Highlights
-
•
Previously institutionalized (PI) youth exhibit altered frontolimbic connectivity.
-
•
Resting-state functional connectivity is associated with IQ in early adolescence.
-
•
PI and comparison youth differ in dorsal attention network connectivity.
-
•
Between-system connectivity is associated with internalizing in early adolescence.
Keywords: Resting-state fMRI, Early life stress, Institutional care, Graph theory
Abstract
Psychosocial acceleration theory and other frameworks adapted from life history predict a link between early life stress and accelerated maturation in several physiological systems. Those findings led researchers to suggest that the emotion-regulatory brain circuits of previously-institutionalized (PI) youth are more mature than youth raised in their biological families (non-adopted, or NA, youth) during emotion tasks. Whether this accelerated maturation is evident during resting-state fMRI has not yet been established. Resting-state fMRI data from 83 early adolescents (Mage = 12.9 years, SD = 0.57 years) including 41 PI and 42 NA youth, were used to examine seed-based functional connectivity between the amygdala and ventromedial prefrontal cortex (vmPFC). Additional whole-brain analyses assessed group differences in functional connectivity and associations with cognitive performance and behavior. We found group differences in amygdala – vmPFC connectivity that may be consistent with accelerated maturation following early life stress. Further, whole-brain connectivity analyses revealed group differences associated with internalizing and externalizing symptoms. However, the majority of whole-brain results were not consistent with an accelerated maturation framework. Our results suggest early life stress in the form of institutional care is associated with circuit-specific alterations to a frontolimbic emotion-regulatory system, while revealing limited differences in more broadly distributed networks.
1. Introduction
Institutional rearing is a form of early life stress (ELS) characterized by caregiver deprivation that has the potential to alter a child’s developmental trajectory. The behavioral sequelae of institutionalization have been well-documented (Gunnar and Van Dulman, 2007), with atypical patterns of cognitive and emotional development evident across childhood and into adolescence (Beckett et al., 2010; Hawk and McCall, 2010; Merz et al., 2016; Zeanah et al., 2009). Importantly, adoption into families is an effective intervention, rendering institutional care a time-limited stressor and mitigating many of the adverse effects of institutional rearing (Nelson et al., 2007) van Ijzendoorn and Juffer, 2006). However, persistent effects remain, including some sleeper effects that emerge in adolescence, such as an increase in internalizing and externalizing disorders in post-institutionalized (PI) youth compared to youth raised in their biological families (Hawk and McCall, 2010).
One possible explanation for the emergence of psychopathology in post-institutionalized (PI) youth during early adolescence is stress-induced alteration of brain circuitry underlying cognitive and emotional processing. The presence of a caregiving relationship has been described as an experience-expectant process of early development that supports emotion regulation, among other processes (Nelson et al., 2011). As a result, variations in caregiving during the first years of life influence the subsequent developmental trajectories of brain circuits underlying emotion regulation and attention. For example, the amygdala and medial prefrontal cortex (mPFC) are two brain regions that interact to form an emotion regulation circuit. The amygdala has a diverse range of cortical and subcortical connections (Phelps and LeDoux, 2005; Saygin et al., 2015) including with the mPFC, a heterogeneous region associated with a wide spectrum of executive functions that serves a regulatory role for the amygdala (Etkin et al., 2011; Miller and Cohen, 2001). Importantly, both the amygdala and mPFC are sensitive to ELS and have been shown to develop differently in individuals with early institutionalized histories than individuals without (Tottenham, 2014).
Functional neuroimaging studies have shown increased amygdala activation in PI children, suggesting a heightened sensitivity to threatening stimuli (e.g. Tottenham et al., 2011). Task-based connectivity studies have reported altered amygdala – PFC connectivity in PI youth during negative emotion processing (Gee et al., 2013) and aversive learning (Silvers et al., 2016) tasks. In comparison to children reared in their birth families, PI children have shown a more developmentally mature profile of connectivity during an emotional face processing task (Gee et al., 2013). This finding is consistent with theories suggesting that early psychosocial stress leads to accelerated biological maturation (e.g. earlier age at menarche; (Belsky et al., 1991; Del Giudice et al., 2011) and subsequent findings linking stress-related early pubertal maturation with the development of psychopathology (Colich et al., 2019). Similar theories have been offered in the context of brain function, such as the stress acceleration hypothesis, which posits premature maturation of emotional behaviors and brain circuitry following early stress (Callaghan and Tottenham, 2016).
While task-based studies are useful for understanding connectivity in a behavioral context, networks identified in resting state functional MRI are thought to represent intrinsic connectivity at baseline and help to characterize the organization of brain networks in the absence of a task. In normative studies of adults the amygdala exhibits patterns of both positive and negative connectivity (Roy et al., 2009). In an emotion regulation circuit, amygdala activity is positively correlated with activity in the ventromedial PFC (vmPFC), and individual differences in this resting-state connectivity are predictive of adaptive functioning, including decreased internalizing symptoms (Kim et al., 2011).
Though normative developmental studies of resting-state functional connectivity (rsFC) remain sparse, existing data present conflicting evidence regarding the developmental trajectory of amygdala connectivity. Two studies have reported that resting-state amygdala – mPFC connectivity becomes more positive with age using cross-sectional analyses of individuals ranging in age from 4 to 23 years (Gabard-Durnam et al., 2014; Qin et al., 2012). Conversely, a third study, which used a combination of cross-sectional and longitudinal data from 10- to 25-year-old individuals, revealed age related decreases in amygdala – vmPFC rsFC (Jalbrzikowski et al., 2017). Importantly, these studies highlight the heterogeneity of the mPFC, highlighting differences in ventral and even subgenual areas of the vmPFC (Gabard-Durnam et al., 2014), a more rostral portion of the anterior cingulate (Qin et al., 2012), and trends in three distinct subregions of the vmPFC (Jalbrzikowski et al., 2017). While more research is necessary to fully disentangle the developmental trajectories of the subregions of the vmPFC, it is clear that amygdala – vmPFC connectivity is refined from childhood to early adulthood.
In addition to differences in emotion regulation, cognitive and attention-related processes have also been associated with early institutional rearing. One of the most often observed behavioral differences in PI youth relative to comparison youth is increased impulsivity and difficulty inhibiting prepotent responses (Herzberg et al., 2018; Hostinar et al., 2012; McLaughlin et al., 2014). In this context, a framework of accelerated maturation falls short—high impulsivity is unlikely to be the more mature state following institutional care. This observation leads to the possibility of a tradeoff in early brain development in which the accelerated maturation of amygdala – vmPFC connectivity comes at the cost of networks supporting attention and executive function. Given this possibility, it is important to broaden the number of systems under study using more exploratory analytic techniques.
One such exploratory approach is the application of graph theory methods to rsFC data. Graph theory analyses of fMRI data allow researchers to examine a whole-brain network, intermediate sub-networks (e.g. the default mode network), and local patterns of activity within the same empirical framework (Power et al., 2011). In a graph theory analysis, each region of interest in the brain is a “node” (or vertex) in the network and the connections between these nodes are “edges” (Fornito et al., 2016). How edges are defined varies by imaging modality and analysis approach, but in the case of fMRI the most common is the Pearson correlation in the BOLD signal between two nodes. The combination of nodes and edges identified from the data can then be used to create a large matrix, or graph, representing functional connections throughout the brain. These matrices serve as the input for calculation of a number of graph metrics, including the detection of highly similar clusters or communities, the relative importance or centrality of a given node, and the average within- and between-system connectivity of specific networks or across the whole brain (Fornito et al., 2016). Developmental studies have shown that as individuals age, variance in the size of communities increases, the centrality of limbic subcortical structures decreases, and between-system connectivity decreases (Betzel et al., 2014; Gu et al., 2015; Sato et al., 2015). Within-system connectivity has shown less consistent patterns to date, though it has also been suggested to increase from childhood into adolescence, followed by a decrease in early adulthood and beyond (Betzel et al., 2014).
Functional connectivity research in PI samples to date has focused largely on frontolimbic connectivity in the context of emotion processing tasks. This study contributes to this literature by examining amygdala – vmPFC connectivity in the resting-state context and diversifying the networks under investigation to include systems involved in the additional cognitive and behavioral processes known to be altered following early institutional care. We used an a priori region of interest approach to investigate amygdala – vmPFC connectivity and predicted more positive connectivity in PI youth and comparison youth. An exploratory whole-brain graph theory analysis was then completed to evaluate preliminary evidence for a developmental tradeoff between emotion-regulation systems and higher order cognitive networks following early caregiver deprivation. Given that a more top-down organization of brain activity centered on the hippocampus is thought to be the more mature state of cortico-limbic function (e.g. Casey, 2015), we predicted that PI youth would exhibit more mature subcortical – cortical connectivity as measured by lower centrality of the amygdala and hippocampus. Conversely, given the possibility of a developmental tradeoff between frontolimbic circuits and higher order cognitive networks, we expected less mature higher order cognitive network and whole brain connectivity, indexed by more between-system connectivity in executive control and attention-related networks and less variance in community size than comparison youth. Behavioral and parent-reported measures of IQ, internalizing symptoms, and externalizing symptoms were examined in association with rsFC outcomes to examine the behavioral relevance of differences in brain function following institutional care.
2. Materials and methods
2.1. Participants
Resting-state fMRI data were collected from 108 participants as part of a larger study investigating the neurobehavioral effects of early institutional care (Gunnar et al., 2012; Hodel et al., 2015). The study included PI youth who were internationally adopted between 4 and 62 months of age from diverse countries of origin. Comparison adolescents raised in their biological families (non-adopted, NA) were recruited from a community participant pool in the United States with similar demographics to the adopting families. Exclusion criteria for all participants included serious illness (e.g. cancer), known genetic conditions, Fetal Alcohol Spectrum Disorder, neurological conditions (e.g. epilepsy), developmental disorders, known IQ below 80, or MRI exclusions (e.g. metal in body). In addition, NA participants were excluded for diagnosed and/or treated psychological/psychiatric disorders. Participants and their parents provided verbal and written assent and consent, respectively, and were compensated for their efforts in the study. All procedures were approved by the University of Minnesota’s Institutional Review Board.
Of the initial sample of 108 youth, twenty-five of the participants (17 PI, 8 NA) were excluded from analysis due to excessive head motion during resting state fMRI due to our stringent criteria, resulting in a final sample of 83 youth (see Section 2.4: MRI Preprocessing). The final sample included in this analysis therefore consisted of 41 adopted or post-institutionalized youth and 42 non-adopted (NA) youth. Further demographic information for these groups is presented in Table 1. The PI youth were internationally adopted between 4 and 62 months of age (M = 15.83 months) from these countries of origin: Russia (34.1 %), China (19.5 %), India (9.8 %), Romania (9.8 %), Ukraine (7.3 %), Vietnam (7.3 %), Ecuador (2.4 %), Ethiopia (2.4 %), Guatemala (2.4 %), Mexico (2.4 %), and Slovakia (2.4 %).
Table 1.
Demographics, mean cognitive and behavioral scores, and adoption history of post-institutionalized (PI) and non-adopted (NA) youth. Test statistics were generated using t-tests for continuous dependent variables and chi-squared tests for categorical measures. * indicates significant group difference.
| Previously-institutionalized (PI) N = 41 |
Non-Adopted (NA) N = 42 |
Test Statistic p-value |
|
|---|---|---|---|
| Sex | 26 Female | 28 Female | 0.006 0.94 |
| Mean Age at Assessment in Years (Range) | 13.0 (12.18–14.09) | 12.8 (12.04–13.96) | −1.530.13 |
| Median Family Income in Thousands (Range) | $80.5 ($30 – $500) | $100 ($20 – $200) | 37.76 0.33 |
| Mean IQ (SD) | 106.83 (12.86) | 118.07 (12.5) | 4.04 0.0001* |
| Mean Internalizing Score (SD) | 0.29 (0.24) | 0.18 (0.18) | −2.54 0.01 |
| Mean Externalizing Score (SD) | 0.18 (0.18) | 0.12 (0.15) | −1.67 0.10 |
| Adoption History | |||
| Age at adoption in months, M (SD); range | 15.83 (12.84); 4−62 | NA | |
| Institutional care in months, M (SD); range | 13.65 (8.91); 3.5−48 |
NA | |
| Percent of care in institution, M (SD); range | 92.35 (14.22); 50−100 |
NA | |
| Time since adoption in years, M (SD); range | 11.68 (1.23) 7.9−13.4 |
NA |
2.2. IQ measurement
Participants completed the Wechsler Abbreviated Scale of Intelligence (WASI) as a measure of general cognitive function (Wechsler, 1999). The WASI is made up of four subtests, including Vocabulary, Block Design, Similarities, and Matrix Reasoning. Full-scale scores were used in this analysis.
2.3. Parent report of problem behavior
Internalizing and externalizing symptoms were obtained using the MacArthur Health and Behavior Questionnaire (HBQ) via primary parent report (Essex et al., 2002). The HBQ has been established as a valid and reliable measure of mental health symptomatology in childhood and early adolescence.
2.4. MRI acquisition
MRI scanning was completed using a Siemens 3 T Trio whole body scanner with a 12-channel head coil. A T1-weighted 3D MPRAGE anatomical scan (TR =2530 ms, TE =3.65 ms, flip angle = 7°, FOV =256 mm, matrix = 256 × 256, 240 sagittal slices, slice thickness =1 mm; 10 min, 49 s) was used for registration of functional images. Resting-state functional data were acquired during eyes-open rest (EPI BOLD, T2*-weighted scan [TR =2500 ms, TE =30 ms, flip angle = 80°, FOV =240 mm, matrix 64 × 64, 40 transverse slices, slice thickness =3.5 mm with no skip, 140 time points; 5 min, 57 s]). Participants were instructed to keep their eyes focused on the blank computer screen and to think of nothing in particular. A field map sequence was collected prior to the functional scan with the same slice prescription and scan parameters for use in distortion correction.
2.5. MRI preprocessing
Preprocessing steps were completed using FMRIB’s Software Library (FSL v5.0.8; Jenkinson et al., 2012) and the Analysis of Functional Neuroimages software package (AFNI v16.0.00; Cox, 1996). For each individual, MPRAGE and field map images were skull stripped. Raw EPI data were slice time corrected, and framewise displacement was estimated prior to correcting the data for motion. Data were then corrected for motion using FSL’s MCFLIRT. Twenty-four motion confound predictors were generated, including predictors for linear translation and rotation estimates (6), the first temporal derivative of these motion estimates (6), the square of the original motion estimates (6), and the first temporal derivative of those squares (6) (Satterthwaite et al., 2013). The root mean square (RMS) summary of translation and rotation was used to estimate absolute and framewise motion, and DVARS, a measure of frame-to-frame variation in signal intensity, was also calculated (Power et al., 2012). Individual data points (volumes) were marked for censoring if they exhibited absolute motion greater than 3.5 mm from the middle volume, framewise displacement greater than 0.5 mm (the previous and subsequent TR were also censored), or a DVARS value above a given individual’s 75th percentile plus two times their interquartile range. Due to findings indicating large effects of head motion on resting state data (e.g. Power et al., 2012; Satterthwaite et al., 2012), we used stringent motion exclusion criteria that resulted in the exclusion of 25 participants (17 PI, 8 NA). Participants were excluded if the total number of TRs that exceeded these combined motion thresholds was greater than 25 % of the resting state scan resulting in a final sample of 83 participants with an average of 5 min, 21 s of data (range =4 min 31 s – 5 min 57 s, SD = 0.4).
EPI data were unwarped based on the associated fieldmap using FSL’s prelude and fugue tools and then detrended (linear and quadratic trends). The stripped MPRAGE data were parcellated into grey matter, white matter, and cerebrospinal fluid using FSL’s fast tool. These parcellations were thresholded at 75 % probability and binarized to create masks for use in generating mean time series estimates for each tissue segmentation. Linear confound regressions were then run on the EPI data that included estimates from the 24 motion confound predictors, gray matter, white matter, CSF, global signal (defined as the mean time course from all sampled voxels), and predictors for each volume censored due to motion. Finally, residuals from the regression analysis were band-pass filtered (0.009 – 0.08 Hz) and spatially smoothed using a 6 mm full-width half-maximum (FWHM) kernel, providing the data for subsequent analyses.
2.6. Seed-based analysis
Bilateral amygdala masks derived from FreeSurfer (Fischl, 2012) were back-projected into participant’s unwarped EPI space. The mean bilateral amygdala signal time course for each participant was then extracted from the residual images produced by the preprocessing pipeline. Back-projected voxels exhibiting greater than 10 % signal loss in the corresponding EPI field map were removed from the individual level amygdala masks in order to obtain more accurate estimates of amygdala signal. The extracted mean amygdala time courses were submitted as predictors for each individual’s general linear model (GLM) to identify voxels in the brain that were correlated over time with signal in the amygdala.
Higher-level analyses were conducted using a random effects GLM to compare group differences in connectivity during resting state. All higher-level seed-based analyses included age at test, sex, and IQ as covariates of non-interest. For the vmPFC, as the a priori region of interest, non-parametric permutation tests using threshold-free cluster enhancement (TFCE) were conducted using Randomise in FSL, within a vmPFC mask (derived from the combination of bilateral Harvard-Oxford frontal medial cortex and subcallosal cortex anatomical masks), using 5000 permutations at p < 0.05. For clusters identified by the permutation test, ROIs were back-projected into each individual’s unwarped EPI space. Individual z-stat maps were transformed into correlation coefficients using the inverse Fisher z transform and mean correlation coefficients were extracted for each individual using the back-projected ROI mask. This produced a mean correlation with the amygdala across voxels within significant ROIs for each individual. An exploratory whole-brain connectivity analysis was also completed using a bilateral amygdala seed and cluster-based correction for multiple comparisons (voxelwise p < 0.005, cluster corrected p < 0.05). The seed-based analysis was also completed using a functionally defined bilateral amygdala ROI for comparison, see the supplementary materials.
2.7. Graph creation
Two hundred sixty four ROIs (5 mm radius spheres), previously identified by Power et al. (2011), were created in MNI standard space using fslmaths and back-projected into each participant’s unwarped EPI space. Each of the ROIs belonged to one of six previously identified resting state networks (RSNs) which included cingulo-opercular, cerebellar, default mode, frontoparietal, occipital, and sensorimotor networks. Additionally, four subcortical ROIs (bilateral amygdala and hippocampus) were created using coordinates retrieved from the association test function of neurosynth.org using the search terms “amygdala” and “anterior hippocampus” (Yarkoni et al., 2011). For each participant, the mean signal intensity within each of the ROIs was extracted from the residualized data for every TR, producing 268 separate time series. Correlation matrices were created for each participant, such that the time series of every ROI was correlated with the time series of every other ROI using Pearson correlation. The resulting 268 × 268 correlation matrices were Fisher z(r) transformed. Finally, because the calculation of some graph metrics (e.g. eigenvector centrality) require that the largest eigenvalue of the matrix be positive, all values in the correlation matrices were made positive using the absolute value of every cell for the calculation of graph metrics using weighted graphs (Fornito et al., 2016). This approach was taken to eliminate arbitrary thresholding of the graph and to include information from every connectivity estimate in subsequent analysis. Graphs were created using MATLAB (version 2017a, MathWorks) and graph theory metrics were calculated using the Brain Connectivity Toolbox (BCT; Rubinov and Sporns, 2010).
2.8. Graph metrics
A number of graph metrics were used in this study, including variance in community size, eigenvector centrality, and within- and between-system connectivity. Community detection was completed by calculating a Newman-Girvan modularity matrix using the modularity_f function of the BCT and Louvain-like community detection using the genlouvain function of the BCT (Jeub et al., 2016). The variance in community size was then calculated for each individual participant and used in further analysis. Eigenvector centrality was calculated to investigate the relative importance of the amygdala and hippocampus in PI and NA youth (Bonacich, 2007; Newman, 2008). Importantly, eigenvector centrality is a measure of node importance to the whole brain network, with limited relation to the strength of connectivity between the node of interest (here amygdala and hippocampus) and other single nodes in the network. Finally, within- and between-system connectivity values were generated by calculating the mean connectivity values of each node in a system with all the other nodes in its network (for within-system connectivity) or all other nodes in the brain excluding those in its network (for between-system connectivity) and averaging across all nodes in the system, similar to the procedure in Gu et al. (2015).
2.9. Data analysis strategy
The weighted correlation matrices created from the preprocessed resting state data were used to calculate variance in community size, subcortical eigenvector centrality, and within- and between-system connectivity. Following the calculation of these metrics, a set of a priori linear regression models were run to evaluate the effects of group and age in the whole-brain network and in a subset of the networks established in the set of ROIs used in this analysis (Power et al., 2011). Sex was also included as a potential covariate of non-interest. Akaike information criterion was used to determine the best-fitting final model. Specifically, group differences in variance in community size and within- and between-system connectivity were evaluated at the whole-brain level. Group differences in within- and between-system connectivity were also evaluated for five networks involved in attention and cognition: the frontoparietal, cingulo-opercular, dorsal attention, ventral attention, and salience networks (Power et al., 2011). Finally, the relative importance of the amygdala and hippocampus, as indicated by eigenvector centrality was compared across groups and as a function of age. Given the exploratory nature of the graph theory analysis, raw significance values are presented without correction for multiple comparisons. Methods and significance thresholds of the a priori seed-based resting-state analysis can be found above (see Section 2.6 Seed-based Analysis).
Behavioral differences between groups were evaluated with two sample t-tests. Internalizing and externalizing symptoms were log-transformed due to right skewness. Brain-behavior relationships were investigated using whole-brain averages of within- and between-system connectivity and for networks exhibiting significant effects between groups. These analyses were intended only to provide preliminary evidence of behavioral relevance for these rsFC measures. Raw significance values without correction for multiple comparisons are presented given the exploratory nature of these associations. Each planned model was run with predictors of group and age, with sex considered as a possible covariate of non-interest. Akaike information criterion was used to determine the best-fitting final model.
3. Results
3.1. Seed-based amygdala resting state functional connectivity
Overall patterns of amygdala connectivity in the full sample are presented in Supplemental Fig. 1. Patterns of connectivity were consistent with those commonly described in the rsFC literature.
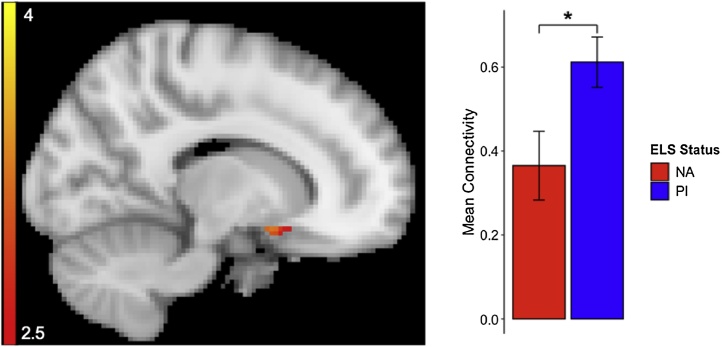
Fig. 1.
Seed-based amygdala – vmPFC resting state analysis revealed a significant group difference in the subgenual ACC (sgACC; x = 38). As seen in the bar graph, PI youth exhibited significantly stronger positive connectivity between amygdala and sgACC than NA youth. PI youth had greater positive connectivity than the NA comparison group. Region survived a voxelwise significance threshold of p < 0.005 and region of interest permutation tests p < 0.05 after 5,000 permutations.
3.2. Group differences in seed-based amygdala connectivity
Group differences were evident within the a priori vmPFC mask used in this analysis. Specifically, PI youth exhibited significantly greater positive amygdala connectivity in the subgenual anterior cingulate cortex (sgACC; see Fig. 1). Extracted connectivity estimates (mean correlation coefficients by group) indicated positive connectivity between the amygdala and sgACC in both groups, while the PI group showed a significantly stronger positive correlation than the NA group (MPI = 0.61 SD = 0.38; MNA = 0.37 SD = 0.53).
Exploratory whole brain analyses did not reveal any additional regions showing supra-threshold group differences in amygdala connectivity.
3.3. Whole-brain connectivity using graph theory metrics
3.3.1. Subcortical eigenvector centrality
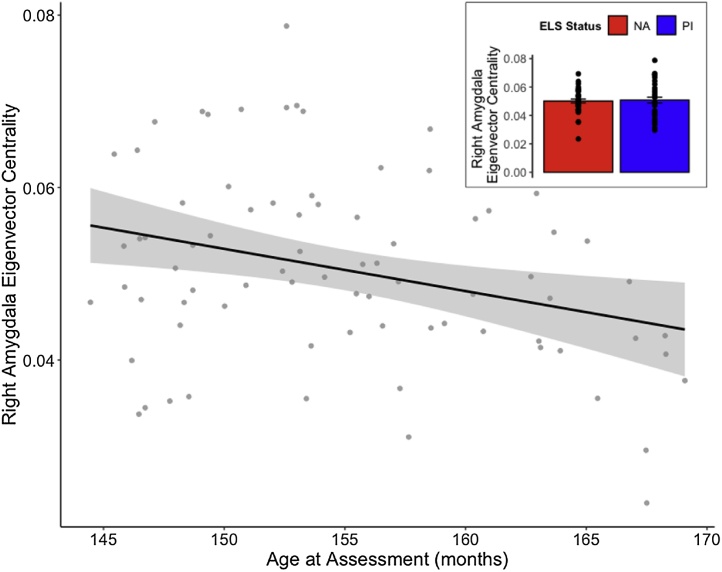
Consistent with our expectations, there was a negative association between age at assessment and right amygdala eigenvector centrality (EVC; F(1,81) = 4.87, p = 0.03; Fig. 2). Left and aggregated amygdala EVC also indicated a negative association with age, but were not statistically significant (F(1,81) = 1.31, p = 0.26 and F(1,81) = 3.55, p = 0.06, respectively). There were no differences in left or right amygdala EVC between PI and NA youth.
Fig. 2.
Right amygdala eigenvector centrality decreased significantly with age in the full sample. Gray shading represents 95 % confidence interval. NA and PI youth did not differ on right amygdala eigenvector centrality (inset).
Analysis of left and right hippocampal EVC revealed no significant effects of age or group.
3.3.2. Variance in community size
There were no significant effects of age or group on the variance in community size.
3.3.3. Within- and between-system connectivity
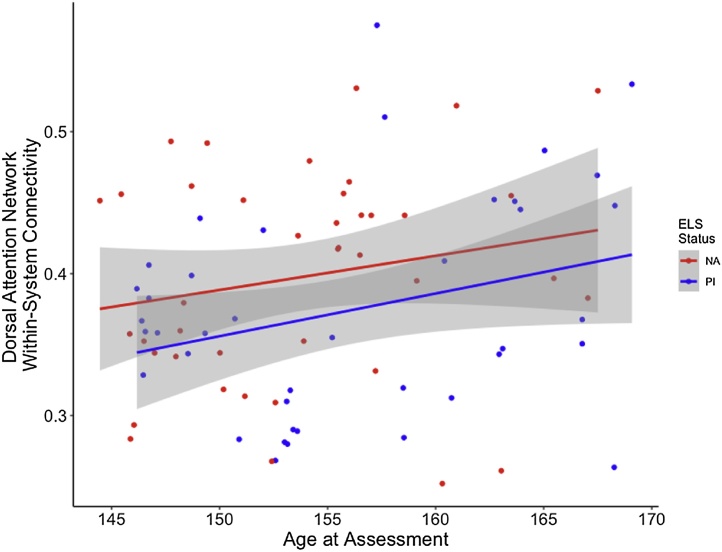
Within- and between-system connectivity were calculated for each of the five higher-order cognitive networks outlined above (see Section 2.9 Data Analysis Strategy). Significant main effects of age and group emerged for within-system connectivity in the dorsal attention network (F(2,77) = 7.60, p = 0.001; Fig. 3). Specifically, within-system connectivity of the dorsal attention network increased with age, and the NA group displayed more within-system connectivity than the PI group (t(77) = 3.06, p = 0.003 and t(77) = 3.00, p = 0.004, respectively). Within-system connectivity in the other four networks examined was not significantly predicted by age or group. No significant effects of age or group were found for between-system connectivity in any of the networks investigated.
Fig. 3.
NA youth exhibited greater dorsal attention network within-system connectivity compared to PI youth. Dorsal attention network within-system connectivity was also associated with age at assessment in the full sample and each group. Gray shading indicates 95 % confidence intervals.
3.4. Behavior and functional connectivity
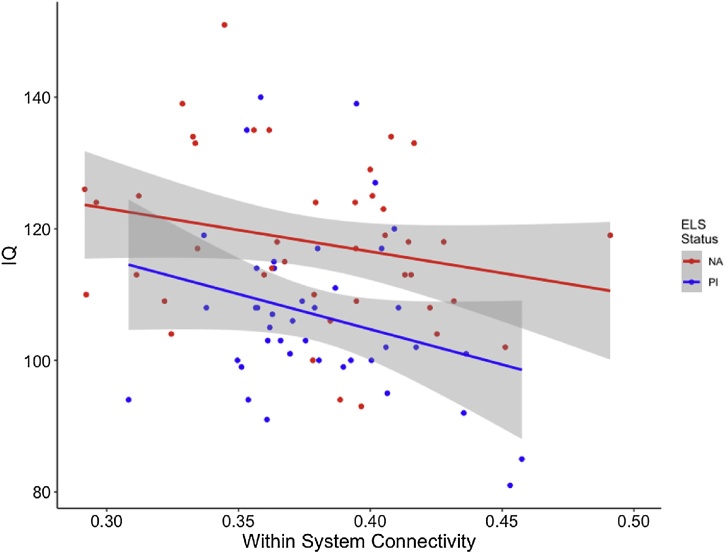
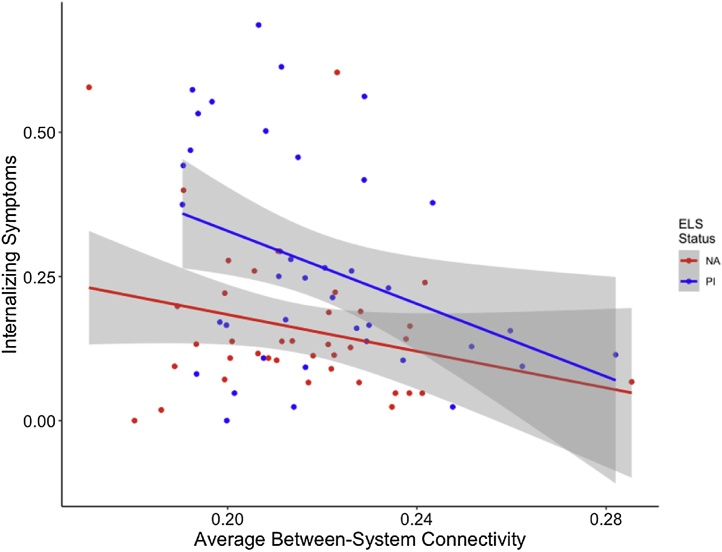
Average within-system connectivity combined across all networks was significantly related to IQ such that more within-system connectivity was related to lower IQ above and beyond the effect of group (F (1, 81) = 5.09, p = 0.03; Fig. 4). No associations were observed between average within-system connectivity and internalizing or externalizing scores. However, between-system connectivity averaged across the whole-brain was associated with behavior. Stronger between-system connectivity was associated with lower internalizing symptoms, above and beyond the effects of group, when controlling for age and sex (F(4,72) = 4.562, p = 0.002; Fig. 5).
Fig. 4.
Higher within-system connectivity is associated with lower IQ scores in the full sample, above and beyond a main effect of group. Gray shading indicates 95 % confidence interval.
Fig. 5.
PI youth exhibited higher levels of internalizing symptoms compared to NA youth, on average. Higher levels of between-system connectivity across the whole brain were associated with fewer internalizing symptoms above and beyond effects of group. Gray shading indicates 95 % confidence intervals.
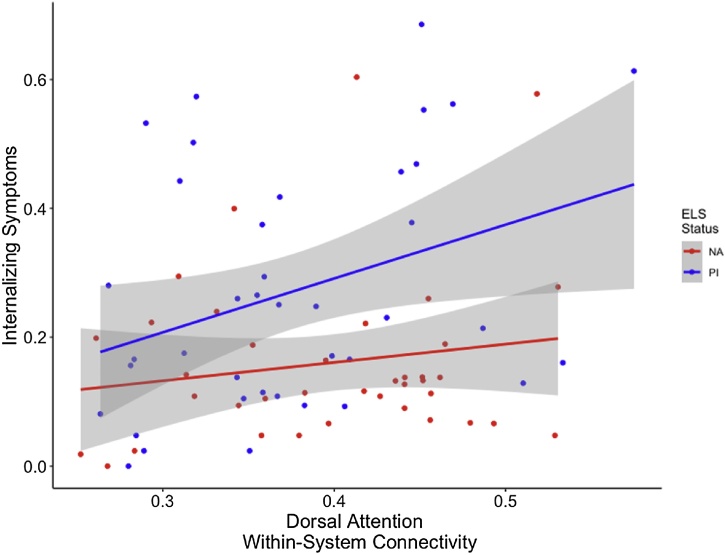
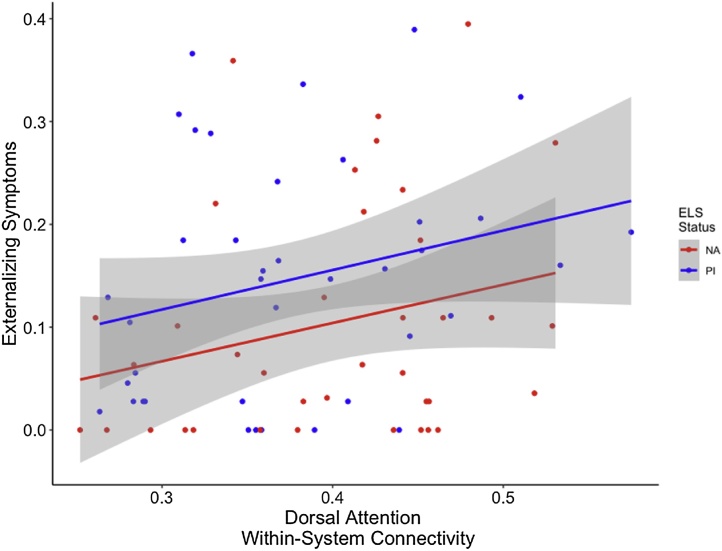
Network-specific differences in dorsal attention network connectivity were associated with both internalizing and externalizing symptoms. Greater dorsal attention network within-system connectivity was associated with more internalizing symptoms, above and beyond the effect of group, when controlling for age and sex (F(4,74) = 3.023, p = 0.02; Fig. 6). Finally, higher levels of externalizing were associated with greater dorsal attention network within-system connectivity when controlling for group, age, and sex (F(4,72) = 4.603, p = 0.002; Fig. 7).
Fig. 6.
PI youth exhibited higher levels of internalizing symptoms compared to NA youth, on average. Higher levels of dorsal attention network within-system connectivity are associated with more internalizing symptoms above and beyond effects of group. Gray shading indicates 95 % confidence intervals.
Fig. 7.
Greater dorsal attention network within-system connectivity was associated with higher levels of externalizing controlling for group, age, and sex. Gray shading indicates 95 % confidence intervals.
4. Discussion
The purpose of this study was to extend the examination of functional connectivity in post-institutionalized youth to the resting-state context and to diversify the brain networks under examination. We confirmed that seed-based amygdala connectivity with the mPFC, which has previously been shown to be associated with early institutional care in the context of a behavioral task (e.g. Gee et al., 2013), is also significantly different between PI and NA youth in our resting-state data. Further, we found significant decreases in the eigenvector centrality of the right amygdala across age in the full sample, though no associations with early institutional care were evident. Additional whole-brain graph theory analyses revealed group and age effects in the within-system connectivity of the dorsal attention network and a number of brain-behavior associations with IQ, internalizing symptoms, and externalizing symptoms. Each of these results has developmental implications that will serve as an important foundation for future research, though the inconsistent findings reported in the current literature require that they be interpreted with care.
Basic research addressing the normative developmental trajectory of amygdala – vmPFC connectivity has produced conflicting evidence that has a direct impact on the interpretation of the results presented here. PI youth in our study exhibited greater positive amygdala – vmPFC connectivity than NA youth, which has been reported to be the more mature state. In the context of resting-state functional connectivity results from Gabard-Durnam et al. (2014) and Qin et al. (2012), this result is consistent with an accelerated maturation framework in which emotional behavior and brain circuitry mature more quickly in individuals who have experienced early caregiver deprivation. However, more recent work has suggested the opposite developmental trend: that resting-state amygdala – vmPFC connectivity decreases through adolescence and into early adulthood (Jalbrzikowski et al., 2017). There are a number of factors that may contribute to the inconsistent results, including differences in sample size, the age range of participants, and the use of different amygdala ROIs (e.g. including whole and subsegmented amygdalae). Given the early adolescent age range of our sample (age 12.04–14.1 years), it could be that the participants in our study were more similar to those in late childhood rather than those in later adolescence. Alternatively, if the connectivity of the entire amygdala with vmPFC becomes more positive with age, our results would be consistent with accelerated maturation. In the context of theoretical work addressing early caregiver deprivation (i.e., neglect), our results seem to support stress-accelerated maturation of emotion-regulatory circuitry consistent with adaptation to a deprived environment (Blair and Raver, 2012; Callaghan and Tottenham, 2016). Given the varying results in the literature, further research is necessary to confirm that the results reported here are an indication of more mature resting-state frontolimbic connectivity in PI youth.
Developmental trends in the relative importance of subcortical structures (as measured by graph theory centrality metrics), variance in community size, and between-system connectivity have been more consistent. A shift from subcortically-dominated patterns of brain activity to more balanced patterns has been suggested by a large body of developmental research that characterizes a shift from co-regulation, in which caregivers aid in responses to stressful or conflicting environments, to a more independent form of regulation dependent upon the top-down control of subcortical limbic structures (e.g., Casey, 2015; Sameroff, 2010). Prior research has demonstrated that the centrality of subcortical limbic structures, including the amygdala, becomes less central to brain network organization as individuals age (Sato et al., 2015). Consistent with this finding, we found age-related decreases in right amygdala eigenvector centrality in the full sample, though no differences were found as a function of early institutional care. The lack of significant differences related to early institutional deprivation in models evaluating subcortical limbic centrality may be due to a lack of whole-brain differences between groups, as eigenvector centrality is normalized to the importance of all nodes in the network. Similarly, decreases in between-system connectivity and increased variance in community size were not evident in our full sample, nor between PI and NA groups, despite such decreases having been well established across adolescent development as whole-brain networks become increasingly segregated (Gu et al., 2015). Overall, the lack of group effects is inconsistent with the possibility that early institutional deprivation has a large impact on broadly distributed networks; this may be preliminary evidence against a developmental tradeoff precipitated by accelerated maturation of frontolimbic circuits. However, while the groups did not differ in average between-system connectivity, a significant association with internalizing symptoms was found. This result lends support to the notion that decreasing between-system connectivity is a normative developmental trajectory across adolescence and may provide a piece to the puzzle when considering the relationships between functional connectivity and adaptive or maladaptive behavior.
Our lack of group differences in variance in community size and measures of between-system connectivity has two additional implications. It is possible that the effects of early institutional care do not affect whole-brain organization in adolescence but are instead more circuit specific. This explanation is consistent with previous research suggesting that frontolimbic circuitry is particularly susceptible to early psychosocial deprivation and adverse caregiving experiences (Callaghan et al., 2014). In other words, a lack of responsive caregiving early in life may result in a canalization of development toward improved self-regulated emotion processing in the absence of a co-regulator. As a result, the relative size of communities across the whole brain, or interactions between disparate networks may not be impacted by psychosocial deprivation, particularly when limited to the first years of life. An alternative explanation for the lack of whole-brain group differences could include protective effects afforded by the post-adoption environment. Transitioning from early caregiver deprivation into well-resourced and supportive homes may result in a reorganization of brain networks that ameliorates differences that may have existed between the groups initially. Longitudinal imaging studies will be able to tease apart these possibilities, including the possibility of sleeper effects that could emerge later in adolescence or adulthood.
Interpreting effects of within-system connectivity is more difficult than subcortical centrality or between-system connectivity due to a lack of prior results in the literature. Despite this difficulty, our within-system connectivity results are interesting in the context of established behavioral differences between PI and NA youth. When examining dorsal attention network within-system connectivity, which differed between groups, increased network connectivity was associated with increased internalizing and externalizing symptoms. Given that the dorsal attention network has been associated with directed attention to external stimuli (Corbetta and Shulman, 2002; Fornito et al., 2012; Fox et al., 2005) and that research in adults has shown that more positive connectivity among regions in the dorsal attention network is associated with ADHD (Sidlauskaite et al., 2016), our results suggest that higher levels of dorsal attention network within-system connectivity during early adolescence are generally associated with maladaptive outcomes. Interestingly, increased within-system connectivity averaged across all networks was negatively associated with IQ in the full sample. While connectivity metrics averaged across networks lack system-specific information about the brain-behavior relationship under study, the association between within-system connectivity and IQ further suggests that connectivity among nodes of the same network has predictive potential. Future research probing within-system connectivity—particularly dorsal attention network within-system connectivity—following ELS may improve our understanding of the neural mechanisms underlying behavioral differences observed in these populations.
Although our results may support current theories regarding circuit-specific accelerated maturation while demonstrating that a more diverse set of networks does not follow a similar pattern, they must be interpreted in the context of several limitations. Most importantly, the study in which these data were collected was not designed to investigate age-related change. As such, age at assessment effects found in subcortical limbic centrality and within-system connectivity of the dorsal attention network are best considered in the context of the group differences (or lack thereof) in these metrics. The amount of resting-state data constitutes an additional limitation as only 5 min and 57 s of data were acquired from each subject. Further, the single timepoint nature of the data limits our analysis to simple associations between brain and behavior without the possibility of causal attribution or directional specificity. It may well be that a transactional relationship exists such that internalizing or externalizing symptoms modulate the environment in ways that shape the development of functional connectivity in these circuits. Notwithstanding this possibility, the findings reported here provide guidance for future research with designs better suited to investigating developmental change.
An additional limitation of this and other studies of extreme groups concerns the heterogeneity present in the risk (PI) group. For example, the participants who survive the stringent motion correction thresholds set in rsFC research may be among the highest functioning individuals in the population, biasing results toward fewer group differences than may actually exist. The possibility for heterogeneity also extends to unmeasured factors prior to entry into the institution. Among these factors are possible genetic confounds that render some individuals more stress sensitive than others, prenatal factors such as maternal stress or substance use, and trauma occurring before institutionalization (McGuinness and Dyer, 2006). Finally, age at adoption varies across the PI group, which affects not only the amount of deprivation experienced but also the developmental timing of transition into an enriched environment. While this study was underpowered to examine age at adoption, future research with larger PI groups should consider such timing effects to elucidate the potential impact of this limitation. In each case, these sources of variation contribute unmeasured signal that may affect the presence or absence of group differences in adoption research.
The work reported here lays the groundwork for future studies despite the limitations discussed above. Recent research has characterized a recalibration of physiological systems across the pubertal period in PI youth (Gunnar et al., 2019). Whether or not such reorganization occurs at the level of the brain is not yet known. Future research examining the effects of ELS on functional connectivity throughout the adolescent period using large, longitudinal datasets may aid in addressing this gap in the literature. Further, the graph theory analyses presented here were largely exploratory in nature. More specific designs targeting neural mechanisms that may be involved in the development of behavioral differences following ELS are needed for application to prevention or intervention efforts. Specifically, investigating associations between the organization of pairs of networks over time could shed light on how altered trajectories of functional connectivity development in one system may influence the subsequent development of another system. Targeting the interactions between networks as predictors of behavioral outcomes has the potential to further our understanding of brain-behavior relationships following environmental insult. Studies of protective factors will also provide new avenues for intervention and bolster mechanistic understandings of brain development following ELS. For example, a recent review of interventions in adoptive families found that interventions using attachment-based strategies in combination with strategies based on psychological theory ranging from family systems to bio-psycho-social models were most effective for improving youth emotional and behavioral functioning (Ní Chobhthaigh and Duffy, 2019). Interventions targeting attachment and other protective factors should continue to be an important part of ELS research focused on the biological consequences of stress.
In sum, we report differences in circuit-specific and whole-brain functional connectivity between previously institutionalized and non-adopted youth in the resting-state context. Circuit-specific differences in frontolimbic connectivity suggest that experiences of ELS modify the developmental trajectory of emotion-regulation systems toward what may be a more mature state. While limited by an inconsistent literature, our results suggest that early institutional care may be associated with accelerated maturation in emotion processing circuitry. Such a developmental pattern could be an adaptive response to deprived caregiver environments. In contrast, despite the possibility of a developmental trade-off in which accelerated maturation of frontolimbic connectivity comes at the cost of higher-order cognition and attention-related networks, our results do not suggest these networks are heavily impacted by early caregiver deprivation. While there was a group difference evident in the within-system connectivity of the dorsal attention network, it remains unclear whether this is consistent with a more or less mature neural profile in PI youth. Despite the lack of support for a developmental tradeoff, this work extends prior task-based connectivity research into the resting-state context and emphasizes the importance of examining a diverse set of brain networks in future research with populations who have experienced early life stress. Continuing to build a more complete picture of the associations between early life stress and resting-state functional connectivity will be an important step toward understanding risk and resilience factors critical to effective intervention.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by a National Institute of Mental Health – United States; P50-MH79513; T32-MH73129; T32-MH015755;National Institute of Child Health and Human Development – United States; T32-HD007151, National Centers for Research Resources – United StatesP41 RR008079, National Institute of Biomedical Imaging and Bioengineering – United StatesP41 EB015894, National Institute of Neurological Disorders and Stroke – United StatesP30 NS076408, National Center for Advancing Translational Sciences – United StatesTL1R002493 and UL1TR002494, University of Minnesota Graduate School Fellowship – United States, Doris Duke Fellowship for the Promotion of Child Well-Being – United States. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences. The authors thank collaborators at the Center for Brain, Genes, and Behavioral Research Across Development located at the Weill-Cornell Medical Center, the Minnesota Supercomputing Institute (MSI) at the University of Minnesota for providing resources that contributed to the research results reported within this paper (http://www.msi.umn.edu), and members of Kathleen M. Thomas’ Cognitive Development & Neuroimaging Lab and Megan R. Gunnar’s Human Developmental Psychobiology Lab for assistance with participant recruitment, scheduling, and testing.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100922.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Beckett C., Castle J., Rutter M., Barke E. VI: institutional deprivation, specific cognitive functions, and scholastic achievement: English and Romanian adoptee (ERA) study findings. Monogr. Soc. Res. Child Dev. 2010;75(1):125–142. doi: 10.1111/j.1540-5834.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- Belsky J., Steinberg L., Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev. 1991;62(4):647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Betzel R.F., Byrge L., He Y., Goñi J., Zuo X.N., Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage. 2014;102(P2):345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- Blair C., Raver C.C. Child development in the context of adversity: experiential canalization of brain and behavior. Am. Psychol. 2012;67(4):309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacich P. Some unique properties of eigenvector centrality. Soc. Networks. 2007;29(4):555–564. doi: 10.1016/j.socnet.2007.04.002. [DOI] [Google Scholar]
- Callaghan B.L., Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Sullivan R.M., Howell B., Tottenham N. The international society for developmental psychobiology Sackler symposium: early adversity and the maturation of emotion circuits-a cross-species analysis. Dev. Psychobiol. 2014;56(8):1635–1650. doi: 10.1002/dev.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu. Rev. Psychol. 2015;66(1):295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Colich N.L., Platt J.M., Keyes K.M., Sumner J.A., Allen N.B., Mclaughlin K.A. Earlier age at menarche as a transdiagnostic mechanism linking childhood trauma with multiple forms of psychopathology in adolescent girls. Psychol. Med. 2019 doi: 10.1017/S0033291719000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(29):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Del Giudice M., Ellis B.J., Shirtcliff E.A. The adaptive calibration model of stress responsivity. Neurosci. Biobehav. Rev. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex M.J., Boyce W.T., Goldstein L.H., Armstrong J.M., Kraemer H.C., Kupfer D.J., Group, T.M.A.B.W The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(5):580–587. doi: 10.1097/00004583-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Harrison B.J., Zalesky A., Simons J.S. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc. Natl. Acad. Sci. U. S. A. 2012;109(31):12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Bullmore E. Fundamentals of Human Imaging Connectomics. 2016. Fundamentals of brain network analysis. [DOI] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E. The development of human amygdala functional connectivity at rest from 4 to 23years: a cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H. Early developmental emergence of human amygdala – prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Satterthwaite T.D., Medaglia J.D., Yang M., Gur R.E., Gur R.C., Bassett D.S. Emergence of system roles in normative neurodevelopment. Proc. Natl. Acad. Sci. 2015;112(44):13681–13686. doi: 10.1073/pnas.1502829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M.R., Van Dulman M.H.M. Behavior problems in postinstitutionalized internationally adopted children. Dev. Psychopathol. 2007;19(01):129–148. doi: 10.1017/S0954579407070071. [DOI] [PubMed] [Google Scholar]
- Gunnar M.R., Wenner J.A., Thomas K.M., Glatt C.E., Mckenna M.C., Clark A.G. The brain-derived neurotrophic factor Val66Met polymorphism moderates early deprivation effects on attention problems. Dev. Psychopathol. 2012;24:1215–1223. doi: 10.1017/S095457941200065X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M.R., DePasquale C.E., Reid B.M., Donzella B. Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proc. Natl. Acad. Sci. U. S. A. 2019;116(48):23984–23988. doi: 10.1073/pnas.1909699116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk B., McCall R.B. CBCL behavior problems of post-institutionalized international adoptees. Clin. Child Fam. Psychol. Rev. 2010;13(2):199–211. doi: 10.1007/s10567-010-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M.P., Hodel A.S., Cowell R.A., Hunt R.H., Gunnar M.R., Thomas K.M. Risk taking, decision-making, and brain volume in youth adopted internationally from institutional care. Neuropsychologia. 2018;119:262–270. doi: 10.1016/J.NEUROPSYCHOLOGIA.2018.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel A.S., Hunt R.H., Cowell R.A., Van Den Heuvel S.E., Gunnar M.R., Thomas K.M. Duration of early adversity and structural brain development in post-institutionalized adolescents. NeuroImage. 2015;105:112–119. doi: 10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C.E., Stellern S.A., Schaefer C., Carlson S.M., Gunnar M.R. Associations between early life adversity and executive function in children adopted internationally from orphanages. Proc. Natl. Acad. Sci. 2012;109(Supplement_2):17208–17212. doi: 10.1073/pnas.1121246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M., Larsen B., Hallquist M.N., Foran W., Calabro F., Luna B. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol. Psychiatry. 2017:511–521. doi: 10.1016/j.biopsych.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. Fsl. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jeub L.G.S., Bazzi M., Jutla I.S., Mucha P.J. 2016. A Generalized Louvain Method for Community Detection Implemented in MATLAB.http://netwiki.amath.unc.edu/GenLouvain Retrieved from. [Google Scholar]
- Kim M.J., Loucks R.A., Palmer A.L., Brown A.C., Solomon K.M., Marchante A.N., Whalen P.J. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness T.M., Dyer J.G. International adoption as a natural experiment. Journal of Pediatric Nursing. 2006;21(4):276–288. doi: 10.1016/j.pedn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Winter W., Fox N.A., Zeanah C.H., Nelson C.A. Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2014;76(8):629–638. doi: 10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz E.C., Harlé K.M., Noble K.G., McCall R.B. Executive function in previously institutionalized children. Child Dev. Perspect. 2016;10(2):105–110. doi: 10.1111/cdep.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nelson C.A., Bos K., Gunnar M.R., Sonuga-Barke E.J.S. The neurobiological toll of early human deprivation. Monogr. Soc. Res. Child Dev. 2011;76(4):127–146. doi: 10.1111/j.1540-5834.2011.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Zeanah C.H., Fox N.A., Marshall P.J., Smyke A.T., Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318(5858):1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Newman M.E.J. The New Palgrave Encyclopedia of Economics. 2008. The mathematics of networks; pp. 1–12. [DOI] [Google Scholar]
- Ní Chobhthaigh S., Duffy F. The effectiveness of psychological interventions with adoptive parents on adopted children and adolescents’ outcomes: a systematic review. Clin. Child Psychol. Psychiatry. 2019;24(1):69–94. doi: 10.1177/1359104518786339. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion orocessing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Young C.B., Supekar K., Uddin L.Q., Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proc. Natl. Acad. Sci. 2012;109(20):7941–7946. doi: 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M.C., Uddin L.Q., Gotimer K. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sameroff A. A unified theory of development: a dialectic integration of nature and nurture. Child Dev. 2010;81(1):6–22. doi: 10.1111/j.1467-8624.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- Sato J.R., Salum G.A., Gadelha A., Vieira G., Zugman A., Picon F.A. Decreased centrality of subcortical regions during the transition to adolescence: a functional connectivity study. NeuroImage. 2015;104:44–51. doi: 10.1016/j.neuroimage.2014.09.063. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., Ruparel K., Loughead J., Calkins M.E. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin Z.M., Osher D.E., Koldewyn K., Martin R.E., Finn A., Saxe R. Structural connectivity of the developing human amygdala. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0125170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidlauskaite J., Sonuga-Barke E., Roeyers H., Wiersema J.R. Altered intrinsic organisation of brain networks implicated in attentional processes in adult attention-deficit/hyperactivity disorder: a resting-state study of attention, default mode and salience network connectivity. Eur. Arch. Psychiatry Clin. Neurosci. 2016;266(4):349–357. doi: 10.1007/s00406-015-0630-0. [DOI] [PubMed] [Google Scholar]
- Silvers J.A., Lumian D.S., Gabard-Durnam L., Gee D.G., Goff B., Fareri D.S. Previous institutionalization is followed by broader amygdala–hippocampal–PFC network connectivity during aversive learning in human development. J. Neurosci. 2016;36(24):6420–6430. doi: 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. The importance of early experiences for neuro-affective development. Curr. Top. Behav. Neurosci. 2014;16:109–129. doi: 10.1007/7854_2013_254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Millner A., Gilhooly T., Zevin J.D., Casey B.J. Elevated amygdala response to faces following early deprivation. Dev. Sci. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ijzendoorn M.H., Juffer F. The Emanuel Miller Memorial Lecture 2006: adoption as intervention. Meta-analytic evidence for massive catch-up and plasticity in physical, socio-emotional, and cognitive development. J. Child Psychol. Psychiatry Allied Discipl. 2006;47(12):1228–1245. doi: 10.1111/j.1469-7610.2006.01675.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1999. Manual for the Wechsler Abbreviated Intelligence Scale (WASI) [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah C.H., Egger H.L., Smyke A.T., Nelson C.A., Fox N.A., Marshall P.J., Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry. 2009;166(7):777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.