in APP/PS1 transgenic mice: A novel mechanism mediated by the activation of Nrf2. Pharmacol. Biochem. Behav. 150-151, 207–216 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Xuan A., et al. , Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in β-amyloid rat model of Alzheimer’s disease. J. Neuroinflammation 9, 202 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oddo S., et al. , Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular abeta and synaptic dysfunction. Neuron 39, 409–421 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Takuma H., Arawaka S., Mori H., Isoforms changes of tau protein during development in various species. Brain Res. Dev. Brain Res. 142, 121–127 (2003). [DOI] [PubMed] [Google Scholar]
- 46.McMillan P., et al. , Tau isoform regulation is region- and cell-specific in mouse brain. J. Comp. Neurol. 511, 788–803 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanger D. P., Anderton B. H., Noble W., Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 15, 112–119 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Anderton B. H., et al. , Sites of phosphorylation in tau and factors affecting their regulation. Biochem. Soc. Symp. 73–80 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y., Wang H., Xian M., Cysteine-activated hydrogen sulfide (H2S) donors. J. Am. Chem. Soc. 133, 15–17 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L., et al. , Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 117, 2351–2360 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Whiteman M., et al. , Phosphinodithioate and phosphoramidodithioate hydrogen sulfide donors. Handb. Exp. Pharmacol. 230, 337–363 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Alexander B. E., et al. , Investigating the generation of hydrogen sulfide from the phosphonamidodithioate slow-release donor GYY4137. MedChemComm 6, 1649–1655 (2015). [Google Scholar]
- 53.Latorre E., Torregrossa R., Wood M. E., Whiteman M., Harries L. W., Mitochondria-targeted hydrogen sulfide attenuates endothelial senescence by selective induction of splicing factors HNRNPD and SRSF2. Aging (Albany NY) 10, 1666–1681 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner J. M., et al. , Analysis of motor function in the tg4-42 mouse model of Alzheimer’s disease. Front. Behav. Neurosci. 13, 107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vöglein J. et al.; Dominantly Inherited Alzheimer Network , Clinical, pathophysiological and genetic features of motor symptoms in autosomal dominant Alzheimer’s disease. Brain 142, 1429–1440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sbodio J. I., Snyder S. H., Paul B. D., Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington’s disease. Proc. Natl. Acad. Sci. U.S.A. 115, 780–785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blackstone E., Morrison M., Roth M. B., H2S induces a suspended animation-like state in mice. Science 308, 518 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Paul B. D., Snyder S. H., Kashfi K., Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 38, 101772 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Enokido Y., et al. , Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J. 19, 1854–1856 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Ichinohe A., et al. , Cystathionine beta-synthase is enriched in the brains of Down’s patients. Biochem. Biophys. Res. Commun. 338, 1547–1550 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Panagaki T., Randi E. B., Augsburger F., Szabo C., Overproduction of H2S, generated by CBS, inhibits mitochondrial Complex IV and suppresses oxidative phosphorylation in Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 116, 18769–18771 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davoli A., et al. , Evidence of hydrogen sulfide involvement in amyotrophic lateral sclerosis. Ann. Neurol. 77, 697–709 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Kamoun P., Belardinelli M. C., Chabli A., Lallouchi K., Chadefaux-Vekemans B., Endogenous hydrogen sulfide overproduction in Down syndrome. Am. J. Med. Genet. A. 116A, 310–311 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Giuliani D., et al. , Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 104, 82–91 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Vandini E., et al. , Mechanisms of hydrogen sulfide against the progression of severe Alzheimer’s disease in transgenic mice at different ages. Pharmacology 103, 50–60 (2019). [DOI] [PubMed] [Google Scholar]
- 66.He X. L., et al. , Hydrogen sulfide improves spatial memory impairment and decreases production of Aβ in APP/PS1 transgenic mice. Neurochem. Int. 67, 1–8 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Zhao F. L., et al. , AP39, a mitochondria-targeted hydrogen sulfide donor, supports cellular bioenergetics and protects against Alzheimer’s disease by preserving mitochondrial function in APP/PS1 mice and neurons. Oxid. Med. Cell. Longev. 2016, 8360738 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu M. M., et al. , Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the Kinetics of response to DNA virus. Immunity 45, 555–569 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Cheng X. J., et al. , Tacrine-hydrogen sulfide donor hybrid ameliorates cognitive impairment in the aluminum chloride mouse model of Alzheimer’s disease. ACS Chem. Neurosci. 10, 3500–3509 (2019). [DOI] [PubMed] [Google Scholar]
- 70.He X. L., et al. , Hydrogen sulfide down-regulates BACE1 and PS1 via activating PI3K/Akt pathway in the brain of APP/PS1 transgenic mouse. Pharmacol. Rep. 68, 975–982 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Cao L., et al. , Hydrogen sulfide inhibits ATP-induced neuroinflammation and Aβ1-42 synthesis by suppressing the activation of STAT3 and cathepsin S. Brain Behav. Immun. 73, 603–614 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Guzmán R., et al. , Protective effect of sulfurous water in peripheral blood mononuclear cells of Alzheimer’s disease patients. Life Sci. 132, 61–67 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Hayes J. D., Dinkova-Kostova A. T., The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 39, 199–218 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Yang G., et al. , Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 18, 1906–1919 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Kumar A., Vaish M., Ratan R. R., Transcriptional dysregulation in Huntington’s disease: A failure of adaptive transcriptional homeostasis. Drug Discov. Today 19, 956–962 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sedlak T. W., et al. , The glutathione cycle shapes synaptic glutamate activity. Proc. Natl. Acad. Sci. U.S.A. 116, 2701–2706 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forman H. J., Zhang H., Rinna A., Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 30, 1–12 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hou Y., et al. , Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.