Abstract
Introduction
The aim of our study was to evaluate whether a biopsy from the tumor base after transurethral resection of bladder tumor (TURBT) has an impact on subsequent management of patients with bladder tumors. While tumor base biopsy at the completion of TURBT is a common practice, there is no definition of its role within the major international professional guidelines.
Material and methods
We retrospectively reviewed the records of consecutive patients undergoing TURBT between 2015 and 2019 at our institution. We recorded demographic and tumor characteristics of initial TURBT, tumor base biopsy and restaging TURBT pathology outcomes. The pathologic outcomes were correlated to assess the additional value of a separate tumor base biopsy.
Results
A total of 532 patients underwent TURBT. A separate tumor base biopsy after completion of TURBT was performed in 154 patients. The mean patient's age was 72.8 ±11.7 years (range 48–94) and 119 (77.2%) were men. In 40 patients (25.9%) muscle was absent in the pathological specimen of the tumor resection. Muscle was present in all but 6 (3.9%) tumor base biopsies. Of the 33 patients who underwent repeated transurethral resection for pT1 tumors, 2 had residual low-grade pTa, 1 had residual high-grade pT1, and 3 patients were upstaged to pT2.
Conclusions
Although tumor base biopsy at the completion of TURBT is a common practice, our analysis fails to demonstrate any tangible benefit in the staging of bladder tumors. In our experience tumor base biopsy did not change the management in patients with superficial or muscle invasive disease.
Keywords: bladder cancer, biopsy, transurethral resection, staging, evaluation study, tumor base
INTRODUCTION
Worldwide, bladder cancer (BCa) is the ninth most frequently diagnosed cancer [1]. Transurethral resection of bladder tumor (TURBT) is the initial diagnosis and treatment for all macroscopic bladder lesions. Approximately 75% of newly diagnosed BCa present with a non-invasive disease confined to the bladder mucosa (stage pTa, carcinoma in situ [CIS]) or submucosa (stage pT1) [2]. Clinical staging including histological type and grade of the tumor, as well as the presence, depth, and type of the tumor invasion are crucial for the subsequent management and prognosis.
Accurate initial staging is paramount. Although TURBT is a well-established procedure, incomplete resection remains a significant concern [3]. The risk of residual disease after TURBT has been reported to be as high as 33–55% in disease that is confined to submucosa (pT1) [4–7]. Moreover, the degree of tumor invasiveness may be underestimating on initial resection, with probability of finding invasive disease on repeated resection reaching 25%, and as high as 45% in cases whereby initial resection did not contain muscle tissue in the pathological specimen [8]. There is significant evidence in the literature to support repeated transurethral resection (re-TURBT) for high-risk non-muscle-invasive bladder cancer in order to limit rates of residual disease as well as the risk of under staging [9–12].
At the completion of TURBT, an additional separate warm or a cold-cup biopsy from the tumor base has been proposed in order to determine the presence of muscle invasiveness [13]. This approach of a separate tumor base biopsy is, indeed, a common practice [14, 15]. However, the national and international guidelines do not discuss the role of tumor base biopsy [16]. The primary endpoint of this study was to evaluate whether a biopsy from the tumor base after resection has an impact on subsequent decision-making and management of patients with BCa.
MATERIAL AND METHODS
Data were obtained retrospectively from a prospectively collected institutional registry of patients who underwent TURBT between January 2015 and March 2019. For this analysis, only patients whereby a separate biopsy was taken from the tumor base after initial resection were included. All patients underwent standard TURBT using the white-light cystoscopy and standard monopolar resection equipment. It is our standard practice to attempt to obtain detrusor muscle in all resections, regardless of tumor appearance.
Taking random biopsies (single, multiple, cold or warm) from the tumor base was done based on the subjective evaluation of the attending surgeon at the completion of TURBT. A general guiding principle was not to obtain tumor base biopsy cases of low-grade (LG) appearing tumors up to 1 cm in size. Conversely, in tumors over 1 cm in size or high-grade (HG) appearing on cystoscopy, a separate tumor base biopsy was generally obtained after visually complete tumor resection. In the events where the tumor was multifocal, a tumor base biopsy was performed from the largest or most aggressive-appearing lesion. All patients who were not suspected having an invasive disease during the surgery received a single post-operative installation of mitomycin C (40 mg/50 ml).
Re-TURBT was offered in the setting of superficial high-grade tumors, T1 of any grade and incomplete initial tumor resection. Re-TURBT was defined as a repeat resection which involved the site of the initial TURBT within 1–6 weeks from the previous resection. Patients in whom the initial TURBT was not visually complete, those with synchronous upper tract urothelial carcinoma or metastatic BCa at the time of surgery were excluded from the study.
Demographic and clinical data, including tumor characteristics, pathological features and subsequent treatments were collected. Specifically, pathology from the initial resection as well as tumor base were recorded separately. In addition, we recorded pathology in patients who underwent re-TURBT. Pathologic outcomes were analyzed to assess whether tumor base biopsy had an impact on management. This work was approved by our institutional ethics committee.
Statistical analysis
Continuous variables are presented as a mean ± standard deviation (range) and categorical variables are expressed as the number (percent). Chi-square test and Fischer's exact test was used to assess whether the tumor base biopsy has an impact on subsequent management, and the relationship between single mitomycin C instillation to the presence of tumor on second resection. P value <0.05 was considered statistically significant. Analyses were performed using IBM SPSS statistics for Windows, Version 23.0. Armonk, NY: IBM CORP.
RESULTS
A total of 532 patients underwent TURBT and in 154 cases a separate biopsy was obtained from the tumor base after completion of tumor resection. The mean age of the patients was 72.8 ±11.7 years (range 48–94) and 119 (77.2%) were men. The tumor was multifocal in 66 (42.8%) patients. Demographic features and distribution of the histopathology from initial TURBT are summarized in Table 1. Overall, 52.5% were pTa, 29.9% were pT1 and 14.9% were pT2. In 8.6% of patients CIS of the bladder was present.
Table 1.
Demographic features and initial TURBT pathologies of the study group
| Variable | No. (%) |
|---|---|
| Total of patients included in analysis, N | 154 |
| Patient mean ±SD, age (y) | 72.8 ±11.7 |
| Gender Male Female |
119 (77.2) 35 (22.8) |
| Tumor multifocality Unifocal Multifocal |
88 (57.2) 66 (42.8) |
| pT stage T0 Tis Ta T1 T2 |
4 (2.5) 13 (8.6) 81 (52.5) 46 (29.9) 23 (14.9) |
| Grade (WHO 2004) Low High |
77 (51.3) 73 (48.7) |
TURBT – transurethral resection of bladder tumor; Tis – carcinoma in situ; SD – standard deviation
In 40 (25.9%) patients muscle was absent in the pathological specimen of initial tumor resection. In all but 6 (3.9%) tumor base biopsies, muscle was present. In these 6 cases final pathology showed pTa LG and pT1 HG in 5 and 1 patients, respectively. The latter underwent re-TURBT showing no residual malignancy.
Tumor base biopsies were negative for malignancy in 133 patients (86.3%), positive in 19 patients (12.3%), and non-diagnostic in 2 (1.2%). All positive tumor base biopsies were pT2 on initial resection, except in one patient where tumor resection did not contain muscle, and the tumor bed biopsy proved invasive bladder disease. Table 2 shows the distribution of tumor base biopsies based on tumor resection pathology.
Table 2.
Distribution of tumor base biopsies based on tumor resection pathology
| Final pathology | DM presence in initial resection (%) | DM presence in tumor base (%) |
|---|---|---|
| pTa LG | 47/75 (62.6%) | 70/75 (93.3%) |
| pTa HG | 5/6 (83%) | 6/6 (100%) |
| pT1 LG | 1/2 (50%) | 2/2 (100%) |
| pT1 HG | 35/44 (79.5%) | 43/44 (97.7%) |
| pT2 HG | 22/23 (95.6%) | 23/23 (100 %) |
DM – detrusor muscle; HG – high-grade; LG – low-grade
Out of 77 patients who were diagnosed with LG disease limited to the mucosal layer of urothelium, 38 (49.3%) and 39 (50.7%) were low and intermediate risk groups, respectively. In 29 (37.6%) of these patients, muscle was absent from the pathological specimen. These patients with non-muscle invasive BCa were managed with surveillance, with or without intravesical chemotherapy or immunotherapy based on risk stratification. Tumor base biopsy was negative in these patients and did not appear to contribute to staging or management decisions (p = 0.431).
Overall re-TURBT was performed in 35 (73%) patients with pTa/T1 HG pathology and in 2 patients who were diagnosed with muscle invasive disease (pT2) as a part of the bladder sparing management. Out of 6 patients with pTa HG, 4 patents refused re-TURBT and were referred for intravesical treatment, in 2 who underwent re-TURBT no residual disease was found.
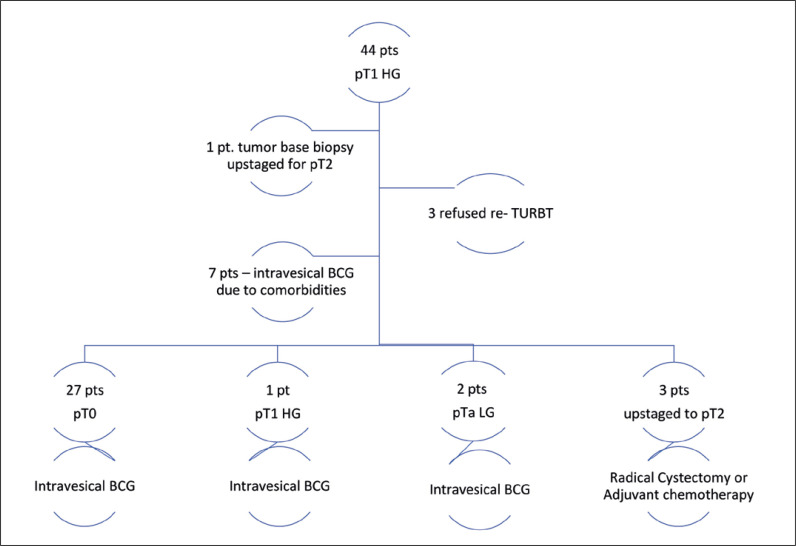
In patients with pT1 HG group, 3 refused re-TURBT and 7 were considered elevated surgical risk due to comorbidities and were referred directly to intravesical therapy. In 7 and 1 patients where detrusor muscle was absent at initial TURBT, tumor base biopsy was negative, and pathology on re-TURBT was pT0 and residual pTa LG, respectively. Detailed pathology results of initial resection, tumor base, and when available re-TURBT, are summarized in Figure 1. In patients with pTa/T1 HG, tumor base biopsy results did not provide additional staging benefit or contribute to management decisions with the exception of one patient (0.6%) whereby tumor bed biopsy upstaged to pT2 (p = 0.632).
Figure 1.
Initial resection, tumor base, and the restage transurethral resection of bladder (TURBT) for pT1 high-grade.
TURBT – transurethral resection of bladder tumor; DM – detrusor muscle; HG – high-grade; LG – low-grade; BCG – Bacillus Calmette-Guérin; pt – patient
Overall, 106 (68.8%) patients received mitomycin C following primary tumor resection. The proportion of patients in the HG group who received mitomycin C and undergone re-TURBT was 63.3%. Post-operative immediate single mitomycin C installation was not associated with pT0 status on re-TURBT (p = 0.582).
DISCUSSION
Complete TURBT is the initial step in the management of bladder cancer. The aim of TURBT is two-fold, both therapeutic (potential curative intent) and diagnostic (accurate staging) [17]. While tumor base biopsy is a common practice and is supported the Campbell-Walsh textbook of Urology [13], the role of this practice is not defined by the major professional guidelines and the impact of tumor base biopsy on management is largely unknown. In addition, there is no consensus on the appropriate technique or indication for a separate tumor base biopsy.
Most reports on the management of bladder cancer with TURBT lack a description of a standardized method on how the procedure should be performed, nor is there a clear indication on the value of tumoral base biopsy/template in order to achieve a more precise staging which ultimately leads to treatment strategy. In a study on pathologic staging, Kurth et al. [18] reported that the most important risk factor for pT1 tumor understating was the absence of detrusor muscle in the specimen obtained by TURBT, reinforcing the importance of a well-performed initial resection.
Mariappan et al. [19] demonstrated the absence of detrusor muscle in the specimen to be associated with a significant higher risk of residual tumor, early recurrence and tumor under staging. Residual tumor rate was 44.4% and 21.7%, when detrusor muscle was absent and present, respectively. Furthermore, detrusor muscle presence in the initial TURBT, emphasized by Czech et al. [20] to be an independent prognostic factor for the outcome of the second resection, and was associated with 3 times higher odds of pT0. Our study demonstrates 11.1% residual tumor rate upon re-TURBT; in all these patients initial tumor resection contained muscle.
In our study 74% had muscularis propria at the tumor resection, where tumor base biopsy was negative for 96.1%. Tumor base biopsy was positive for invasive disease in 19 patients, 18 and 1 patients had pT2 and pT1 HG, respectively. Therefore, in our experience, in pT2 disease, tumor base biopsy did not provide additional diagnostic benefit.
All pTa/T1 HG were offered re-TURBT. In these cases specifically, a separate tumor base biopsy did not change management. Three patient with pT1 HG who underwent re-TURBT were upstaged to pT2, in these patients the initial tumor resection contained muscularis propria, which was free of tumor and negative tumor base biopsies were negative for tumor.
For patients who underwent re-TURBT, immediate post-operative mitomycin C installation was not a predictive factor associated with pT0 status in our data set. Our study reflects real life situations and our results underscore ablative effect of intravesical chemotherapy in pT1 tumors.
Tumor base biopsy after a complete TURBT could theoretically increase the risk of bladder perforation; the incidence of this remains unknown. For instance, Balbay et al. [21] found extraperitoneal leaks on cystography in 58.3% of otherwise asymptomatic patients following TURBT. This suggests that the practice of tumor base biopsies may increase the morbidity of TURBT.
We would submit that there is a need to establish proper nomenclature, define the technique and inquire into the potential benefits for tumor base sampling, biopsy or resection. In our opinion, the not well-defined random sampling of the crater was not beneficial to clinical decision-making. Moreover, the pathologic concordance between tumor specimen and tumor base were excellent thus questioning whether a separate specimen of tumor base may assist in a more accurate pathological assessment of the tumor.
Because of its retrospective nature, this study has certain limitations, such as single institution design, small sample and when a tumor bed biopsy was taken at the discretion of the surgeon without standardized protocol. At our institution, tumor base biopsy was omitted in small tumors with low grade appearance.
In our study, tumor base biopsy was concordant with primary resection specimen in most cases without the added value for staging and prognosis. In the three cases that were upstaged to pT2 disease on re-TURBT, tumor base biopsy was negative and failed to capture the muscle invasive component. A prospective study is required to better determine the value of tumoral base biopsy after a complete transurethral resection.
CONCLUSIONS
Although tumor base biopsy at the completion of transurethral resection of bladder tumor (TURBT) is a common practice, our analysis fails to demonstrate any tangible benefit in the staging of bladder tumors. In our experience a separate random tumor base biopsy after resection did not appear to contribute additional information to staging or aid in management decision-making in patients with superficial (Ta, T1) or muscle invasive disease. While complete TURBT remains the mainstay of diagnosis and management of bladder tumors, tumor base biopsy appears to have limited utility.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Brausi M, Collette L, Kurth K, van der Meijden AP, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol. 2002;41:523–531. doi: 10.1016/s0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 4.Divrik RT, Yildirim U, Zorlu F, Ozen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycIn: a prospective, randomized clinical trial. J Urol. 2006;175:1641–1644. doi: 10.1016/S0022-5347(05)01002-5. [DOI] [PubMed] [Google Scholar]
- 5.Grimm MO, Steinhoff C, Simon X, Spiegelhalder P, Ackermann R, Vogeli TA. Effect of routine repeat transurethral resection for superficial bladder cancer: a long-term observational study. J Urol. 2003;170(2 Pt 1):433–437. doi: 10.1097/01.ju.0000070437.14275.e0. [DOI] [PubMed] [Google Scholar]
- 6.Lazica DA, Roth S, Brandt AS, Bottcher S, Mathers MJ, Ubrig B. Second transurethral resection after Ta high-grade bladder tumor: a 4.5-year period at a single university center. Urol Int. 2014;92:131–135. doi: 10.1159/000353089. [DOI] [PubMed] [Google Scholar]
- 7.Nieder AM, Brausi M, Lamm D, et al. Management of stage T1 tumors of the bladder: International Consensus Panel. Urology. 2005;66(6 Suppl 1):108–125. doi: 10.1016/j.urology.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 8.Dalbagni G, Vora K, Kaag M, et al. Clinical outcome in a contemporary series of restaged patients with clinical T1 bladder cancer. Eur Urol. 2009;56:903–910. doi: 10.1016/j.eururo.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miladi M, Peyromaure M, Zerbib M, Saighi D, Debre B. The value of a second transurethral resection in evaluating patients with bladder tumours. Eur Urol. 2003;43:241–245. doi: 10.1016/s0302-2838(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 10.Schwaibold HE, Sivalingam S, May F, Hartung R. The value of a second transurethral resection for T1 bladder cancer. BJU Int. 2006;97:1199–1201. doi: 10.1111/j.1464-410X.2006.06144.x. [DOI] [PubMed] [Google Scholar]
- 11.Herr HW, Donat SM, Dalbagni G. Can restaging transurethral resection of T1 bladder cancer select patients for immediate cystectomy? J Urol. 2007;177:75–79. doi: 10.1016/j.juro.2006.08.070. [DOI] [PubMed] [Google Scholar]
- 12.Dangi AD, Kumar RM, Kodiatte TA, et al. Is there a role for second transurethral resection in pTa high-grade urothelial bladder cancer? Cent European J Urol. 2018;71:287–294. doi: 10.5173/ceju.2018.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson C, Weber R, Patel D, et al. A 10-Item Checklist Improves Reporting of Critical Procedural Elements during Transurethral Resection of Bladder Tumor. J Urol. 2016;196:1014–1020. doi: 10.1016/j.juro.2016.03.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zabell JMD, Konety BRMDMBA. In: Management Strategies for Non-Muscle-Invasive Bladder Cancer (Ta, T1, and CIS) Partin AWMDP, Dmochowski RRMDMF, Kavoussi LRMDMBA, Peters CAMD, editors. Campbell-Walsh Urology; 2021. pp. 3091–3111.e3098. [Google Scholar]
- 15.Babjuk M, Burger M, Compérat EM, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Power NE, Izawa J. Comparison of Guidelines on Non-Muscle Invasive Bladder Cancer (EAU, CUA, AUA, NCCN, NICE) Bladder Cancer. 2016;2:27–36. doi: 10.3233/BLC-150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richterstetter M, Wullich B, Amann K, et al. The value of extended transurethral resection of bladder tumour (TURBT) in the treatment of bladder cancer. BJU Int. 2012;110(2 Pt 2):E76–79. doi: 10.1111/j.1464-410X.2011.10904.x. [DOI] [PubMed] [Google Scholar]
- 18.Kurth KH, Bouffioux C, Sylvester R, van der Meijden AP, Oosterlinck W, Brausi M. Treatment of superficial bladder tumors: achievements and needs. The EORTC Genitourinary Group. Eur Urol. 2000;37(Suppl 3):1–9. doi: 10.1159/000052386. [DOI] [PubMed] [Google Scholar]
- 19.Mariappan P, Zachou A, Grigor KM. Detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience. Eur Urol. 2010;57:843–849. doi: 10.1016/j.eururo.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 20.Czech AK, Gronostaj K, Frydrych J, et al. Identification of potential prognostic factors for absence of residual disease in the second resection of T1 bladder cancer. Cent European J Urol. 2019;72:252–257. doi: 10.5173/ceju.2019.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balbay MD, Cimentepe E, Unsal A, Bayrak O, Koc A, Akbulut Z. The actual incidence of bladder perforation following transurethral bladder surgery. J Urol. 2005;174:2260–2262. doi: 10.1097/01.ju.0000181811.61199.35. [DOI] [PubMed] [Google Scholar]