Abstract
Background
Global assessment of antimicrobial agents prescribed to infants in the neonatal intensive care unit (NICU) may inform antimicrobial stewardship efforts.
Methods
We conducted a one-day global point prevalence study of all antimicrobials provided to NICU infants. Demographic, clinical, and microbiologic data were obtained including NICU level, census, birth weight, gestational/chronologic age, diagnoses, antimicrobial therapy (reason for use; length of therapy), antimicrobial stewardship program (ASP), and 30-day in-hospital mortality.
Findings
On July 1, 2019, 26% of infants (580/2,265; range, 0–100%; median gestational age, 33 weeks; median birth weight, 1800 g) in 84 NICUs (51, high-income; 33, low-to-middle income) from 29 countries (14, high-income; 15, low-to-middle income) in five continents received ≥1 antimicrobial agent (92%, antibacterial; 19%, antifungal; 4%, antiviral). The most common reasons for antibiotic therapy were “rule-out” sepsis (32%) and “culture-negative” sepsis (16%) with ampicillin (40%), gentamicin (35%), amikacin (19%), vancomycin (15%), and meropenem (9%) used most frequently. For definitive treatment of presumed/confirmed infection, vancomycin (26%), amikacin (20%), and meropenem (16%) were the most prescribed agents. Length of therapy for culture-positive and “culture-negative” infections was 12 days (median; IQR, 8–14) and 7 days (median; IQR, 5–10), respectively. Mortality was 6% (42%, infection-related). An NICU ASP was associated with lower rate of antibiotic utilization (p = 0·02).
Interpretation
Global NICU antibiotic use was frequent and prolonged regardless of culture results. NICU-specific ASPs were associated with lower antibiotic utilization rates, suggesting the need for their implementation worldwide.
Funding
Merck & Co.; The Ohio State University College of Medicine Barnes Medical Student Research Scholarship
Keywords: Global point prevalence study, Neonatal infection, Neonatal antimicrobial stewardship, Antibiotics, Antifungal
Research in context.
Evidence before this study
Excessive antibiotic use among preterm infants in the neonatal intensive care unit (NICU) has been associated with adverse patient outcomes as well as unit-level and public health consequences such as emergence of multi-drug resistant organisms and higher health-care costs. Antibiotic therapy in NICUs varies widely among institutions irrespective of level of NICU care, medical morbidities, and proven bloodstream infection. Previous global point prevalence studies have included both pediatric and neonatal populations, with little focus on the quantity and reasons for their use. We searched PubMed with the following terms: point prevalence, antimicrobial agent, antibiotic, neonate, NICU with no language restrictions although only English publications subsequently were reviewed. We reviewed publications on NICU antimicrobial stewardship before September 2020.
Added value of this study
We found that 26% of infants in 84 NICUs from 29 countries (14, high-income and 15, low-to-middle income) received at least one antimicrobial agent on July 1, 2019. Antibiotics (92%) were most frequently used while 19% and 4% of infants received antifungal and antiviral agents, respectively. The antimicrobial utilization per NICU in low-to-middle income countries was significantly greater than in NICUs of high-income countries, while centers that had an NICU-specific Antimicrobial Stewardship Program had lower antibiotic utilization rates regardless of the country's income level.
Implications of all the available evidence
The finding that NICU-specific antimicrobial stewardship programs had a positive impact on antibiotic utilization highlights the potential value of such programs to reduce antibiotic consumption and possibly minimize the adverse effects of antimicrobial overuse in high-risk infants. In addition, global assessment of all antimicrobial use provided to infants in the NICU and reasons for their use should inform future antimicrobial stewardship efforts.
Alt-text: Unlabelled box
1. Introduction
Antimicrobial agents are the most prescribed medications in the neonatal intensive care unit (NICU) [1,2]. When used for proven infections, antibiotics have dramatically improved survival in this high-risk population [3,4]. However, by presumed disruption of the infant's bacterial microbiome, antibiotic overuse has been associated with adverse patient outcomes such as late-onset sepsis, invasive candidiasis, necrotizing enterocolitis, bronchopulmonary dysplasia, retinopathy of prematurity, neurodevelopmental impairment, and even death [5], [6], [7], [8], [9], [10], [11], [12], [13]. In addition, antibiotic overuse can result in unit-level and public health consequences such as emergence of multi-drug resistant organisms and higher health-care costs [14,15]. Despite this knowledge and associations, the use of antibiotic therapy in NICUs varies widely among institutions in the United States irrespective of level of NICU care, medical morbidities, and proven bloodstream infection [16], [17], [18]. Although it is likely that similar variation exists among NICUs worldwide [19], [20], [21], [22], [23], [24], there has been little focus on why and how antibiotics are used on a global scale.
Accordingly, the objectives of the NO-More-AntibioticS and Resistance (NO-MAS-R) study was to 1) determine a single day global prevalence of all antimicrobial use and specific agents provided to infants in the NICU; 2) quantify the clinical diagnoses why infants received antimicrobial therapy; and 3) quantify the proportion of antibiotics that were started empirically, targeted to an identified pathogen, or used for prophylaxis. We hypothesized that a substantial proportion of infants in NICUs worldwide would be exposed to antimicrobial therapy. Our ultimate goal was that global assessment of all antimicrobial use provided to infants in the NICU and reasons for their use will inform future antimicrobial stewardship efforts.
2. Methods
2.1. Design and setting
This cross-sectional, observational study enrolled all infants who were in the NICU and prescribed at least one antimicrobial agent as of 8 AM or later (local time) on July 1, 2019, the birthday of Ignaz Semmelweiss, MD, the “Father of Hand Hygiene” and “Savior of Mothers and Newborns [25].” Pediatricians, neonatologists, pediatric infectious diseases specialists, and pediatric pharmacists from around the world were queried to determine their interest in participating in a survey of antibiotic practices in infants admitted to Level II, III, or IV NICUs. These classifications of neonatal inpatient care consist of increasing level of specialty care (Level II) and subspecialty intensive care (Levels III and IV) [26]. Infants in Level II NICUs are born at ≥32 weeks’ gestation weighing ≥1500 g with medical problems that are expected to resolve rapidly. Level III NICUs are able to provide sustained life support for the most complex and critically ill infants <32 weeks’ gestation, weighing <1500 g at birth, or have medical or surgical conditions regardless of gestational age. Level IV NICUs have the capabilities of a Level III NICU plus provide on-site surgical repair of serious congenital or acquired malformations.
2.2. Participants and study procedures
The following data were collected on each infant: pertinent demographic and clinical information including birth weight, gestational and chronologic age, diagnoses, name of antimicrobial agent(s) prescribed and received, routes of administration, reason for its use, planned length of therapy, and culture results. In addition, information was obtained on the geographic location of the NICU, NICU level, NICU census, referral or delivery NICU, and existence of an antimicrobial stewardship program (ASP) either in the hospital or specific to the NICU. An NICU-specific ASP consisted of dedicated personnel in the NICU who supervise antibiotic use and/or had specific guidelines on antimicrobial use for the NICU. Finally, a 30-day follow-up assessment was performed to determine actual length of antimicrobial therapy for each agent prescribed as well as infant outcomes (discharged home, transferred to another facility, or in-hospital death). In addition, the infection-related in-hospital mortality was assessed at 30 days by review of the attending physician's documentation in the medical record and/or autopsy report if available. The 30-day mortality has been the standard used by the Centers for Medicare & Medicaid Services of the United States to assess quality of medical and surgical care and has been used in pediatric patients for assessment of sepsis mortality [27,28]. Antibiotic use was compared among infants who were less than three days of age versus those three days of age or older in keeping with evaluations for early vs. late-onset infections and sepsis, respectively.
Diagnostic terms were defined to assure consistency among sites. An antimicrobial agent was defined as a drug that exhibits activity against a bacterial, fungal, or viral pathogen. Prophylactic therapy was defined as an antimicrobial agent prescribed to prevent an infection. Empiric therapy was defined as initiation of an antimicrobial agent for suspected infection with the intent of discontinuing such therapy once infection was ruled out. Definitive therapy was defined as antimicrobial therapy that was continued to cure an infection that was substantiated by clinical and/or microbiologic diagnostics. “Rule-out sepsis” was defined as initiation of antimicrobial therapy for infants who were undergoing evaluation for a possible bloodstream infection with intent to discontinue therapy if the culture(s) were sterile at 24 to 72 h. “Culture-negative” sepsis or meningitis was defined as a clinical diagnosis of a bloodstream or central nervous system infection, respectively, with sterile bacterial cultures and no viral or fungal pathogen identified.
Antibiotics were grouped by the Access, Watch, Reserve (“AWaRe”) classification on the basis of the World Health Organization's (WHO) Essential Medicines List for Children and their use compared among NICUs in Asia, Europe, Africa, North America, Central America, and South America [21,29,30]. The Access group contains more narrow-spectrum antibiotics, the Watch group contains broader spectrum antibiotic classes, and the Reserve group consists of antibiotics reserved for multidrug resistant infections.
2.3. Data sources
The study survey and data were collected and managed using REDCap (Research Electronic Data Capture), a secure, web-based software platform hosted at Nationwide Children's Hospital [31,32]. A study investigator at each site entered de-identified data either real time or retrospectively if real time entry was not feasible. In case of technical difficulties with REDCap, local site investigators entered de-identified data using an Excel spreadsheet that was subsequently entered into REDCap by the study coordinator at Nationwide Children's Hospital. The data collection tool was piloted on December 12, 2018 with 12 international sites to identify any data collection challenges. Modifications were made based on participants’ feedback.
Although we were not able to perform external data validation at each site, procedures were implemented to minimize data entry errors. Each participating site was provided a Manual of Operations with standard definitions, the REDCap data entry had restriction on numbers entered, all names of antimicrobial agents were selected from a drop-down menu, and the Excel database had prefilled parameters to facilitate data acquisition. In addition, we queried sites if there was concern for entered data.
2.4. Ethics statement
The study was approved by the Institutional Review Board (IRB) at Nationwide Children's Hospital under the following designated number: STUDY00000208. Invitation letter, study protocol in English or Spanish, and the Nationwide Children's Hospital IRB approval letter were provided to each participating site for submission to the local IRB. Each site was responsible for obtaining ethics approval if required by their institution. Since all data were anonymized without patient identifiers and there was no direct contact with patients, the study at all sites was exempt from the need to obtain informed consent.
2.5. Statistical analysis
Descriptive statistics and graphical presentation of the data were done by means of Microsoft Excel. Comparative statistical analyses were performed using IBM SPSS Statistics for Windows, Version 26·0. Armonk, NY: IBM Corp. For categorical data, chi-squared or Fisher exact tests were used for comparison as well as analysis of dependence. For normally distributed continuous data, means with standard deviation were derived for descriptive statistics and analyzed with two-sample t-test [33]. All nonparametric data were reported as median with interquartile range (IQR) and analyzed with independent-samples Mann-Whitney U test. For accuracy, missing data were not imputed.
2.6. Role of the funding source
The funding agency had no influence on study design, data collection, or analysis. The corresponding author had full access to the data and is responsible for the decision to submit for publication.
3. Results
3.1. Demographics
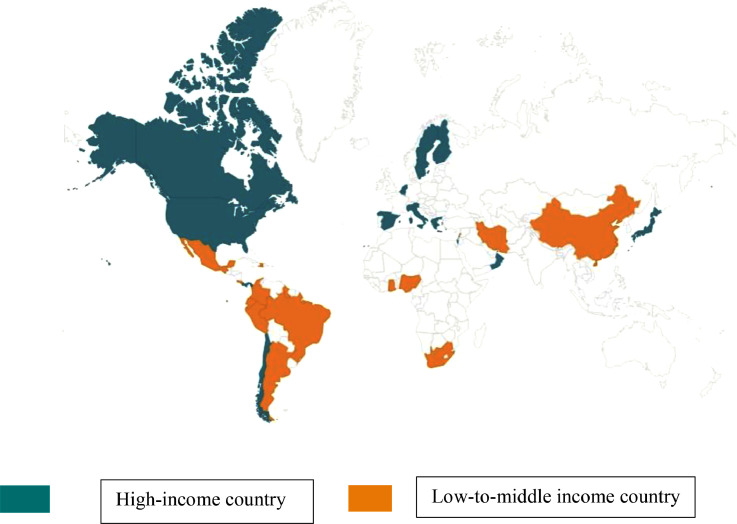
On July 1, 2019, 580 (26%) of 2265 infants in 84 hospital NICUs in 29 countries (14 high and 15 low-to-middle income countries, Fig. 1) received at least one antimicrobial agent. Of the 84 NICUs, 51 (61%) and 33 (39%) were in high and low-to-middle income countries, respectively, with a median of 5 infants per site in both high (IQR, 2–9) and low-to-middle (IQR, 3–9) income countries. Sixty-two (74%) NICUs were in hospitals that had a labor and delivery service and the rest in referral centers that cared for outborn infants. All but 4 NICUs were Level 3 (n = 42, 50%) or Level 4 (n = 38; 46%). The antimicrobial utilization per NICU in low-to-middle income countries (31%, median; IQR, 17%−48%) was significantly greater than in NICUs of high-income countries (18%, median; IQR, 10%−36%; p = 0·0013).
Fig. 1.
Map of the 29 countries that participated in the NO-More-AntibioticS and Resistance (NO-MAS-R) study, a point prevalence study of all infants in the neonatal intensive care unit (NICU) who received at least one antimicrobial agent on July 1, 2019, the birthday of Ignaz Semmelweiss, MD. Participating countries (number of NICUs; total number of infants on antimicrobial therapy) by level of income were: High income, Belgium (n = 1; 3), Canada (n = 2; 5), Chile (n = 4; 38), Finland (n = 1; 10), Greece (n = 1; 5), Israel (n = 2; 14), Italy (n = 13; 77), Japan (n = 3; 12), Netherlands (n = 1; 9), Oman (n = 1; 3), Panama (n = 1; 1), Spain (n = 5; 35), Sweden (n = 1; 4), United States (n = 15; 84); Middle-to-Low Income, Argentina (n = 2; 10), Brazil (n = 1; 13), China (n = 1; 21), Colombia (n = 12; 43), Costa Rica (n = 1; 5), Ecuador (n = 3; 12), Ghana (n = 1; 10), Guyana (n = 1; 9), Haiti (n = 1; 29), Iran (n = 1; 3), Lebanon (n = 1; 2), Mexico (n = 1; 8), Nigeria (n = 3; 43), Peru (n = 2; 14), South Africa (n = 2; 58).
Of the 580 infants who received an antimicrobial agent, 83% were ≥3 days of age, the median gestational age was 33 weeks (IQR, 28–37 weeks), median birth weight was 1800 g (IQR, 1060–2840 g), and median postnatal age was 11 days (IQR, 4–33 days) (Table 1). Most of the infants were male (59%) and 23% (n = 133) were <28 weeks’ gestation (Table 1). Their characteristics and outcome by global region are provided in Supplementary Table 1. Antibacterials were the most frequently used antimicrobial agents with 92% (n = 531) of infants receiving 940 antibiotics on the assessment day (Table 2), 19% (n = 108) receiving 108 antifungal agents, and 4% (n = 25) received 25 antiviral medications.
Table 1.
Characteristics and outcome of the 580 infants who received antimicrobial therapy in the neonatal intensive care unit on July 1, 2019.
| Infants in Neonatal Intensive Care Unit |
|||||||
|---|---|---|---|---|---|---|---|
| <3 days old |
≥3 days old |
Total | |||||
| Country income | Low-to-Middle Income | High-Income | Total | Low-to-Middle Income | High-Income | Total | |
| No. of infants | 60 (60%) | 40 (40%) | 100 (17%) | 220 (46%) | 260 (54%) | 480 (83%) | 580 |
| Birth weight (grams, IQR) | 2680 (1720–3110) | 2208 (1575–3078) | 2400 (1680–3100) | 2253, 1200–2914 | 1350, 800–2460 | 1680 (995–2771) | 1800 (1060–2835) |
| ≤1000 | 3 (50%) | 3 (50%) | 6/100 (6%) | 23 (18%) | 102 (82%) | 125/477 (26%) | 131/577 (23%) |
| 1001–1500 | 5 (42%) | 7 (58%) | 12/100 (12%) | 50 (54%) | 43 (46%) | 93/477 (20%) | 105/577 (18%) |
| 1501–2500 | 20 (63%) | 12 (38%) | 32/100 (32%) | 54 (51%) | 51 (49%) | 105/477 (22%) | 137/577 (24%) |
| ≥2501 | 32 (64%) | 18 (36%) | 50/100 (50%) | 91 (59%) | 63 (41%) | 154/477 (32%) | 204/577 (35%) |
| Chronologic age (days, IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 10 (5–27) | 21 (9–61) | 16 (6–40) | 11 (4–33) |
| Gestational age (weeks, IQR) | 36 (32–39) | 34 (31–37) | 34 (32–38) | 34 (30–36) | 30 (26–36) | 32 (27–37) | 33 (28–37) |
| ≤28 weeks | 3 (33%) | 6 (67%) | 9/93 (10%) | 38 (26%) | 109 (74%) | 147/474 (31%) | 156/567 (28%) |
| 29–33 weeks | 14 (56%) | 11 (44%) | 25/93 (27%) | 59 (50%) | 58 (50%) | 117/474 (25%) | 142/567 (25%) |
| 34–36 weeks | 14 (54%) | 12 (46%) | 26/93 (28%) | 39 (50%) | 39 (50%) | 78/474 (16%) | 104/567 (18%) |
| ≥37 weeks | 22 (67%) | 11 (33%) | 33/93 (35%) | 79 (60%) | 53 (40%) | 132/474 (28%) | 165/567 (29%) |
| Sex, male | 33 (63%) | 19 (37%) | 52 (52%) | 132 (46%) | 156 (54%) | 288 (60%) | 340 (59%) |
| Race/ethnicity | |||||||
| White | 1 (4%) | 25 (96%) | 26 (26%) | 1 (1%) | 138 (99%) | 139 (29%) | 165 (28%) |
| Black | 29 (100%) | 0 | 29 (29%) | 106 (82%) | 23 (18%) | 129 (27%) | 158 (27%) |
| Hispanic | 16 (76%) | 5 (24%) | 21 (21%) | 81 (64%) | 45 (36%) | 126 (26%) | 147 (25%) |
| Asian | 5 (50%) | 5 (50%) | 10 (10%) | 12 (50%) | 12 (50%) | 24 (5%) | 34 (6%) |
| Other | 4 (57%) | 3 (43%) | 7 (7%) | 14 (33%) | 29 (67%) | 43 (9%) | 50 (9%) |
| Unknown | 5 (71%) | 2 (29%) | 7 (7%) | 6 (32%) | 13 (68%) | 19 (4%) | 26 (4%) |
| MDRO colonization | |||||||
| MRSA | 0 | 0 | 0 | 1 (25%) | 3 (75%) | 4 (1%) | 4 (1%) |
| VRE | 0 | 0 | 0 | 0 | 1 (100%) | 1 (1%) | 1 (1%) |
| CRE | 0 | 0 | 0 | 1 (100%) | 0 | 1 (1%) | 1 (1%) |
| ESBL-Enterobacteriaceae | 0 | 0 | 0 | 7 (88%) | 8 (12%) | 15 (3%) | 15 (3%) |
| Respiratory support | |||||||
| None | 26 (62%) | 16 (38%) | 42/91 (46%) | 92 (56%) | 71 (44%) | 163/454 (36%) | 204/545 (38%) |
| Any | 27 (55%) | 22 (45%) | 49/91 (54%) | 112 (38%) | 179 (62%) | 291/454 (64%) | 340/545 (62%) |
| Nasal cannula | 15 (94%) | 1 (6%) | 16 (18%) | 45 (61%) | 29 (39%) | 74 (15%) | 90 (17%) |
| CPAP | 4 (29%) | 10 (71%) | 14 (15%) | 29 (37%) | 49 (63%) | 78 (16%) | 92 (16%) |
| Mechanical ventilation | 8 (42%) | 11 (58%) | 19 (21%) | 37 (28%) | 94 (72%) | 131 (27%) | 150 (26%) |
| Tracheostomy | 0 | 0 | 0 (0%) | 1 (13%) | 7 (88%) | 8 (2%) | 8 (1%) |
| Central venous catheter | 8 (32%) | 18 (72%) | 26 (26%) | 61 (29%) | 151 (72%) | 212 (44%) | 238 (41%) |
| Urinary catheter | 0 | 4 (100%) | 4 (4%) | 6 (19%) | 25 (81%) | 31 (6%) | 35 (6%) |
| Probiotic | 1 (25%) | 3 (75%( | 4 (4%) | 4 (3%) | 27 (87%) | 31 (6%) | 35 (6%) |
| Lactoferrin | 0 | 1 (100%) | 1 (1%) | 0 | 8 (100%) | 8 (2%) | 9 (2%) |
| Acid suppression medication | 4 (100%) | 0 | 4 (4%) | 11 (26%) | 31 (74%) | 42 (9%) | 46 (8%) |
| Mortality-30 day | 3 (60%) | 2 (40%) | 5 (5%) | 13 (46%) | 15 (54%) | 28 (6%) | 33 (6%) |
| Infection-related | 1 (33%) | 2 (67%) | 3 (60%) | 6 (55%) | 5 (45%) | 11 (39%) | 14 (42%) |
IQR, interquartile range; MDRO, multi-drug resistant organism; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; CRE, carbapenem-resistant Enterobacteriaceae; ESBL, extended-spectrum beta-lactamase; CPAP, continuous positive airway pressure.
Table 2.
Antibacterial therapy (n = 940) provided to 531 (23%) of 2265 infants in the neonatal intensive care unit of 84 hospitals in 29 countries grouped by the World Health Organization AWaRe classification.*
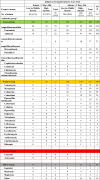 |
*Antibiotics were grouped by the AWaRE (Access, Watch, and Reserve) classification on the basis of the WHO Essential Medicines List for Children. The Access group contains narrower-spectrum antibiotics, the Watch group contains broader spectrum antibiotic classes, and the Reserve group consist of antibiotics reserved for multidrug resistant infections.
3.2. Antibacterial therapy
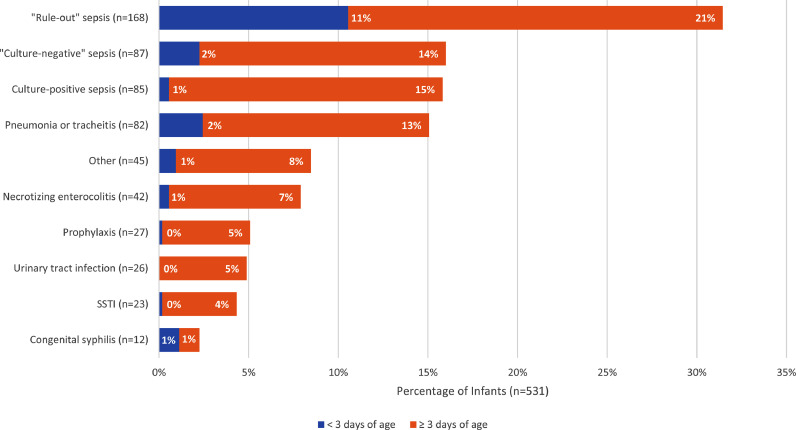
The majority of infants (55%, 293/531) received an antibacterial agent(s) as empiric therapy for possible infection while 38% (n = 204/531) received antibiotic(s) for a specific infection-related diagnosis and 6% (n = 34/531) for prophylaxis. The most frequent reason for infants receiving antibiotics was for possible sepsis (“rule-out sepsis”; 32%; n = 168/531) with the majority (66%; n = 111) of these infants being ≥3 days of age (Fig. 2). The second most frequent reason was “culture-negative” sepsis/meningitis (16%; n = 87). Only 20% of infants received antibacterial therapy for culture-confirmed infection (15%; n = 80, culture-proven sepsis/meningitis; 4%; n = 21, culture-proven urinary tract infection (UTI); 1%; n = 5 had both; Table 3). The most common single organisms detected in blood were coagulase-negative staphylococci (n = 32; 29%) and Klebsiella pneumoniae (n = 24; 22%) while in urine, Klebsiella spp. (n = 12; 39%) and Escherichia coli (n = 7; 23% Table 3) were the most frequent pathogens. Other indications for antibacterial therapy were pneumonia/tracheitis (15%; n = 82), NEC (8%; n = 42), surgical site infection (4%; n = 23), and congenital syphilis (2%; n = 12).
Fig. 2.
Indications for antibiotic use among the 531 infants, with the percentage of infants who received antibacterial therapy for that indication on the x-axis.
Table 3.
Pathogens detected in blood and urine of the 580 infants who received antimicrobial therapy in the neonatal intensive care unit on July 1, 2019.
| Infants in Neonatal Intensive Care Unit |
|||||||
|---|---|---|---|---|---|---|---|
| <3 Days Old |
≥3 Days Old |
Total | |||||
| Country income | Low-to-Middle Income | High Income | Total | Low-to-Middle Income | High Income | Total | |
| No. of infants | 60 (60%) | 40 (40%) | 100 (17%) | 220 | 260 | 480 (83%) | 580 |
| No. of pathogens in blood: | 3 (50%) | 3 (50%) | 6 (6%) | 46 | 59 | 105 (22%) | 111 (19%) |
| Gram-positive | 2 (50%) | 2 (50%) | 4 (67%) | 17 | 31 | 48 (46%) | 52 (47%) |
| Coagulase-negative staphylococci (CoNS)* | 0 | 2 | 2 (33%) | 8 | 22 | 30 (29%) | 32 (29%) |
| Streptococcus pneumoniae | 0 | 0 | 0 | 8 | 0 | 8 (8%) | 8 (7%) |
| Staphylococcus aureus | 2 | 0 | 2 (33%) | 1 | 4 | 5 (5%) | 7 (6%) |
| Group B Streptococcus | 0 | 0 | 0 | 0 | 2 | 2 (2%) | 2 (2%) |
| Bacillus cereus | 0 | 0 | 0 | 0 | 2 | 2 (2%) | 2 (2%) |
| Enterococcus faecalis | 0 | 0 | 0 | 0 | 1 | 1 (1%) | 1 (1%) |
| Gram-negative | 1 | 1 | 2 (33%) | 23 | 20 | 43 (47%) | 45 (41%) |
| Klebsiella spp* | 1 | 0 | 1 (17%) | 11 | 12 | 23 (22%) | 24 (22%) |
| E. coli | 0 | 1 | 1 (17%) | 3 | 3 | 6 (6%) | 7 (6%) |
| Pseudomonas aeruginosa | 0 | 0 | 0 | 3 | 1 | 4 (4%) | 4 (4%) |
| Enterobacter cloacae | 0 | 0 | 0 | 1 | 2 | 3 (3%) | 3 (3%) |
| Serratia marcescens | 0 | 0 | 0 | 1 | 1 | 2 (2%) | 2 (2%) |
| Acinetobacter species | 0 | 0 | 0 | 2 | 0 | 2 (2%) | 2 (2%) |
| Stenotrophomonas maltophilia | 0 | 0 | 0 | 0 | 1 | 1 (1%) | 1 (1%) |
| Pseudomonas fluorescens | 0 | 0 | 0 | 1 | 0 | 1 (1%) | 1 (1%) |
| Alcaligenes faecalis | 0 | 0 | 0 | 1 | 0 | 1 (1%) | 1 (1%) |
| Fungi | 0 | 0 | 0 | 4 | 1 | 5 (%) | 5 (5%) |
| Candida parapsilosis | 0 | 0 | 0 | 2 | 1 | 3 (3%) | 3 (3%) |
| Candida albicans | 0 | 0 | 0 | 1 | 0 | 1 (1%) | 1 (1%) |
| Yeast not identified | 0 | 0 | 0 | 1 | 0 | 1 (1%) | 1 (1%) |
| Polymicrobial | 0 | 0 | 0 | 2 | 6 | 8 (8%) | 8 (7%) |
| E. coli, CoNS | 0 | 0 | 0 | 1 | 1 | 2 (2%) | 2 (2%) |
| C. albicans, C. lusitaniae | 0 | 0 | 0 | 0 | 1 | 1 (1%) | 1 (1%) |
| S. maltophilia, CoNS | 0 | 0 | 0 | 0 | 1 | 1 (1%) | 1 (1%) |
| C. albicans, K. pneumoniae* | 0 | 0 | 0 | 0 | 1 | 1 (1%) | 1 (1%) |
| E. faecalis, K. pneumoniae | 0 | 0 | 0 | 0 | 1 | 1 (1%) | 1 (1%) |
| K. pneumoniae, P. aeruginosa, CoNS | 0 | 0 | 0 | 0 | 1 | 1 (1%) | 1 (1%) |
| B. pertussis, S. pneumoniae | 0 | 0 | 0 | 1 | 0 | 1 (1%) | 1 (1%) |
| Enterovirus* | 0 | 0 | 0 | 0 | 1 | 1 (1%) | 1 (1%) |
| No. of pathogens in urine | 0 | 0 | 0 | 12 | 19 | 31 (6%) | 31 (5%) |
| Gram-positive | 0 | 0 | 0 | 0 | 1 | 1 (3%) | 1 (3%) |
| Enterococcus spp. | 0 | 0 | 0 | 0 | 1 | 1 (3%) | 1 (3%) |
| Gram-negative | 0 | 0 | 0 | 12 | 13 | 25 (81%) | 25 (81%) |
| Klebsiella spp. | 0 | 0 | 0 | 6 | 6 | 12 (39%) | 12 (39%) |
| E. coli | 0 | 0 | 0 | 3 | 4 | 7 (23%) | 7 (23%) |
| Enterobacter spp. | 0 | 0 | 0 | 0 | 3 | 3 (10%) | 3 (10%) |
| P. aeruginosa | 0 | 0 | 0 | 2 | 0 | 2 (6%) | 2 (6%) |
| Gram negative bacilli, not yet identified | 0 | 0 | 0 | 1 | 0 | 1 (3%) | 1 (3%) |
| Fungi | 0 | 0 | 0 | 0 | 1 | 1 (3%) | 1 (3%) |
| C. albicans | 0 | 0 | 0 | 0 | 1 | 1 (3%) | 1 (3%) |
| Polymicrobial | 0 | 0 | 0 | 0 | 4 | 4 (13%) | 4 (13%) |
| Klebsiella. spp., Enterococcus spp., S. aureus | 0 | 0 | 0 | 0 | 1 | 1 (3%) | 1 (3%) |
| Klebsiella spp., Enterococcus spp. | 0 | 0 | 0 | 0 | 1 | 1 (3%) | 1 (3%) |
| E. coli, CoNS | 0 | 0 | 0 | 0 | 1 | 1 (3%) | 1 (3%) |
| E. coli, Enterococcus spp. | 0 | 0 | 0 | 0 | 1 | 1 (3%) | 1 (3%) |
Same pathogen also was detected in cerebrospinal fluid: 2, Klebsiella pneumoniae; 1, CoNS; 1, enterovirus
~Pathogens in polymicrobial cultures are not included in the listing of single isolates.
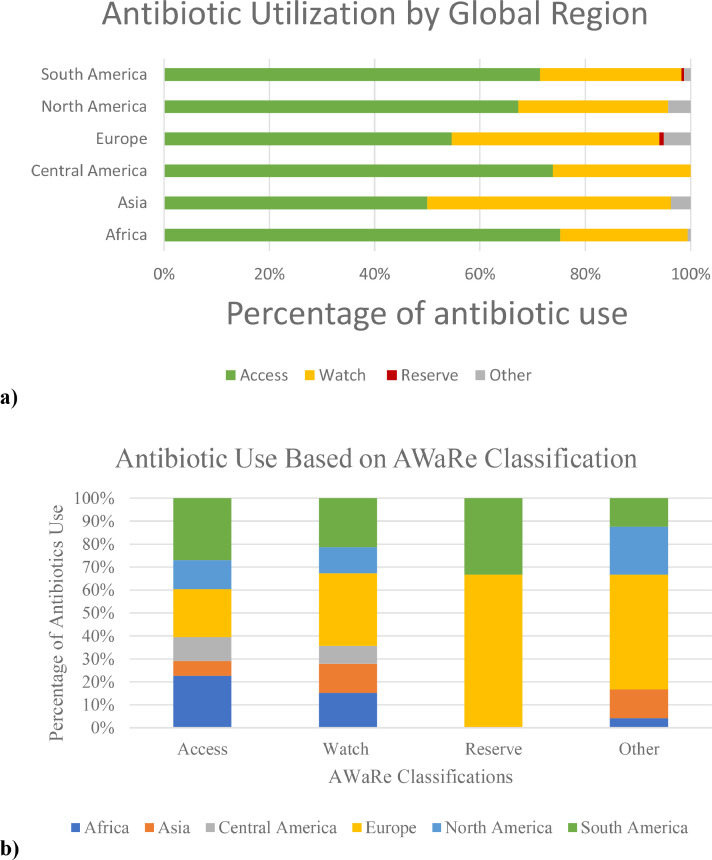
Among the 531 infants who received antibacterial therapy, ampicillin (40%, n = 211), gentamicin (35%, n = 185), and amikacin (19%, n = 101) were most frequently prescribed (Table 2). Among the 38% (204/531) of infants on definitive antibacterial therapy, vancomycin (26%, n = 53), amikacin (n = 20%, n = 40), and meropenem (16%, n = 32) were the most prescribed agents. An additional 35 (17%) infants received third and fourth generation cephalosporins. Access antibiotics were used more frequently by Africa, South America, North America, and Central America while Europe and Asia used more Watch antibiotics. Reserve antibiotics were used only by Europe and South America (Fig. 3).
Fig. 3.
Percentage of total antibiotic use among infants in the neonatal intensive care unit as determined by the World Health Organization AWaRe classification* by a) global region and b) AWaRe group. *AWaRe = Access, Watch, and Reserve.
At the initial assessment, the planned length of treatment was reported in the medical record for 475 (89%) of the 531 infants on antibiotics. The planned length of therapy was indicated as indefinite (15%, n = 72), 1 to 3 days (24%, n = 113), 4 to 6 days (10%, n = 49), 7 days (23%, n = 109), 10 days (12%, n = 59), 14 days (10%, n = 48), 21 days (3%, n = 16), or 4 to 6 weeks (2%, n = 9) for planned length of antibiotic therapy. The final length of therapy was reported on 405 (85%) of the 475 infants when assessed on follow-up at 30 days. The actual length of therapy was 7 days (median; IQR, 5–12 days) overall, with infants who received definitive treatment receiving 10 days (median; IQR, 7–15 days). Final length of therapy for culture-positive and culture-negative infections without concomitant diagnosis of meningitis was 12 days (median; IQR, 8–14 days) and 7 days (median; IQR, 5–10 days), respectively. Length of therapy for pneumonia was 7 days (median; IQR, 5–10 days), for UTI was 7 days (median; IQR, 7–14 days), and for NEC, 9 days (median; IQR, 7–15 days).
Length of antibiotic therapy for any indication did not differ between NICUs with or without NICU-specific ASPs (7 days [median; IQR, 5–13 days] vs. 8 days [median; IQR, 5–12], respectively; p = 0·99) nor was it different between high or low-to-middle income countries (7 days [median, [IQR, 4–12 days] vs. 9 days [median, IQR 5–13], respectively; p = 0·29). Similarly, length of antibiotic therapy for definitive treatment did not differ between NICUs with or without NICU-specific ASPs (10 days [median; IQR, 7–14 days] vs. 12 days [median; IQR, 7–16 days], respectively; p = 0·50) or between high vs. low-to-middle income countries (10 days [median; IQR, 7–14 days] vs. 10 days [median; IQR, 7–15 days], respectively; p = 0·50) 7 (IQR, 6–12). For infants >72 h of age, duration of empiric therapy was 7 days (median; IQR, 5–10) and 10 days for definitive treatment (median; IQR, 7–14). Duration of therapy with antibacterial agents for prophylaxis among infants was 16 days (median; IQR, 7–56).
3.3. Antifungal therapy
Of the 108 infants who received antifungal agents (fluconazole [59%, n = 64], amphotericin B deoxycholate [19%, n = 21], nystatin [18%, n = 19], amphotericin B liposomal [2%, n = 2], caspofungin [1%, n = 1], unknown [1%, n = 1]), the main indication was prophylaxis (62%, n = 67). Of the remaining 41 infants who received antifungal therapy, “rule-out sepsis” (54%; n = 22), sepsis (17%; n = 7), and skin/soft tissue infections (10%; n = 4) were the most common indications. Definitive antifungal treatment for culture-confirmed fungal infection consisted mainly of fluconazole and amphotericin B deoxycholate therapy. Infants on definitive therapy with antifungal agents accounted for 17% (n = 18) of their use. Of the seven (n = 580; 1%) infants treated for systemic disease and had positive cultures for yeast (6, Candida spp. and 1 yeast not identified), 4 received fluconazole and 3 received amphotericin B deoxycholate. Of 67 patients who were on antifungal prophylaxis, the majority were extremely low birth weight infants, less than 1000 gs (69%; n = 46). Overall, 24 (29%) of the 84 hospital NICUs utilized fluconazole prophylaxis.
3.4. Antiviral therapy
Among the 25 infants who received antiviral agents, the main indication was prophylaxis (72%, n = 18) for exposure to maternal infection with human immunodeficiency virus and all received zidovudine monotherapy. Of the remaining seven infants, 5 received acyclovir (2, empiric; 3, treatment), 1 received ganciclovir for cytomegalovirus infection, and in one infant the specific antiviral agent was not known.
3.5. Other analyses
A hospital-wide ASP that included the NICU was present in 62% (n = 50/81) of hospitals (31 high-income and 19 low-to-middle income countries; p = 0·95). An NICU-specific ASP was present in 52% (40/77) of NICUs (23 high-income and 17 low-to-middle income countries; p = 0·47). Eight hospitals with NICU-specific ASP did not have a hospital-wide ASP. NICUs with their own ASP had significantly lower median rates of antibiotic utilization compared to those without one (18% vs. 29%, respectively; p-value=0·02). Similarly, these NICUs with ASP also had fewer antibiotic utilization per patient (1·4 vs. 1·7 antibiotics/patient) and used less Access antibiotics (0·89 vs. 1·2 antibiotic/patient) but similar Watch antibiotics (0·53 vs. 0·45 antibiotic/patient).
Eleven (2%) infants received probiotics and three had bloodstream infections with coagulase-negative staphylococci and two with Staphylococcus aureus, while one had NEC. Three (0·05%) infants received lactoferrin and two had bloodstream infections with coagulase-negative staphylococci and one with S. aureus while the third had staphylococcal osteomyelitis. Among the 46 (8%) infants who received acid suppression medications, 14 had bloodstream infection (7, coagulase-negative staphylococci; 2, S. aureus; 3, Klebsiella pneumoniae; 1, Streptococcus pneumoniae; 1 polymicrobial with Stenotrophomonas maltophilia and coagulase-negative staphylococci).
3.6. Outcomes
Mortality at 30 days was 6% (33/580) and did not differ between infants who received antimicrobial agents in the first 48 h of age (5%; n = 5) versus those who were ≥72 h of age (6%; n = 28; Table 1). The percentage of deaths that were assessed as infection-related also was not different between the two groups (60% [3/5] vs. 39% [11/28], respectively). Of the 33 deaths, 42% (n = 14) were assessed as being related to infection.
4. Discussion
On July 1, 2019, 26% of high-risk infants in NICUs worldwide received at least one antimicrobial agent, mostly antibacterials and in those cared for in low-to-middle income countries. The majority of the infants received antibiotic therapy as empiric coverage for possible sepsis, although a substantial number were treated for “culture-negative” sepsis/meningitis and only a minority for culture-confirmed infection. Importantly, hospitals that had an NICU-specific ASP had significantly lower antibiotic utilization rates. The importance of such a program cannot be over-emphasized as it ultimately may help to decrease antimicrobial resistance in NICUs worldwide [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46].
Infants often received antimicrobial therapy based on clinical suspicion of a serious bacterial infection rather than on positive bacterial culture results. After excluding antibiotics provided for prophylaxis and empiric therapy, 49% of infants received prolonged antibiotic therapy without microbiologic evidence of infection. The notion of prolonged antibiotic therapy for “culture-negative” sepsis should be dispelled from our NICUs [47,48]. Infants in the NICU often experience prematurity-related events that mimic signs and symptoms of infection. Performance of full sepsis evaluations (e.g. blood, urine, cerebrospinal fluid) before initiation of antibiotic therapy, obtaining sufficient quantity of blood for culture, and investigation of viral and fungal etiologies should allow comfort in discontinuation of antibiotics if cultures are sterile [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]. Ultimately, genomic methods for detection of microbial pathogens in body fluids may allow optimal identification of infected infants and more appropriate use of antimicrobial therapy [59,60].
Overall length of antibiotic therapy was often prolonged (i.e., > 72 h) as 80% (325/405) of infants received a median of 7 days with definitive treatment for presumed or culture-positive infection being a median of 10 days. Final length of therapy for culture-positive and culture-negative infections without concomitant diagnosis of meningitis was 12 days (median; IQR, 8–14 days) and 7 days (median; IQR, 5–10 days), respectively. Although length of antibiotic therapy remains an unresolved issue, single center studies have suggested shorter courses of five total days are safe and effective for “culture-negative” sepsis and pneumonia [40,[61], [62], [63]]. Our study did not find any differences in length of therapy between centers irrespective of country, income level, or presence of ASP, confirming the need for robust research to address this knowledge gap.
Analysis of antibiotic use utilizing the WHO AWaRe classification revealed similar use of Access antibiotics across NICUs in South America, North America, and Central America, but higher Watch antibiotics in Europe and Asia (Fig. 3). As some broad spectrum agents such as meropenem are included in the Watch group, further assessment utilizing an antibiotic spectrum index may be a more optimal tool [30,64,65]. Overall use of Reserve antibiotics was low and these agents were only used in Europe and South America (Fig. 3).
Fewer infants received antiviral agents in our study with the majority prescribed for HIV prophylaxis. Similarly, the majority of antifungal agents were used for prophylaxis, although it varied by center as only 29% of NICUs utilized prophylactic fluconazole. The majority of treatment courses were for oral candidiasis, and only 1% (7/580) of infants were treated for systemic disease with either amphotericin B deoxycholate (n = 3) or fluconazole (n = 4). A more targeted assessment of antifungal prophylaxis and treatment is needed to address needed stewardship in this area [66].
Our novel, single-day, cross-sectional study has limitations. First of all and inherent in its design, a single-day prevalence study did not allow assessment of day-to-day variability or longitudinal trends, and thus the resulting wide uncertainty in antimicrobial use was demonstrated by the large range (0–100%) of infants exposed to antimicrobial agents in the 84 NICUs. The generalizability of the results was limited by 48% of the NICUs being from three countries, namely Colombia, Italy, and the United States. Recruitment of study sites was a convenient sample done through personal contact with individuals and centers, many of which have a specific interest in neonatal infectious diseases. It is therefore possible that our study may underestimate the actual use of antimicrobials globally especially among centers not devoted to antimicrobial stewardship. Similar studies performed at a national level in Greece and Australia showed higher prevalence of antimicrobial use that ranged from 40% to 46%, respectively [19,22]. We also did not conduct personal interviews with prescribers to fully understand the rationale for initial and continuation of antimicrobial use beyond what was stated in the infant's medical record. Specific antibiotic mean inhibitory concentrations for detected pathogens also was not obtained in order to optimally assess use of more broad-spectrum agents in the Watch and Reserve groups. We did not query sites concerning antibiotic shortages or supply that could have influenced the type of antimicrobial agent(s) used. Finally, the proportion of infants who received probiotics and lactoferrin could not be ascertained since the number of infants who received them without receiving antimicrobial agents was not obtained.
In conclusion, we found that more than a quarter of infants in NICUs globally received at least one antimicrobial agent. Although the antibiotic utilization was lower in high-income countries, centers that had an NICU-specific ASP had lower antibiotic utilization rates regardless of the country's income level. The finding that NICU-specific ASP had a positive impact on utilization highlights the potential value of such a program to reduce antibiotic consumption and possibly minimize the adverse effects associated with dysbiosis in high-risk infants.
Declaration of Competing Interest
Dr. Pablo J. Sánchez has received research grant support from Merck & Co. during the conduct of the study, and grant from MedImmune, Inc - AstraZeneca, outside of the submitted work.
Dr. Pavel Prusakov has received research grant support from Merck & Co. and Pfizer.
Dr. Debra A. Goff has received research grant support from Merck & Co. and Pfizer.
Dr. Landgrave reports other support from GSK, outside the submitted work. Dr. Kekomäki reports grants and personal fees from Sanofi, grants and personal fees from Merck Sharp & Dome, other support from Pfizer, all outside of the submitted work.
Dr. Mesa reports speaker fees from Pfizer and GlaxoSmithKline, outside of the submitted work.
Mr. Wozniak received a Barnes Medical Student Research Scholarship grant from The Ohio State University College of Medicine.
The other authors have nothing to disclose.
Acknowledgments
Data sharing
All data will be available upon reasonable request to the corresponding author, and it will be shared according to the standards of ethical policies regulating data sharing of human subjects.
Funding
Merck & Co. (PJS, PP, DAG); The Ohio State University College of Medicine Barnes Medical Student Research Scholarship (PSW)
Authors’ contributions
Pavel Prusakov (PP), Debra A. Goff, and Pablo J. Sánchez (PJS) conceptualized and designed the study and analyzed the data set. PP wrote the first draft of the manuscript. All authors are members of the Global NEO-ASP Study Group and obtained the local data, contributed to the interpretation of the data, and made critical revision of the manuscript for important intellectual content. All authors have read and agreed to the final version of the manuscript. PJS as the corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgements
Additional members of the Global NEO-ASP Study Group who contributed to the study: Uchechukwu Obiora Ezomike MBBS, University of Nigeria Teaching Hospital; Kenechukwu K Iloh MBBS, University of Nigeria Teaching Hospital; Carlos Fajardo MD, EpicLatino Neonatal Network; Alejandro Jordan-Villegas, MD, Arnold Palmer Hospital for Children; Ana A. Garcia Robles, PharmD, La Fe University and Polytechnic Hospital; Ana Ruth Mejía-Elizondo MD, Hospital Central Dr. Ignacio Morones Prieto and Universidad Autonoma de San Luis Potosi; Angeliki Kontou, MD, Hippokration Hospital; Ashley Casper, PharmD, Norton Children's Hospital; Ayah Al Bizri MPH, American University of Beirut; Belén Fernández Monteagudo MD, Gregorio Marañon University Hospital; Benedict Nwomeh, MD, Nationwide Children's Hospital; Carlo Pietrasanta, MD, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico; Carlos Andrés Espinosa Rivas MD, Hospital General San Francisco; Carolina Guerra, MD, Division of Neonatology Hospital Barros Luco Trudeau, Santiago, Chile; Claudia R. Hentges, MD, Universidade Federal do Rio Grande do Sul. Hospital de Clinicas de Porto Alegre; David A Kaufman, MD, University of Virginia School of Medicine; Diana Singh, MBBS, University of Guyana, School of Medicine; Efrain Gabriel Suarez Concha MD, Hospital General San Francisco; Eilon Shany, MD, Soroka University Medical Center; Elias Iosifidis, MD, Hippokration Hospital; Elisavet Chorafa, MD, Hippokration Hospital; Emmanuel A. Ameh, MBBS, National Hopital, Abuja, Nigeria; Felix Alakaloko, FMCS, Lagos University Teaching Hospital; Hilary White, DO, St. Vincent Women's Hospital; Imad Kassis MD, The Ruth Rappaport Children's Hospital; Jack Long MD, The Robert Larner College of Medicine at The University of Vermont; Jennifer Bowes, MSc, Children's Hospital of Eastern Ontario; Kosmas Sarafidis, MD, Hippokration Hospital; Laura Piedad Simbaña Guachamin MD, Hospital General San Francisco; Lorenza Pugni, MD, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico; Laszlo Markasz, MD, Uppsala University, Uppsala University Children's Hospital; Louis Bont, MD, Wilhelmina Children's Hospital; Mame Y. Nyarko, MBChB, Princess Marie Louise Children's Hospital; Maranatha Persaud MBBS, University of Guyana, School of Medicine; María L. Avila-Aguero MD, Hospital Nacional de Niños “Dr Carlos Sáenz Herrera” and Yale School of Public Health; Mariya Mukhtar-Yola. MBBS, National Hopital, Abuja, Nigeria; Meirav Sela MSc, The Ruth Rappaport Children's Hospital; Navjyot K. Vidwan, MD, Norton Children's Hospital; Pinky Lea Chirwa MBChB, Nelson Mandela Children's Hospital; Renato S. Procianoy MD, Universidade Federal do Rio Grande do Sul. Hospital de Clinicas de Porto Alegre; Rocío Inojosa MD, Pontificia Universidad Catolica; Ulanda Kilanya Haynes MD, University of Guyana, School of Medicine; Valentina Favero MD, TrevisoHospital; Wilmer Orlando Sánchez Escalante, Hospital General San Francisco; Zaid Alhinai MD, Sultan Qaboos University Hospital
We thank the EpicLatino Neonatal Network for assistance in recruitment of study sites. The study was supported by a grant from Merck & Co. (PP, DAG, and PJS) and The Ohio State University College of Medicine Barnes Medical Student Research Scholarship (PSW). These sponsors had no role in the design and conduct of the study; collection, analysis, and interpretation of data; in the writing of the study; and in the decision to submit the paper for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100727.
Contributor Information
Pavel Prusakov, Email: pavel.prusakov@nationwidechildrens.org.
Debra A. Goff, Email: Debbie.Goff@osumc.edu.
Phillip S. Wozniak, Email: phillip.wozniak@osumc.edu.
Azraa Cassim, Email: ladyaz786@gmail.com.
Catherine E.A. Scipion, Email: cscipion@haitihealth.org.
Soledad Urzúa, Email: soleurzua@gmail.com.
Andrea Ronchi, Email: a.ronchi83@gmail.com.
Lingkong Zeng, Email: freeman315@163.com.
Oluwaseun Ladipo-Ajayi, Email: yawy2@yahoo.com.
Noelia Aviles-Otero, Email: naviote7f@gmail.com.
Chisom R. Udeigwe-Okeke, Email: adaobisom@yahoo.com.
Rimma Melamed, Email: rimmam@bgu.ac.il.
Rita C. Silveira, Email: drarita.c.s@gmail.com.
Cinzia Auriti, Email: cinzia.auriti@opbg.net.
Claudia Beltrán-Arroyave, Email: claumd77@hotmail.com.
Elena Zamora-Flores, Email: zamoraflores@hotmail.com.
Maria Sanchez-Codez, Email: mscodez1990@gmail.com.
Eric S. Donkor, Email: ericsdon@hotmail.com.
Satu Kekomäki, Email: satu.kekomaki@hus.fi.
Nicoletta Mainini, Email: nicmainini@yahoo.it.
Rosalba Vivas Trochez, Email: rossyviv@hotmail.com.
Jamalyn Casey, Email: Jamalyn.Casey@ascension.org.
Juan M. Graus, Email: juan.m.graus@gmail.com.
Mallory Muller, Email: Mallory.Muller@orlandohealth.com.
Sara Singh, Email: sara.divah@gmail.com.
Yvette Loeffen, Email: Y.G.T.Loeffen-2@umcutrecht.nl.
María Eulalia Tamayo Pérez, Email: eulalia.tamayo@udea.edu.co.
Gloria Isabel Ferreyra, Email: glorietaferrey@yahoo.com.ar.
Victoria Lima-Rogel, Email: limamv@hotmail.com.
Barbara Perrone, Email: barbara.perrone7@gmail.com.
Giannina Izquierdo, Email: gianninai@yahoo.es.
María Cernada, Email: mariacernada@gmail.com.
Sylvia Stoffella, Email: Sylvia.Stoffella@ucsf.edu.
Sebastian Okwuchukwu Ekenze, Email: sebekenze@gmail.com, sebastian.ekenze@unn.edu.ng.
Concepción de Alba-Romero, Email: concha.dealbaromero@gmail.com.
Chryssoula Tzialla, Email: c.tzialla@smatteo.pv.it.
Jennifer T. Pham, Email: Tran@uic.edu.
Kenichiro Hosoi, Email: hosoi-k@ks.kyorin-u.ac.jp.
Magdalena Cecilia Calero Consuegra, Email: magdacalero@yahoo.com.
Pasqua Betta, Email: mlbetta@yahoo.it.
O. Alvaro Hoyos, Email: alvaromicro@hotmail.com.
Emmanuel Roilides, Email: roilides@gmail.com.
Gabriela Naranjo-Zuñiga, Email: gabinarzu@gmail.com.
Makoto Oshiro, Email: makoto@nagoya-1st.jrc.or.jp.
Victor Garay, Email: garayvic@gmail.com.
Vito Mondì, Email: vito.mondi@mail.it.
Danila Mazzeo, Email: danilamazzeo@outlook.it.
James A. Stahl, Email: James.Stahl@nortonhealthcare.org.
Joseph B. Cantey, Email: cantey@uthscsa.edu.
Juan Gonzalo Mesa Monsalve, Email: jgmesa1981@yahoo.com.
Erik Normann, Email: erik.normann@akademiska.se.
Lindsay C. Landgrave, Email: Lindsay.c.landgrave@gsk.com.
Ali Mazouri, Email: mazouriali@yahoo.com.
Claudia Alarcón Avila, Email: claudiaalarconavila@gmail.com.
Fiammetta Piersigilli, Email: Fiammetta.piersigilli@uclouvain.be.
Monica Trujillo, Email: trupitv@gmail.com.
Sonya Kolman, Email: Sonya.Kolman@nmch.org.za.
Verónica Delgado, Email: veritamd@icloud.com.
Veronica Guzman, Email: draverolui@gmail.com.
Mohamed Abdellatif, Email: mabdelmonem2015@gmail.com.
Luis Monterrosa, Email: Luis.Monterrosa@HorizonNB.ca.
Lucia Gabriella Tina, Email: gabriella.tina@tiscali.it.
Khalid Yunis, Email: kayunis@aub.edu.lb.
Marco Antonio Belzu Rodriguez, Email: drmarcobr@gmail.com.
Nicole Le Saux, Email: Lesaux@cheo.on.ca.
Valentina Leonardi, Email: valentinaleo83@yahoo.it.
Alessandro Porta, Email: alessandro-porta@tiscali.it.
Giuseppe Latorre, Email: g.latorre@miulli.it.
Hidehiko Nakanishi, Email: n0n.hide@med.kitasato-u.ac.jp.
Michal Meir, Email: MI_MEIR@rambam.health.gov.il.
Paolo Manzoni, Email: paolomanzoni@hotmail.com.
Ximena Norero, Email: xnorero@gmail.com.
Angela Hoyos, Email: ahoyos@clinicadelcountry.com.
Diana Arias, Email: dianaarias@hotmail.com.
Rubén García Sánchez, Email: rubennigue@hotmail.com.
Alexandra K. Medoro, Email: Alexandra.Medoro@nationwidechildrens.org.
Pablo J. Sánchez, Email: Pablo.Sanchez@nationwidechildrens.org.
Appendix. Supplementary materials
References
- 1.Hsieh E.M., Hornik C.P., Clark R.H., Laughon M.M., Benjamin D.K., Jr., Smith P.B. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31(9):811–821. doi: 10.1055/s-0033-1361933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grohskopf L.A., Huskins W.C., Sinkowitz-Cochran R.L., Levine G.L., Goldmann D.A., Jarvis W.R. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr Infect Dis J. 2005;24(9):766–773. doi: 10.1097/01.inf.0000178064.55193.1c. [DOI] [PubMed] [Google Scholar]
- 3.Shane A.L., Sanchez P.J., Stoll B.J. Neonatal sepsis. Lancet. 2017;390(10104):1770–1780. doi: 10.1016/S0140-6736(17)31002-4. [DOI] [PubMed] [Google Scholar]
- 4.Patel R.M., Kandefer S., Walsh M.C., Bell E.F., Carlo W.A., Laptook A.R. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–340. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotten C.M., Taylor S., Stoll B., Goldberg R.N., Hansen N.I., Sanchez P.J. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander V.N., Northrup V., Bizzarro M.J. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–397. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantey J.B., Pyle A.K., Wozniak P.S., Hynan L.S., Sanchez P.J. Early antibiotic exposure and adverse outcomes in preterm, very low birth weight infants. J Pediatr. 2018;203:62–67. doi: 10.1016/j.jpeds.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 8.Kuppala V.S., Meinzen-Derr J., Morrow A.L., Schibler K.R. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–725. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novitsky A., Tuttle D., Locke R.G., Saiman L., Mackley A., Paul D.A. Prolonged early antibiotic use and bronchopulmonary dysplasia in very low birth weight infants. Am J Perinatol. 2015;32(1):43–48. doi: 10.1055/s-0034-1373844. [DOI] [PubMed] [Google Scholar]
- 10.Ting J.Y., Roberts A., Sherlock R., Ojah C., Cieslak Z., Dunn M. Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics. 2019;143(3) doi: 10.1542/peds.2018-2286. [DOI] [PubMed] [Google Scholar]
- 11.Ting J.Y., Synnes A., Roberts A., Deshpandey A., Dow K., Yoon E.W. Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr. 2016;170(12):1181–1187. doi: 10.1001/jamapediatrics.2016.2132. [DOI] [PubMed] [Google Scholar]
- 12.Ting J.Y., Synnes A., Roberts A., Deshpandey A.C., Dow K., Yang J. Association of antibiotic utilization and neurodevelopmental outcomes among extremely low gestational age neonates without proven sepsis or necrotizing enterocolitis. Am J Perinatol. 2018;35(10):972–978. doi: 10.1055/s-0038-1632390. [DOI] [PubMed] [Google Scholar]
- 13.Cantey J.B., Huffman L.W., Subramanian A., Marshall A.S., Ballard A.R., Lefevre C. Antibiotic exposure and risk for death or bronchopulmonary dysplasia in very low birth weight infants. J Pediatr. 2017;181:289–293. doi: 10.1016/j.jpeds.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 14.de Man P., Verhoeven B.A., Verbrugh H.A., Vos M.C., van den Anker J.N. An antibiotic policy to prevent emergence of resistant bacilli. Lancet. 2000;355(9208):973–978. doi: 10.1016/s0140-6736(00)90015-1. [DOI] [PubMed] [Google Scholar]
- 15.Johnston K.J., Thorpe K.E., Jacob J.T., Murphy D.J. The incremental cost of infections associated with multidrug-resistant organisms in the inpatient hospital setting-A national estimate. Health Serv Res. 2019;54(4):782–792. doi: 10.1111/1475-6773.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulman J., Benitz W.E., Profit J., Lee H.C., Duenas G., Bennett M.V. Newborn antibiotic exposures and association with proven bloodstream infection. Pediatrics. 2019;144(5) doi: 10.1542/peds.2019-1105. [DOI] [PubMed] [Google Scholar]
- 17.Schulman J., Profit J., Lee H.C., Duenas G., Bennett M.V., Parucha J. Variations in neonatal antibiotic use. Pediatrics. 2018;142(3) doi: 10.1542/peds.2018-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantey J.B., Wozniak P.S., Sanchez P.J. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatr Infect Dis J. 2015;34(3):267–272. doi: 10.1097/INF.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 19.Osowicki J., Gwee A., Noronha J., Britton P.N., Isaacs D., Lai T.B. Australia-wide point prevalence survey of antimicrobial prescribing in neonatal units: how much and how good? Pediatr Infect Dis J. 2015;34(8):e185–e190. doi: 10.1097/INF.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 20.Versporten A., Sharland M., Bielicki J., Drapier N., Vankerckhoven V., Goossens H. The antibiotic resistance and prescribing in European Children project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr Infect Dis J. 2013;32(6):e242–e253. doi: 10.1097/INF.0b013e318286c612. [DOI] [PubMed] [Google Scholar]
- 21.Hsia Y., Lee B.R., Versporten A., Yang Y., Bielicki J., Jackson C. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Health. 2019;7(7):e861–ee71. doi: 10.1016/S2214-109X(19)30071-3. [DOI] [PubMed] [Google Scholar]
- 22.Gkentzi D., Kortsalioudaki C., Cailes B.C., Zaoutis T., Kopsidas J., Tsolia M. Epidemiology of infections and antimicrobial use in Greek Neonatal Units. Arch Dis Child Fetal Neonatal Ed. 2019;104(3):F293–F2F7. doi: 10.1136/archdischild-2018-315024. [DOI] [PubMed] [Google Scholar]
- 23.Shipp K.D., Chiang T., Karasick S., Quick K., Nguyen S.T., Cantey J.B. Antibiotic stewardship challenges in a referral neonatal intensive care unit. Am J Perinatol. 2016;33(5):518–524. doi: 10.1055/s-0035-1569990. [DOI] [PubMed] [Google Scholar]
- 24.Versporten A., Bielicki J., Drapier N., Sharland M., Goossens H. The worldwide antibiotic resistance and prescribing in European children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother. 2016;71(4):1106–1117. doi: 10.1093/jac/dkv418. [DOI] [PubMed] [Google Scholar]
- 25.Citrome L. Happy birthday ignac semmelweiss! now, let's all wash our hands! Int J Clin Pract. 2018;72(10):e13256. doi: 10.1111/ijcp.13256. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Pediatrics Committee on F Newborn. Levels of neonatal care. Pediatrics. 2012;130(3):587–597. doi: 10.1542/peds.2012-1999. [DOI] [PubMed] [Google Scholar]
- 27.Krumholz H.M., Normand S.L. Public reporting of 30-day mortality for patients hospitalized with acute myocardial infarction and heart failure. Circulation. 2008;118(13):1394–1397. doi: 10.1161/CIRCULATIONAHA.108.804880. [DOI] [PubMed] [Google Scholar]
- 28.Scott H.F., Brou L., Deakyne S.J., Kempe A., Fairclough D.L., Bajaj L. Association between early lactate levels and 30-day mortality in clinically suspected sepsis in children. JAMA Pediatr. 2017;171(3):249–255. doi: 10.1001/jamapediatrics.2016.3681. [DOI] [PubMed] [Google Scholar]
- 29.WHO . World Health Organization; Geneva: 2019. The 2019 WHO AWaRe classification of antibiotics for evaluation and monitoring of use. WHO/EMP/IAU/2019.11). . 2019. [Google Scholar]
- 30.Sharland M., Pulcini C., Harbarth S., Zeng M., Gandra S., Mathur S. Classifying antibiotics in the WHO Essential Medicines List for optimal use-be AWaRe. Lancet Infect Dis. 2018;18(1):18–20. doi: 10.1016/S1473-3099(17)30724-7. [DOI] [PubMed] [Google Scholar]
- 31.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lumley T., Diehr P., Emerson S., Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 34.Araujo da Silva A.R., Marques A., Di Biase C., Faitanin M., Murni I., Dramowski A. Effectiveness of antimicrobial stewardship programmes in neonatology: a systematic review. Arch Dis Child. 2020;105(6):563–568. doi: 10.1136/archdischild-2019-318026. [DOI] [PubMed] [Google Scholar]
- 35.Cantey J.B., Patel S.J. Antimicrobial stewardship in the NICU. Infect Dis Clin North Am. 2014;28(2):247–261. doi: 10.1016/j.idc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Dukhovny D., Buus-Frank M.E., Edwards E.M., Ho T., Morrow K.A., Srinivasan A. A collaborative multicenter QI initiative to improve antibiotic stewardship in newborns. Pediatrics. 2019;144(6) doi: 10.1542/peds.2019-0589. [DOI] [PubMed] [Google Scholar]
- 37.Johnson C.L., Saiman L. A blueprint for targeted antimicrobial stewardship in neonatal intensive care units. Infect Control Hosp Epidemiol. 2017;38(10):1144–1146. doi: 10.1017/ice.2017.183. [DOI] [PubMed] [Google Scholar]
- 38.Cantey J.B. Optimizing the use of antibacterial agents in the neonatal period. Paediatr Drugs. 2016;18(2):109–122. doi: 10.1007/s40272-015-0161-1. [DOI] [PubMed] [Google Scholar]
- 39.Dramowski A., Velaphi S., Reubenson G., Bekker A., Perovic O., Finlayson H. National neonatal sepsis task force launch: supporting infection prevention and surveillance, outbreak investigation and antimicrobial stewardship in neonatal units in South Africa. S Afr Med J. 2020;110(5):360–363. doi: 10.7196/SAMJ.2020.v110i5.14564. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy K.N., Hawke A., Dempsey E.M. Antimicrobial stewardship in the neonatal unit reduces antibiotic exposure. Acta Paediatr. 2018;107(10):1716–1721. doi: 10.1111/apa.14337. [DOI] [PubMed] [Google Scholar]
- 41.Rueda M.S., Calderon-Anyosa R., Gonzales J., Turin C.G., Zea-Vera A., Zegarra J. Antibiotic overuse in premature low birth weight infants in a developing country. Pediatr Infect Dis J. 2019;38(3):302–307. doi: 10.1097/INF.0000000000002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez P.J., Moallem M., Cantey J.B., Milton A., Michelow I.C. Empiric therapy with vancomycin in the neonatal intensive care unit: let's "get smart" globally! J Pediatr (Rio J) 2016;92(5):432–435. doi: 10.1016/j.jped.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Steinmann K.E., Lehnick D., Buettcher M., Schwendener-Scholl K., Daetwyler K., Fontana M. Impact of empowering leadership on antimicrobial stewardship: a single center study in a neonatal and pediatric intensive care unit and a literature review. Front Pediatr. 2018;6:294. doi: 10.3389/fped.2018.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ting J.Y., Shah P.S. Antibiotic stewardship in neonates: challenges and opportunities. Transl Pediatr. 2020;9(3):198–201. doi: 10.21037/tp-20-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ting J.Y., Paquette V., Ng K., Lisonkova S., Hait V., Shivanada S. Reduction of inappropriate antimicrobial prescriptions in a tertiary neonatal intensive care unit after antimicrobial stewardship care bundle implementation. Pediatr Infect Dis J. 2019;38(1):54–59. doi: 10.1097/INF.0000000000002039. [DOI] [PubMed] [Google Scholar]
- 46.Urzua S., Ferres M., Garcia P., Sanchez A., Luco M. Strategies to reduce infections and antibiotic use and its effects in a neonatal care unit. Rev Chilena Infectol. 2017;34(2):99–107. doi: 10.4067/S0716-10182017000200001. [DOI] [PubMed] [Google Scholar]
- 47.Cantey J.B., Sanchez P.J. Prolonged antibiotic therapy for "culture-negative" sepsis in preterm infants: it's time to stop! J Pediatr. 2011;159(5):707–708. doi: 10.1016/j.jpeds.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 48.Cantey J.B., Baird S.D. Ending the culture of culture-negative sepsis in the neonatal ICU. Pediatrics. 2017;140(4) doi: 10.1542/peds.2017-0044. [DOI] [PubMed] [Google Scholar]
- 49.Moallem M., Song E., Jaggi P., Conces M.R., Kajon A.E., Sanchez P.J. Adenovirus and "culture-negative sepsis" in a preterm neonate. AJP Rep. 2016;6(4):e417–ee20. doi: 10.1055/s-0036-1597266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ronchi A., Doern C., Brock E., Pugni L., Sanchez P.J. Neonatal adenoviral infection: a seventeen year experience and review of the literature. J Pediatr. 2014;164(3):529–535. doi: 10.1016/j.jpeds.2013.11.009. e1-4. [DOI] [PubMed] [Google Scholar]
- 51.Ronchi A., Michelow I.C., Chapin K.C., Bliss J.M., Pugni L., Mosca F. Viral respiratory tract infections in the neonatal intensive care unit: the VIRIoN-I study. J Pediatr. 2014;165(4):690–696. doi: 10.1016/j.jpeds.2014.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ronchi A., Ouellette C.P., Mejias A., Salamon D., Leber A., Pugni L. Detection of cytomegalovirus in saliva from infants undergoing sepsis evaluation in the neonatal intensive care unit: the VIRIoN-C study. J Perinat Med. 2018;47(1):90–98. doi: 10.1515/jpm-2018-0021. [DOI] [PubMed] [Google Scholar]
- 53.Lanzieri T.M., Dollard S.C., Josephson C.D., Schmid D.S., Bialek S.R. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics. 2013;131(6):e1937–e1945. doi: 10.1542/peds.2013-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett N.J., Tabarani C.M., Bartholoma N.M., Wang D., Huang D., Riddell S.W. Unrecognized viral respiratory tract infections in premature infants during their birth hospitalization: a prospective surveillance study in two neonatal intensive care units. J Pediatr. 2012;161(5):814–818. doi: 10.1016/j.jpeds.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cantey J.B. The spartacus problem: diagnostic inefficiency of neonatal sepsis. Pediatrics. 2019;144(5) doi: 10.1542/peds.2019-2576. [DOI] [PubMed] [Google Scholar]
- 56.Klingenberg C., Kornelisse R.F., Buonocore G., Maier R.F., Stocker M. Culture-negative early-onset neonatal sepsis - at the crossroad between efficient sepsis care and antimicrobial stewardship. Front Pediatr. 2018;6:285. doi: 10.3389/fped.2018.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamba V., D'Souza S., Carafa C., Zepf A., Bassel C.L., Gutierrez M. Standardizing the approach to late onset sepsis in neonates through antimicrobial stewardship: a quality improvement initiative. J Perinatol. 2020 doi: 10.1038/s41372-019-0577-5. [DOI] [PubMed] [Google Scholar]
- 58.Ho T., Buus-Frank M.E., Edwards E.M., Morrow K.A., Ferrelli K., Srinivasan A. Adherence of newborn-specific antibiotic stewardship programs to CDC recommendations. Pediatrics. 2018;142(6) doi: 10.1542/peds.2017-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blauwkamp T.A., Thair S., Rosen M.J., Blair L., Lindner M.S., Vilfan I.D. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4(4):663–674. doi: 10.1038/s41564-018-0349-6. [DOI] [PubMed] [Google Scholar]
- 60.Witt R.G., Blair L., Frascoli M., Rosen M.J., Nguyen Q.H., Bercovici S. Detection of microbial cell-free DNA in maternal and umbilical cord plasma in patients with chorioamnionitis using next generation sequencing. PLoS ONE. 2020;15(4) doi: 10.1371/journal.pone.0231239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engle W.D., Jackson G.L., Sendelbach D., Ford D., Olesen B., Burton K.M. Neonatal pneumonia: comparison of 4vs 7 days of antibiotic therapy in term and near-term infants. J Perinatol. 2000;20(7):421–426. doi: 10.1038/sj.jp.7200416. [DOI] [PubMed] [Google Scholar]
- 62.Engle W.D., Jackson G.L., Sendelbach D.M., Stehel E.K., Ford D.M., McHugh K.M. Pneumonia in term neonates: laboratory studies and duration of antibiotic therapy. J Perinatol. 2003;23(5):372–377. doi: 10.1038/sj.jp.7210949. [DOI] [PubMed] [Google Scholar]
- 63.Cantey J.B., Wozniak P.S., Pruszynski J.E., Sanchez P.J. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis. 2016;16(10):1178–1184. doi: 10.1016/S1473-3099(16)30205-5. [DOI] [PubMed] [Google Scholar]
- 64.Lahart A.C., McPherson C.C., Gerber J.S., Warner B.B., Lee B.R., Newland J.G. Application of an antibiotic spectrum index in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2019;40(10):1181–1183. doi: 10.1017/ice.2019.221. [DOI] [PubMed] [Google Scholar]
- 65.Gerber J.S., Hersh A.L., Kronman M.P., Newland J.G., Ross R.K., Metjian T.A. Development and application of an antibiotic spectrum index for benchmarking antibiotic selection patterns across hospitals. Infect Control Hosp Epidemiol. 2017;38(8):993–997. doi: 10.1017/ice.2017.94. [DOI] [PubMed] [Google Scholar]
- 66.Johnson M.D., Lewis R.E., Dodds Ashley E.S., Ostrosky-Zeichner L., Zaoutis T., Thompson G.R. Core recommendations for antifungal stewardship: a statement of the mycoses study group education and research consortium. J Infect Dis. 2020;222(Supplement_3):S175–SS98. doi: 10.1093/infdis/jiaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.