Abstract
The objective of this study is to determine the therapeutic efficacy of allicin against Candida albicans (C. albicans) and Staphylococcus aureus (S. aureus), the common etiological agents for denture stomatitis (DS). The minimum inhibitory concentration (MICs), minimum bactericidal concentrations (MBCs) and minimum fungicidal concentration (MFCs) of allicin were determined by the broth microdilution method followed by checkerboard microdilution method for a synergistic interaction between allicin + nystatin and allicin + CHX. The potential of allicin to eradicate C. albicans and S. aureus biofilms was assessed by treating biofilm formed on self- polymerized acrylic resin with allicin at a sub-MIC concentration for 5 min. The commercial denture cleanser (brand X) was used as a positive control. A Kruskal-Wallis test followed by the post-hoc Mann-Whitney U test was applied (SPSS 20.0), and the level of significance was set at P < 0.05. Allicin exhibited antimicrobial activity against C. albicans (MIC:8 µg/ml and MFC:16 µg/ml) and S. aureus (MIC:8 µg/ml and MBC:8 µg/ml). A synergistic interaction was observed between allicin + nystatin and allicin + CHX (FICI ≤ 0.5). Allicin exhibited significant biofilm eradication against C. albicans and S. aureus biofilms with percentages of 50.0% and 52.6%, respectively. The results of this study suggest a possible application of allicin in treating C. albicans and S. aureus infection in DS.
Keywords: Allicin, Denture stomatitis, Candida albicans, Staphylococcus aureus, Biofilm
1. Introduction
Denture stomatitis (DS) or also known as chronic erythematous candidiasis, is a type of disease that mostly affects the palatal mucosa. (Akpan and Morgan, 2002, Aoun and Berberi, 2017). The DS is characterized by inflamed mucosa, burning sensation, and pain, particularly under the upper denture and alteration in taste sensation. The risk of developing DS in those who are wearing a denture is up to 36.7% (Emami et al., 2012) and increase up to 65% in those who wear complete dentures (Frenkel, 2000, Lemos et al., 2003). The predisposing factors are continuous denture wearing, dental trauma, decreased salivary flow, denture hygiene, type of denture base material, denture age, cellular immunity of the wearer, smoking, dietary factors and changes in oral microbiota (Coco et al., 2008, Gasparoto et al., 2009).
One of the etiological factors that trigger DS is the presence of biofilm on the surface of the denture. Denture biofilm composed of a complex mixture of pathogenic and opportunistic bacteria, fungi, desquamated epithelial cells and biofilm matrix (Glass et al., 2001). This biofilm matrix created a physical barrier and acted as a reservoir of protection for oral microorganisms like C. albicans that is considered to be the primary etiological agent in the pathogenesis of DS (Ramage et al., 2004). The C.albicans has an adhesive property towards the acrylic resin (Salerno et al., 2011). Furthermore, the anaerobic microenvironment between the denture and palatal mucosa further supplements the growth of Candida (Budtz-Jörgensen, 1990). The other significant colonization factors of Candida are due to coaggregation with pre-attached microorganisms on the denture and oral mucosa, such as S. aureus, which also known as bacterial-yeast interaction (Coco et al., 2008). S. aureus was usually isolated together with Candida from dental prostheses of individuals with DS (Monroy et al., 2005, Pereira et al., 2013). The C.albicans and S.aureus will not only colonize the surface that has contact with oral mucosa but also adhere to the cracks and imperfections of the denture material (Ramage et al., 2004). Ultimately, dentures may act as a pathogen reservoir and provide a source of continued mucosal exposure to microorganisms (Gendreau and Loewy, 2011).
The mutualistic C. albicans–S. aureus relationship was reported to alter phenotypic of the microorganisms, induce drug tolerance (Todd and Peters, 2019, Kean et al., 2017), and form a unique biofilm architecture (Peters et al., 2010), which enhance the disease severity. It was reported that the C. albicans-S.aureus biofilm displayed synergistic pathogenicity and also reduced the sensitivity to antifungal miconazole (Kean et al., 2017). The mechanism for the increased tolerance to the antibiotic was reported to cause by the upregulation of drug efflux pumps in S.aureus following sequential exposure to farnesol the quorum sensing (QS) molecule of C. albicans (Todd and Peters, 2019). Zago et al., (2015) also reported the enhanced secretion of hydrolytic enzymes, secreted aspartyl proteinase (SAP) in co-culture of C.albicans and S.aureus compared to the monospecies culture of C. albicans. SAP is one of the C. albicans' virulence factors that contribute to their proteolytic activity and play an essential role in host tissue adherence and colonization (Kumar et al., 2015).
DS has been recognized for long periods; however, no treatments have been suggested as the best treatment of choice due to the reported relapse cases (Martins and Gontijo, 2017). DS has a multifactorial aetiology; thus, treatments are done by elimination of predisposing factors. For DS caused by Candida infection, brushing, washing with commercial cleanser products and using disinfectants such as chlorhexidine and sodium hypochlorite are highly recommended for denture cleaning procedures. However, the efficacy of commercial denture cleansers in the removal of fungi is doubtful (Felton et al., 2011). Failure in ensuring denture hygiene contributes to DS recurrent case even following the treatment regimen. The specific antifungal agents such as amphotericin B, nystatin, miconazole or clotrimazole also used locally or systemically to cease candida from developing. (Walsh et al., 2015).
Nystatin is the most commonly prescribed antifungal agent for the treatment of oral candidiasis and DS (Tay et al., 2014) besides other antifungals such as miconazole and fluconazole. However, there have been some side effects related to the use of anti-fungal agents, such as nausea, vomiting, diarrhoea (Walsh et al., 2015), fungal resistance, drug toxicity (Tay et al., 2014, Lyu et al., 2016) and relapse cases (Martins and Gontijo, 2017). Meanwhile, CHX is the common antimicrobial agent that has been widely prescribed in dentistry as an antiseptic mouthwash (Ellepola and Samaranayake, 2000). CHX drug prescription is available in the dental clinic or can be purchased over-the-counter (OTC) at the pharmacies (Ingram et al., 2008). The formulation of mouth rinse, which consists of 0.2% chlorhexidine gluconate, was successfully used to treat Candida-associated denture stomatitis as well as acute pseudomembranous candidiasis. CHX with higher concentration which is 2%, is recommended for overnight denture disinfectant (Ellepola and Samaranayake, 2000).
Researchers are actively investigating the alternative therapeutic agents for management and treatment of DS derived from natural sources such as plant extract or phytocompounds. Consideration of the natural products to be investigated are; elicit the same therapeutic effects as commercial drugs, safe and fewer side effects to the patients (Ramage et al., 2004, Bakri and Douglas, 2005). Garlic (Allium sativum L.) is one of herbal medicine which is known to have medication benefits, such as antibacterial, anti-fungal, and antiviral properties (Bakri and Douglas, 2005). For this study we used, allicin a phytocompound derived from garlic, which was reported to exhibit prominent antifungal effects (Kim et al., 2012).
This study aimed to assess the synergistic/additive antimicrobial effect of allicin with nystatin on C. albicans and allicin with CHX on S. aureus. We also investigated the therapeutic efficacy of allicin in removing C. albicans, and S. aureus preformed biofilm on self-cured resin cubes within 5 min exposure to mimic the clinical application of using denture cleanser (express formula) as denture disinfectant.
2. Material and methods
2.1. Preparation for standard C. albicans and of S. aureus inoculum
The stock culture of C. albicans ATCC 14053 was inoculated initially into 30 mL of sterilized Yeast Peptone Dextrose (YPD) broth (Oxoid Ltd, Basingstoke, Hampshire, UK), accordingly, to form the C. albicans suspension. The concentration of the C. albicans in the study was adjusted to a final concentration of 0.5 McFarland (1.5 × 106 CFU/mL) before the experiment.
The S.aureus suspension was prepared by inoculating the stock culture of S. aureus ATCC 29213 into 30 mL of sterilised Brain Heart Infusion (BHI) broth (Oxoid Ltd, Basingstoke, Hampshire, UK). The concentration of the S. aureus in the study was adjusted to a final concentration of 0.5 McFarland (1.5 × 108 CFU/mL) before the experiment.
2.2. Preparation of allicin and other reagents
Allicin was purchased from AK Scientific, Inc (US). A stock solution of 2048 µg/ml of allicin was prepared in 5% dimethyl sulfoxide (DMSO) and stored at 4°C until further use. The 5-Min Express Denture Cleanser (Brand X) in the form of a tablet was purchased from the local pharmacy (OTC) and was freshly prepared according to manufacturer’s instruction on the packaging, 10 min before the test. The Brand X denture cleanser contained potassium monopersulfate as one of the active ingredients which also acts as a disinfectant. The CHX ≤ 99.5% and nystatin were purchased from Sigma-Aldrich and were diluted for the application in the experiment.
2.3. Minimum inhibitory concentration (MIC), minimum fungicidal concentration (MFC) and minimum bactericidal concentration (MBC)
The MIC, MBC, and MFC of allicin against C. albicans and S. aureus were determined using the broth microdilution method outlined by the Clinical and Laboratory Standard Institute (CLSI) with minor modifications (CLSI, 2008, CLSI, 2010).
2.4. Checkerboard microdilution assay
The synergism activity between allicin + nystatin and allicin + CHX was assessed using a checkerboard microdilution assay according to the method described elsewhere (Cuenca-Estrella, 2004, Haroun and Al-Kayali, 2016). The final concentration after the addition of 100 µL of inoculum in 96-well U-bottom microplates ranged between 0.03% and 1.92% for CHX, 2 µg/mL to 128 µg/mL for nystatin and 1 µg/mL to 128 µg/mL for allicin. The inoculum was prepared at a final concentration of 5 × 106 CFU/mL per well. The cells were incubated at 35°C for 24–48 h. The effects of the combinations of antimicrobial agents were interpreted by the fractional inhibitory concentration index (FICI) based on Loewe additivity (LA) theory as synergistic (FICI ≤ 0.5), additive or indifferent (0.5 < FICI ≤ 4.0) or antagonistic (FICI > 4.0).
2.5. Biofilm eradication assay
2.5.1. Fabrication of self-cured acrylic resin cubes
The self-curing (SC) acrylic resin cubes used in this study were also used by Lee et al. (2009). Ninety acrylic resin cubes (8 mm square by 2 mm thick) were prepared for biofilm eradication assay of C. albicans (n = 45) and S. aureus (n = 45). The SC acrylic resin cubes were prepared following the manufacturer’s recommendations ((PalaXpress auto polymerising acrylic resin, Heraeus Kulzer). The resins were allowed to polymerise in a pressure pot at temperature 55°C (131°F), pressure: 200 kPa for 30 min. After polymerization, the cubes were removed from the matrix mould and were polished using 150-, 300-, 600-, and 1200-grit sandpapers (3 M/ESPE, Sumaré, Brazil). The acrylic resin cubes were then stored in distilled water at 37°C for 48 h to remove excess monomer. After storage for 48 h, the quality of the polishing of acrylic resin cubes was checked by a profilometer (Ambios Technology) and specimens size were measured by digital calliper to standardize the specimens and to homogenize the groups. The cubes that had roughness between 2.7 and 3.7 μm were selected for the following tests (Panariello et al., 2015). Immediately before use in the experiment, cubes were sterilized using an ultraviolet light unit for 10 min.
2.5.2. Biofilm eradication assay
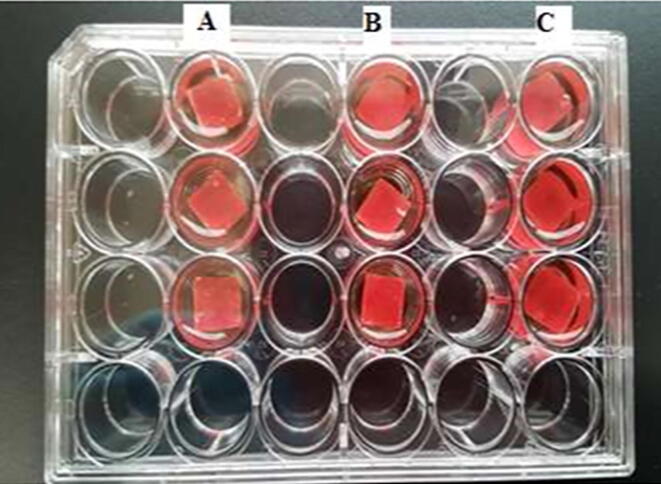
The subMIC concentration allicin (4 µg/mL) was used in the biofilm eradication assay. The cubes were randomly divided into three groups, and fifteen cubes were subjected to each group. For the formation of preformed biofilm, cubes were placed in 6-well flat-bottom plate and immersed with 6 mL of standard S. aureus suspension (0.5 McFarland) and incubated at 37°C for 24 h. After 24 h, using a sterile forceps, the cubes with preformed biofilm were taken out from the incubation well. Remaining broth and loosely attached bacteria were removed by gently tapping on a sterile absorbent paper in an empty well. The resin cubes were then placed in a new 24-well flat-bottom plate (one cube per well). 1 mL of allicin, denture cleanser (brand X) and sterile distilled water (control) were pipetted into the well according to the group, and the cubes were incubated for 5 min (Fig. 1). The solutions were then removed, and 1 mL of 0.1% crystal violet was pipetted into the wells and left for 15 min. After staining, the cubes were slowly immersed in distilled water to remove loosely-bound cells and placed on a paper towel to be air-dried for 15 min or more. Next, the stained cubes were transferred into a new 24 wells plate, and the bound dye was extracted from the stained cells using 1 mL of 95% ethanol. 700 µL of the de-staining solution was then transferred to the next well. By using the crystal violet (CV) assay, biofilm disruption was determined and quantified by measuring the absorbance of the solution at 550 nm in a microplate reader (Tecan′s Infinite ® 200 PRO).
Fig. 1.
Self-cured acrylic resin cubes (8 mm × 8 mm × 2 mm) with S. aureus biofilm were treated for 5 min in 24-well flat-bottom microplate containing 1 mL of sterile distilled water (A), 1 mL of 4 µg/mL allicin (B) and 1 mL of denture cleanser (Brand X) (C). Similar steps were repeated for C. albicans.
The percentage of biofilm eradication (on established biofilm) was calculated as:
The similar procedures were repeated for C. albicans. Each species was tested for biofilm eradication in triplicates, and the assay was repeated five times.
2.6. Statistical analysis
Statistical analysis was carried out using the Statistical Package for Social Sciences Software (SPSS version 20). The differences between groups were evaluated using Kruskal Wallis and Mann Whitney test. Data obtained was expressed as the mean ± standard deviation. The significance level was set up at p < 0.001.
3. Results
3.1. Minimum inhibitory concentration (MIC), minimum fungicidal concentration (MFC) and minimum bactericidal concentration (MBC)
The antimicrobial activity of allicin against C. albicans and S. aureus was shown in Table 1. The MIC or the lowest concentration of allicin, which prevents visible growth of C. albicans after 24 h incubation in microwell culture plates were 8 µg/mL. The MFC or lowest allicin dilutions causing approximately 99–99.5% killing activity of C. albicans was 16 µg/mL. The MIC and MBC of allicin against S. aureus were recorded at the same concentration, 8 µg/mL. Thus, the MIC/MBC ratio of allicin against S. aureus was calculated as 1.
Table 1.
The antimicrobial activities (MIC, MBC, and MFC) of allicin against S. aureus and Candida albicans.
| Organism | C. albicans ATCC 14053 | S. aureus ATCC 29213 | ||
|---|---|---|---|---|
| Measurement parameter | MIC | MFC | MIC | MBC |
| Antimicrobial concentration (µg/mL) | 8 | 16 | 8 | 8 |
3.2. Checkerboard microdilution assay
The synergism between allicin and nystatin against C. albicans, allicin, and CHX against S. aureus was assessed via checkerboard microdilution assay. The following standard classified the effect of the combinations of antimicrobial agents (1) FICI ≤ 0.5, synergistic effect; (2) 0.5 < FICI ≤ 4.0, additive or indifferent; and (3) FICI > 4.0, antagonistic. The results show that allicin and nystatin, in combination, expressed a synergistic activity against C. albicans with FICI = 0.5. (Table 2) The synergistic activity was also recorded between allicin and CHX against S. aureus with FICI = 0.375. (Table 2)
Table 2.
Antimicrobial activity of allicin, nystatin, and CHX and combination of allicin/nystatin against C. albicans and combination of allicin/CHX against S.aureus showing minimum inhibitory concentration (MIC), fractional inhibiting concentrations (FIC) and FIC index (FICI).
| Microorganism | Agent | The MIC of each agent (µg/mL) |
FIC | FICI | Outcome | |
|---|---|---|---|---|---|---|
| Alone | Combination | |||||
| S. aureus | CHX | 0.96 | 0.12 | 0.125 | 0.375 | Synergism |
| allicin | 8 | 2 | 0.25 | |||
| C.albicans | nystatin | 8 | 2 | 0.25 | 0.5 | Synergism |
| allicin | 32 | 8 | 0.25 | |||
3.3. Biofilm eradication assay
As for the biofilm eradication assay, the results are shown in Fig. 2. The percentage of C. albicans biofilm eradication by allicin (4 µg/mL) was 50.00 ± 1.45%, whereas for C. albicans biofilm treated with Brand X was 41.99 ± 1.69%. For S. aureus, the biofilm eradication following 5-minutes exposure to allicin and Brand X was 52.56 ± 0.9% and 44.46 ± 1.1%, respectively. Similar to the activity against C. albicans biofilm, allicin also significantly dispersed S. aureus biofilm on self-cured acrylic resin cubes compared to a positive control (brand X denture cleanser).
Fig. 2.
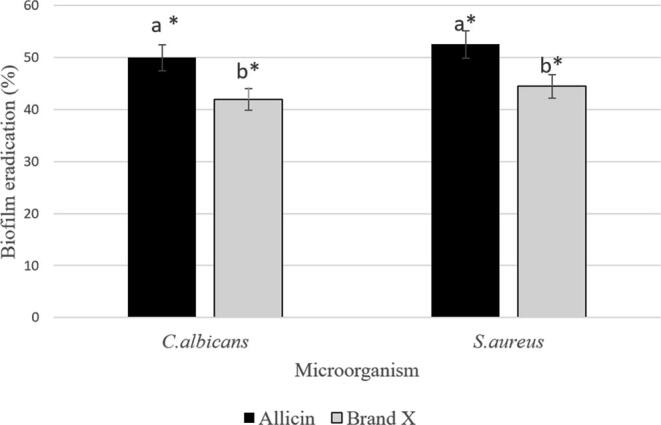
The efficacy of allicin to eradicate C. albicans and S. aureus biofilm at 4 µg/mL concentration (sub-MIC). The biofilm eradication percentage was denoted as the mean ± standard deviation (SD). An asterisk indicates significant differences in mean percentages were compared to the negative control (p < 0.001) according to the non-parametric Kruskal Wallis test with Mann Whitney. Different letters indicate statistically significant differences between groups.
4. Discussion
In this study, an approach was made to determine the therapeutics value of allicin, one of the phytocompounds in garlic (Allium sativum L.). The use of plant extracts as antimicrobial agents was widely reported; however, there is still limitations of studies using phytocompounds against C.albicans and S.aureus. Among the phytocompounds reported exhibiting antimicrobial activity against oral microorganisms were thymol (Belato et al., 2018), glabridin and licochalcone A (Messier and Grenier, 2011).
The antimicrobial and antibiofilm activities of allicin, against C. albicans and S. aureus biofilm, were initially evaluated by determination MIC + MFC and MIC + MBC values. The broth microdilution method is a quantitative miniaturization concept with lower cost, yet still, it provides reproducible results (Reller et al.,2009). MIC is the gold standard method to measure the lowest concentration of antimicrobial components to kill animals and human pathogenic bacteria (Andrews, 2001).
The results showed that allicin exhibited excellent antimicrobial activity against C. albicans and S. aureus with a MIC of 8 µg/mL for both C. albicans and S. aureus, MBC of 8 µg/mL for S. aureus and MFC of 16 µg/mL for C. albicans. MIC and MBC values obtained indicate the bactericidal property of allicin against S. aureus. This is supported by Tripathi (2013). Furthermore, our results are in agreement with a previous study done by Khodavandi et al. (2010). As for MFC, the values obtained in this study are consistent with a study conducted by Shadkchan et al., (2004), the antifungal ability of allicin against Aspergillus spp. A previous study also documented the antibacterial activity of allicin against other bacterial species such as Salmonella, Escherichia coli, Streptococcus spp., certain parasites, and fungi (Ankri and Mirelman, 1999). Allicin has a chemical property to react with free thiol groups, through the thiol-disulfide exchange. Therefore, the antimicrobial activity of allicin is thought to be mainly due to interaction with thiol-containing enzymes, including alcohol dehydrogenases and cysteine proteases. These enzymes are essential for the nutrition and metabolism of bacteria.
Allicin's antifungal activity was thought to be due to the ability of allicin to invade the cellular membrane of C. albicans and organelles. Under electron microscope observation, this penetration results in host cell destruction and death (Lee et al.,2011). Apart from the cell-killing effect towards C. albicans, Shadkchan et al. (2004) reported the significant (P < 0.001) antifungal ability of allicin, which caused ten times inhibition of Aspergillus fumigatus in infected mice.
A synergistic interaction was recorded between allicin + nystatin and allicin + CHX. Allicin’s synergistic effects with other antifungal agents were also reported in other studies such as azoles (Khodavandi et al.,2010) and amphotericin B (An et al.,2009).
In the present work, we have evaluated the performance of allicin to eradicate S. aureus, and C. albicans preformed biofilm in monospecies culture. Commercial denture cleanser (Brand X) was used as a positive control because it is easily purchased at the pharmacy and one of the common brands in the market. In this study, the self-cured resin was used because the attachment of Candida to self-cured acrylic resin was reported higher compared to heat-cured acrylic resin (Kalla et al.,2011)
The antibiofilm efficacy of allicin against C. albicans and S. aureus biofilm was confirmed because the compound had significantly (p < 0.001) dispersed >50% of the biofilm upon short exposure (5 min). Interestingly, in this study, from the post-hoc statistical analysis, we managed to demonstrate that biofilm eradication by allicin is significantly more effective than Brand X cleanser. The findings of another study also showed the ability of allicin to disperse biofilm and exhibited antibiofilm activity against E. coli (Barbieri et al.,2007). The concrete and exact modes of biofilm eradication by allicin are not yet understood. However, since allicin is a hydrophobic compound, it can easily penetrate the structure of exopolysaccharides (EPS) in the biofilm and interact with microbial molecules (Miron et al., 2000).
Based on the results, we determined the antimicrobial and antibiofilm activity of allicin, which might have beneficial effects on DS alternative treatment. Due to antimicrobial activity, allicin has the potential to be used as a topical treatment on the infected mucosa surface. It can also be included as one of the active ingredients in the denture cleanser due to its ability to remove the biofilm following short exposure. Further tests are required to explore the anti-biofilm property of allicin by employing a multi-species biofilm model and determination of toxicity dose against the oral mucosal cell. Future exploration could also include determination of safe dosage of allicin applicable for a clinical application through in vivo study.
5. Conclusions
In conclusion, our findings indicated the antimicrobial effects of allicin against S. aureus and C.albicans and the efficacy to disperse the preformed biofilms. Thus, their antimicrobial and antibiofilm activities could present them as a promising alternative for future clinical application of managing and treating oral candidiasis and S. aureus infection related to DS patients.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgments
Acknowledgements
This study was conducted in Research Laboratory, Faculty of Dentistry, UiTM Sungai Buloh, Selangor, Malaysia. We acknowledge all the research team members as the pioneer of the purposed work and backbone throughout the study. We would also like to extend the acknowledgement to the undergraduate students from the Faculty of Dentistry (Mohammad Rasydan Bin Mohammad Rafee, Mohamad Nur Syafiq Bin Abdul Latif, Nur Afiqah Binti Mohamad, and Nur Adibah Binti Zullkafle) who were involved with the project.
Funding
This work was funded by the Lestari Grant, Universiti Teknologi MARA (UiTM), Malaysia [600-IRMI/DANA 5/3/LESTARI (0068/2016)].
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mukarramah Zainal, Email: mukarramah@uitm.edu.my.
Nurhayati Mohamad Zain, Email: nurhayati8370@uitm.edu.my.
Indah Mohd Amin, Email: indahma@uitm.edu.my.
Vivi Noryati Ahmad, Email: vivi_noryati@uitm.edu.my.
References
- Akpan A., Morgan R. Oral candidiasis. Postgrad. Med. J. 2002;78(922):455–459. doi: 10.1136/pmj.78.922.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J.M. Determination of minimum inhibitory concentrations. J. Antimicro. Chemother. 2001;48(Suppl. S1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Ankri S., Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1(2):125–129. doi: 10.1016/S1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- An M.M., Shen H., Cao Y., Zhang J., Cai Y., Wang R., Jiang Y. Allicin enhances the oxidative damage effect of amphotericin B against Candida albicans. Int. J. Antimicrob. Agents. 2009;33(3):258–263. doi: 10.1016/j.ijantimicag.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Aoun G., Berberi A. Prevalence of chronic erythematous candidiasis in lebanese denture wearers: a clinico-microbiological study. Materia Socio-Medica. 2017;29(1):26–29. doi: 10.5455/msm.2017.29.26-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakri I.M., Douglas C.W. Inhibitory effect of garlic on oral bacteria. Arch. Oral Biol. 2005;50(7):645–651. doi: 10.1016/j.archoralbio.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Barbieri D.S.A.V., Vicente V.A., Fraiz F.C., Lavoranti O.J., Svidzinski T.I.E., Pinheiro R.L. Analysis of the in vitro adherence of Streptococcus mutans and Candida albicans. Brazilian J. Microbiol. 2007;38:624–631. doi: 10.1590/S1517-83822007000400009. [DOI] [Google Scholar]
- Belato K.K., de Oliveira J.R., de Oliveira F.S., de Oliveira L.D., Camargo S.E.A. Cytotoxicity and genotoxicity of thymol verified in murine macrophages (RAW 264.7) after antimicrobial analysis in Candida albicans, Staphylococcus aureus, and Streptococcus mutans. J. Funct. Foods. 2018;40:455–460. [Google Scholar]
- Budtz-Jörgensen E. Etiology, pathogenesis, therapy, and prophylaxis of oral yeast infections. Acta Odontol. Scand. 1990;48(1):61–69. doi: 10.3109/00016359009012735. [DOI] [PubMed] [Google Scholar]
- CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2010. Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement. CLSI document M100–S20. [Google Scholar]
- CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition. CLSI document M27–A3. [Google Scholar]
- Coco B.J., Bagg J., Cross L.J., Jose A., Cross J., Ramage G. Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral Microbiol. Immunol. 2008;23:377–383. doi: 10.1111/j.1399-302X.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- Cuenca-Estrella M. Combinations of antifungal agents in therapy. What value are they? J. Antimicrob. Chemother. 2004;54(5):854–869. doi: 10.1093/jac/dkh434. [DOI] [PubMed] [Google Scholar]
- Ellepola A.N., Samaranayake L.P. Antimycotic agents in oral candidosis: an overview: 1. Clinical variants. Dental Update. 2000;3:111–116. doi: 10.12968/denu.2000.27.3.111. [DOI] [PubMed] [Google Scholar]
- Emami E., Taraf H., de Grandmont P., Gauthier G., de Koninck L., Lamarche C., de Souza R.F. The association of denture stomatitis and partial removable dental prostheses: a systematic review. Int. J. Prosthodont. 2012;25(2):113–119. [PubMed] [Google Scholar]
- Felton D., Cooper L., Duqum I., Minsley G., Guckes A., Haug S., Meredith P., Solie C., Avery D., Deal Chandler N. Evidence-based guidelines for the care and maintenance of complete dentures: a publication of the American college of prosthodontists. J. Prostho.: Implant Esthetic Reconstruct. Dentist. 2011;20:S1–S12. doi: 10.1111/j.1532-849X.2010.00683.x. [DOI] [PubMed] [Google Scholar]
- Frenkel H. Oral health care among nursing home residents, Oral health care among nursing home residents in Avon. Gerodontology. 2000;17(1):33–38. doi: 10.1111/j.1741-2358.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- Gasparoto T.H., Dionísio T.J., de Oliveira C.E., Porto V.C., Gelani V., Santos C.F., Campanelli A.P., Lara V.S. Isolation of Candida dubliniensis from denture wearers. J. Med. Microbiol. 2009;58:959–962. doi: 10.1099/jmm.0.008391-0. [DOI] [PubMed] [Google Scholar]
- Gendreau L., Loewy Z.G. Epidemiology and etiology of denture stomatitis. J. Prostho. 2011;20(4):251–260. doi: 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- Glass R.T., Bullard J.W., Hadley C.S., Mix E.W., Conrad R.S. Partial spectrum of microorganisms found in dentures and possible disease implications. J. Am. Osteopath. Associat. 2001;101:65–66. [PubMed] [Google Scholar]
- Haroun M.F., Al-Kayali R.S. Synergistic effect of Thymbra spicata L. extracts with antibiotics against multidrug-resistant Staphylococcus aureus and Klebsiella pneumoniae strains. Iranian J. Basic Med. Sci. 2016;19(11):1193–1200. [PMC free article] [PubMed] [Google Scholar]
- Ingram D.M., Bosse G.M., Baldwin R. Ingestion of a denture cleanser: did it cause gastric perforation? J. Med. Toxicol. 2008;4(1):21–24. doi: 10.1007/BF03160946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla R., Rao H., Kumar S. Surface adherence of Candida Albicans to different polymethylmethacrylate denture base resins: an in-vitro-study. Int. J. Prosthet. Dent. 2011;2(1):2–7. [Google Scholar]
- Kean R., Rajendran R., Haggarty J., Townsend E.M., Short B., Burgess K.E., Lang S., Millington O., Mackay W.G., Williams C., Ramage G. Candida albicans mycofilms support Staphylococcus aureus colonization and enhances miconazole resistance in dual-species interactions. Front. Microbiol. 2017;8:258. doi: 10.3389/fmicb.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodavandi A., Alizadeh F., Aala F., Sekawi Z., Chong P.P. In vitro investigation of antifungal activity of allicin alone and in combination with azoles against candida species. Mycopathologia. 2010;287–295 doi: 10.1007/s11046-009-9251-3. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Kim K.S., Han I., Kim M.H., Jung M.H., Park H.K. Quantitative and qualitative analysis of the antifungal activity of allicin alone and in combination with antifungal drugs. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Saraswat D., Tati S., Edgerton M. Novel aggregation properties of Candida albicans secreted aspartyl proteinase Sap6 mediate virulence in oral candidiasis. Infect. Immun. 2015;83(7):2614–2626. doi: 10.1128/IAI.00282-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.E., Li C.Y., Chang H.W., Yang Y.H., Wu J.H. Effects of different denture cleaning methods to remove Candida albicans from acrylic resin denture-based material. J. Dent. Sci. 2011;6(4):216–220. doi: 10.1016/j. [DOI] [Google Scholar]
- Lee D., Howlett J., Pratten J., Mordan N., McDonald A., Wilson M., Ready D. Susceptibility of MRSA biofilms to denture-cleansing agents. FEMS Microbiol. Lett. 2009;291(2):241–246. doi: 10.1111/j.1574-6968.2008.01463.x. [DOI] [PubMed] [Google Scholar]
- Lemos M.M.C., Miranda J.L., Souza M.S.G.S. Clinic, microbiologic and histophato-logic study of the denture stomatitis. Revista Brasileira De Patologia Oral. 2003;2(1):3–10. [Google Scholar]
- Lyu X., Zhao C., Yan Z.M., Hua H. Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Des. Develop. Therapy. 2016;10:1161–1171. doi: 10.2147/DDDT.S100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins K.V., Gontijo S.M.D.L. Treatment of denture stomatitis: literature review. Revista Brasileira De Odontologia. 2017;74(3):215–220. [Google Scholar]
- Messier C., Grenier D. Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans. Mycoses. 2011;54(6):e801–e806. doi: 10.1111/j.1439-0507.2011.02028.x. [DOI] [PubMed] [Google Scholar]
- Miron T., Rabinkov A., Mirelman D., Wilchek M., Weiner L. The mode of action of allicin: its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta. 2000;1463:20–30. doi: 10.1016/S0005-2736(99)00174-1. [DOI] [PubMed] [Google Scholar]
- Monroy T.B., Maldonado V.M., Martínez F.F., Barrios B.A., Quindós G., Vargas L.O.S. Candida albicans, staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med. Oral Patol. Oral y Cirugia. 2005;10:27–39. [PubMed] [Google Scholar]
- Panariello B.H., Izumida F.E., Moffa E.B., Pavarina A.C., Jorge J.H., Giampaolo E.T. Effects of short-term immersion and brushing with different denture cleansers on the roughness, hardness, and color of two types of acrylic resin. Am. J. Dent. 2015;28(3):150–156. [PubMed] [Google Scholar]
- Pereira C.A., Toledo B.C., Santos C.T., Pereira Costa A.C., Back-Brito G.N., Kaminagakura E., Jorge A.O. Opportunistic microorganisms in individuals with lesions of denture stomatitis. Diagn. Microbiol. Infect. Dis. 2013;76(4):419–424. doi: 10.1016/j.diagmicrobio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Peters B.M., Jabra-Rizk M.A., Scheper M.A., Leid J.G., Costerton J.W., Shirtliff M.E. Microbial interactions and differential protein expression in Staphylococcus aureus–Candida albicans dual-species biofilms. FEMS Immunol. Med. Microbiol. 2010;59(3):493–503. doi: 10.1111/j.1574-695X.2010.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G., Tomsett K., Wickes B.L., López-Ribot J.L., Redding S.W. Denture stomatitis, a role for Candida biofilms., oral surgery oral medicine oral pathology oral radiology. Endodontology. 2004;98(1):53–59. doi: 10.1016/S1079210404002872. [DOI] [PubMed] [Google Scholar]
- Reller L.B., Weinstein M., Jorgensen J.H., Ferraro M.J. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin. Infect. Dis. 2009;49(11):1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- Salerno C., Pascale M., Contaldo M., Esposito V., Busciolano M., Milillo L., Guida A., Petruzzi M., Serpico R. Candida-associated denture stomatitis. Med. Oral Patol. Oral y Cirugía Bucal. 2011;16(2):139–143. doi: 10.4317/medoral.16.e139. [DOI] [PubMed] [Google Scholar]
- Shadkchan Y., Shemesh E., Mirelman D., Miron T., Rabinkov A., Wilchek M., Osherov N. Efficacy of allicin, the reactive molecule of garlic, in inhibiting Aspergillus spp. in vitro, and in a murine model of disseminated aspergillosis. J. Antimicrob. Chemother. 2004;53(5):832–836. doi: 10.1093/jac/dkh174. [DOI] [PubMed] [Google Scholar]
- Tay L.Y., Jorge J.H., Herrera D.R., Campanha N.H., Gomes B.P., dos Santos F.A. Evaluation of different treatment methods against denture stomatitis: a randomized clinical study. Oral Medicine Oral Pathology Oral Radiol. 2014;118(1):72–77. doi: 10.1016/j.oooo.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Todd O.A., Peters B.M. Candida albicans and Staphylococcus aureus Pathogenicity and polymicrobial interactions: lessons beyond koch’s postulates. J. Fungi. 2019;5(3):81. doi: 10.3390/jof5030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi K.D. seven ed. India, Jaypee Brothers Medical Publishers; New Delhi: 2013. Essentials of Medical Pharmacology; pp. 696–697. [Google Scholar]
- Walsh T., Riley P., Veitz-Keenan A. Interventions for managing denture stomatitis. Cochrane Datab. System. Rev. 2015 [Google Scholar]
- Zago C.E., Silva S., Sanitá P.V., Barbugli P.A., Dias C.M.I., Lordello V.B., Vergani C.E. Dynamics of biofilm formation and the interaction between Candida albicans and methicillin-susceptible (MSSA) and-resistant Staphylococcus aureus (MRSA) PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0123206. [DOI] [PMC free article] [PubMed] [Google Scholar]