Abstract
Background
Invasive mechanical ventilation of hypoxaemic coronavirus disease 2019 (COVID-19) patients is associated with mortality rates of >50%. We evaluated clinical outcome data of two hospitals that agreed on a predefined protocol for restrictive use of invasive ventilation where the decision to intubate was based on the clinical presentation and oxygen content rather than on the degree of hypoxaemia.
Method
Data analysis was carried out of patients with positive PCR-testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), typical history, and symptoms and pulmonary infiltrates who exhibited oxygen saturation values of <93%.
Results
We identified 78 patients who met the inclusion criteria. The oxygen saturation nadir was 84.4±6.5% for the whole group. 53 patients (68%) received nasal oxygen (group 1), 17 patients (22%) were treated with nasal high-flow continuous positive airway pressure (CPAP), noninvasive ventilation or a combination thereof (group 2), and eight patients (10%) were intubated (group 3). The Horovitz index was 216±8 for group 1, 157±13 for group 2 and 106±15 for group 3. Oxygen content was 14.5±2.5, 13.4±1.9 and 11.6±2.6 mL O2·dL−1 for the three respective groups. Overall mortality was 7.7%; the mortality of intubated patients was 50%. Overall, 93% of patients could be discharged on room air.
Conclusion
Permissive hypoxaemia where decisions for the level of respiratory therapy were based on the clinical presentation and oxygen content resulted in low intubation rates, low overall mortality and a low number of patients who require oxygen after discharge.
Short abstract
Permissive hypoxaemia where the decision to intubate is based on the clinical picture and oxygen content is feasible in the acute phase of #COVID19 https://bit.ly/35Xj9LO
Introduction
Although coronavirus disease 2019 (COVID-19) is asymptomatic to mild in about 80% of cases, about 15% of patients show a severe and about 5% a critical course, which is usually based on lung involvement with respiratory failure [1]. The first therapeutic recommendations therefore addressed hypoxaemia in particular with the aim of keeping oxygen saturation above at least 90% [2]. Some authors even advise caution against the use of high-flow oxygen administration (NHF) and noninvasive ventilation (NIV) in acute hypoxaemic respiratory insufficiency in the context of COVID-19 and call for early intubation [3], and others recommend intubation and invasive ventilation if the Horovitz index [4] is 200 or lower [5]. In general, hypoxaemia is an accepted indication for intubation in COVID-19 patients [6–8]. The work of Raoof et al. [9] provides a good overview of the respiratory support recommendations of different countries and societies in the context of COVID-19 disease. The prognosis for invasively ventilated COVID-19 patients however is poor, and mortality is somewhere around 50% and even higher, especially in older patients [10–12]. The fact that there is a large discrepancy between oxygen saturation and the extent of dyspnoea has given rise to the term “happy hypoxaemia” [13] in COVID-19 patients. The pathophysiological mechanisms of this phenomenon are being discussed currently [13–17] as well as the impact of happy hypoxaemia on respiratory management [14, 18]. A recent Cochrane review did not find evidence that higher oxygen targets benefit patients with respiratory failure [19]. Hypoxaemia can either be caused by intrapulmonary shunting or ventilation–perfusion mismatch, which in COVID-19 is mainly caused by diffusion impairment. Only the latter is responsive to supplemental oxygen [20]. Dyspnoea on the other hand poorly correlates to hypoxaemia [21]. In the presence of inflammatory diseases (such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections), dyspnoea is frequently being caused by other mechanisms such as stimulation of irritant, stretch and J receptors [22] or activation of respiratory muscles [23]. Focusing on tissue hypoxia oxygen delivery, determined by oxygen content (CaO2) and cardiac output is crucial. Critical values for CaO2 with signs of anaerobic metabolism occur in animal models at levels below 9 mL O2·dL−1 blood, which corresponds to an oxygen saturation of ∼50% at normal haemoglobin levels and normal cardiac output [24]. Experiments in healthy humans have shown that the critical level for oxygen content is lower than 6.6 mL O2·dL−1) [25]. In particular, lung tissue is vulnerable to oxygen concentrations of >21%, and it is well known that oxygen per se is toxic if given in high concentrations and can cause acute respiratory distress syndrome (ARDS) in animal models [26]. This leads to the question as to whether a higher tolerance of hypoxaemia and a preferred use of noninvasive respiratory support instead of intubation and decision-making based on the clinical presentation as previously suggested should be preferred [18] and whether this can improve outcome in COVID-19 patients. Based on these considerations, we analysed the data for all hypoxaemic COVID-19 patients in two hospitals that had previously agreed on such a protocol for the treatment of respiratory failure in COVID-19 pneumonia.
Method
Treatment protocol
At the beginning of the pandemic, we developed a predefined protocol for COVID-19 therapy in our hospitals. For the treatment of respiratory insufficiency, we have defined a strategy that provides invasive ventilation only when other measures have failed to stabilise the patient and intubation appears to be vital. The primary goal is to maintain the patient's spontaneous breathing for as long as possible. Positioning techniques such as prone or lateral position are to be used at each therapy stage – even under room air, oxygen therapy or NIV. Furthermore, we follow the principle of Hippocrates’ “primum non nocere” and have excluded the employment of experimental procedures such as the use of hydroxychloroquine, lopinavir/ritonavir, tocilizumab or remdesivir. Patients received a pneumococcal active antibiotic (ampicillin/sulbactam) in combination with a macrolide as well as prophylactic doses of heparin. Whenever possible patients were mobilised as early as possible.
The two participating pulmonary facilities (Kloster Grafschaft, facility 1 and Bethanien Moers, facility 2) agreed on the following protocol: Respiratory support was to be given in the escalating sequence shown in figure 1. Escalation to the next level was made if the patients’ clinical work of breathing required the next level of support or the oxygen content was determined to be below 9 mL O2·dL−1. Hypoxaemia per se was not an indication to escalate (permissive hypoxaemia).
FIGURE 1.
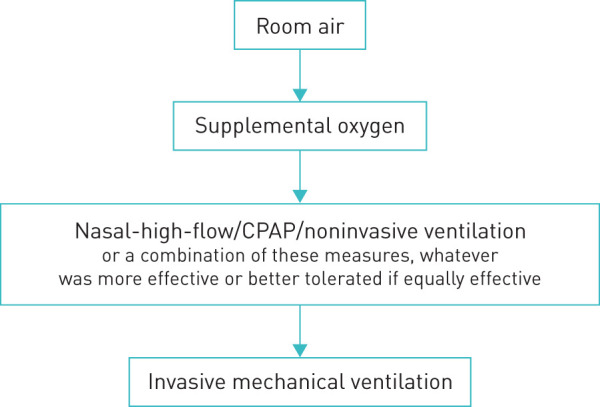
Escalation sequence. CPAP: continuous positive airway pressure.
Inclusion criteria
Patients had to be primarily admitted to one of the investigational sites to be included. Patients who were transferred from other hospitals with prior treatment (e.g. who were already intubated) were excluded.
Patients had to be Sars-CoV-2 PCR positive and had to have infiltrates on conventional chest radiograph or computed tomography (CT) scan. Only patients with an oxygen saturation measured by pulse oximetry (SpO2) of <93% were included in the analysis. All patients were treated in an intermediate care or intensive care setting with continuous monitoring of oxygen saturation and heart rate by pulse oximetry. Patients were seen at least daily by a senior physician, and laboratory tests were done at least every other day. Respiratory therapists were readily available to deliver respiratory support.
Data were retrospectively collected by chart review and transferred into a concerted Excel database at the respective site.
The ethics commission responsible approved the retrospective analysis (AEKWL 2020-897-f-S).
Statistics
Continuous variables are presented as means (±sd), and categorical variables as numbers and frequency (percentages). All data was transferred to SPSS (version 27, IBM, Armonk, NY, USA) for further analysis. Multiple comparison of continuous variables was performed by means of ANOVA. For post hoc analysis we used a t-test with Bonferroni adjustment for multiple comparisons. A p-value of <0.05 was used as the significance threshold. Missing data were handled by pairwise deletion.
Results
We identified a total of 78 patients who met the inclusion criteria (26 from facility 1 and 52 from facility 2). Mean hospital length of stay was 14.5±13.5 days. Basic demographic data and pre-existing comorbidities are shown in table 1.
TABLE 1.
Basic demographic data and pre-existing comorbidities
| Patients n | 78 |
| Male | 56.4 |
| Caucasian | 100 |
| Age years | 65±14 |
| BMI kg·m−2 | 29.4±4.9 |
| Active smoker | 16.7 |
| Former smoker | 46.2 |
| Nonsmoker | 37.2 |
| Hypertension | 70.5 |
| Diabetes | 19.2 |
| Coronary artery disease | 21.8 |
| Asthma | 17.9 |
| COPD | 10.3 |
| Malignancy | 21.8 |
Data are presented as % or mean±sd, unless otherwise stated. BMI: body mass index.
On admission 87.2% of the patients experienced dyspnoea. Anosmia was present in 30.8% of patients and 84.6% reported fatigue. Bilateral infiltrates on chest radiograph or CT scan were present in 97.5% of patients; only 2.5% had unilateral infiltrates. Overall admission SpO2 was 92±5.8%; the lowest reported oxygen saturation during the hospital course was 84.4±6.5%.
For further analysis we grouped patients according to the maximal respiratory treatment as shown in figure 1. This group distribution is shown in figure 2. The lowest reported oxygen saturation within these groups is shown in figure 3. The lowest reported Horovitz index is shown in figure 4. The lowest measured oxygen content is shown in figure 5.
FIGURE 2.
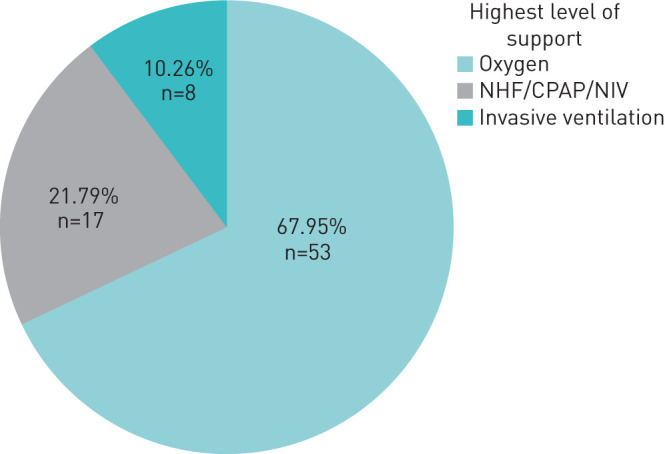
Distribution of the highest level of respiratory support that was delivered to the patient. NHF: nasal high-flow; CPAP: continuous positive airway pressure; NIV: noninvasive ventilation.
FIGURE 3.
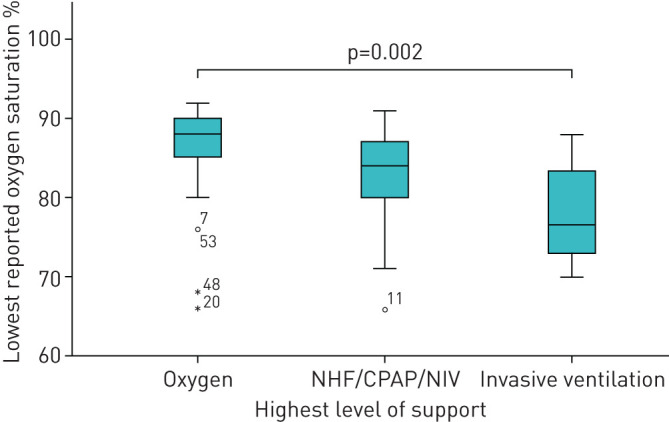
Oxygen saturation nadir of the respective treatment groups. The thick line in the middle is the median. The top and bottom box lines show the first and third quartiles. The whiskers show the maximum and minimum values, with the exceptions of outliers (circles) and extremes (asterisks). Outliers are at least 1.5 box lengths from the median and extremes are at least three box lengths from the median. The case numbers are given for outliers and extremes. NHF: nasal high-flow; CPAP: continuous positive airway pressure; NIV: noninvasive ventilation.
FIGURE 4.
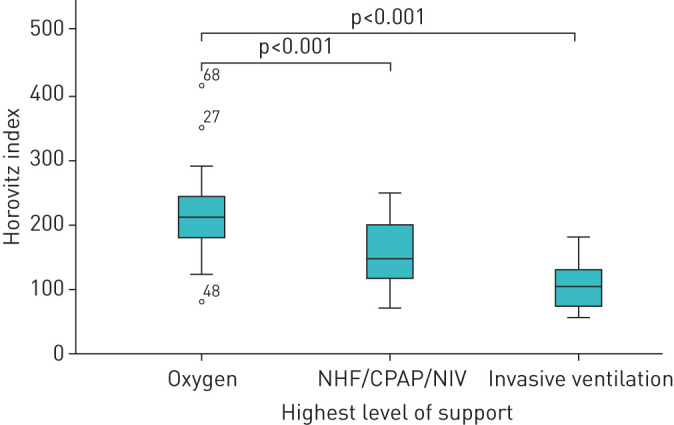
Lowest reported Horovitz index (arterial oxygen tension/inspiratory oxygen fraction ratio) of the respective treatment groups. The thick line in the middle is the median. The top and bottom box lines show the first and third quartiles. The whiskers show the maximum and minimum values, with the exceptions of outliers (circles). Outliers are at least 1.5 box lengths from the median. The case numbers are given for outliers. NHF: nasal high-flow; CPAP: continuous positive airway pressure; NIV: noninvasive ventilation.
FIGURE 5.
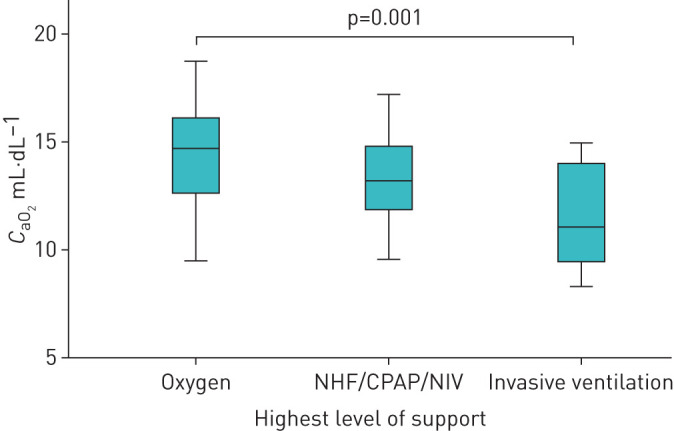
Lowes measured arterial oxygen content (CaO2) of the respective treatment groups. NHF: nasal high-flow; CPAP: continuous positive airway pressure; NIV: noninvasive ventilation.
Laboratory values according to maximal treatment are shown in table 2.
TABLE 2.
Demographic, physiological and laboratory data according to treatment groups
| Oxygen alone | NHF/CPAP/NIV | Invasive MV | |
| Subjects n | 53 | 17 | 8 |
| Age years | 65±15 | 62±13 | 73±9 |
| BMI kg·m−2 | 29.4±5.2 | 29.6±5.1 | 29.1±2.5 |
| LOS days | 10.1±6.7*,# | 19.3±11.8#,¶ | 34±26.9*,# |
| Temperature on admission °C | 38.3±1* | 39.1±0.9¶ | 39.1±0.5 |
| SpO2 on admission % | 93.1±3.7# | 91.6±6# | 85.4±10.6*,¶ |
| Respiratory rate on admission min−1 | 20.6±4.4* | 25.7±6.7¶ | 23.5±7.6 |
| Heart rate on admission min−1 | 90±20 | 99±16 | 95±21 |
| Systolic arterial blood pressure on admission mmHg | 118±21 | 110±22 | 103±23 |
| Diastolic arterial blood pressure on admission mmHg | 69±11 | 68±10 | 63±13 |
| Haemoglobin nadir mg·dL−1 | 12.4±1.9 | 12.1±2 | 11±2.4 |
| Leukocyte nadir µL−1 | 5745±2732 | 5724±2602 | 5512±1629 |
| Lymphocyte nadir µL−1 | 986±2015 | 553±262 | 469±342 |
| LDH maximum U·L−1 | 405±129*,# | 562±260#,¶ | 741±190*,¶ |
| BNP maximum pg·mL−1 | 1773±3086 | 669±895# | 5258±7659* |
| CRP maximum mg·dL−1 | 10.9±7.1*,# | 21.6±9.2¶ | 26.4±12.3¶ |
| PCT maximum ng·mL−1 | 0.9±2# | 0.8±1.2# | 3±2.9*,¶ |
| Creatinine maximum mg·dL−1 | 1.25±0.62# | 1±0.3# | 2.6±1.4*,¶ |
| D-dimer maximum ng·mL−1 | 1530±1575# | 2698±2464 | 6574±5321¶ |
| Troponin maximum µg·L−1 | 28.5±39# | 25.9±33.1# | 467±1047*,¶ |
| PO2 nadir mmHg | 54.5±11 | 47.8±10.5 | 45.9±6.8 |
Data are presented as mean±sd unless otherwise stated. NHF: nasal high-flow; CPAP: continuous positive airway pressure; NIV: noninvasive ventilation; MV: mechanical ventilation; BMI: body mass index; LOS: length of (hospital) stay; SpO2: oxygen saturation measured by pulse oximetry; LDH: lactate dehydrogenase; BNP: brain natriuretic peptide; CRP: C-reactive protein; PCT: procalcitonin; PO2: oxygen tension. *: p<0.05 versus NHF/CPAP/NIV; #: p<0.05 versus invasive MV; ¶: p<0.05 versus oxygen alone; +: on admission.
All patients received β-lactam antibiotics; 97.4% of patients received an additional macrolide. Systemic anticoagulation was given in 88.5% of patients. Since our data were collected before data from the RECOVERY trial were published [27], dexamethasone was not given as the standard of care. Outcome at discharge according to the maximal respiratory support received is shown in figure 6. The overall mortality was 7.7%, and the mortality rate of intubated patients was 50%. The two patients who died on oxygen treatment were 86 and 96 years of age and had declared to refuse any form of respiratory support beyond oxygen administration in their living will. Reasons for intubation in eight patients were septic shock on admission in one patient, cardiac arrest due to AV-block 3 in one patient and NIV failure according to our protocol in six patients.
FIGURE 6.
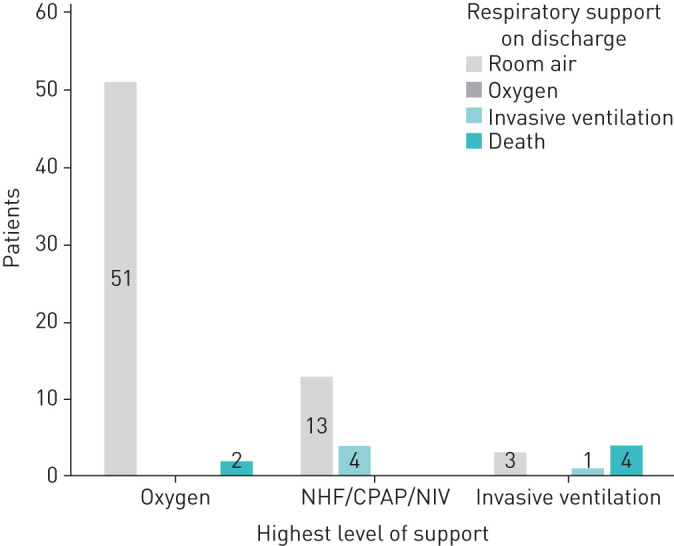
Outcome/respiratory support at discharge according to the maximal respiratory support received. NHF: nasal high-flow; CPAP: continuous positive airway pressure; NIV: noninvasive ventilation.
Discussion
Our escalation protocol of respiratory support measures that was based on the clinical presentation and oxygen content rather than on markers of oxygenation or the Horovitz index resulted in an intubation rate of only 10.3% and an overall mortality of 7.7%. Our mortality rate of 50% in intubated patients is comparable to previously published data [10–12]. The overall mortality of patients hospitalised for SARS-CoV-2 infections in Germany is 22% [11] and is thus much higher than in our study in which only hypoxaemic patients were included. Raoof et al. [9] have already pointed out the various therapeutic approaches of different countries and societies with regard to the treatment of respiratory insufficiency. Randomised trials on this issue are unlikely to be conducted during the current pandemic. Thus, comparisons of different treatment strategies of different cohorts within the same healthcare system can be helpful to judge on treatment efficiency. Roedl et al. [28] reported retrospective data on a large cohort in the city of Hamburg. They reported that 167 (75%) of intensive care unit (ICU) patients received invasive mechanical ventilation within a median of 1 day after admission, while NHF or NIV was only used in 18% of patients prior to intubation with high failure rates. In addition, partial pressure of oxygen levels were higher (70 mmHg in survivors and 64 mmHg in non-survivors of mechanical ventilation) compared to our cohort (table 2) indicating that the decision to intubate was probably done more progressively. ICU mortality rates were 44% in ventilated and 35% in nonventilated patients and thus were much higher than the rate we reported (7.7%). Burns and et al. [29] found an improved survival when NIV was given to frail patients who were not deemed appropriate for invasive mechanical ventilation and proposed a general integrated escalation strategy of noninvasive respiratory therapies to avoid intubation. Patel et al. [30] worked with a respiratory escalation scheme in moderate to severe hypoxaemic COVID-19 patients that was comparable to ours. Their decision to intubate was also based on clinical presentation, but the oxygen saturation goal was 94% and the ratio of intubated patients was 36%. Our protocol did not call for an oxygen saturation goal, which might explain the lower intubation rate of 10.3%. Brusasco et al. [31] reported a continuous positive airway pressure (CPAP) success rate of 83% in COVID-19 patients with a mean arterial oxygen tension/inspiratory oxygen fraction ratio of 119. Oranger et al. [32] reduced the combined outcome of intubation and/or death significantly from 57% to 23% after a protocol of routine CPAP use was introduced to hypoxaemic COVID-19 patients. The patients’ good tolerance of hypoxaemia has led to the term “happy hypoxaemia” [13]. Hypoxaemia rarely causes dyspnoea [21]; dyspnoea is more often related to hypercapnia, acidosis [33] or activation of the respiratory muscles [23]. Hypoxaemia as measured by arterial blood gas analysis or pulse oximetry does not equal tissue hypoxia.
Oxygen delivery to the cells is determined by the oxygen content (1.34×haemoglobin (mg·dL−1)×oxygen saturation (%)/100+0.0031×partial pressure of oxygen (mmHg)) times the cardiac output. The majority of oxygen (98%) is bound to haemoglobin (represented by bold type in the equation above), while the amount of freely dissolved oxygen (underlined part of the equation) is negligible. Lowering the saturation by x% has the same effect as lowering the haemoglobin by x%. So why we are more concerned about severe hypoxaemia than about severe anaemia? The latter was not present in our patients, as shown in table 2, which might explain in part the good tolerance of hypoxaemia.
Elevated body temperature (fever) as seen in our patients shifts the oxygen dissociation curve to the right, which facilitates the release of oxygen in the periphery [34]. A lack of oxygen on the cellular level does not occur until the oxygen delivery has decreased to 25% of the normal value [35]. In animal models anaerobic metabolism occurred if the oxygen content fell below 9 mL O2·dL−1 [24]. Lieberman et al. [25] had shown that lowering the oxygen content to as low as 6.6 mL O2·dL−1 did not cause signs of anaerobic metabolism in healthy volunteers. Our threshold of 9 mL O2·dL−1 appeared to be safe in our patient cohort.
Based on these considerations, the clinical importance of hypoxaemia should not be overestimated especially since invasive ventilation might correct hypoxaemia in the short term but may inflict ventilator-associated lung injury [36] or oxygen-induced ARDS [26]. A more restrictive use of invasive mechanical ventilation and oxygen, as suggested recently [18], might be advised. Our data suggest that such a strategy is more beneficial for COVID-19 patients.
We observed the typical laboratory abnormalities as seen in previous investigations [30, 37].
Previous investigations have shown that there is great potential to recover from COVID-19 with very little sequelae [38]. It should be particularly emphasised that the majority (93%) of the patients who survived could be discharged without oxygen. Only four patients treated with NHF/CPAP/NIV required oxygen on discharge, and one patient who was intubated required tracheostomy and continued on invasive ventilation. Our data suggest that the lungs recover well from COVID-19 if they are denied the stress of invasive ventilation and over-oxygenation.
Conclusion
A respiratory support escalation scheme based on clinical appearance and oxygen content rather than on the level of oxygenation (permissive hypoxaemia) is feasible and has a favourable outcome in our retrospective analysis. Hypoxaemia per se should not be an indication for invasive mechanical ventilation. The vast majority of patients recover well from COVID-19 if such a strategy is pursued.
Footnotes
Data availability: Data will be shared upon request.
Conflict of interest: T. Voshaar has nothing to disclose.
Conflict of interest: P. Stais has nothing to disclose.
Conflict of interest: D. Köhler has nothing to disclose.
Conflict of interest: D. Dellweg has nothing to disclose.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected. Geneva, WHO, 2020. [Google Scholar]
- 3.Cheung JCH, Ho LT, Cheng JV, et al. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med 2020; 8: e19. doi: 10.1016/S2213-2600(20)30084-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luterman A, Horovitz JH, Carrico CJ, et al. Withdrawal from positive end-expiratory pressure. Surgery 1978; 83: 328–332. [PubMed] [Google Scholar]
- 5.Kluge S, Janssens U, Welte T, et al. German recommendations for critically ill patients with COVID-19. Med Klin Intensivmed Notfmed 2020; 115: 111–114. doi: 10.1007/s00063-020-00689-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region: case series. N Engl J Med 2020; 382: 2012–2022. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323: 1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raoof S, Nava S, Carpati C, et al. High-flow, noninvasive ventilation and awake (Nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest 2020; 158: 1992–2002. doi: 10.1016/j.chest.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amit M, Sorkin A, Chen J, et al. Clinical course and outcomes of severe Covid-19: a national scale study. J Clin Med 2020; 9: 2282. doi: 10.3390/jcm9072282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med 2020; 8: 853–862. doi: 10.1016/S2213-2600(20)30316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim ZJ, Subramaniam A, Reddy MP, et al. Case fatality rates for COVID-19 patients requiring invasive mechanical ventilation: a meta-analysis. Am J Respir Crit Care Med 2021; 203: 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couzin-Frankel J. The mystery of the pandemic's ‘happy hypoxia’. Science 2020; 368: 455–456. doi: 10.1126/science.368.6490.455 [DOI] [PubMed] [Google Scholar]
- 14.Dhont S, Derom E, Van Braeckel E, et al. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res 2020; 21: 198. doi: 10.1186/s12931-020-01462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobin MJ, Jubran A, Laghi F. Misconceptions of pathophysiology of happy hypoxemia and implications for management of COVID-19. Respir Res 2020; 21: 249. doi: 10.1186/s12931-020-01520-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobin MJ, Laghi F, Jubran A. Reply to Jounieaux et al.: On happy hypoxia and on sadly ignored “acute vascular distress syndrome” in patients with COVID-19. Am J Respir Crit Care Med 2020; 202: 1599–1600. doi: 10.1164/rccm.202007-2940LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med 2020; 202: 356–360. doi: 10.1164/rccm.202006-2157CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobin MJ. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med 2020; 201: 1319–1320. doi: 10.1164/rccm.202004-1076ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cumpstey AF, Oldman AH, Smith AF, et al. Oxygen targets in the intensive care unit during mechanical ventilation for acute respiratory distress syndrome: a rapid review. Cochrane Database Syst Rev 2020; 9: CD013708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobin MJ, Laghi F, Jubran A. Ventilatory failure, ventilator support, and ventilator weaning. Compr Physiol 2012; 2: 2871–2921. [DOI] [PubMed] [Google Scholar]
- 21.Dellweg D, Schmitten J, Kerl J, et al. Impact of hypobaric flight simulation on walking distance and oxygenation in COPD patients. Respir Physiol Neurobiol 2019; 260: 1–7. doi: 10.1016/j.resp.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 22.Tobin MJ. Principles and Practice of Intensive Care Monitoring. New York, McGraw-Hill Professional, 1998. [Google Scholar]
- 23.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, et al. An official ATS clinical policy statement: congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med 2010; 181: 626–644. doi: 10.1164/rccm.200807-1069ST [DOI] [PubMed] [Google Scholar]
- 24.Mark AH, Van Der Hoeven BM, Maertzdorf WJ, et al. Mixed venous oxygen saturation and biochemical parameters of hypoxia during progressive hypoxemia in 10- to 14-day-old piglets. Pediatr Res 1997; 42: 878–884. doi: 10.1203/00006450-199712000-00026 [DOI] [PubMed] [Google Scholar]
- 25.Lieberman JA, Weiskopf RB, Kelley SD, et al. Critical oxygen delivery in conscious humans is less than 7.3 ml O2·kg−1·min−1. Anesthesiology 2000; 92: 407–413. doi: 10.1097/00000542-200002000-00022 [DOI] [PubMed] [Google Scholar]
- 26.Santos R DL, Seidenfeld JJ, Anzueto A, et al. One hundred percent oxygen lung injury in adult baboons. Am Rev Respir Dis 1987; 136: 657–661. doi: 10.1164/ajrccm/136.3.657 [DOI] [PubMed] [Google Scholar]
- 27.RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19: preliminary report. N Engl J Med 2021; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roedl K, Jarczak D, Thasler L, et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: a multicentric study in Germany. Aust Crit Care 2020; in press [ 10.1016/j.aucc.2020.10.009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns GP, Lane ND, Tedd HM, et al. Improved survival following ward-based non-invasive pressure support for severe hypoxia in a cohort of frail patients with COVID-19: retrospective analysis from a UK teaching hospital. BMJ Open Respir Res 2020; 7: e000621. doi: 10.1136/bmjresp-2020-000621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel M, Gangemi A, Marron R, et al. Retrospective analysis of high flow nasal therapy in COVID-19-related moderate-to-severe hypoxaemic respiratory failure. BMJ Open Respir Res 2020; 7: e000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brusasco C, Corradi F, Di Domenico A. Continuous positive airway pressure in COVID-19 patients with moderate-to-severe respiratory failure. Eur Respir J 2021; 57: 2002524. 10.1183/13993003.02524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oranger M, Gonzalez-Bermejo J, Dacosta-Noble P, et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur Respir J 2020; 56: 2001692. doi: 10.1183/13993003.01692-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012; 185: 435–452. doi: 10.1164/rccm.201111-2042ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barcroft J, King WOR. The effect of temperature on the dissociation curve of blood. J Physiol 1909; 39: 374–384. doi: 10.1113/jphysiol.1909.sp001345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronco JJ, Fenwick JC, Tweeddale MG, et al. Identification of the critical oxygen delivery for anaerobic metabolism in critically ill septic and nonseptic humans. JAMA 1993; 270: 1724–1730. doi: 10.1001/jama.1993.03510140084034 [DOI] [PubMed] [Google Scholar]
- 36.Finfer SR, Vincent J-L, Slutsky AS, et al. Critical care medicine ventilator-induced lung injury. N Engl J Med 2013; 369: 2126. doi: 10.1056/NEJMe1304035 [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sung HK, Kim JY, Heo J, et al. Clinical course and outcomes of 3,060 patients with coronavirus disease 2019 in Korea, January-May 2020. J Korean Med Sci 2020; 35: e280. doi: 10.3346/jkms.2020.35.e280 [DOI] [PMC free article] [PubMed] [Google Scholar]


