Abstract
Background
Anticorrelated resting state connectivity between task-positive and task-negative networks in adults supports flexible shifting between externally focused attention and internal thought. Findings suggest that children show positive correlations between task-positive (frontoparietal; FP) and task-negative (default mode; DMN) networks. FP-DMN connectivity also associates with intellectual functioning across the lifespan. We investigated whether FP-DMN connectivity in healthy children varied with age and intelligence quotient (IQ).
Methods
We utilized network-based statistics (NBS) to examine resting state functional connectivity between FP and DMN seeds in N = 133 7−25-year-olds (Mage = 15.80). Linear regression evaluated FP-DMN associations with IQ.
Results
We detected NBS subnetworks containing both within- and between-network connections that were inversely associated with age. Four FP-DMN connections showed more negative connectivity between FP (inferior frontal gyrus and precentral gyrus) and DMN regions (frontal medial cortex, precuneus, and frontal pole) among older participants. Frontal pole-precentral gyrus connectivity inversely associated with IQ.
Conclusions
FP-DMN connectivity was more anticorrelated at older ages, potentially indicating dynamic network segregation of these circuits from childhood to early adulthood. Youth with more mature (i.e., anticorrelated) FP-DMN connectivity demonstrated higher IQ. Our findings add to the growing body of literature examining neural network development and its association with IQ.
Keywords: Child, Network connectivity, Typical development, Intelligence, Frontoparietal, Default Mode
Functional magnetic resonance imaging (fMRI) studies of healthy individuals at rest have identified canonical brain networks associated with broad aspects of psychological functioning (Cole et al., 2014; Arbabshirani et al., 2013; Di et al., 2013; Smith et al., 2009; Power et al., 2011; Yeo et al., 2011). Task positive networks include regions where activity typically increases during task performance (Fox et al., 2005; Cabeza and Nyberg, 2000) and with increased cognitive demand (Klingberg et al., 1997; Rietschel et al., 2012). Functional connectivity between these regions at rest associates with better performance on cognitive control tasks (Marek et al., 2015; Sheffield et al., 2015). These task positive networks include the fronto-parietal (FP), cingulo-opercular, dorsal attention, salience, and ventral attention networks, which are implicated in cognitive control and attention modulation. The default mode network (DMN), in contrast, is thought to be task negative as it is typically engaged during undirected thought or passive mental states and disengaged during task performance (Raichle et al., 2001). Regions in the DMN are active when the brain is not engaged in a specific task (Harrison et al., 2008), and activity typically increases when an individual engages in task irrelevant thoughts, mind wandering, and autobiographical thinking (Harrison et al., 2008; Spreng and Grady, 2010; Mason et al., 2007).
Resting state connectivity between these task positive (FP, cingulo-opercular, dorsal attention) and task negative (DMN) networks is anticorrelated (i.e., negative correlations) in adults (Fox et al., 2005; Gopinath et al., 2015; Keller et al., 2013, 2015; Parente and Colosimo, 2020; Uddin et al., 2009). Moreover, regions comprising these networks show anticorrelated task-induced activations that increase with increasingly demanding cognitive load (Hampson et al., 2010; Persson et al., 2007; Douw et al., 2016; Leech et al., 2011; Grady et al., 2010; Hugdahl et al., 2019; Amer et al., 2016; Avelar-Pereira et al., 2017). Similar results are observed at rest, such that greater anticorrelation between task positive and negative networks is associated with improved cognitive control capacity measured outside of the scanner (Keller et al., 2015; Kelly et al., 2008; Reineberg et al., 2018; Kim and Kang, 2018; James et al., 2016; Medaglia et al., 2018). Such anticorrelation suggests that when task positive networks are more active, e.g., during a cognitively demanding task, the task negative network is less active, and vice-versa. This balance is thought to underlie the ability to remain focused on a task and not become distracted by internal thoughts (Fair et al., 2010; Mills et al., 2018; Posner et al., 2014a). Anticorrelation between task positive and negative networks in adults has been well documented; however, the functional connectivity between these networks in children remains understudied.
Recent evidence from cross-sectional studies of resting-state functional connectivity between task positive and negative networks in children, and specifically between the FP and DMN, suggests that children may not show adult-like patterns of between-network connectivity. Rather, in children, patterns of anticorrelation between these networks may develop over time, possibly mirroring children’s increasing ability to focus on and execute tasks independently with increasing age (Gur et al., 2012; Roalf et al., 2014; Piaget, 1952; Marsh et al., 2008; Luna et al., 2015). Prior findings show that the FP (task positive) and DMN (task negative) show positive connectivity in children ages 7–12 (Margolis et al., 2019; Koyama et al., 2013; Chai et al., 2014). Moreover, the FP and DMN are more anticorrelated at older ages, such that children (ages 8–12) show positive connectivity, adolescents (ages 13–17) show mixed positive and negative connectivity, and adults (ages 18–24) show negative (anticorrelated) connectivity (Chai et al., 2014). In contrast, one longitudinal study of 176 typically developing youth showed negative connectivity between the right frontal pole (FP) and DMN regions (left posterior middle temporal gyrus, left paracingulate gyrus) at age 7 that did not change with increasing age (Mills et al., 2018). Positive connectivity between other task positive networks (cingulo-opercular, dorsal attention) and the DMN has not been reported in children, but increasing age is associated with increasingly negative connectivity between DMN regions and regions in the dorsal attention network (Chai et al., 2014). Thus, much of the extant data point to a pattern of age-related change in FP-DMN connectivity with connectivity between these regions shifting toward more negative correlations in young adulthood. Notably, there are methodological differences between studies (e.g., exploratory seed-to-voxel analytic methods [Chai et al., 2014; Langeslag et al., 2013; Sherman et al., 2014] versus independent components analysis [Mills et al., 2018]) and some had relatively small sample sizes. Given that prior findings are mixed, more targeted work examining FP-DMN connectivity is needed, particularly among young children.
Precisely characterizing age-related patterns of FP-DMN connectivity is important to understanding intellectual functioning in youth. Specifically, performance on measures of intelligence relies heavily on in-the-moment attentional control during testing as well as on an individuals’ ability to pay attention to and integrate information learned across their lifetime. In children ages 6–13, increased FP-DMN anticorrelation is associated with higher intellectual functioning (Langeslag et al., 2013; Sherman et al., 2014). To our knowledge, no work has evaluated FP-DMN resting state functional connectivity correlates of intellectual functioning in adolescents. Such a study would facilitate our understanding of how brain network connectivity during childhood and adolescence impacts global functioning, such as that associated with intellectual functioning.
Herein, we examine associations between age and FP-DMN connectivity and how these associations relate to child intellectual functioning. As prior findings show positive connectivity between the DMN and the FP (Margolis et al., 2019; Koyama et al., 2013; Chai et al., 2014), we specifically focused our analyses on FP-DMN connectivity in a sample of 133 healthy youth ranging from 7 to 25 years old. We hypothesized that FP-DMN connectivity would be more anticorrelated at older ages, consistent with prior findings (Margolis et al., 2019; Koyama et al., 2013; Chai et al., 2014; Sherman et al., 2014; Fair et al., 2008). Further, we hypothesized that youth with more adult-like FP-DMN connectivity (i.e., more anticorrelated) would demonstrate higher intellectual functioning than youth with less adult-like FP-DMN connectivity (i.e., less anticorrelated).
1. Methods
1.1. Participants
MRI data from 133 healthy youth who were recruited as comparison participants for case-control studies (Cha et al., 2015; Marsh et al., 2011; Posner et al., 2014b; Tau et al., 2014) were included in the current study. Youth in these parent studies were recruited between 2011–2017 from the greater New York City area (see Table S1 for recruitment strategies). The case-control studies required that healthy comparison participants did not meet criteria for any current psychiatric disorders on the basis of a semi-structured diagnostic interview [KSADS-PL (Kaufman et al., 1997); SCID DSM-IV (First and Gibbon, 2004)] completed by a trained research assistant and confirmed by a licensed psychologist or psychiatrist. Children with MRI contraindications, including history of concussion, metal in the body, etc. were excluded from the parent studies. Of the initial 133 participants, eight were excluded for excessive head motion (described below; see Table S2 for detailed demographic information). All parent studies were approved by the Institutional Review Board of the New York State Psychiatric Institute, and all participants provided informed consent and assent. Participants who completed an MRI scan session with at least one structural and one resting state scan were included in the current study.
1.2. Measures
All participants completed interview assessments for psychiatric disorders (K-SADS-PL or SCID DSM-IV diagnostic interview). A subset of youth (N = 93) additionally completed measures of intellectual functioning [Wechsler Abbreviated Scales of Intelligence, WASI (Wechsler, 1999)].
1.3. Imaging data acquisition
All participants were scanned on the same General Electric Signa 3-Tesla LX scanner (Milwaukee, WI). Included participants completed at least one T1-weighted fast field echo (FFE) structural scan and at least 5 min of concatenated resting state axial echo-planar imaging (EPI) scan time using a standard quadrature 32-channel head coil. Specific scan parameters varied slightly across studies (see Table S1); statistical analyses included pulse sequence as a covariate to control for differences across studies. Participants were instructed to rest quietly and let their minds wander while focusing on a white fixation cross for the duration of resting state scans.
1.4. Preprocessing and motion correction
Preprocessing was completed using Statistical Parametric Mapping 12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and CONN-fMRI Functional Connectivity Toolbox v 18b [http://www.nitric.org/projects/conn/, (Whitfield-Gabrieli and Nieto-Castanon, 2012)], with MATLAB version R2018a. Resting runs were concatenated prior to preprocessing. Preprocessing consisted of functional realignment and unwarping, slice timing correction, scrubbing, and simultaneous segmentation and normalization to the Montreal Neurological Institute (MNI) template. Head motion outliers were identified using the ART tools (>0.5 mm framewise displacement or Z > 3 change in global signal). Frames with head motion outliers were regressed in participant-level models along with anatomical nuisance regressors [aCompCor; (Behzadi et al., 2007)] from white matter (10 components) and cerebrospinal fluid (10 components). Participants with more than 5 min of useable functional data were included in final analyses. Functional images were band-pass filtered (0.01−0.1 Hz).
1.5. Network definition
Brain networks have been previously defined by a small number of representative regions of interest [ROIs (Fox et al., 2005; Chai et al., 2014; Sherman et al., 2014; Fair et al., 2008)], neural network templates (e.g. (Yeo et al., 2011; Desikan et al., 2006), or data-driven network analytic methods, such as independent components analysis (ICA) or principal components analysis [PCA (Arbabshirani et al., 2013; Avelar-Pereira et al., 2017; Kelly et al., 2008; Qian et al., 2019)]. We defined the FP and DMN using 25 frontoparietal and 58 default mode seeds as 5 mm spheres from the Power 264 atlas (Power et al., 2011) in order to examine associations between age and FP-DMN connectivity across multiple network ROIs. The blood-oxygen level dependent signal (BOLD) time course of each ROI was defined as the average of its voxels’ time courses. Resting-state functional connectivity between each pair of ROIs was calculated as the Fisher r-to-Z transformed Pearson’s correlation between their time courses. Connectivity values were used to create an 83 × 83 connectivity matrix with 3403 unique edges ((83*82)/2) for each participant.
1.6. Network based statistics
Traditional methods for controlling for multiple comparisons when using many ROIs to define neural networks, such as false discovery rate, can artificially limit the statistical power of a study (Zalesky et al., 2010). One alternative to traditional multiple comparison correction is to use nonparametric methods for controlling for family-wise error rate (or the probability of making any Type 1 errors), rather than false discovery rate (expected proportion of false rejections out of total rejections), such as with network-based statistics [NBS (Zalesky et al., 2010)]. NBS conserves power while still controlling for multiple comparison corrections across numerous ROIs and sensitivity thresholds (see Supplementary Methods).
1.7. Statistical analysis
We used NBS to examine how FP-DMN connectivity varied with age (Zalesky et al., 2010). This was implemented in three steps. First, NBS performed a multiple regression with each edge in the connectivity matrix as the dependent variable, age as the predictor of interest, and sex, mean motion (framewise displacement in mm), and pulse sequence as nuisance covariates. An edge is defined here as the connectivity between two ROIs, similar to graph theory (Zalesky et al., 2010). Second, subnetworks of interconnected edges with positive or negative age effects corresponding to T-test statistics above a prechosen edge-level threshold were retained (i.e., NBS identified subnetworks of brain regions where the strength of the edges was associated with child age above the pre-chosen sensitivity threshold). Observed subnetworks change with changing sensitivity thresholds thereby revealing different information about the structure of these subnetworks across multiple sensitivity thresholds. For example, subnetworks that are significant only at liberal thresholds (e.g., p < 0.05) are likely to be subtle but large; whereas effects present only at conservative thresholds are characterized by strong but focal associations. Subnetworks that retain their significance across a range of thresholds are characterized by strong, topologically extended effects. In order to compare findings across multiple thresholds and following methods in recent publications (Zalesky et al., 2010; Fornito et al., 2016; Nelson et al., 2017; Pua et al., 2018; Wagner et al., 2019), we examined sensitivity thresholds of t>2.5 (p < .006), t>3.0 (p < .002), and t>3.5 (p < .0001). Finally, NBS performed bootstrapping with 5000 simulations to determine whether the size of the retained subnetworks were larger than expected by chance. In primary analyses, we defined the size of a subnetwork as its extent (number of constituent edges). Size was defined by intensity (sum of the test statistics of its constituent edges) in confirmatory analyses. Because NBS performs one-sided testing, we considered networks with a corrected analysis-level p < .025 as significant. BrainNet Viewer (Xia et al., 2013) was used to visualize significant networks.
FP-DMN functional connectivity was extracted for all FP-DMN edges that were significant across multiple thresholds. Linear regression models evaluated associations between age and FP-DMN resting state connectivity at each extracted edge and Full-Scale Intelligence Quotient (FSIQ) score, controlling for sex, mean in-scanner motion, and pulse sequence. Missing data were excluded listwise from secondary analyses.
2. Results
2.1. Sample characteristics
The final sample included 124 youth (58 boys, 35.5 % non-Hispanic Caucasian, 16.1 % Hispanic) ranging in age from 7 to 25 years (M = 16.28, SD = 4.73; Table 1). Mean framewise displacement (F (3, 121) = 4.85, p = 0.003) and volumes scrubbed (F (3, 121) = 3.80, p = 0.012) differed across age groups, such that younger children showed greater framewise displacement and number of volumes scrubbed than children in older groups (see Supplementary Table 2). As a result, excluded youth were younger than included youth (F (1, 131) = 11.90, p = 0.001); excluded youth did not differ from included youth on any other variables (see Supplementary Table 3). Ninety-two youth had complete imaging and WASI data (see Supplementary Table 4).
Table 1.
Sample Characteristics.
| N = 124 | Mean (SD) / N (%) |
|---|---|
| Age | 16.33 (4.71) |
| 7−10 years | 20 (16.1) |
| 11–13 years | 21 (16.9) |
| 14–17 years | 28 (22.6) |
| 18–25 years | 55 (44.3) |
| Sex (% male) | 58 (46.8) |
| Race | |
| White | 38 (35.5) |
| Black | 29 (27.1) |
| Asian | 12 (11.2) |
| American Indian | 1 (0.8) |
| Other/Mixed | 27 (25.2) |
| Hispanic | 20 (16.1) |
| FSIQ | 114.06 (15.66) |
| Framewise Displacement | 0.21 (0.24) |
Notes. Means and standard deviations are presented for all continuous variables. Number of participants and percentages are presented for all categorical variables. Race/Ethnicity data was missing for 19 participants. FSIQ = Full Scale IQ.
Table 2.
Frontoparietal-default mode network edges with significant age-related connectivity across multiple NBS thresholds.
| FP Node | DMN Node | β | t-value |
|---|---|---|---|
| Left Inferior Frontal Gyrus, pars opercularis (x = −47, y = 11, z = 23) | Right Frontomedial Cortex (x = 8, y = 48, z = −15) |
−0.01 | −3.33 |
| Left Inferior Frontal Gyrus, pars opercularis (x = −47, y = 11, z = 23) | Right Precuneus Cortex (x = 15, y = −63, z = 26) |
−0.01 | −3.22 |
| Left Inferior Frontal Gyrus, pars opercularis (x = −47, y = 11, z = 23) | Right Frontal Pole (x = 22, y = 39, z = 39) |
−0.01 | −3.64 |
| Right Precentral Gyrus (x = 47, y = 10, z = 33) |
Right Frontal Pole (x = 22, y = 39, z = 39) |
−0.01 | −3.20 |
Displays connections and t-values for all frontoparietal-default mode network edges with significant age-related connectivity across multiple NBS thresholds.
2.2. Age and frontoparietal – default mode network connectivity
No positive associations between age and within network (FP or DMN) or between network (FP-DMN) functional connectivity were observed at any threshold.
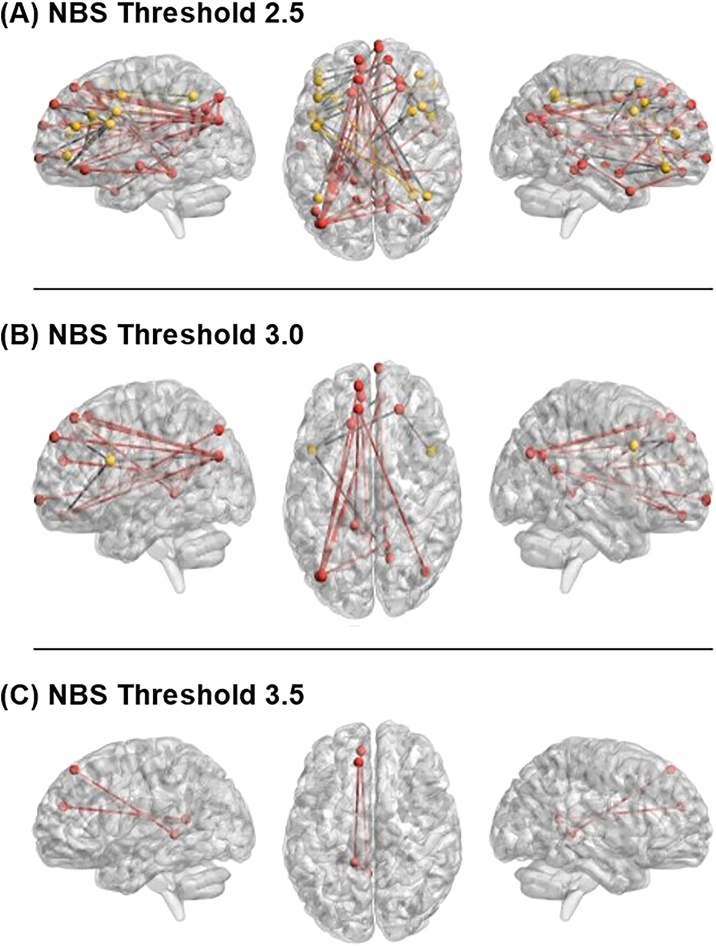
Negative associations between age and connectivity were found at threshold t = 2.5 with one subnetwork containing 53 edges across 43 nodes (Fig. 1, Table S4; p(extent) = .01; p(intensity) = .009). Of these, 20 edges were FP-DMN connections across 30 unique nodes. Specifically, the functional connectivity between FP regions (precentral gyrus, inferior frontal gyrus, middle frontal gyrus, and angular gyrus) and DMN regions (precuneus, frontal pole, frontal medial cortex, superior frontal gyrus, and the posterior cingulate) was inversely associated with age, such that connectivity was more anticorrelated among older participants.
Fig. 1.
Negative age effects for connectivity in FP/DMN Networks at A) NBS threshold 2.5 (p < .006) B) NBS threshold 3.0 (p < .002) and C) NBS threshold 3.5 (p < .0001). FP/DMN Network seeds were defined using Power 264 atlas seeds (Power et al., 2011) for DMN and FP networks. DMN nodes and within network edges are red. FP nodes and within network edges are gold. Significant between network edges are black. Covariates included sex, mean motion, and pulse sequence. Multiple comparison correction was completed using 5000 bootstrapped samples with alpha set to p < .025 to correct for inclusion of both positive and negative contrasts.
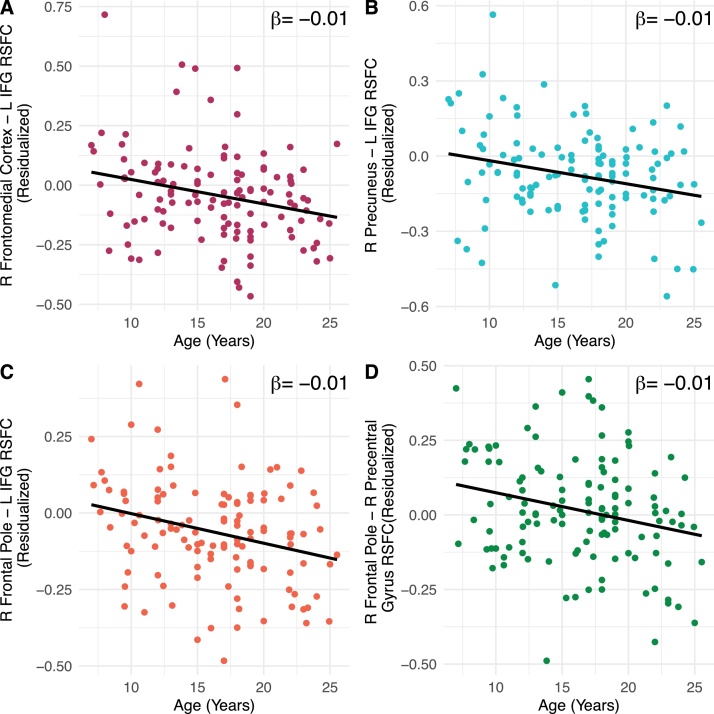
At threshold t = 3.0, one subnetwork containing 15 edges across 16 nodes was negatively associated with age (Fig. 1; Table S5; p(extent) = .003; p(intensity) = .007). Of these, 4 edges were FP-DMN connections across 5 nodes (Table 2). Specifically, functional connectivity between FP regions (inferior frontal gyrus and precentral gyrus) and DMN regions (frontal medial cortex, precuneus, and frontal pole) was inversely associated with age (Fig. 1). Mean connectivity values at the youngest ages (7–10 years) were near-zero (frontomedial cortex – IFG M = 0.05, SD = .22; precuneus – IFG M = 0.07, SD = 0.24; frontal pole-IFG M= −0.2, SD = 0.22; frontal pole-precentral gyrus M = 0.03, SD = 0.22) and were negative at the oldest ages (18+; frontomedial cortex – IFG M = −0.10, SD = 0.18, p = 0.02; precuneus – IFG M= −.14, SD = 0.16; frontal pole-IFG M= −0.09, SD = 0.16; frontal pole-precentral gyrus M= −.001, SD = 0.17). For every one-year increase in participant age, we observed a 0.01 decrease in the resting state functional connectivity values of these four FP-DMN edges.
At threshold t = 3.5, one subnetwork containing 3 edges across 4 nodes was negatively associated with age (Fig. 1; Table S6; p(extent) = . 024; p(intensity) = . 019). This subnetwork was composed only of DMN-DMN connections.
FP-DMN functional connectivity was extracted for all four FP-DMN edges that were significant across multiple thresholds. Across these edges (Table 2), FP-DMN connectivity was inversely associated with age (Fig. 2).
Fig. 2.
Associations between frontoparietal-default mode network functional connectivity and age. Age was inversely associated with functional connectivity between A-maroon) right frontomedial cortex – left inferior frontal gyrus, B-teal) precuneus- left inferior frontal gyrus, C-orange) R frontal pole – left inferior frontal gyrus and d-green) right frontal pole - right precentral gyrus. Full sample is included in all of the analyses (N = 124).
2.3. Frontoparietal-default mode network connectivity and intellectual functioning
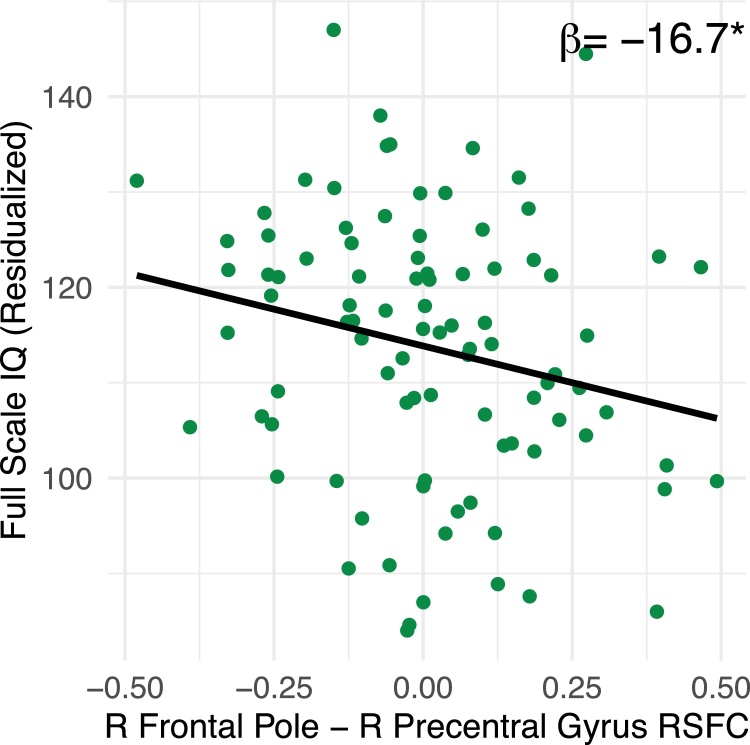
Precentral gyrus (FP) – frontal pole (DMN) connectivity was negatively associated with FSIQ (F (5,91) = 4.65, p < 0.01; β = −.22, t = −2.18, p = 0.03; Fig. 3), such that more negative connectivity between FP-DMN (i.e., more anticorrelation) was associated with higher FSIQ. For every 0.01 point decrease in functional connectivity between the precentral gyrus and the frontal pole, we observed a 1.6 point increase in IQ. Other edges showing significant age effects were not associated with FSIQ (p’s>.24, Supplementary Fig. 1).
Fig. 3.
Resting state functional connectivity (right frontal pole - right precentral gyrus) was associated with FSIQ score such that more negative connectivity (greater anticorrelation) was associated with higher FSIQ scores (N = 92).
3. Discussion
We used NBS analysis to examine how FP-DMN connectivity varied with age in a large cross-sectional sample of healthy children, youth, and young adults. FP-DMN connectivity was more anticorrelated and negative among older versus younger participants. Healthy young children demonstrated near-zero FP-DMN connectivity between FP regions (inferior frontal gyrus and precentral gyrus) and DMN regions (frontal medial cortex, precuneus, and frontal pole) that was highly variable, whereas healthy older adolescents showed negative connectivity. More mature FP-DMN connectivity (i.e., more anticorrelation) between the frontal pole and the precentral gyrus was associated with greater intellectual functioning (higher FSIQ scores) in healthy youth across all ages.
We show that child FP-DMN connectivity begins near-zero and becomes increasingly anticorrelated with increasing cross-sectional age. Like prior findings, we observed an inverse association between age and FP-DMN network connectivity (Chai et al., 2014). However, in contrast to prior findings (Margolis et al., 2019; Koyama et al., 2013), we observed near-zero, rather than positive connectivity values in the youngest age group. Specifically, our findings indicate that youth in early childhood demonstrate highly variable connectivity that averages to near-zero between some regions in the DMN and FP (frontomedial cortex-IFG, precuneus-IFG, frontal pole – precentral gyrus) while other regions show early anticorrelations (frontal pole – IFG). Connectivity values near-zero represent stochastic or random associations between brain regions, possibly indicating that the crosstalk between these networks is largely unpredictable in early childhood. Given prior findings of positive connectivity between regions in these networks at younger ages and that the mean age of our sample was slightly older than previous work (Koyama et al., 2013; Chai et al., 2014), the near-zero connectivity we observed may represent an intermediate phase in FP-DMN connectivity as it shifts from positive to near-zero to negative correlations across development. In line with this idea, prior findings point to children spending less time in these task positive and negative network states relative to older adolescents (Medaglia et al., 2018). Potentially, less time spent in primary network states (either task positive or task negative) may underlie the near-zero correlations we observed. Considerable variability in network connectivity may reflect individual differences in developmental timing, which varies widely in healthy children (Atun-Einy et al., 2012; Fenson and Dale, 1994). Further, although global signal regression is useful in controlling for physiologic and movement noise, it also shifts the mean of connectivity values towards zero (Murphy et al., 2009) making it difficult to interpret negative values. As we were specifically interested in how FP-DMN connectivity might become more anticorrelated (negative) with age, we used aCompCor to regress out signal from white matter and cerebrospinal fluid. Two of the prior studies that reported positive FP-DMN findings in young children used aCompCor (Margolis et al., 2019; Chai et al., 2014). One used global signal regression (Koyama et al., 2013) but nevertheless still reported positive FP-DMN connectivity. Taken together, these findings suggest that youth in early childhood demonstrate positive connectivity that becomes increasingly negative with increasing age. Future longitudinal studies examining FP-DMN associations in even younger children are needed to better understand the development of these networks.
As expected, the subnetworks in our NBS analyses that varied with age contracted across increasingly conservative edge-level sensitivity thresholds. As there is no optimal NBS sensitivity threshold, we selected three thresholds to offer a comprehensive examination of how FP-DMN connectivity varied with age. At the most lenient edge-level threshold, t>2.5, we detected the most network connections (including connections both between and within networks), we detected between and within network connections in similar, albeit fewer, nodes at threshold 3.0, and only within network connections at threshold 3.5. Although there were additional edges showing inverse associations with age between FP and DMN at the lowest sensitivity threshold, we discuss only the edges that were identified at more than one sensitivity threshold. Notably, we detected overlapping nodes at each threshold, rather than the subnetworks re-structuring completely. These retained connections represent strong, focal effects (Zalesky et al., 2010). Of these retained connections many were in IFG, consistent with prior findings that IFG connectivity is critical for effective cognitive control (Bos et al., 2017) and is disrupted in youth who have difficulty remaining focused and avoiding distraction, such as in attention deficit hyperactivity disorder (Chen et al., 2019a; Hong et al., 2017; Dickstein et al., 2006). In addition, findings from task fMRI studies show age-related changes in IFG functional activation are mirrored by improvement in verbal fluency (Luna et al., 2010; Scherf et al., 2006). Thus, age-related change in between network anticorrelations may contribute to age-related gains in executive functioning.
Greater anticorrelation between one FP-DMN edge (frontal pole – precentral gyrus) was associated with higher intellectual functioning. FP-DMN connectivity in youth (ages 10–13) and adults has been associated with cognitive task performance (Mills et al., 2018) and IQ (Sherman et al., 2014; Song et al., 2008). Our results extend these findings to even younger ages. IQ, as a proxy for global intellectual functioning, is an important predictor of youth’s future educational and occupational attainment, as well as risk for early mortality and justice involvement (Gur et al., 2012; Roalf et al., 2014; Loeber et al., 2012; Whalley and Deary, 2001; DiRago and Vaillant, 2006). FP-DMN anticorrelations likely support intellectual functioning through the mechanism of cognitive control capacity (Cochrane et al., 2019; Chen et al., 2019b). Specifically, greater anticorrelation between the frontal pole and the precentral gyrus may support enhanced efficiency between these networks and underlie improvements in attentional control, facilitating better integration of learned information over time and better performance on tests of intelligence.
Our study has some limitations. Our cross-sectional sample allows us to examine associations between age and FP-DMN connectivity, but we are unable to draw conclusions about network maturation. Longitudinal studies are required to better understand the temporal relationship between development, FP-DMN connectivity, and IQ. Our data suggest that the strongest, most focal connections were within network, rather than between networks; however following conventions for defining the two networks we studied, we used a different number of nodes for each. Our finding that the within network connection is strongest may thus be an artifact of the number of nodes used to define each network. Like many pediatric neuroimaging studies, we had fewest children in our youngest age group (7–10 years old). This is a common challenge facing investigators because early child samples are particularly vulnerable to motion artifacts, study attrition, and low recruitment when compared to older populations (Raschle et al., 2009, 2012). Despite this, we have as many or more children in this age group than comparable studies (Margolis et al., 2019; Koyama et al., 2013; Chai et al., 2014). Participants in our sample demonstrated higher than average FSIQ scores thereby limiting the generalizability of our findings to healthy youth with relatively high FSIQ. Additionally, our finding that connectivity was associated with FSIQ was only present for one edge. Future studies should examine children with a broader range of FSIQ scores. Although we controlled statistically for the effects of small differences between study scan parameters, our results may have been affected by differences in pulse sequences. As the field moves toward an emphasis on “big data,” future data collections must include children in early and middle childhood. Our study used resting state methods to examine FP-DMN connectivity. Future work should integrate task-based methodology to examine functional activation of FP and DMN networks during task performance.
4. Conclusions
Children demonstrated associations between FP-DMN network functional connectivity and age; younger children demonstrated FP-DMN connectivity near-zero whereas adolescents and young adults showed greater FP-DMN anticorrelation. Moreover, greater FP-DMN anticorrelation was associated with higher intellectual functioning. Intervention programs that foster earlier development and maturity of FP-DMN anticorrelation, such as enhanced early education, may thus contribute to positive global outcomes (Ramey and Campbell, 1984; Campbell et al., 2012, 2002).
Data statement
Data will be shared upon request. All data requests must be submitted to:
Amy Margolis, Ph.D.
CUIMC/Herbert Pardes Building of the New York State Psychiatric Institute, 1051 Riverside Drive, New York, NY 10032.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Lauren Thomas, Sarah Banker, David Semanek, Susie Hong, and Martine Fontaine for their support in data management and participant recruitment and care. The authors would also like to extend special thanks to the participants and their families. This work was presented virtually under the title “Resting State Connectivity of Task Positive and Task Negative Networks in Young Children” as a poster at the Society of Biological Psychiatry's 75th Annual Scientific Program and Convention in April 2020. This study was supported by NIEHSK23ES026239 to A.M.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100928.
Contributor Information
Mariah DeSerisy, Email: mdeserisy@fordham.edu.
Amy E. Margolis, Email: amy.margolis@nyspi.columbia.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Amer T., Anderson J.A.E., Campbell K.L., Hasher L., Grady C.L. Age differences in the neural correlates of distraction regulation: a network interaction approach. Neuroimage. 2016;139:231–239. doi: 10.1016/j.neuroimage.2016.06.036. [DOI] [PubMed] [Google Scholar]
- Arbabshirani M.R., Havlicek M., Kiehl K.A., Pearlson G.D., Calhoun V.D. Functional network connectivity during rest and task conditions: a comparative study. Hum. Brain Mapp. 2013;34:2959–2971. doi: 10.1002/hbm.22118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atun-Einy O., Berger S.E., Scher A. Pulling to stand: common trajectories and individual differences in development. Dev. Psychobiol. 2012;54:187–198. doi: 10.1002/dev.20593. [DOI] [PubMed] [Google Scholar]
- Avelar-Pereira B., Bäckman L., Wåhlin A., Nyberg L., Salami A. Age-related differences in dynamic interactions among default mode, frontoparietal control, and dorsal attention networks during resting-state and interference resolution. Front. Aging Neurosci. 2017:9. doi: 10.3389/fnagi.2017.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos D.J., Oranje B., Achterberg M., Vlaskamp C., Ambrosino S., de Reus M.A. Structural and functional connectivity in children and adolescents with and without attention deficit/hyperactivity disorder. J. Child Psychol. Psychiatry. 2017;58:810–818. doi: 10.1111/jcpp.12712. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Campbell F.A., Ramey C.T., Pungello E., Sparling J., Miller-Johnson S. Early childhood education: young adult outcomes from the abecedarian project. Appl. Dev. Sci. 2002;6:42–57. [Google Scholar]
- Campbell F.A., Pungello E.P., Burchinal M., Kainz K., Pan Y., Wasik B.H. Adult outcomes as a function of an early childhood educational program: an Abecedarian Project follow-up. Dev. Psychol. 2012;48:1033–1043. doi: 10.1037/a0026644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J., Fekete T., Siciliano F., Biezonski D., Greenhill L., Pliszka S.R. Neural correlates of aggression in Medication-Naive Children with ADHD: multivariate analysis of morphometry and tractography. Neuropsychopharmacology. 2015;40:1717–1725. doi: 10.1038/npp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Ofen N., Gabrieli J.D., Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cogn. Neurosci. 2014;26:501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Huang X., Wu M., Li K., Hu X., Jiang P. Disrupted brain functional networks in drug-naive children with attention deficit hyperactivity disorder assessed using graph theory analysis. Hum. Brain Mapp. 2019;40:4877–4887. doi: 10.1002/hbm.24743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Spagna A., Wu T., Kim T.H., Wu Q., Chen C. Testing a cognitive control model of human intelligence. Sci. Rep. 2019;9:2898. doi: 10.1038/s41598-019-39685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane A., Simmering V., Green C.S. Fluid intelligence is related to capacity in memory as well as attention: evidence from middle childhood and adulthood. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole Michael W., Bassett Danielle S., Power Jonathan D., Braver Todd S., Petersen Steven E. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Di X., Gohel S., Kim E., Biswal B. Task vs. Rest—different network configurations between the coactivation and the resting-state brain networks. Front. Hum. Neurosci. 2013:7. doi: 10.3389/fnhum.2013.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein S.G., Bannon K., Xavier Castellanos F., Milham M.P. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J. Child Psychol. Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- DiRago A.C., Vaillant G.E. Resilience in inner city youth: childhood predictors of occupational status across the lifespan. J. Youth Adolesc. 2006;36:61. [Google Scholar]
- Douw L., Wakeman D.G., Tanaka N., Liu H., Stufflebeam S.M. State-dependent variability of dynamic functional connectivity between frontoparietal and default networks relates to cognitive flexibility. Neuroscience. 2016;339:12–21. doi: 10.1016/j.neuroscience.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U., Church J.A., Miezin F.M., Barch D.M. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Posner J., Nagel B.J., Bathula D., Dias T.G., Mills K.L. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L., Dale P.S. Variability in early communicative development. Monogr. Soc. Res. Child Dev. 1994;59:1–173. [PubMed] [Google Scholar]
- First M.B., Gibbon M. The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II) In: Hilsenroth M.J., Segal D.L., Hersen M., editors. Comprehensive Handbook of Psychological Assessment, Volume 2: Personality Assessment. John Wiley and Sons, Inc.; Hoboken, NJ: 2004. p. 134. [Google Scholar]
- Fornito A., Zalesky A., Bullmore E. Elsevier Academic Press; San Diego, CA: 2016. Fundamentals of Brain Network Analysis. [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath K., Krishnamurthy V., Cabanban R., Crosson B.A. Hubs of anticorrelation in high-resolution resting-state functional connectivity network architecture. Brain Connect. 2015;5:267–275. doi: 10.1089/brain.2014.0323. [DOI] [PubMed] [Google Scholar]
- Grady C.L., Protzner A.B., Kovacevic N., Strother S.C., Afshin-Pour B., Wojtowicz M. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb. Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.C., Richard J., Calkins M.E., Chiavacci R., Hansen J.A., Bilker W.B. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26:251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Driesen N., Roth J.K., Gore J.C., Constable R.T. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn. Reson. Imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., Lopez-Sola M., Hernandez-Ribas R., Deus J., Ortiz H. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci U S A. 2008;105:9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J., Park B.Y., Cho H.H., Park H. Age-related connectivity differences between attention deficit and hyperactivity disorder patients and typically developing subjects: a resting-state functional MRI study. Neural Regen. Res. 2017;12:1640–1647. doi: 10.4103/1673-5374.217339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K., Kazimierczak K., Beresniewicz J., Kompus K., Westerhausen R., Ersland L. Dynamic up- and down-regulation of the default (DMN) and extrinsic (EMN) mode networks during alternating task-on and task-off periods. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G.A., Kearney-Ramos T.E., Young J.A., Kilts C.D., Gess J.L., Fausett J.S. Functional independence in resting-state connectivity facilitates higher-order cognition. Brain Cogn. 2016;105:78–87. doi: 10.1016/j.bandc.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keller C.J., Bickel S., Honey C.J., Groppe D.M., Entz L., Craddock R.C. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J. Neurosci. 2013;33:6333. doi: 10.1523/JNEUROSCI.4837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J.B., Hedden T., Thompson T.W., Anteraper S.A., Gabrieli J.D.E., Whitfield-Gabrieli S. Resting-state anticorrelations between medial and lateral prefrontal cortex: association with working memory, aging, and individual differences. Cortex. 2015;64:271–280. doi: 10.1016/j.cortex.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.M.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim J., Kang E. Strength of resting-state functional connectivity associated with performance-adjustment ability. Behav. Brain Res. 2018;347:377–384. doi: 10.1016/j.bbr.2018.02.024. [DOI] [PubMed] [Google Scholar]
- Klingberg T., O’Sullivan B.T., Roland P.E. Bilateral activation of fronto-parietal networks by incrementing demand in a working memory task. Cereb. Cortex. 1997;7:465–471. doi: 10.1093/cercor/7.5.465. [DOI] [PubMed] [Google Scholar]
- Koyama M.S., Di Martino A., Kelly C., Jutagir D.R., Sunshine J., Schwartz S.J. Cortical signatures of dyslexia and remediation: an intrinsic functional connectivity approach. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeslag S.J.E., Schmidt M., Ghassabian A., Jaddoe V.W., Hofman A., van der Lugt A. Functional connectivity between parietal and frontal brain regions and intelligence in young children: the Generation R study. Hum. Brain Mapp. 2013;34:3299–3307. doi: 10.1002/hbm.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Kamourieh S., Beckmann C.F., Sharp D.J. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. 2011;31:3217. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R., Menting B., Lynam D.R., Moffitt T.E., Stouthamer-Loeber M., Stallings R. Findings from the Pittsburgh youth study: cognitive impulsivity and intelligence as predictors of the age–crime curve. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:1136–1149. doi: 10.1016/j.jaac.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Marek S., Larsen B., Tervo-Clemmens B., Chahal R. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 2015;38:151–170. doi: 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Hwang K., Foran W., Hallquist M.N., Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002328. e1002328-e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis A.E., Pagliaccio D., Davis K.S., Thomas L., Banker S.M., Cyr M. Neural correlates of cognitive control deficits in children with reading disorder. Brain Imaging Behav. 2019 doi: 10.1007/s11682-019-00083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R., Gerber A.J., Peterson B.S. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:1233–1251. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R., Horga G., Wang Z., Wang P., Klahr K.W., Berner L.A. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am. J. Psychiatry. 2011;168:1210–1220. doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M.F., Norton M.I., Van Horn J.D., Wegner D.M., Grafton S.T., Macrae C.N. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medaglia J.D., Satterthwaite T.D., Kelkar A., Ciric R., Moore T.M., Ruparel K. Brain state expression and transitions are related to complex executive cognition in normative neurodevelopment. Neuroimage. 2018;166:293–306. doi: 10.1016/j.neuroimage.2017.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills B.D., Miranda-Dominguez O., Mills K.L., Earl E., Cordova M., Painter J. ADHD and attentional control: impaired segregation of task positive and task negative brain networks. Netw Neurosci. 2018;2:200–217. doi: 10.1162/netn_a_00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B.G., Bassett D.S., Camchong J., Bullmore E.T., Lim K.O. Comparison of large-scale human brain functional and anatomical networks in schizophrenia. Neuroimage Clin. 2017;15:439–448. doi: 10.1016/j.nicl.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente F., Colosimo A. Functional connections between and within brain subnetworks under resting-state. Sci. Rep. 2020;10:3438. doi: 10.1038/s41598-020-60406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J., Lustig C., Nelson J.K., Reuter-Lorenz P.A. Age Differences in Deactivation: A Link to Cognitive Control? J. Cogn. Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Piaget J. International Universities Press [©1952]; New York: 1952. The Origins of Intelligence in Children. [Google Scholar]
- Posner J., Park C., Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol. Rev. 2014;24:3–15. doi: 10.1007/s11065-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Siciliano F., Wang Z., Liu J., Sonuga-Barke E., Greenhill L. A multimodal MRI study of the hippocampus in medication-naive children with ADHD: what connects ADHD and depression? Psychiatry Res. 2014;224:112–118. doi: 10.1016/j.pscychresns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua E.P.K., Malpas C.B., Bowden S.C., Seal M.L. Different brain networks underlying intelligence in autism spectrum disorders. Hum. Brain Mapp. 2018;39:3253–3262. doi: 10.1002/hbm.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Castellanos F.X., Uddin L.Q., Loo B.R.Y., Liu S., Koh H.L. Large-scale brain functional network topology disruptions underlie symptom heterogeneity in children with attention-deficit/hyperactivity disorder. Neuroimage Clin. 2019;21 doi: 10.1016/j.nicl.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey C.T., Campbell F.A. Preventive education for high-risk children: cognitive consequences of the Carolina Abecedarian Project. Am. J. Ment. Defic. 1984;88:515–523. [PubMed] [Google Scholar]
- Raschle N.M., Lee M., Buechler R., Christodoulou J.A., Chang M., Vakil M. Making MR Imaging Child’s Play - Pediatric Neuroimaging Protocol, Guidelines and Procedure. JoVE. 2009:e1309. doi: 10.3791/1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N., Zuk J., Ortiz-Mantilla S., Sliva D.D., Franceschi A., Grant P.E. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann. N. Y. Acad. Sci. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineberg A.E., Gustavson D.E., Benca C., Banich M.T., Friedman N.P. The relationship between resting state network connectivity and individual differences in executive functions. Front. Psychol. 2018:9. doi: 10.3389/fpsyg.2018.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel J.C., Miller M.W., Gentili R.J., Goodman R.N., McDonald C.G., Hatfield B.D. Cerebral-cortical networking and activation increase as a function of cognitive-motor task difficulty. Biol. Psychol. 2012;90:127–133. doi: 10.1016/j.biopsycho.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Roalf D.R., Gur R.E., Ruparel K., Calkins M.E., Satterthwaite T.D., Bilker W.B. Within-individual variability in neurocognitive performance: age- and sex-related differences in children and youths from ages 8 to 21. Neuropsychology. 2014;28:506–518. doi: 10.1037/neu0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Sweeney J.A., Luna B. Brain basis of developmental change in visuospatial working memory. J. Cogn. Neurosci. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Sheffield J.M., Repovs G., Harms M.P., Carter C.S., Gold J.M., MacDonald A.W., 3rd Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. doi: 10.1016/j.neuropsychologia.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L.E., Rudie J.D., Pfeifer J.H., Masten C.L., McNealy K., Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev. Cogn. Neurosci. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.T.M., Mackay C.E. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., Zhou Y., Li J., Liu Y., Tian L., Yu C. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008;41:1168–1176. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Grady C.L. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Tau G.Z., Marsh R., Wang Z., Torres-Sanchez T., Graniello B., Hao X. Neural correlates of reward-based spatial learning in persons with cocaine dependence. Neuropsychopharmacology. 2014;39:545–555. doi: 10.1038/npp.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Clare Kelly A.M., Biswal B.B., Xavier Castellanos F., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G., de la Cruz F., Köhler S., Pereira F., Richard-Devantoy S., Turecki G. Connectomics-based functional network alterations in both depressed patients with suicidal behavior and healthy relatives of suicide victims. Sci. Rep. 2019;9:14330. doi: 10.1038/s41598-019-50881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence (WASI) [Google Scholar]
- Whalley L.J., Deary I.J. Longitudinal cohort study of childhood IQ and survival up to age 76. BMJ. 2001;322:819. doi: 10.1136/bmj.322.7290.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.